Multiple Melting Temperatures in Glass-Forming Melts
Abstract
1. Introduction
2. The Homogenous Nucleation of Two Glassy States
2.1. The Bulk Glassy State Below Tg
2.2. The Glassy State of Phase 3 up to the Melting Temperature Tn+ above Tm
3. Reduction of the Crystallization Enthalpy at the Crystallization Temperature Tx
4. Gleaning Information in Published Works to Predict the Crystallized Fraction from the Knowledge of Tx or Melting Temperatures of Various Phases 3 above Tm
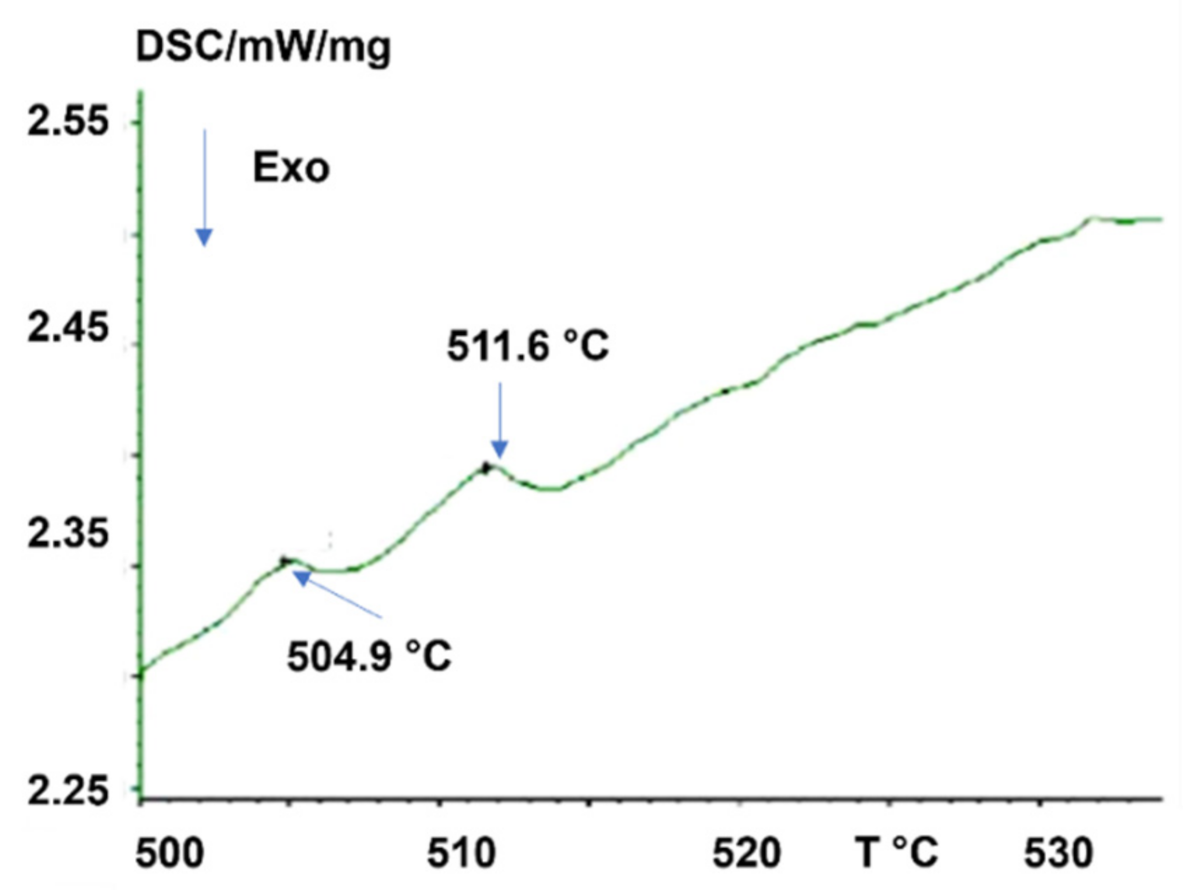
4.1. Three Melting Temperatures of Bismuth Were Observed above Tm in Two Different Experiments
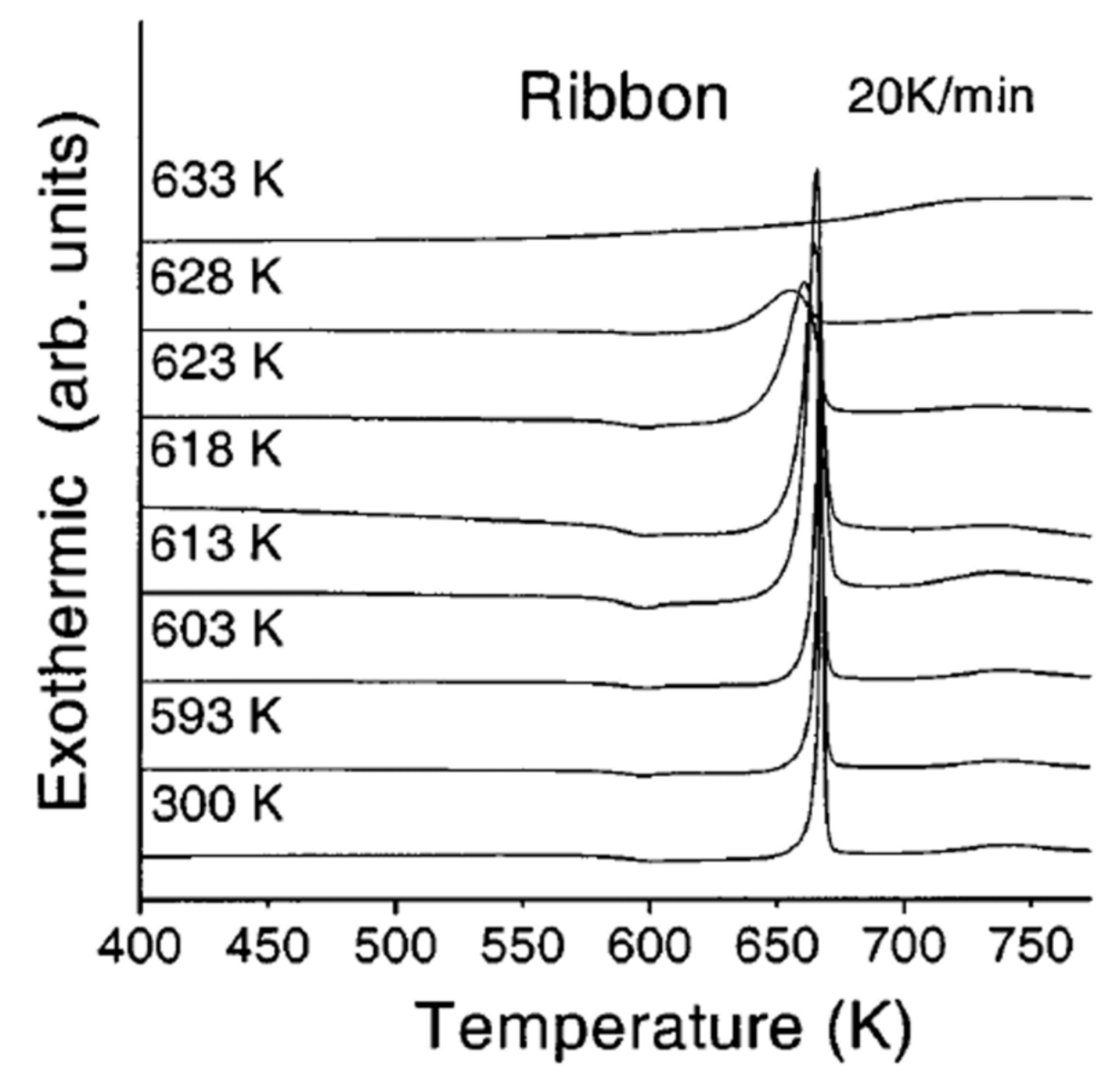
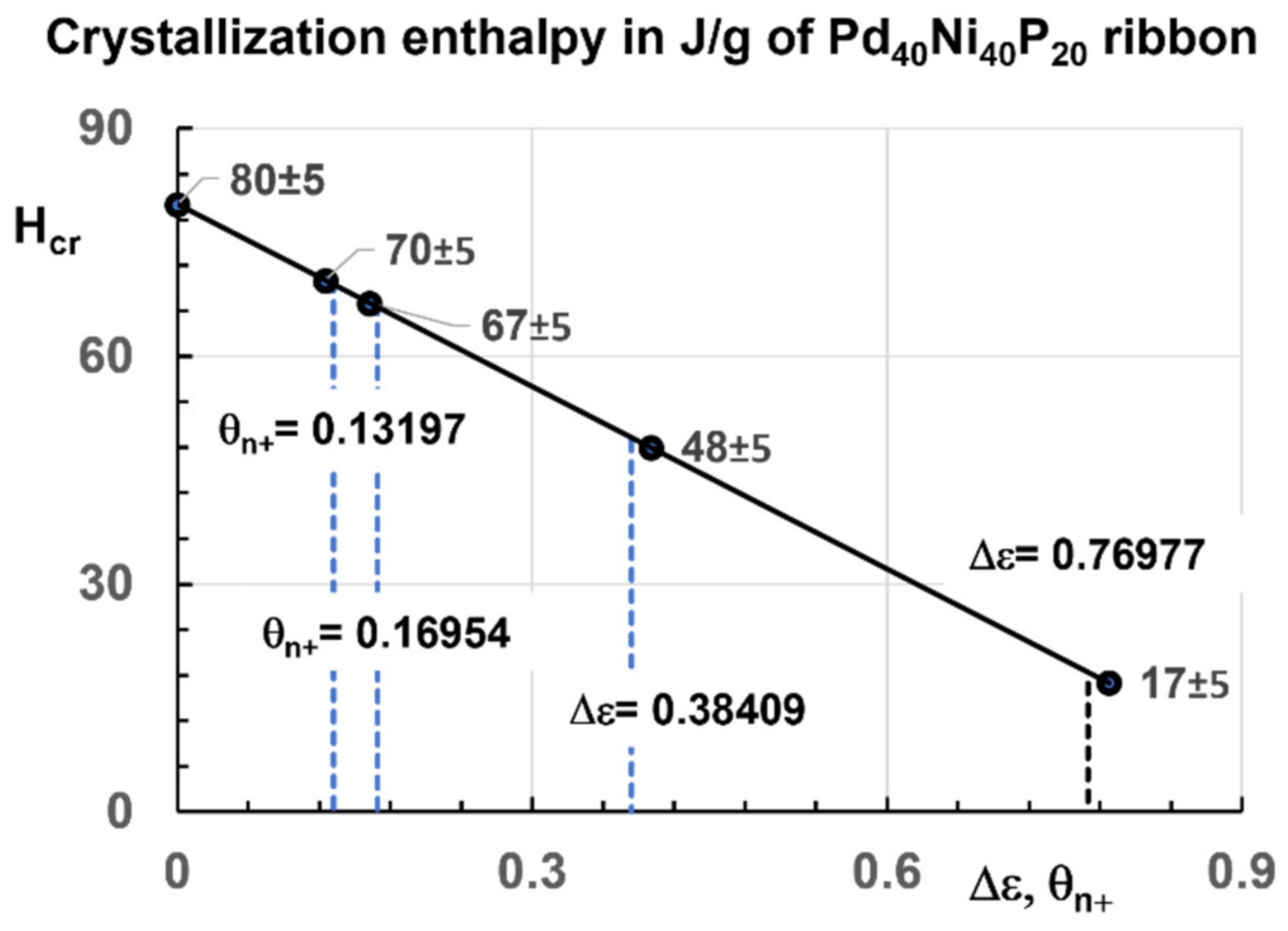
4.2. Crystallization Enthalpy Reduction of Pd40Ni40P20 at the Temperature Tx after Quenching and Various Annealing of the Same Duration at Lower Temperatures
4.3. Melting Temperatures of Co76Sn24 above Tm = 1392 K
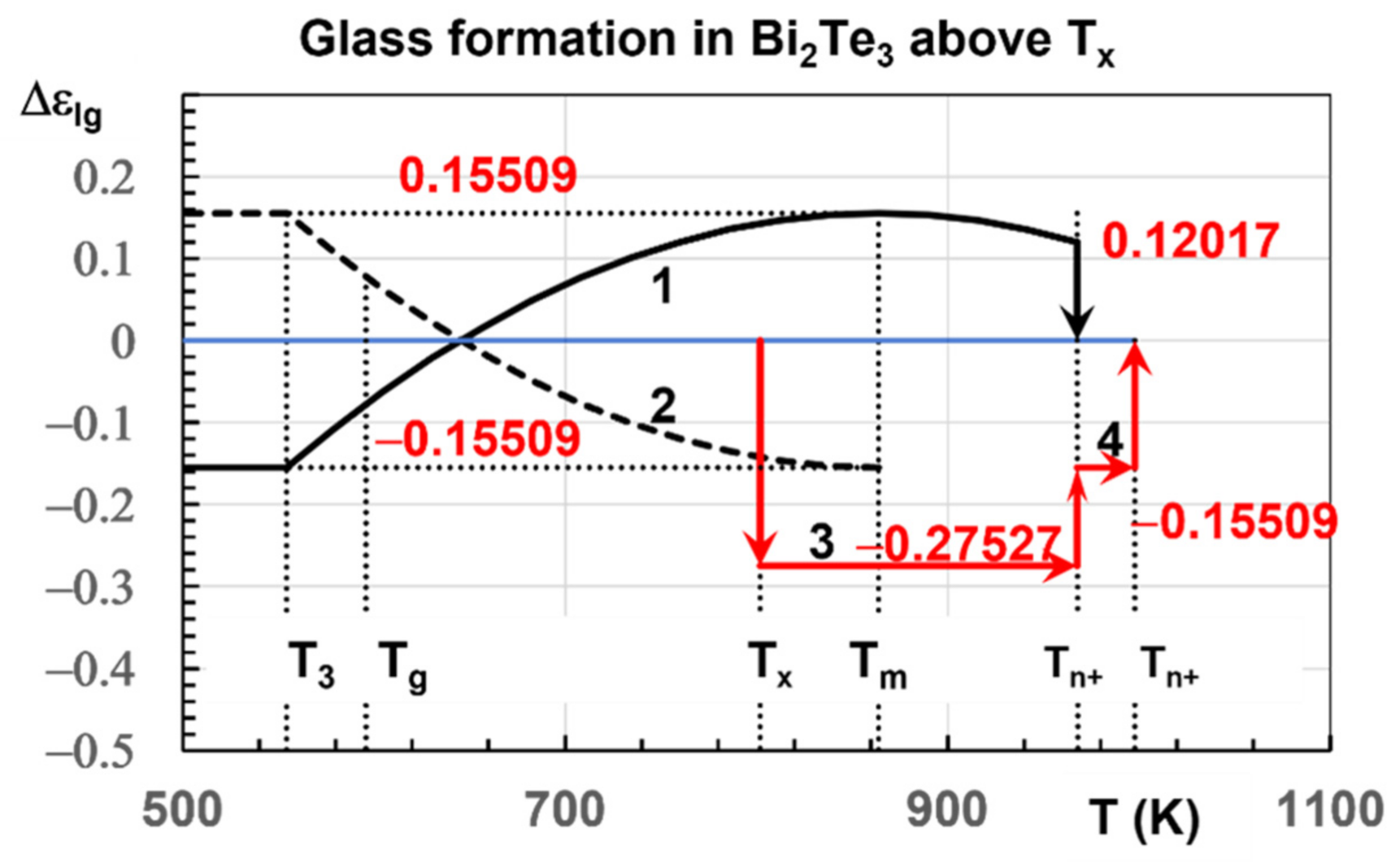
4.4. Melting Temperatures of Bi2Te3 and Its Alloys above Tm = 864 K
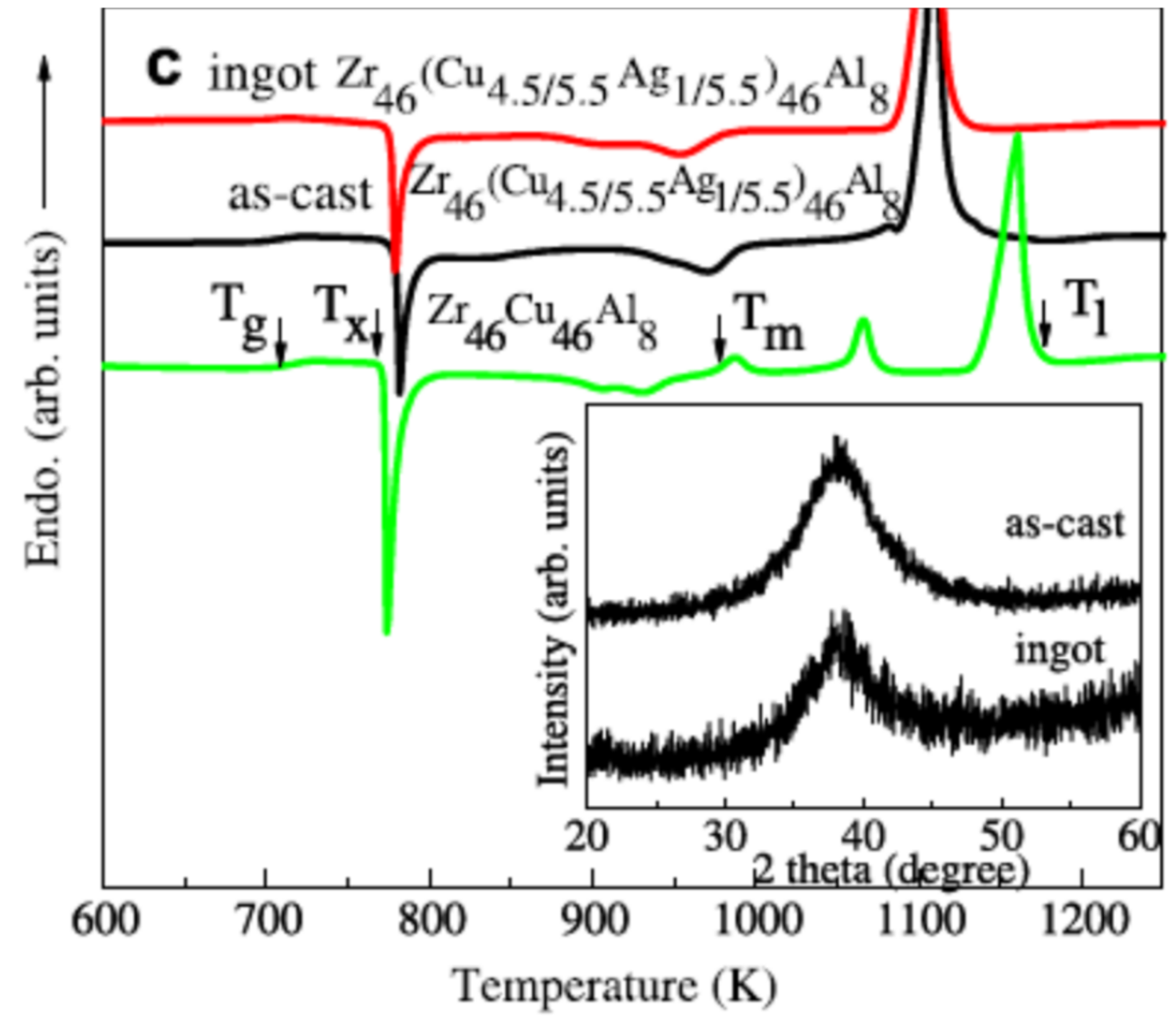
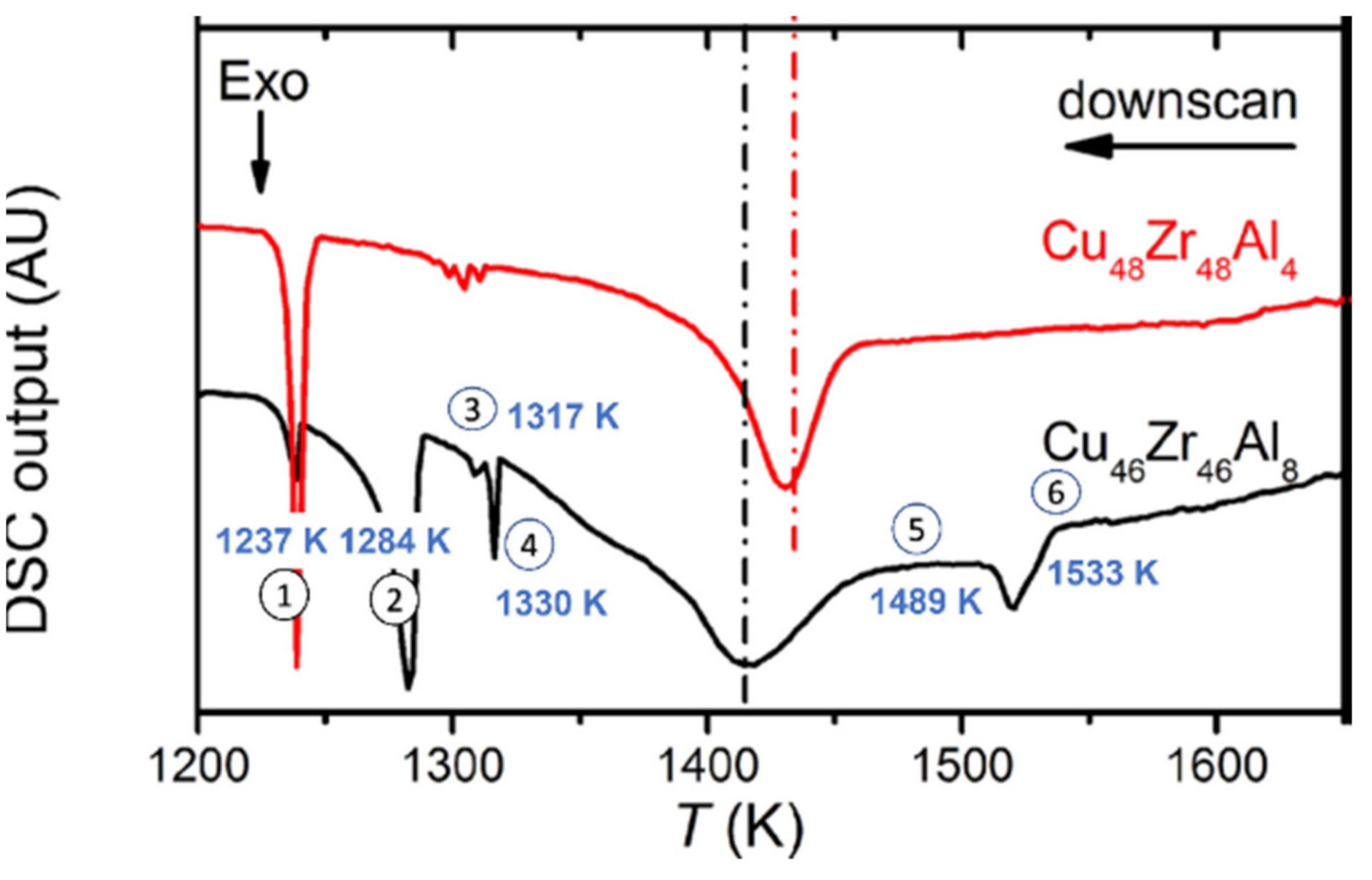
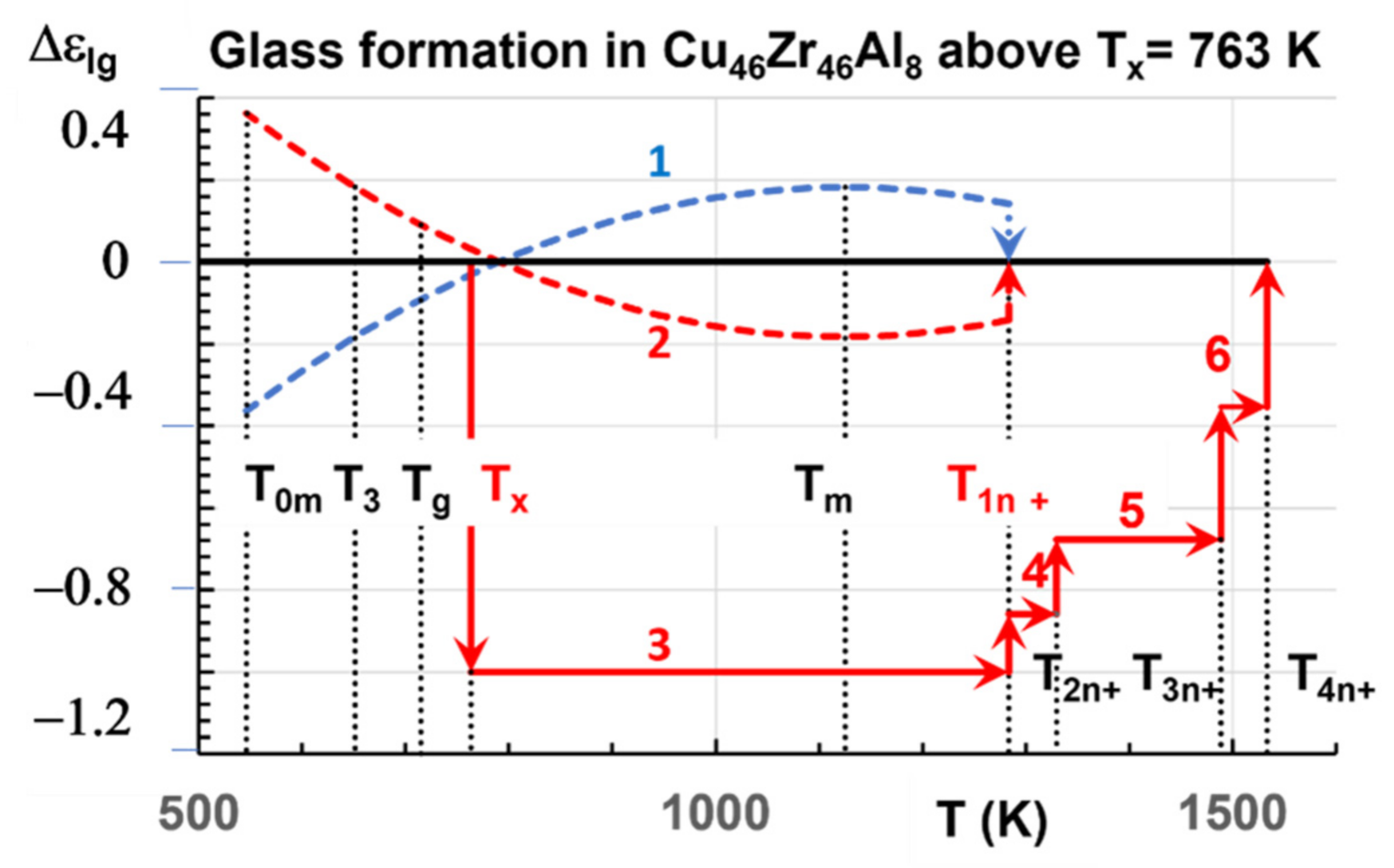
4.5. The Six Melting Temperatures of Zr46Cu46Al8 above 1200 K
5. The Case of a Few Polymers
5.1. Polyvinyle Acetate (C4H6O2)n
5.2. Polymer Called 1,2-Propadeniol
5.3. Poly(ethylene terephthalate)
5.4. Bisphenol-A Polycarbonate (PC) and Poly(3-hydroxybutyrate) (PHB)
5.5. Interaction between Globular Proteins and Proteins Solutions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, P.H.; Grande, T.; Sciorfino, F.; Angel, C.A. Amorphous polymorphism. Comput. Mater. Sci. 1995, 4, 373–382. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Popova, S.V.; Voloshin, R.N. High pressure transformations in simple metals. Int. J. High Press. Res. 1997, 15, 207–305. [Google Scholar]
- Poole, P.H.; Grande, T.; Angell, C.A.; McMillan, P.F. Polymorphic phase transitions in liquids and glasses. Science 1997, 275, 322. [Google Scholar] [CrossRef]
- Mishima, O.; Stanley, H.F. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar] [CrossRef]
- Stillinger, F.H.; Debenedetti, P.G. Supercooled liquids and the glass transition. Nature 2001, 410, 259–267. [Google Scholar]
- Angilella, G.N.; Leys, F.E.; March, N.H.; Pucci, R. Phase transitions in liquids and glasses. Phys. Chem. Liq 2003, 41, 211–226. [Google Scholar] [CrossRef]
- Debenedetti, P.G. Supercooled and glassy water. J. Phys. Condens. Matter. 2003, 15, 1669–1726. [Google Scholar] [CrossRef]
- McMillan, P.F. Polyamorphic transformations in liquids and glasses. J. Mater. Chem. 2004, 14, 1506–1512. [Google Scholar] [CrossRef]
