Biodiesel Production from Waste Oils: A South African Outlook
Abstract
:1. Introduction
2. Pre-Treatment of WCO Feedstock
3. Biodiesel Production
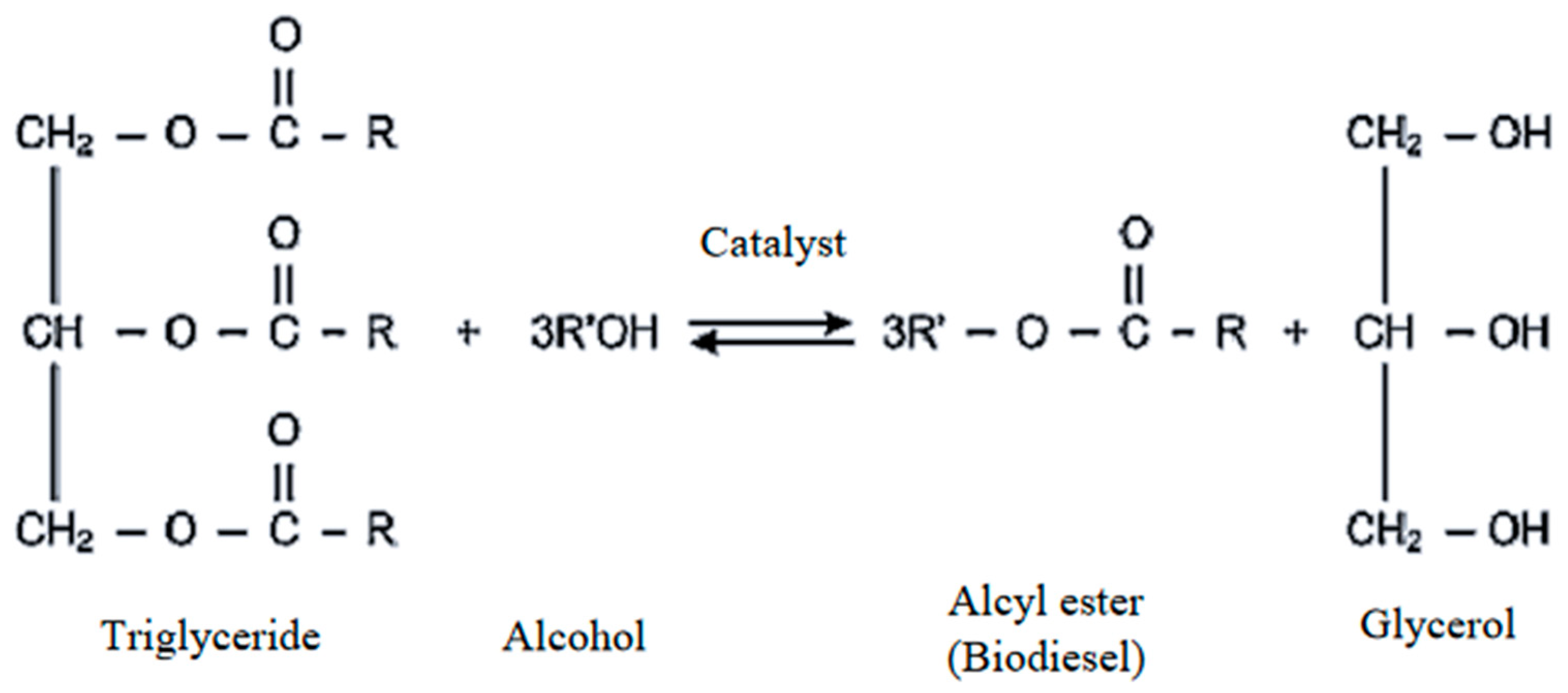
3.1. Transesterification Process
3.2. The Role of Alcohol
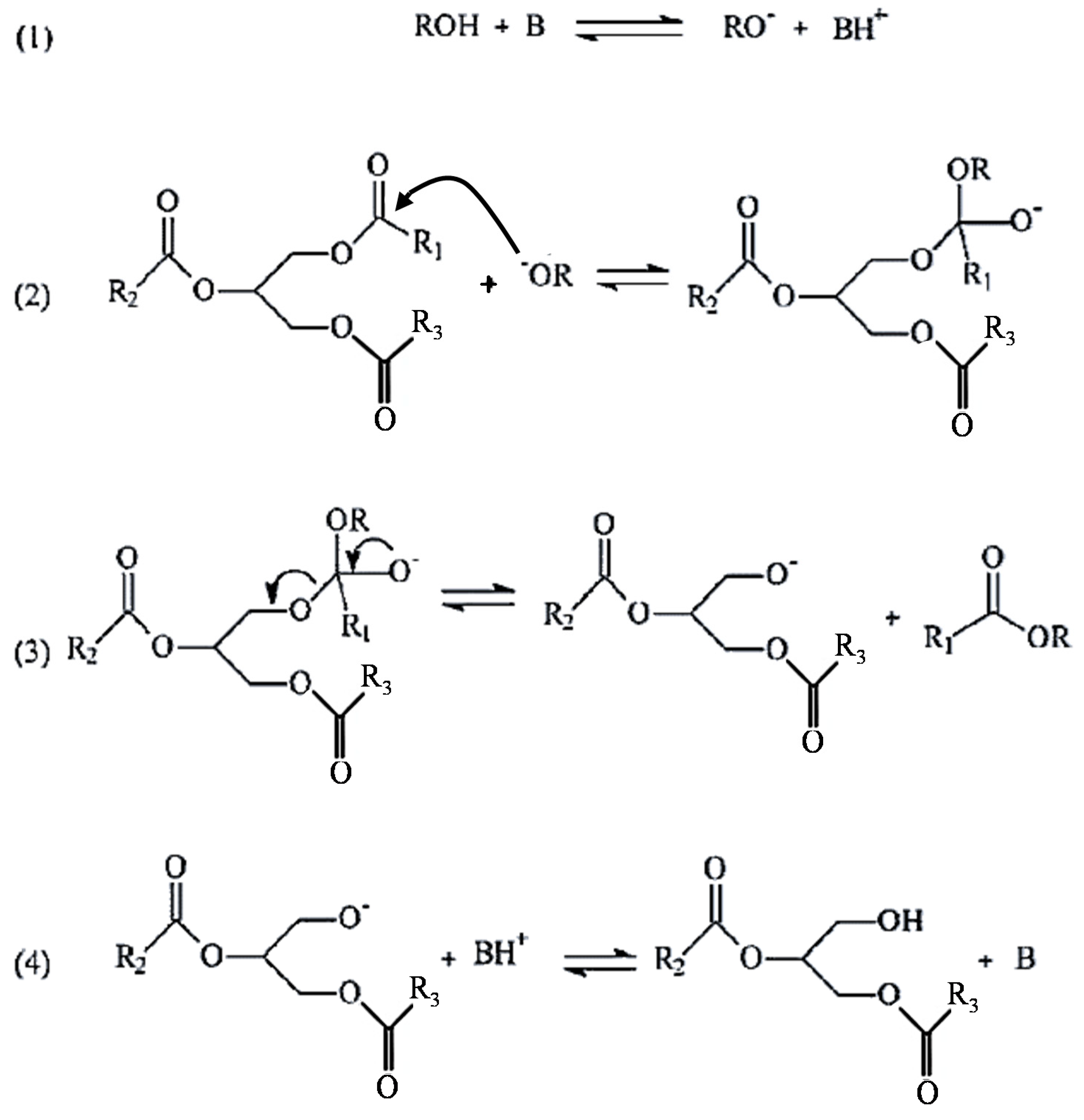
3.3. Catalysis
3.3.1. Homogeneous Acid and Base Catalysts
3.3.2. Ionic Liquids
3.3.3. Biocatalysts
3.3.4. Heterogeneous Catalysts
3.3.5. Carbon Based Catalysts
3.4. Characterization of Biodiesel
3.5. Summary of Important Parameters for the Best Biodiesel Yields
- water and FFA content,
- alcohol to oil ratio.
- Reaction temperature
- Reaction time
- Catalyst concentration
- Purification
4. Technical and Economic Feasibility Overview
4.1. Biodiesel Plant Considerations
4.2. Biofuels Industry, SA
- investments towards renewable energy and environmentally friendly energy sources to reduce global warming,
- empowering the agricultural sector,
- promotion of sustainable development, exertion of downward pressure on global crude oil prices and the need to improve energy security.
4.3. Relevance to the South African Economy and Legislature
4.3.1. Selected Legislation Governing Waste in South Africa
- ❖
- National Environmental Management: Waste Act No. 59 of 2008
- The objects of the National Environmental Management Waste Act 59 of 2008 are to protect health, well-being and the environment by providing reasonable measures for avoiding and minimizing the generation of waste; reducing, re-using, recycling and recovering waste; treating and safely disposing of waste as a last resort; promoting and ensuring the effective delivery of waste services; and generally, to give effect to Section 24 of the Constitution in order to secure an environment that is not harmful to health and well-being.
