Effects of Hydrostatic Dissolution and Seepage on the Transport and Mechanical Properties of Glauberite
Abstract
1. Introduction
2. Methods
2.1. Seepage and Dissolution in Glauberite
2.2. Equipment
2.3. Test Specimens
2.4. Test Procedure
3. Test Results
3.1. Dissolution Characteristics of Glauberite Rock
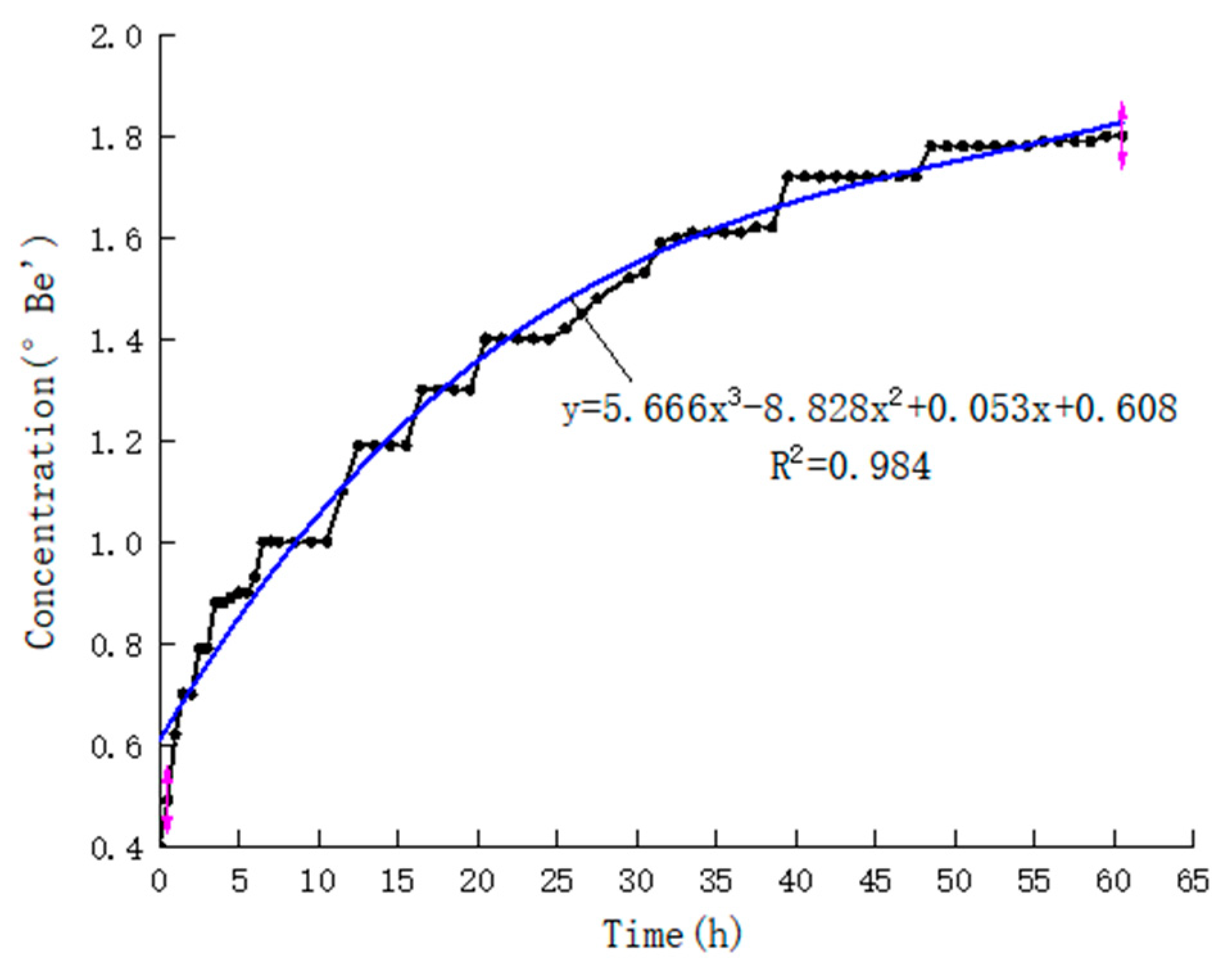
3.1.1. Continuous Hydrostatic Leaching Test