- McMillan, P.F.; Wilson, M.; Wilding, M.C.; Daisenberger, D.; Mezouar, M.; Greaves, G.N. Polyamorphism and liquid-liquid phase transitions: Challenges for experiment and theory. J. Phys. Condens. Matter. 2007, 19, 415101. [Google Scholar] [CrossRef]
- Berthier, L.; Biroli, G. Theoretical perspective on the glass transition and amorphous materials. Rev. Mod. Phys. 2011, 83, 587. [Google Scholar] [CrossRef]
- Stanley, H.E. Liquid Polymorphism; Wiley Online Library: Hoboken, NJ, USA, 2013; Volume 152. [Google Scholar]
- Gallo, P.; Amann-Winkel, K.; Angell, C.A.; Anisimov, M.A.; Caupin, F.; Chakravarty, C.; Lascaris, E.; Loerting, T.; Panagiotopoulos, A.Z.; Russo, J.; et al. A tale of two liquids. Chem. Rev. 2016, 116, 7463–7500. [Google Scholar] [CrossRef]
- Handle, H.; Loerting, T.; Sciortino, F. Supercooled and glassy water: Metastable liquid(s), amorphous solid(s), and a no-man’s land. Proc. Natl. Acad. Sci. USA 2017, 114, 13336–13344. [Google Scholar] [CrossRef]
- Palmer, J.C.; Poole, P.H.; Sciorfino, F.; Debenedetti, P.G. Advances in computational studies of the liquid–liquid transition in water and water-like models. Chem. Rev. 2018, 118, 9129–9151. [Google Scholar] [CrossRef]
- Tanaka, H. Liquid-liquid transition and polyamorphism. J. Chem. Phys. 2020, 153, 130901. [Google Scholar] [CrossRef] [PubMed]
- Kearns, K.L.; Swallen, S.F.; Ediger, M.D.; Wu, T.; Sun, Y.; Yu, L. Hiking down the energy landscape: Progress toward the Kauzmann temperature via vapor deposition. J. Phys. Chem. B 2008, 112, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Kearns, K.L.; Swallen, S.F.; Ediger, M.D.; Wu, T.; Yu, L. Influence of substrate temperature on the stability of glasses prepared by vapor deposition. J. Chem. Phys. 2007, 127, 154702. [Google Scholar] [CrossRef] [PubMed]
- Leon-Gutierrez, E.; Sepulveda, A.; Garcia, G.; Clavaguera-Mora, M.T.; Rodriguez-Vieja, J. Stability of thin film glasses of toluene and ethylbenzene formed by vapor deposition: An in situ nanocalorimetric study. Phys. Chem. Chem. Phys. 2010, 12, 14693–14698. [Google Scholar] [CrossRef]
- Ishii, K.; Nakayama, H.; Hirabayashi, S.; Moriyama, R. Anomalously high-density glass of ethylbenzene prepared by vapor deposition at temperatures close to the glass-transition temperature. Chem. Phys. Lett. 2008, 459, 109–112. [Google Scholar] [CrossRef]
- Ha, A.; Cohen, I.; Zhao, X.; Lee, M.; Kivelson, D. Supercooled liquids and polyamorphism. J. Phys. Chem. Lett. 1996, 100, 1–4. [Google Scholar] [CrossRef]
- Kivelson, D.; Kivelson, S.A.; Zhao, X.; Nussinov, Z.; Tarjus, G. A thermodynamic theory of supercooled liquids. Physica A. 1995, 219, 27–38. [Google Scholar] [CrossRef]