- ❖
- National Waste Management Regulations, 2012
- Published in terms of Section 69 of the National Environmental Management: Waste Act No. 59 of 2008 [124]. The purpose of the regulations is to regulate the collection of data and information to fulfil the objectives of the national waste information system as set out in Section 61 of the Waste Act [125].
4.3.2. Primary Laws and Regulations Governing the Oil and Gas Industry in South Africa
- ❖
- The Mineral and Petroleum Resources Development Act (Act No. 28 of 2002)—this is the framework legislation in terms of which upstream oil and gas rights are granted and controlled, together with prospecting and mining rights. The government has indicated that it will seek to separate the regulation of oil and gas from that of mining by enacting a separate legislative framework in the medium term.
- ❖
- The Petroleum Pipelines Act (Act No. 60 of 2003)—this provides the regulatory framework for the construction and operation of petroleum pipelines, loading facilities and storage facilities.
- ❖
- The Petroleum Products Act (Act No. 120 of 1977)—this regulates the downstream sector, establishing the scheme for the licensing of wholesalers, retailers and manufacturers of petroleum products.
- ❖
- The Gas Act (Act No. 48 of 2001)—this provides the regulatory framework for the construction and operation of gas transmission, storage, distribution, liquefaction and re-gasification facilities, as well as for trading in gas.
- ❖
- The National Environmental Management Act (Act No. 107 of 1999)—this was enacted as framework legislation for environmental management in SA. It subjects various activities to environmental authorisation, including oil and gas exploration, production and decommissioning. Since November 2015, the act has also imposed additional environmental obligations, such as the furnishing of financial provision for environmental obligations relating to the rehabilitation and remediation of areas in which exploration and production activities have been conducted.
- ❖
- The International Trade and Administration Act (Act No. 71 of 2002)—this regulates the import and export of petroleum and petroleum products to SA. A list setting out the commodities that require import and export permits is published by the minister of trade.
4.3.3. Government Bodies Who Are Charged with Regulating the Oil and Gas Industry in South Africa
- Department of Mineral Resources—administers the Mineral and Petroleum Resources Development Act, which is the principal statute governing the exploration for and production of petroleum resources. It is also the competent authority to issue environmental authorisations under the National Environmental Management Act.
- Department of Energy—is the controller of petroleum products within the Department of Energy and is the licensing authority under the Petroleum Products Act.
- Petroleum Agency of South Africa—has been delegated various first-tier functions in terms of the Mineral and Petroleum Resources Development Act relating to the acceptance and consideration of applications for petroleum rights and permits.
- National Energy Regulator of South Africa (NERSA)—administers and is the competent licensing authority under the Petroleum Pipelines Act and the Gas Act.
5. Conclusions and Prospects
- ➢
- The SA government can invest in promoting behavioural change among its residents in order to achieve a better collection of waste oils. This can be achieved by creating WCO collection centres in each municipality and appropriating financial rewards for every litre of WCO collected. This could be especially useful in the rural areas where it is easier for people to deliver WCO when travelling to town for their normal shopping activities than it would be for a waste collection vehicle to travel throughout the different remote municipal regions.
- ➢
- The hotels, restaurants and fast-food chains that produce large quantities of WCO are usually located in easily accessible areas. A well-managed waste collection system including specified travel routes for each municipality would help minimise transport costs. Further, government tax exemption incentives for companies that store and sell WCO could encourage cooperation in the hospitality industry.
- ➢
- Policy can phase-out or reduce the manufacturing and sale of petrol consuming machinery in a bid to promote diesel machinery sales, which can also accommodate biodiesel and biodiesel blends with fossil diesel. This would also help reduce the emission of greenhouse gases.
- ➢
- The Government can increase investments towards renewable energy and specifically expand on the expectations for biofuels.
- ➢
- The Government can promote the design of semi-industrial plants that can be easily accessed by interested entrepreneurs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- South Africa. Available online: https://knoema.com/atlas/South-Africa/topics/Energy/Oil (accessed on 24 January 2022).
- Yasar, F. Comparison of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 1–7. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Wang, T. Global Biodiesel Production by Country 2018. Statista. 2020. Available online: https://www.statista.com/statistics/271472/biodiesel-production-in-selected-countries/ (accessed on 25 May 2020).
- Phan, A.N.; Phan, T.M. Biodiesel Production from Waste Cooking Oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- National Waste Information Regulations. South Africa. 2012. Available online: https://www.ecolex.org/details/legislation/national-waste-information-regulations-no-r-625-of-2012-lex-faoc117326/ (accessed on 18 May 2020).