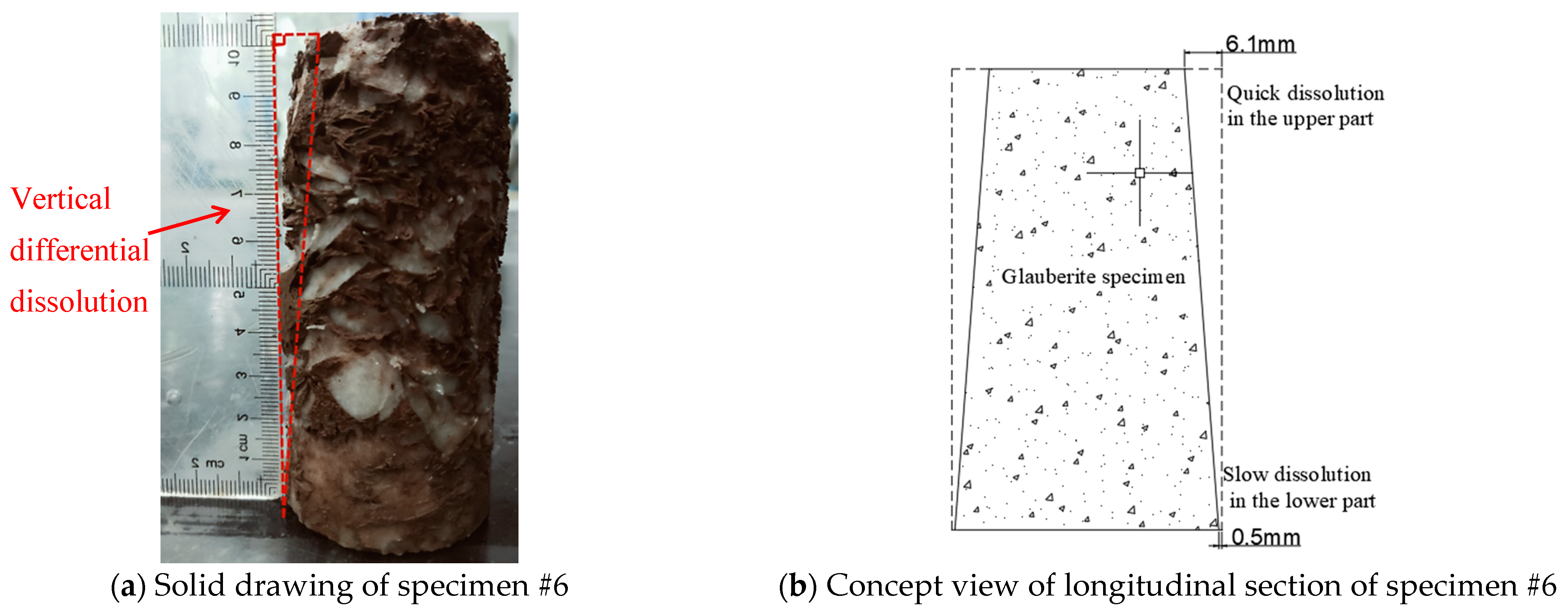
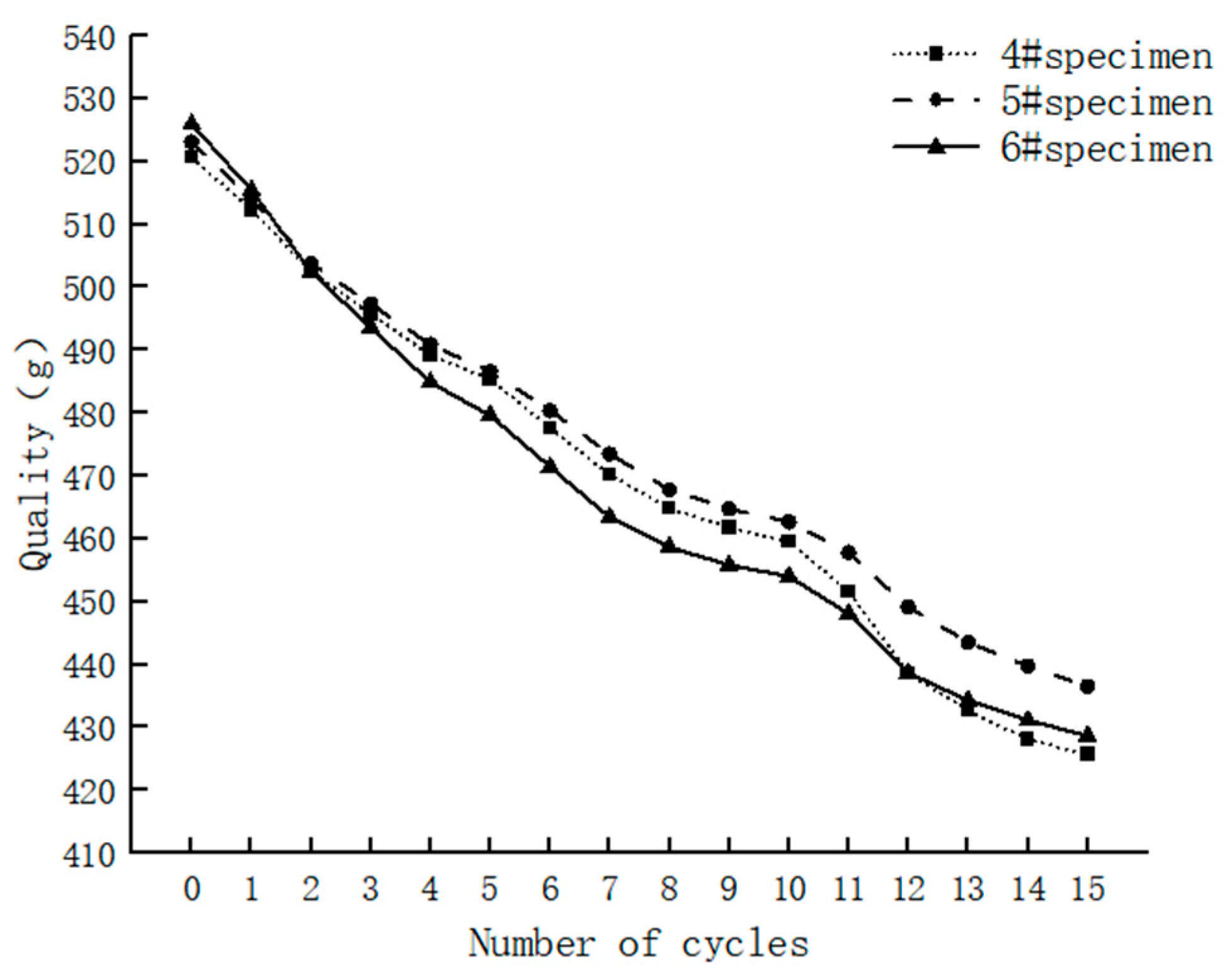
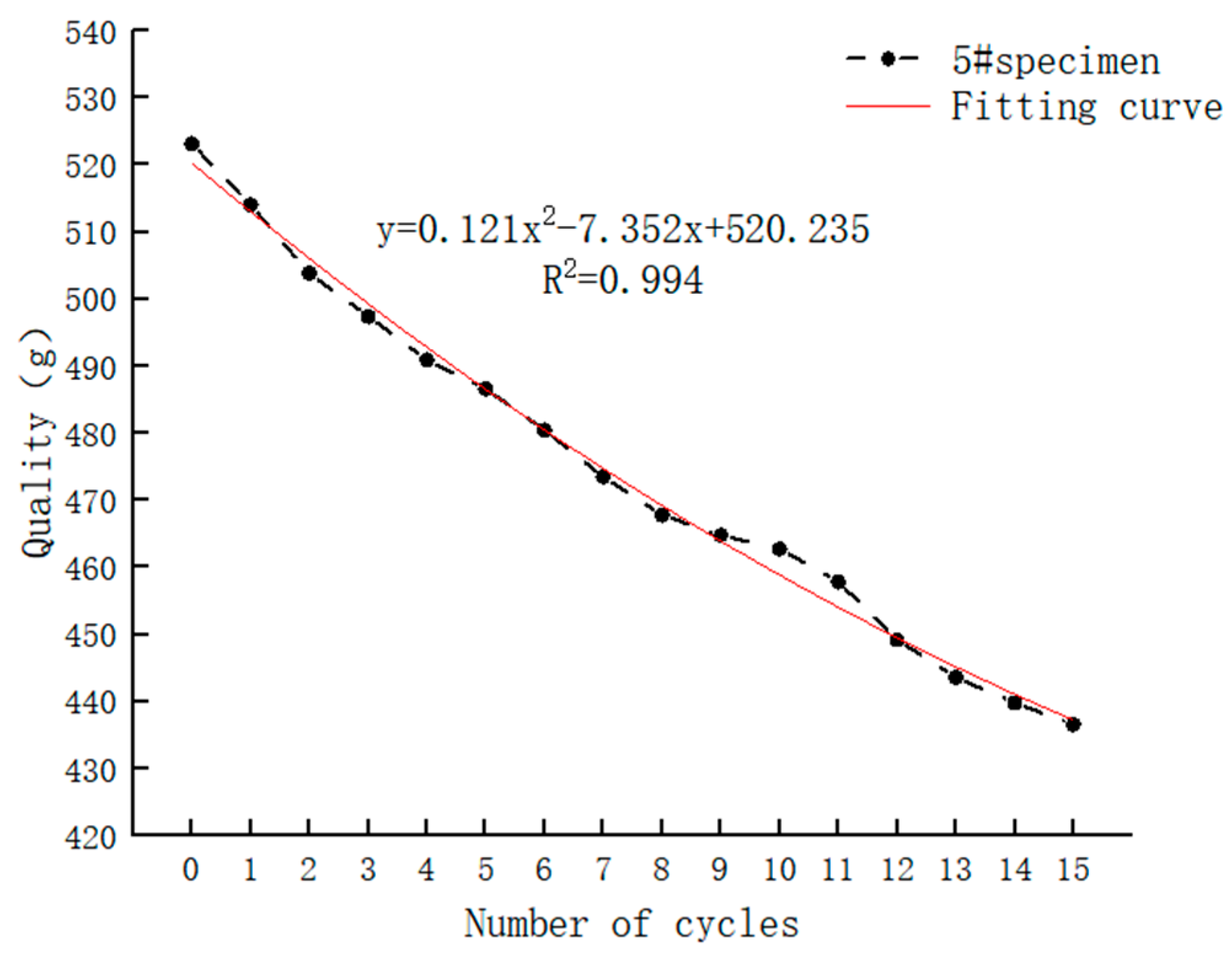