- Miltenburg, K.V.; Blok, K. Calorimetric investigation of a new solid phase in Triphenylphosphite. J. Phys. Chem. 1996, 100, 16457–16459. [Google Scholar] [CrossRef]
- Kurita, R.; Tanaka, H. On the abundance and general nature of the liquid-liquid phase transition in molecular systems. J. Phys. Condens. Matter. 2005, 17, L293–L302. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.-Q.; Perepezko, J.H.; Yu, L. Possible existence of two amorphous phases of D-Mannitol related by a first-order transition. J. Chem. Phys. 2015, 142, 244504. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanaka, H. The reversibility and first-order nature of liquid-liquid transition in a molecular liquid. Nature Comm. 2016, 7, 13438. [Google Scholar] [CrossRef]
- An, Q.; Johnson, W.L.; Samwer, K.; Corona, S.L.; Goddart, W.A., III. Formation of two glass phases in binary Cu-Ag liquid. Acta Mater. 2020, 195, 274–281. [Google Scholar] [CrossRef]
- An, Q.; Johnson, W.L.; Samwer, K.; Corona, S.L.; Goddard, W.A., III. First-order transition in liquid Ag to the heterogeneous G-Phase. J. Phys. Chem. Lett. 2020, 11, 632–645. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Louzguine-Luzguin, D.V. Crystallization of FCC and BCC liquid metals studied by molecular dynamics simulation. Metal. 2020, 10, 1532. [Google Scholar]
- Becker, S.; Devijver, E.; Molinier, R.; Jakse, N. Glass-forming ability of elemental zirconium. Phys. Rev. B 2020, 102, 104205. [Google Scholar] [CrossRef]
- Popel, P.S.; Sidorov, V.E. Microheterogeneity of liquid metallic solutions and its influence on the structure and propertes of rapidly quenched alloys. Mater.Sci. Eng. A 1997, 226, 237–244. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kiraga, K.; Inoue, A.; Masumoto, T.; Jo, H.H. Crystallization and high mechanical strengthof Al-based amorphous alloys. Mater. Trans. 1994, 35, 293–302. [Google Scholar] [CrossRef]
- Hu, Q.; Sheng, H.C.; Fu, M.W.; Zeng, X.R. Influence of melt temperature on the Invar effect in (Fe71.2B.024Y4.8)96Nb4 bulk metallic glasses. J. Mater. Sci. 2019, 48, 6900–6906. [Google Scholar]
- Jiang, H.-R.; Bochtler, B.; Riegler, X.-S.; Wei, S.S.; Neuber, N.; Frey, M.; Gallino, I.; Busch, R.; Shen, J. Thermodynamic and kinetic studies of the Cu-Zr-Al(-Sn) bulk metallic glasses. J. Alloys Compd. 2020, 844, 156126. [Google Scholar] [CrossRef]
- Yue, Y. Experimental evidence for the existence of an ordered structure in a silicate liquid above its liquidus temperature. J. Non-Cryst. Sol. 2004, 345, 523–527. [Google Scholar] [CrossRef]
- Wei, S.; Yang, F.; Bednarcik, J.; Kaban, I.; Shuleshova, O.; Meyer, A.; Busch, R. Liquid-liquid transition in a strong bulk metallic glass-forming liquid. Nat. Commun. 2013, 4, 2083. [Google Scholar] [CrossRef]