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste cooking oil as an alternate feedstock for biodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, L.; Laca, A.; Herrero, M.; Diaz, M. life cycle assessment comparison between centralized and decentralized biodiesel production from raw sunflower oil and waste cooking oils. J. Clean. Prod. 2012, 37, 162–171. [Google Scholar] [CrossRef]
- MKulkarni, G.; Dalai, A.K. Waste cooking oils—An economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Haas, M.J.; Aloon, A.J.M.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef]
- Attari, A.; Abbaszadeh-Mayvan, A.; Taghizadeh-Alisaraei, A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 2022, 158, 1–16. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selvan, V.A.M. A comprehensive review of low cost production from waste chicken fat. Renew. Sustain. Energy Rev. 2018, 83, 390–401. [Google Scholar] [CrossRef]
- Dimian, A.C.; Kiss, A.A. Eco-efficient processes for biodiesel poduction from waste lipids. J. Clean. Prod. 2019, 239, 1–15. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Refaat, A.A. Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2010, 7, 183–213. [Google Scholar] [CrossRef] [Green Version]
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Rahim, R.; Aziz, A.; Shah, L.A. Physical Characteristics of Biodiesel Blend Fuels with Alcohol and Additives. Procedia Eng. 2013, 53, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Thoai, D.N.; Hang, P.T.L.; Lan, D.T. Pre-treatment of waste cooking oil with high free fatty acids content for biodiesel production: An optimization study via response surface methodology. Vietnam J. Chem. 2019, 57, 568–573. [Google Scholar] [CrossRef]
- Sahar; Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Habbib-ur-Rehman; Nasir, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Shalaby, E.A.; El-Gendy, N.S. Two steps alkaline transesterification of waste cooking oil and quality assessment of produced biodiesel. Int. J. Chem. Biochem. Sci. 2012, 1, 30–35. [Google Scholar]
- Asri, N.P.; Sari, D.A.P.; Poedjojono, B.; Suprapto. Pre-treatment of waste frying oils for biodiesel production. Mod. Appl. Sci. 2015, 9, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, N.; Oktar, N.; Tapan, N. Esterification of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel 2008, 87, 1789–7898. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Processes 2013, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2019, 262, 1–15. [Google Scholar] [CrossRef]
- Kapilan, N.; Bayko, D.; Baykov, A. Review on new methods used for the production of biodiesel. Pet. Coal 2014, 56, 62–73. [Google Scholar]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannkarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Saravanaan, C.G. Optimization of the transesterification process for biodiesel production and use of biodiesel in a compression ignition engine. Energy Fuels 2007, 21, 2998–3003. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.K.; Lee, K.T. Mixed methanol-ethanol technology to produce greener biodiesel from waste cooking oil: A breakthrough for SO42−/SnO2-SiO2 catalyst. Fuel Processing Technol. 2011, 92, 1639–1645. [Google Scholar] [CrossRef]
- Issariyakul, T.; Kulkarrni, M.G.; Dalai, A.K.; Bakhshi, N.N. Production of biodiesel from waste fryer grease using mixed methanol/ethanol system. Fuel Processing Technol. 2007, 88, 429–436. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of biodiesel from waste cooking oil using a homogeneous catalyst: Study of semi-industrial pilot of microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Di Bitonto, L.; Pastore, C. Metal hydrated-salts as efficient and reusable catalysts for pre-treating waste cooking oils and animal fats for an effective production of biodiesel. Renew. Energy 2019, 143, 1193–1200. [Google Scholar] [CrossRef]
- Chua, S.Y.; Periasamy, L.A.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E.C. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Selvaraj, R.; Praveenkumar, R.; Moorthy, I. A comprehensive review of biodiesel production methods from various feedstocks. Biofuels 2016, 10, 325–333. [Google Scholar] [CrossRef]