3.1.2. Cyclic Hydrostatic Leaching Test
3.2. Transport Properties of Glauberite
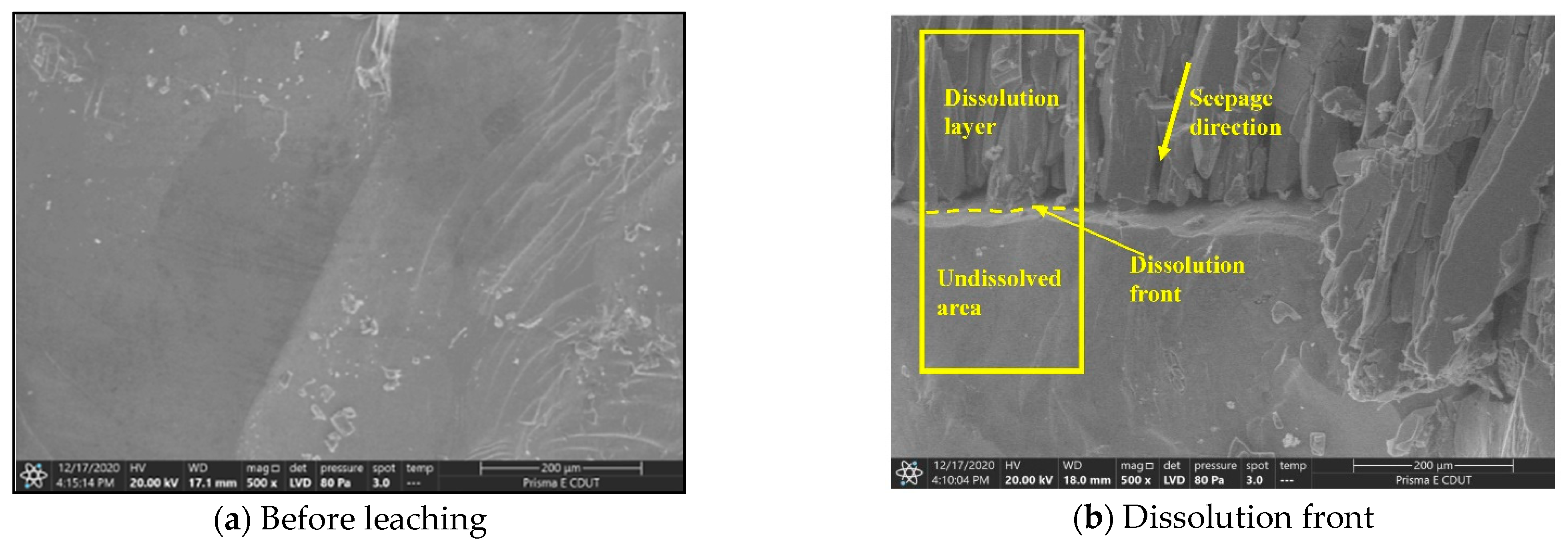
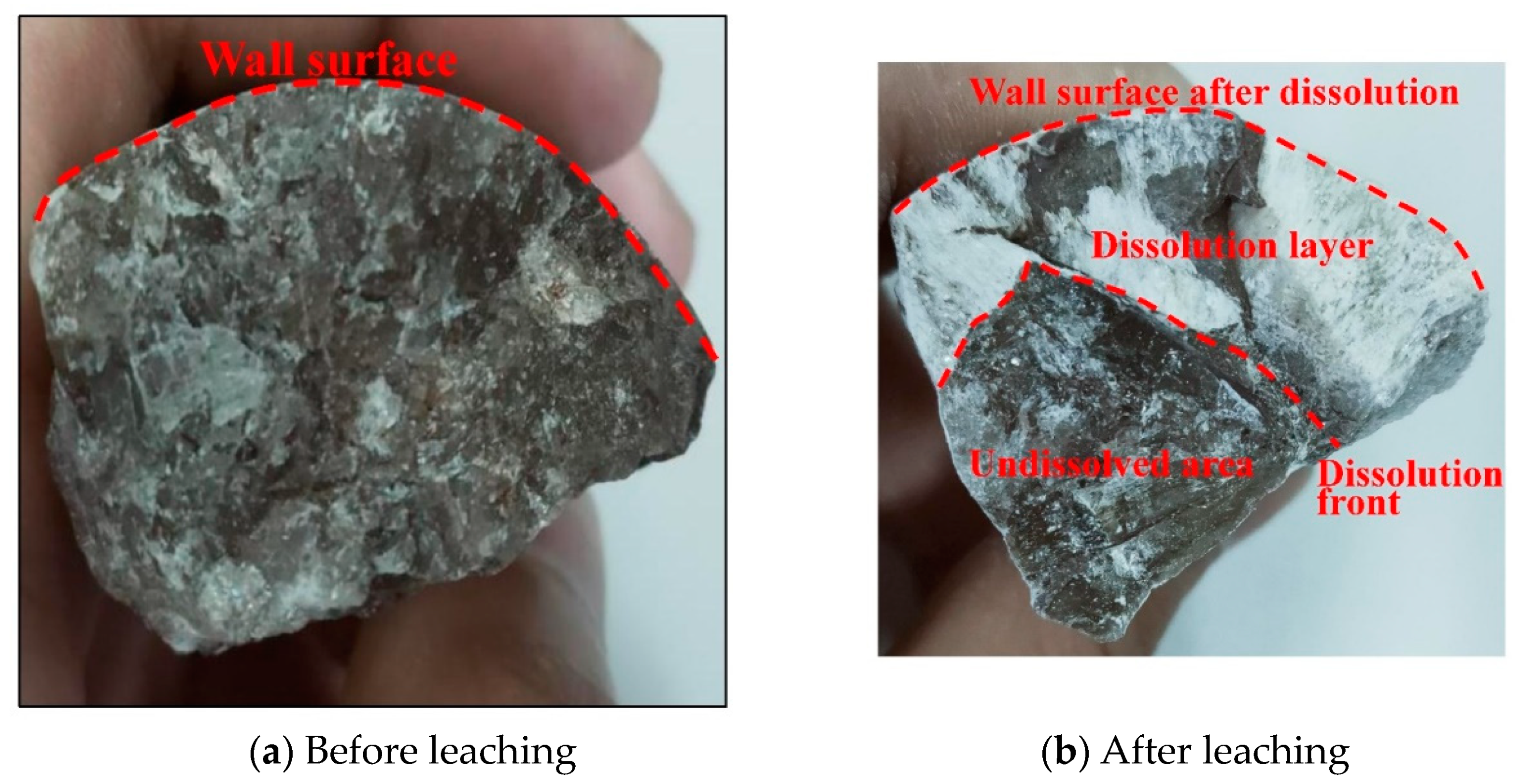