- Way, C.; Wadhwa, P.; Busch, R. The influence of shear rate and temperature on the viscosity and fragility of the Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 metallic-glass-forming liquid. Acta Mater. 2007, 55, 2977–2983. [Google Scholar] [CrossRef]
- Chen, E.-Y.; Peng, S.-X.; Peng, L.; Di Michiel, M.; Vaughan, G.B.M.; Yu, Y.; Yu, H.-B.; Ruta, B.; Wei, S.; Liu, L. Glass-forming ability correlated with the liquid-liquid transition in Pd42.5Ni42.5P15 alloy. Scr. Mater. 2021, 193, 117–121. [Google Scholar] [CrossRef]
- Xu, W.; Sandor, M.T.; Yu, Y.; Ke, H.-B.; Zhang, H.P.; Li, M.-Z.; Wang, W.-H.; Liu, L.; Wu, Y. Evidence of liquid-liquid transition in glass-forming La50Al35Ni15 melt above liquidus temperature. Nat. Commun. 2015, 6, 7696. [Google Scholar] [CrossRef]
- Wang, L.; Bian, X.; Liu, J. Discontinuous structural phase transition of liquid metal and alloys. Phys. Lett. A 2004, 326, 429–435. [Google Scholar] [CrossRef]
- Greenberg, Y.; Yahel, E.; Caspi, E.N.; Benmore, C.; Beuneu, B.; Daniel, M.P.; Markov, G. Evidence for a temperature-driven structural transition in liquid bismuth. EPL 2009, 86, 36004. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, L.; Sun, Q.; Qin, J.; Brian, X.; Yue, Y. Indication of liquid-liquid phase transition in CuZr-based melts. Appl. Phys. Lett. 2013, 103, 171904. [Google Scholar] [CrossRef]
- Tanaka, H. Bond orientational order in liquids: Towards a unified description of water-like anomalies, liquid-liquid transition, glass transition, and crystallization. Eur. Phys. J. 2012, 35, 113. [Google Scholar] [CrossRef]
- He, Y.-X.; Li, J.; Wang, J.; Kou, H.; Beaugnon, E. Liquid-liquid structure transition and nucleation in undercooled Co-B eutectic alloys. Appl. Phys. A 2017, 123, 391. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Z.; Cojocaru-Morédin, O.; Zhu, B.; Wang, X.-Y.; Gao, N.; Zhuang, Y.; Zu, F.-Q. Dependence of solidification for Bi2Te3-xSex alloys on their liquid states. Sci. Rep. 2017, 7, 2463. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, L.; Wang, X.-Y.; Zhu, B.; Huang, Z.-Y.; Zu, F.-Q. Influence of melt overheating treatment on solidification behavior of BiTe-based alloys at different cooling rates. Mater. Des. 2015, 88, 743–750. [Google Scholar] [CrossRef]
- Tournier, R.F.; Beaugnon, E. Texturing by cooling a metallic melt in a magnetic field. Sci. Technol. Adv. Mater. 2009, 10, 014501. [Google Scholar] [CrossRef]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury. J. Chem. Phys. 1952, 20, 411. [Google Scholar] [CrossRef]
- Kelton, K.F. Crystal nucleation in liquids and glasses. Sol. State Phys. 1991, 45, 75–177. [Google Scholar]