- Celante, D.; Schenkel, J.V.D.; de Castilhos, F. Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide. Fuel 2018, 212, 101–107. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Wong, L.P.; Hilaire, D.S.; Kim, J.; Salako, O.; Jean, M.J.; Adeyemi, R.; James, S.; Foster, T.; Pratt, L.M. Biodiesel fuel production from brown grease produced by wastewater treatment plant: Optimization of acid catalyzed reaction conditions. J. Environ. Chem. Eng. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- AbdelDayem, H.M.; Sabil, B.G.; El-Hosiny, F.I. Facile synthesis of hydrothermal stable hierarchically macro-mesoporous hollow microspheres γ-Al2O3-graphene oxide composite: As a new efficient acid-base catalyst for transesterification reaction for biodiesel production. Fuel 2020, 277, 1–10. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 1–15. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. The use of KOH/Clinoptilolite catalyst in pilot of microreactor for biodiesel production from waste cooking oil. Fuel 2020, 263, 1–10. [Google Scholar] [CrossRef]
- Ferreira, R.S.B.; dos Passos, R.M.; Sampaio, K.A.; Batista, E.A.C. Heterogeneous catalysts for biodiesel production: A review. Food Public Health 2019, 9, 125–137. [Google Scholar] [CrossRef]
- Romero, R.; Martinez, S.L.; Nativi, R. Biodiesel production by using heterogeneous catalysts. In Alternative Fuel; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Kamran, E.; Mashhadi, H.; Mohammadi, A.; Ghobadian, B. Biodiesel production from Elaeagnus angustifolia L. seed as a novel waste feedstock using potassium hydroxide catalyst. Biocatal. Agric. Biotechnol. 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Vadery, V.; Cherikkallinmel, S.K.; Ramakrishnan, R.M.; Sugunan, S.; Narayanan, B.N. Green production of biodiesel over waste borosilicate glass derived catalyst and the process up-gradation in pilot scale. Renew. Energy 2019, 141, 1042–1053. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Shine, K.; Agatheeshwaren, A.; Sivakumar, V. Biodiesel production from castor oil using potassium hydroxide as a catalyst: Simulation and validation. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2898–2905. [Google Scholar] [CrossRef]
- Pisitpong, I.; Sotsanan, L.; Luengnaruemitchai, A.; Samai, J.I. Biodiesel production from palm oil using potassium hydroxide loaded on ZrO2 catalyst in a batch reactor. Chiang Mai J. Sci. 2014, 41, 128–137. [Google Scholar]
- Sukasem, N.; Manophan, S. The development of biodiesel production from vegetable oils by using different proportions of lime catalyst and sodium hydroxide. Energy Procedia 2017, 138, 991–997. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Processing Technol. 2010, 91, 1378–1385. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Liu, C.Z.; Wang, F.; Stiles, A.R.; Guo, C. Ionic liquids for biofuel production: Opportunities and challenges. Appl. Energy 2012, 92, 406–414. [Google Scholar] [CrossRef]
- Hosseini, S.; Moradi, G.R.; Bahrami, K. Synthesis of a novel stabilized basic ionic liquid through immobilization on boehmite nanoparticles: A robust nanocatalyst for biodiesel production from soybean oil. Renew. Energy 2019, 138, 70–78. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Li, K.; Zhang, H.; Fan, L.; Lu, Z. Efficient production of biodiesel from ionic liquid catalyzed esterification using ultrasonic-microwave combined intensification. Chem. Eng. Processing—Process. Intensif. 2020, 149, 1–9. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liq. 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2019, 239, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Ullah, Z.; Bustam, M.A.; Man, Z. Biodiesel production from waste cooking oil by acidic ionic liquid as a catalyst. Renew. Energy 2015, 77, 521–526. [Google Scholar] [CrossRef]
- Panchal, B.; Chang, T.; Qin, S.; Sun, Y.; Wang, J.; Bian, K. Optimization of soybean oil transesterification using an ionic liquid and methanol for biodiesel synthesis. Energy Rep. 2019; in press. [Google Scholar] [CrossRef]
- Liang, J.H.; Ren, X.Q.; Wang, J.T.; Jinag, M.; Li, Z.J. Preparation of biodiesel by transesterification from cottonseed oil using the basic dication ionic liquids as catalysts. J. Fuel Chem. Technol. 2010, 38, 275–280. [Google Scholar] [CrossRef]
- Ding, H.; Ye, W.; Wang, Y.; Wang, X.; Li, L.; Liu, D.; Gui, J.; Song, C.; Ji, N. Process intensification of transesterification for biodiesel production from palm oil: Microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy 2018, 144, 957–967. [Google Scholar] [CrossRef]
- Gong, H.; Gao, L.; Nie, K.; Wang, M.; Tan, T. A new reactor for enzymatic synthesis of biodiesel from waste cooking oil: A static-mixed reactor pilot study. Renew. Energy 2020, 154, 270–277. [Google Scholar] [CrossRef]