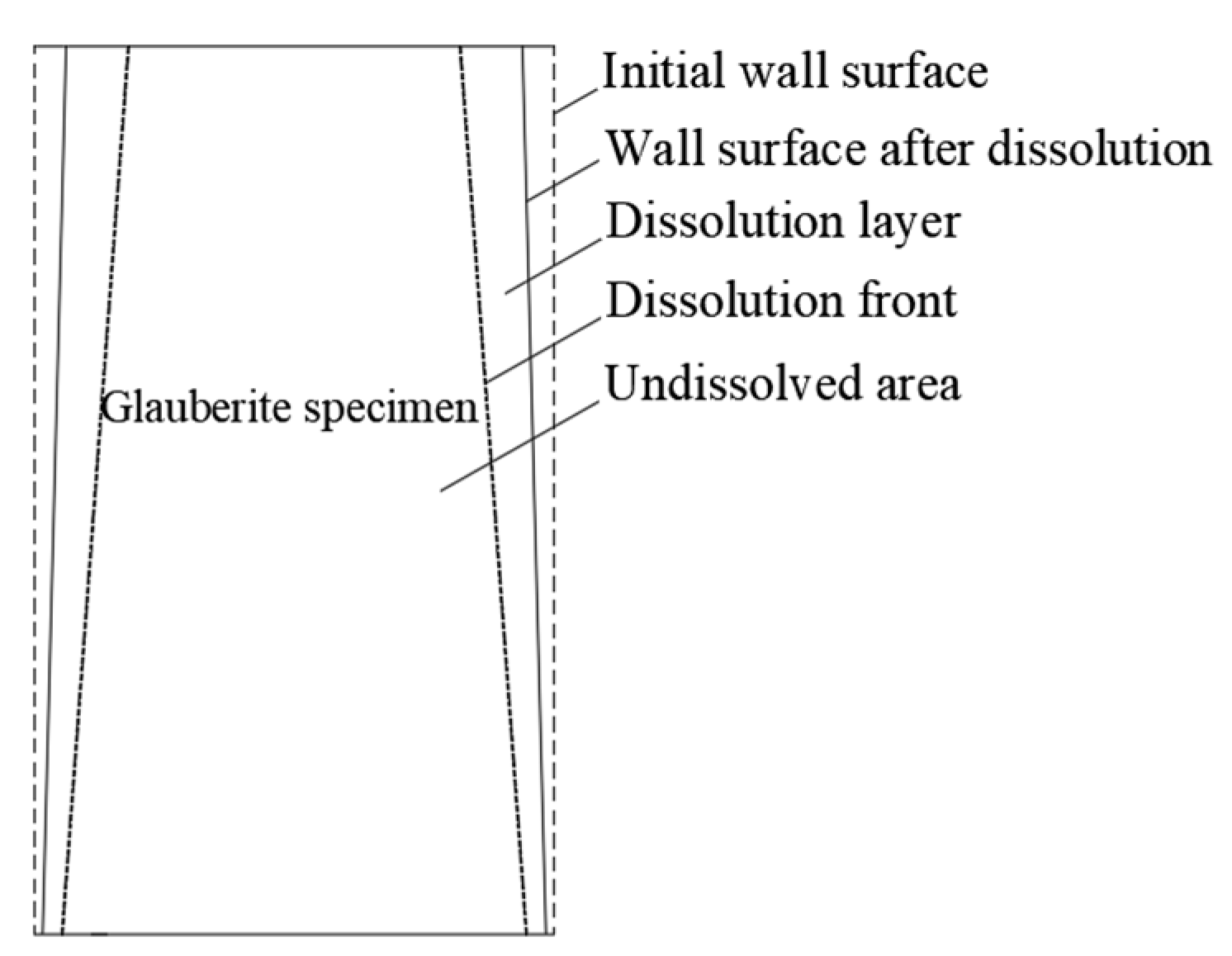
3.2.1. Dissolution and Seepage Mechanism
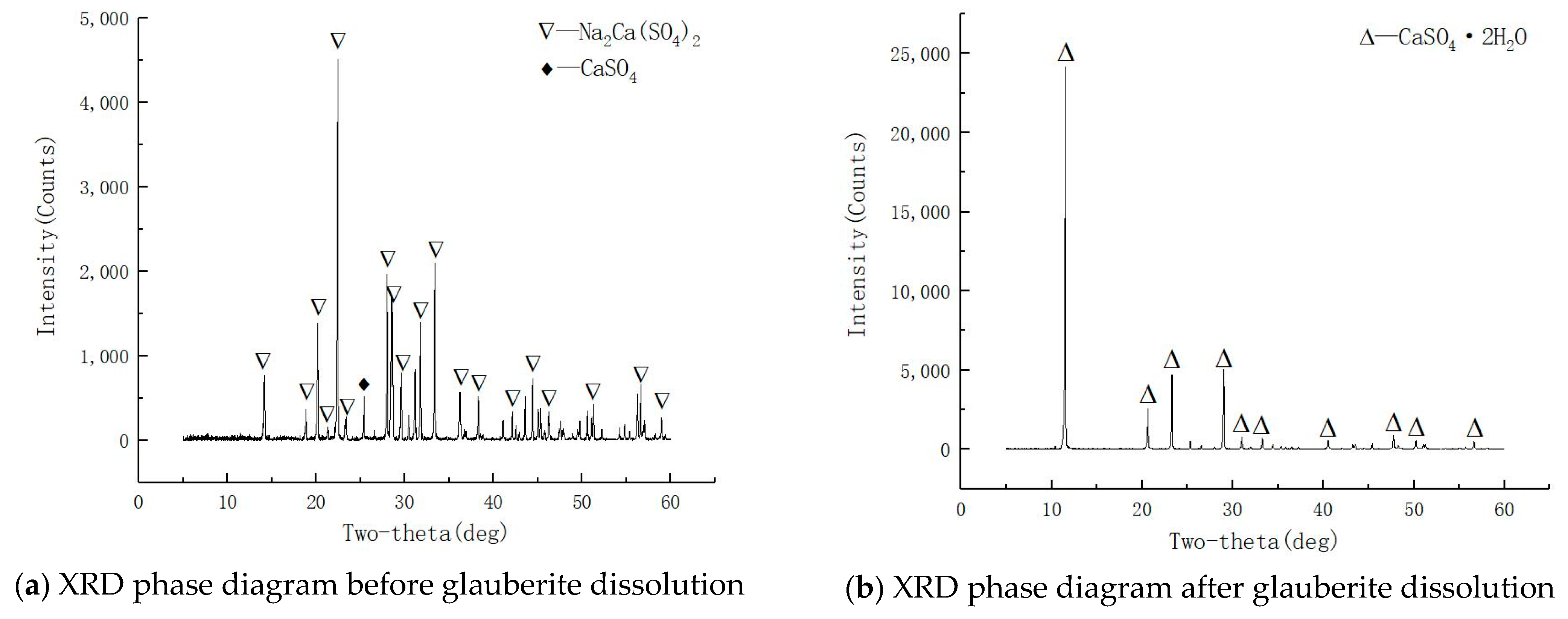