- Tournier, R.F. Presence of intrinsic growth nuclei in overheated and undercooled liquid elements. Physica B. 2007, 392, 79–91. [Google Scholar] [CrossRef]
- Tournier, R.F. Lindemann’s rule applied to the melting of crystals and ultra-stable glasses. Chem. Phys. Lett. 2016, 651, 198–202, Erratum in 2017, 675, 174. [Google Scholar] [CrossRef][Green Version]
- Tournier, R.F. Thermodynamic and kinetic origin of the vitreous transition. Intermetallics 2012, 30, 104–110. [Google Scholar] [CrossRef]
- Tournier, R.F. Glass phase and other multiple liquid-to-liquid transitions resulting from two-liquid competition. Chem. Phys. Lett. 2016, 665, 64–70. [Google Scholar] [CrossRef]
- Tournier, R.F. Crystallization of supercooled liquid elements induced by superclusters containing magic atom numbers. Metals 2014, 4, 359–387. [Google Scholar] [CrossRef]
- Franck, F.C. Supercooling of liquids. Proc. Roy. Soc. Lond. 1952, A215, 43–46. [Google Scholar]
- Tournier, R.F. Homogeneous nucleation of phase transformations in supercooled water. Physica B 2020, 579, 411895. [Google Scholar] [CrossRef]
- Oguni, M.; Maruyama, S.; Wakabayashi, K.; Nagoe, A. Glass transitions of ordinary and heavy water within silica-gel nanopores. Chem. Asian J. 2007, 2, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Speedy, R.J.; Angell, C.A. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 °C. J. Chem. Phys. 1976, 65, 851–858. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Dewetting Temperatures of Prefrozen and Grafted Layers in Ultrathin Films Viewed as Melt-Memory Effects. Physica B 2021, 611, 412796. [Google Scholar] [CrossRef]
- Takaiwa, D.; Atano, I.; Koga, K.; Tanaka, H. Phase diagram of water in carbon nanotubes. Proc. Natl. Acad. Sci. USA 2008, 105, 39–43. [Google Scholar] [CrossRef]
- Assland, S.; McMillan, P.F. Density-driven liquid-liquid phase separation with system Al2O3-Y2O3. Nature 1994, 369, 633–636. [Google Scholar] [CrossRef]
- Tournier, R.F. First-order transitions in glasses and melts induced by solid superclusters nucleated by homogeneous nucleation instead of surface melting. Chem. Phys. 2019, 524, 40–54. [Google Scholar] [CrossRef]
- Hassaine, M.; Jimenez-Rioboo, R.J.; Sharapova, I.V.; Korolynk, O.A.; Krivchikov, A.I.; Ramos, M.A. Thermal properties and Brillouin -scattering study of glass, crystal, and “glacial” states in n-butanol. J. Chem. Phys. 2009, 131, 174508. [Google Scholar] [CrossRef]
- Krivchikov, A.I.; Hassaine, M.; Sharapova, I.V.; Korolyuk, O.A.; Jimenez-Rioboo, R.J.; Ramos, M.A. Low temperature properties of glassy and crystalline states of n-butanol. J. Non-Cryst. Sol. 2011, 357, 524–529. [Google Scholar] [CrossRef]