- Saranya, G.; Ramachandra, T.V. Novel biocatalyst for optimal biodiesel production from diatoms. Renew. Energy 2020, 153, 919–934. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Asl, F.A.; Emampour, M.; Yousefi, M.; Lish, M.P.; Habibi, Z.; Mohammadi, M. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; Production of biodiesel from waste cooking oil. Process. Biochem. 2020, 90, 156–167. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Magro, L.D.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Pizarro, A.V.L.; Park, E.Y. Lipase-catalyzed production of biodiesel fuel from vegetable oils contained in waste activated bleaching earth. Process. Biochem. 2003, 38, 1077–1082. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Iuliano, M. Active biocatalyst for biodiesel production from spent coffee ground. Bioresour. Technol. 2018, 266, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yagiz, F.; Kazan, D.; Akin, A.N. Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem. Eng. J. 2007, 134, 262–267. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, J.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Ferrero, G.O.; Faba, E.M.S.; Rickert, A.A.; Eimer, G.A. Alternatives to rethink tomorrow: Biodiesel production from residual and non-edible oils using biocatalyst technology. Renew. Energy 2020, 150, 128–135. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Yang, S.Y.; Song, H.S.; Park, J.Y.; Park, Y.L.; Han, Y.H.; Choi, Y.K.; et al. Conversion of waste cooking oil into biodiesel using heterogeneous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ambat, I.; Srivastava, V.; Iftekhar, S.; Haapaniemi, E.; Sillanpää, M. Effect of different co-solvents on biodiesel production from various low-cost feedstocks using Sr–Al double oxides. Renew. Energy 2020, 146, 2158–2169. [Google Scholar] [CrossRef]
- Diamantopoulos, N.; Panagiotaras, D.; Nikolopoulos, D. Comprehensive review on the biodiesel production using solid acid heterogeneous catalysts. J. Thermodyn. Catal. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Konwar, L.J.; Das, R.; Thakur, A.J.; Salminen, E.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.P.; Deka, D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J. Mol. Catal. A Chem. 2014, 388–389, 167–176. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Yaşar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel 2019, 255, 1–6. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2020, 151, 295–310. [Google Scholar] [CrossRef]
- Marwaha, A.; Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Waste materials as potential catalysts for biodiesel production: Current state and future scope. Fuel Processing Technol. 2018, 181, 175–186. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Waste materials for production of biodiesel catalysts: Technological status and prospects. J. Clean. Prod. 2020, 263, 1–17. [Google Scholar] [CrossRef]
- Chatterjee, A.; Hu, X.; Lam, F.L.-Y. Modified coal fly ash waste as an efficient heterogeneous catalyst for dehydration of xylose to furfural in biphasic medium. Fuel 2019, 239, 726–736. [Google Scholar] [CrossRef]
- Shabani, J.M.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis of hydroxy sodalite from coal fly ash for biodiesel production from waste-derived maggot oil. Catalysts 2019, 9, 1052. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Qu, R.; Liu, S.; Zhao, H.; Wu, W.; Song, H.; Zheng, C.; Wu, X.; Gao, X. Synthesis of zeolites from coal fly ash for removal of harmful gaseous pollutants: A review. Aerosol Air Qual. Res. 2020, 20, 1127–1144. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.K.; Bajpai, S.; Dewangan, U.K.; Tamrakar, R.K. Suitability of leaching test methods for fly ash and slag: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 523–537. [Google Scholar] [CrossRef] [Green Version]
- Ram, L.; Masto, R. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2013, 128, 52–74. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly ash—Waste management and overview: A review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Go, Y.W.; Yeom, S.H. Fabrication of a solid catalyst using coal fly ash and its utilization for producing biodiesel. Environ. Eng. Res. 2018, 24, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Nurfitri, I.; Maniam, G.P.; Hindryawati, N.; Yusoff, M.M.; Ganesan, S. Potential of feedstock and catalysts from waste in biodiesel preparation: A review. Energy Convers. Manag. 2013, 74, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 1–13. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Khalil, N.N.A.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Mashuri, S.I.S.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 1–8. [Google Scholar] [CrossRef]