- Hays, C.C.; Johnson, W.L. Undercooling of bulk metallic glasses processed by electrostatic levitation. J. Non-Cryst. Sol. 1999, 250, 596–600. [Google Scholar] [CrossRef]
- Yang, B.; Perepezko, J.H.; Schmelzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513. [Google Scholar] [CrossRef]
- Ojovan, M.I. Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects. J. Exp. Theor. Phys. Lett. 2004, 79, 632–634. [Google Scholar] [CrossRef]
- Ozhovan, M.I. Topological characteristics of bonds in SiO2 and GeO. oxide systems at glass-liquid transition. J. Exp. Theor. Phys. 2006, 103, 819–829. [Google Scholar] [CrossRef]
- Ojovan, M.I. Ordering and structural changes at the glass-liquid transition. J. Non-Cryst. Sol. 2013, 382, 79. [Google Scholar] [CrossRef]
- Sanditov, D.S.; Ojovan, M.I. On relaxation nature of glass transition in amorphous materials. Physica B 2017, 523, 96–113. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine Luzgin, D.V. Revealing Structural Changes at Glass Transition via Radial Distribution Functions. J. Phys. Chem. B 2020, 124, 3186–3194. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Undercooled Phase Behind the Glass Phase with Superheated Medium-Range Order above Glass Transition Temperature. Physica B 2021, 602, 412542. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Building and breaking bonds by homogenous nucleation in glass-forming melts leading to three liquid states. Materials 2021, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Tournier, R.F.; Ojovan, M.O. Prediction of Second Melting Temperatures Already Observed in Pure Elements by Molecular Dynamics Simulations. Materials 2021, 14, 6509. [Google Scholar] [CrossRef] [PubMed]
- Vinet, B.; Magnusson, L.; Fredriksson, H.; Desré, P.J. Correlations between surface and interface energies with respect to crystal nucleation. J. Coll. Interf. Sci. 2002, 255, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zhou, Z.; Schroers, J.; Johnson, W.L.; Rhim, W.K. Overheating threshold and its effect on time-temperature-transformation diagrams of zirconium based bulk metallic glasses. Appl. Phys. Lett. 2004, 84, 5010–5012. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Hu, R.; Kou, H.; Beaugnon, E. Evidence for the structure transition in a liquid Co-Sn alloy by in-situ magnetization measurement. Mater. Lett. 2015, 145, 261–263. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Li, L.; Wang, J.; Yildiz, E.; Beaugnon, E. Composition dependent characteristic transition temperatures o Co-B melts. J. Non-Cryst. Sol. 2019, 522, 119583. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Li, J.; Li, C.; Kou, H.; Zhang, P.; Beaugnon, E. Nucleation of supercooled Co melts under a high magnetic field. Mater. Chem. Phys. 2019, 225, 133–136. [Google Scholar] [CrossRef]
- Bian, X.F.; Sun, B.A.; Hu, L.N.; Jia, Y.B. Fragility of superheated melts and glass-forming ability in Al-based alloys. Phys. Lett. A 2005, 335, 61–67. [Google Scholar] [CrossRef]
- Lan, S.; Ren, Y.; Wei, X.Y.; Wang, B.; Gilbert, E.P.; Shibayama, T.; Watanabe, S.; Ohnuma, M.; Wang, X.-L. Hidden amorphous phase and reentrant supercooled liquid in Pd-Ni-P metallic glass. Nat. Commun. 2017, 8, 14679. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Comments about a recent publication entitled entitled “Improving glass forming ability of off-eutectic metallic glass formers by manipulating primary crystallization reactions”. Scr. Mater. 2021, 205, 114039. [Google Scholar] [CrossRef]
- Zeng, Y.Q.; Yu, J.S.; Tian, Y.; Hirata, A.; Fujita, T.; Zhang, X.H.; Nishiyama, N.; Kato, H.; Jiang, J.Q.; Inoue, A.; et al. Response to the commentary by Robert Tournier and Michael Ojovan on our publication entitled “Improving glass forming. Scr. Mater. 2021, 205, 114035. [Google Scholar] [CrossRef]
- Adams, G.; Gibbs, J.H. On the temperatutre dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 1965, 43, 139. [Google Scholar] [CrossRef]
- Liu, C.-Y.; He, J.; Keunings, R.; Bailly, C. new linearized relation for the universal viscosity-temperature behavior of polymer melts. Macromolecules 2006, 39, 8867–8869. [Google Scholar] [CrossRef]
- Vopson, M.M.; Rugers, N.; Hepburn, I. The generalized Lindemann Melting coefficient. Sol. State Commun. 2020, 318, 113977. [Google Scholar] [CrossRef]
- Jiang, J.; Saksl, K.; Nishiyama, N.; Inoue, A. Crystallization in Pd40N0P20 glass. J. Appl. Phys. 2002, 92, 3651–3656. [Google Scholar] [CrossRef]
- Wilde, G.; Görler, G.P.; Willnecker, R.; Dietz, G. The thermodynamic properties of Pd40Ni40P20 in the glassy, liquid, and crystalline states. Appl. Phys. Lett. 1994, 65, 397–399. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J.; Wang, J.; Guo, T.; Kou, H.; Beaugnon, E. Effect of liquid-liquid structure transition on the nucleation in undercooled Co-Sn eutectic alloy. Mater. Chem. Phys. 2016, 170, 261–265. [Google Scholar] [CrossRef]
- He, Y.X.; Li, J.S.; Wang, J.; Beaugnon, E. Liquid-liquid structure transition in metallic melt and its impact on solidification: A review. Trans. Nonferrous Met. Soc. China 2020, 30, 2293–2310. [Google Scholar] [CrossRef]
- Connel, L.C.; Pokhrel, N.; Logan, M.; Voss, C.; Buna, D. Thermal Characterization of Semi-Conductor Bi2Te3 Alloys Using Differential Calorimetry. Available online: https://www.ramapo.edu/scholarsday/files/formidable/9/EngPhys-BunaBi2Te3-DSC-Research-Poster-.pptx (accessed on 19 December 2021).