- Bastos, R.R.C.; Corrêa, A.P.d.; da Luz, P.T.S.; Filho, G.N.d.; Zamian, J.R.; da Conceição, L.R.V. Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Convers. Manag. 2020, 205, 1–12. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Faria, D.N.; Cipriano, D.F.; Schettino, M.A., Jr.; Neto, Á.C.; Cunha, A.G.; Freitas, J.C. Na, Ca-based catalysts supported on activated carbon for synthesis of biodiesel from soybean oil. Mater. Chem. Phys. 2020, 249, 1–9. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Ascaso, S.; Pérez-Rodríguez, S.; Calderón, J.C.; Gálvez, M.E.; Nieto, M.J.; Moliner, R.; Boyano, A.; Sebastián, D.; Alegre, C.; et al. Carbon-based catalysts: Synthesis and applications. Comptes Rendus Chim. 2015, 18, 1229–1241. [Google Scholar] [CrossRef]
- Narowska, B.; Kułażyński, M.; Łukaszewicz, M.; Burchacka, E. Use of activated carbons as catalyst supports for biodiesel production. Renew. Energy 2019, 135, 176–185. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew. Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Chen, Y.; Shi, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification. RSC Adv. 2017, 7, 7250–7258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wei, D.; Ding, W.; Zhang, X. Carbon-based nanostructured catalyst for biodiesel production by catalytic distillation. Catalysis 2014, 43, 121–125. [Google Scholar] [CrossRef]
- Clohessy, J.; Kwapinski, W. Carbon-based catalysts for biodiesel production—A review. Appl. Sci. 2020, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfari, J.; Najafi, B.; Ardabili, S.F.; Shamshirband, S. Limiting factors for the use of palm oil biodiesel in a diesel engine in the context of the ASTM standard. Cogent Eng. 2017, 4, 1–17. [Google Scholar] [CrossRef]
- Specification for Biodiesel (B100)–ASTM D6751-07b 9. Available online: https://www.glycerintraders.com/ASTM%206751%20spec.pdf (accessed on 18 January 2022).
- Biodiesel Standards. Available online: https://www.biofuelsystems.com/biodiesel/specification.htm (accessed on 25 January 2022).
- Demirbas, A. Characterization of biodiesel fuels. Energy Sources Part A 2009, 31, 889–896. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nanocatalyst. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Processing Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, K.S. Biodiesel production process optimization and characterization to assess suitability of product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Farid, M.A.A.; Roslan, A.M.; Hassan, M.A.; Hasan, M.Y.; Othman, M.R.; Shirai, Y. Net energy and techno-economic assessment of biodiesel production from waste cooking oil using a semi-industrial plant: A Malaysia perspective. Sustain. Energy Technol. Assess. 2020, 39, 1–11. [Google Scholar] [CrossRef]
- Ogunkunle, O.; Ahmed, N.A. A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep. 2019, 5, 1560–1579. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Statistical Release (Stats SA). Available online: http://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed on 28 October 2021).
- Statistical Release (Stats SA). Available online: http://www.statssa.gov.za/?p=12056 (accessed on 18 May 2020).
- Fawaz, E.G.; Salam, D.A. Preliminary economic assessment of the use of waste frying oils for biodiesel production in Beirut, Lebanon. Sci. Total Environ. 2018, 637–638, 1230–1240. [Google Scholar] [CrossRef]
- Araujo, V.K.W.S.; Hamacher, S.; Scavarda, L.F. Economic assessment of biodiesel production from waste frying oils. Bioresour. Technol. 2010, 101, 4415–4422. [Google Scholar] [CrossRef]
- Biofuels Industrial Strategy. South Africa. December 2007. Available online: http://www.energy.gov.za/files/esources/renewables/biofuels_indus_strat.pdf(2).pdf (accessed on 17 May 2020).
- Van Zyl, W.H.; Prior, B.A. Chair of Energy Research: Biofuels. 2009. Available online: http://academic.sun.ac.za/biofuels/media%20info/South%20Africa%20Biofuels%20May%202009%20Progress%20Report.pdf (accessed on 18 May 2020).
- Yearbook 2018/2019. Government Communications and Information Systems. South Africa. Available online: https://www.gcis.gov.za/south-africa-yearbook-201819 (accessed on 18 May 2020).
- Mineral and Petroleum Resources Development Act No. 28 of 2002. South Africa. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/a28-020.pdf (accessed on 18 May 2020).
- The Constitution of the Republic of South Africa. 1996. Available online: http://www.saflii.org/za/legis/num_act/cotrosa1996423/ (accessed on 22 January 2020).
- National Energy Act No. 34 of 2008. South Africa. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/316381263.pdf (accessed on 17 May 2020).
- National Environmental Management Waste Act 59 of 2008. South Africa. Available online: http://www.saflii.org/za/legis/consol_reg/fsfcapoafwmltart1085.pdf (accessed on 17 May 2020).
- Directorate. Overview of the Petrol and Diesel Market in South Africa between 2007 and 2016; Energy Data Collection, Management and Analysis 2017; Department of Energy: Pretoria, South Africa, 2017; ISBN 978-1-920435-11-0.