- Jian, Q.K.; Wang, X.D.; Nie, X.P.; Zhang, G.Q.; Ma, H.; Fecht, H.J.; Bendnarck, J.; Franz, H.; Liu, Y.G.; Cao, Q.P.; et al. Zr-(Cu,Ag)-Al bulk metallic glasses. Acta Mater. 2008, 56, 1785–1796. [Google Scholar] [CrossRef]
- Jabroui, H.; Ouaskit, S.; Richard, J.; Garden, J.-L. Determination of entropy production during glass transition: Theory and experiment. J. Non-Cryst. Sol. 2020, 533, 119907. [Google Scholar] [CrossRef]
- Jabrane, S.; Létoffé, J.M.; Claudy, P. Vitrification and crystallization in the R(-)1,2-propanediol-S(+)1,2-propadeniol system. Therm. Chim. Act. 1995, 258, 33–47. [Google Scholar] [CrossRef]
- Righetti, M.C. Crystallization of polymers investigated by temperature-modulated DSC. Materials 2017, 10, 442. [Google Scholar] [CrossRef]
- Okazaki, I.; Wunderlich, B. Reversible melting in polymer crystals detected by temperature-modulateddifferential scanning calorimetry. Macromolecules 1997, 30, 1758–1764. [Google Scholar] [CrossRef]
- Schick, C.; Wurm, A.; Mohamed, A. Vitrification and devitrificationof the rigid fraction amorphous of semi- crystalline polymers revealed from frequency-dependent heat capacity. Colloid Polym. Sci. 2001, 279, 800–806. [Google Scholar] [CrossRef]
- Caccamo, C.; Pellicane, G.; Costa, D. Phase transitions in hard-core Yukawa fluids: Toward a theory of phase stability in protein solutions. J. Phys. Condens. Matter 2000, 12, A437. [Google Scholar] [CrossRef]
- Fiumara, G.; Pandaram, O.D.; Pellicane, G.; Saija, F. Theoretical and computer simulation study of phase coexistence of nonadditive hard-disk mixtures. J. Chem. Phys. 2014, 141, 214508. [Google Scholar] [CrossRef] [PubMed]

| Tg | Tm | θg | εls0 | θom2 | εgs0 | θog2 | Δεlg (θ0m) | Δε= θn+ | Tn+ | Obs. | T′g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 715 | 962 | −025676 | 1.74324 | 0.19893 | 1.61486 | 0.27642 | −0.4527 | 0.06419 | 1209 | ||
| 715 | 962 | −0.25676 | 1.74324 | 0.19893 | 1.61486 | 0.27642 | −0.4527 | 0.09947 | 1057 | 1209 | |
| 715 | 962 | −0.25676 | 1.74324 | 0.19893 | 1.61486 | 0.27642 | −0.4527 | 0.12838 | 1086 | 1209 | |
| 715 | 962 | −0.25676 | 1.74324 | 0.19893 | 1.61486 | 0.27642 | −0.4527 | 0.22785 | 1181 | 1209 | |
| 715 | 1034 | −0.30851 | 1.69149 | 0.23193 | 1.53723 | 0.31617 | −0.40957 | 0.07713 | 1353 | ||
| 715 | 1034 | −0.30851 | 1.69149 | 0.23193 | 1.53723 | 0.31617 | −0.40957 | 0.11953 | 1158 | 1353 | |
| 715 | 1034 | −0.30851 | 1.69149 | 0.23193 | 1.53723 | 0.31617 | −0.40957 | 0.15426 | 1194 | 1353 | |
| 715 | 1034 | −0.30851 | 1.69149 | 0.23193 | 1.53723 | 0.31617 | −0.40957 | 0.19665 | 1237 | Yes | 1353 |
| 715 | 1034 | −0.30851 | 1.69149 | 0.23193 | 1.53723 | 0.31617 | −0.40957 | 0.27378 | 1317 | Yes | 1353 |
| 715 | 1125 | −0.36444 | 1.63556 | 0.26492 | 1.45333 | 0.35311 | −0.36296 | 0.09111 | 1535 | ||
| 715 | 1125 | −0.36444 | 1.63556 | 0.26492 | 1.45333 | 0.35311 | −0.36296 | 0.1412 | 1284 | Yes | 1535 |
| 715 | 1125 | −0.36444 | 1.63556 | 0.26492 | 1.45333 | 0.35311 | −0.36296 | 0.18222 | 1330 | Yes | 1535 |
| 715 | 1125 | −0.36444 | 1.63556 | 0.26492 | 1.45333 | 0.35311 | −0.36296 | 0.32342 | 1489 | Yes | 1535 |
| 715 | 1125 | −0.36444 | 1.63556 | 0.26492 | 1.45333 | 0.35311 | −0.36296 | 0.36296 | 1533 | Yes | 1535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournier, R.F.; Ojovan, M.I. Multiple Melting Temperatures in Glass-Forming Melts. Sustainability 2022, 14, 2351. https://doi.org/10.3390/su14042351
Tournier RF, Ojovan MI. Multiple Melting Temperatures in Glass-Forming Melts. Sustainability. 2022; 14(4):2351. https://doi.org/10.3390/su14042351
Chicago/Turabian StyleTournier, Robert F., and Michael I. Ojovan. 2022. "Multiple Melting Temperatures in Glass-Forming Melts" Sustainability 14, no. 4: 2351. https://doi.org/10.3390/su14042351
APA StyleTournier, R. F., & Ojovan, M. I. (2022). Multiple Melting Temperatures in Glass-Forming Melts. Sustainability, 14(4), 2351. https://doi.org/10.3390/su14042351