- National Development Plan 2030. South Africa. Available online: https://www.gov.za/issues/national-development-plan-2030 (accessed on 17 May 2020).
- Government Notices, Department of Mineral Resources and Energy. Pretoria, South Africa. 7 February 2020. Available online: http://www.energy.gov.za/files/policies/petroleum/Biofuels-Regulatory-Framework-and-National-Biofuels-Feedstock-Protocol.pdf (accessed on 17 May 2020).
| Properties | ASTM D 6751 Limit Method | EN 14214 Limit Method | ||
|---|---|---|---|---|
| Density @ 15 °C | 870–890 kg/m3 | ASTM D4052–91 | 860–900 kg/m3 | EN ISO 3675, EN ISO 121 85 |
| Viscosity @ 40 °C | 1.9–6.0 mm2/s | ASTM D445 | 3.5–5.0 mm2/s | EN ISO 3140 |
| Water content and sediment | 0.050 (%v) max | ASTM D2709 | 500 mg/kg (max) | EN ISO 12937 |
| Flash point | 130 °C (min) | ASTM D93 | >101 °C (min) | EN ISO 3679 |
| Cloud point | Report to customer | ASTM D2500 | Based on national specification | EN ISO 23015 |
| Calorific value | 35 MJ/kg | ASTM D240 | ||
| Cetane number | 47 (min) | ASTM D613 | 51 (min) | EN ISO 5165 |
| Acid value | 0.50 mg KOH/g (max) | ASTM D664 | 0.50 mg KOH/g (max) | EN 14104 |
| Iodine value | 120 g/100 g (max) | EN 14111 | ||
| Total glycerol | 0.24% m/m (max) | ASTM D6548 | 0.25% m/m | EN 14105 |
| Methanol content | 0.20% m/m (max) | EN 14110 | ||
| Carbon residue | 0.05 max wt% | ASTM D4530 | 0.30% m/m (max) | EN ISO 10370 |
| Sulphated ash content | 0.020% m/m (max) | ASTM D874 | 0.02% m/m (max) | EN ISO 3987 |
| Province | Population | % | Square Kilometres | 2017 SA Economy Contribution by Province [116] (%) |
|---|---|---|---|---|
| Gauteng | 15,810,338 | 26.3 | 18,178 | 34 |
| KwaZulu-Natal | 11,513,575 | 19.1 | 94,361 | 16 |
| Western Cape | 7,113,776 | 11.8 | 129,462 | 14 |
| Eastern Cape | 6,676,590 | 11.1 | 168,966 | 8 |
| Limpopo | 5,926,724 | 9.8 | 125,755 | 7 |
| Mpumalanga | 4,743,584 | 7.9 | 76,495 | 8 |
| North West | 4,122,854 | 6.9 | 104,882 | 6 |
| Free State | 2,932,441 | 4.9 | 129,825 | 5 |
| Northern Cape | 1,303,047 | 2.2 | 372,889 | 2 |
| Total: 60,142,949 | Total: 1,220,813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linganiso, E.C.; Tlhaole, B.; Magagula, L.P.; Dziike, S.; Linganiso, L.Z.; Motaung, T.E.; Moloto, N.; Tetana, Z.N. Biodiesel Production from Waste Oils: A South African Outlook. Sustainability 2022, 14, 1983. https://doi.org/10.3390/su14041983
Linganiso EC, Tlhaole B, Magagula LP, Dziike S, Linganiso LZ, Motaung TE, Moloto N, Tetana ZN. Biodiesel Production from Waste Oils: A South African Outlook. Sustainability. 2022; 14(4):1983. https://doi.org/10.3390/su14041983
Chicago/Turabian StyleLinganiso, Ella Cebisa, Boitumelo Tlhaole, Lindokuhle Precious Magagula, Silas Dziike, Linda Zikhona Linganiso, Tshwafo Elias Motaung, Nosipho Moloto, and Zikhona Nobuntu Tetana. 2022. "Biodiesel Production from Waste Oils: A South African Outlook" Sustainability 14, no. 4: 1983. https://doi.org/10.3390/su14041983
APA StyleLinganiso, E. C., Tlhaole, B., Magagula, L. P., Dziike, S., Linganiso, L. Z., Motaung, T. E., Moloto, N., & Tetana, Z. N. (2022). Biodiesel Production from Waste Oils: A South African Outlook. Sustainability, 14(4), 1983. https://doi.org/10.3390/su14041983