3.2.2. Phase Change Characteristics
3.2.3. Transport Properties of Glauberite
3.3. Mechanical Properties of Glauberite Rock
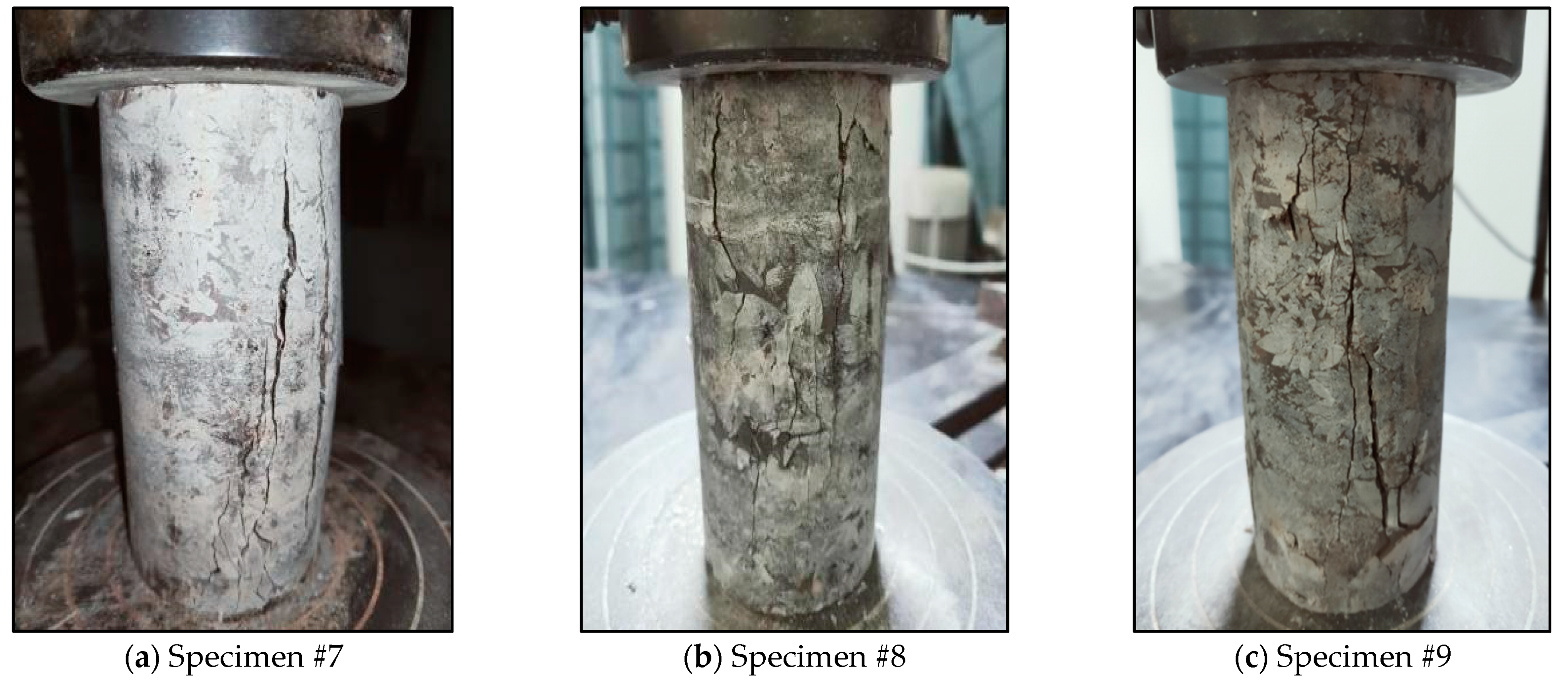
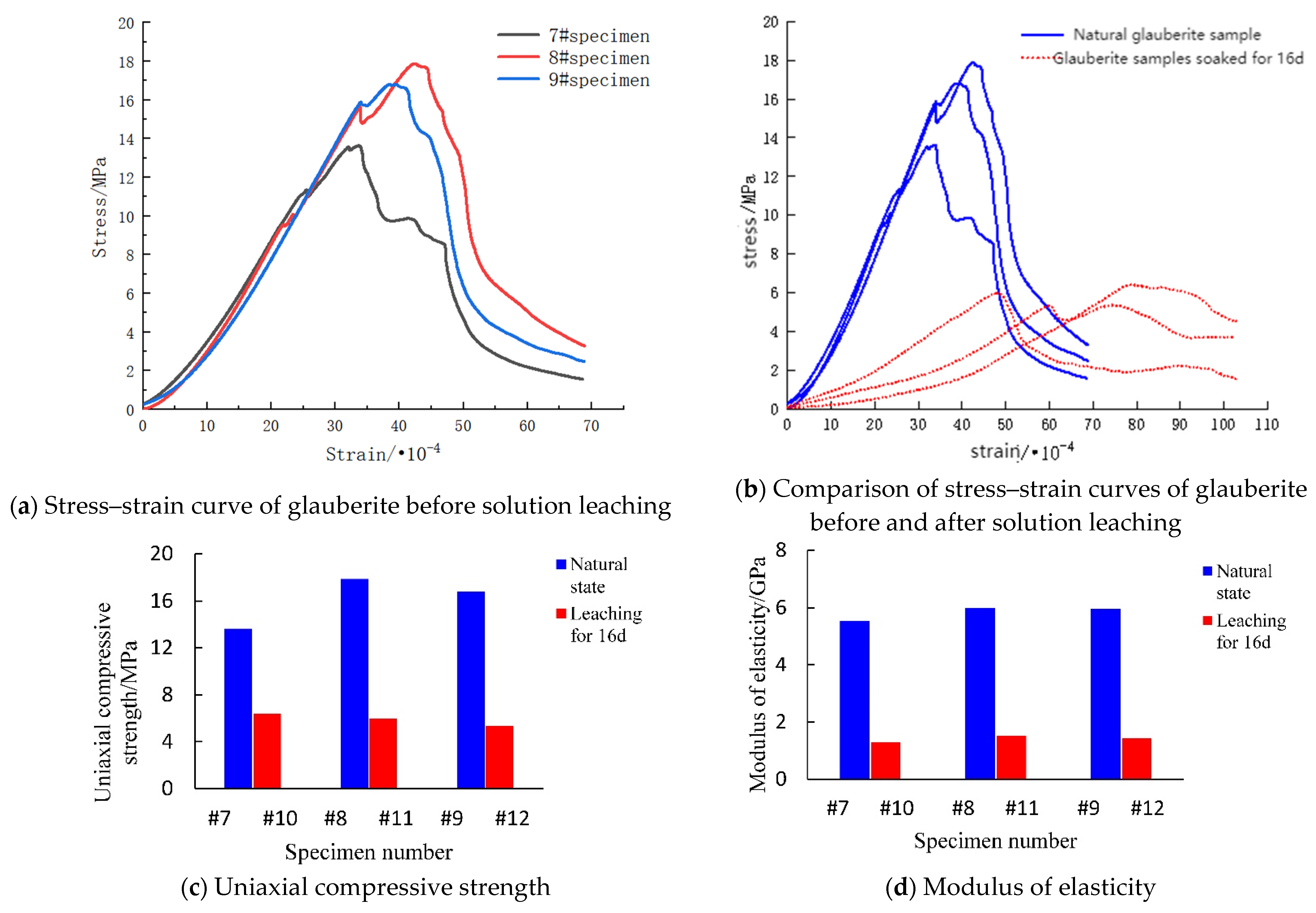
3.3.1. Mechanical Properties before Dissolution
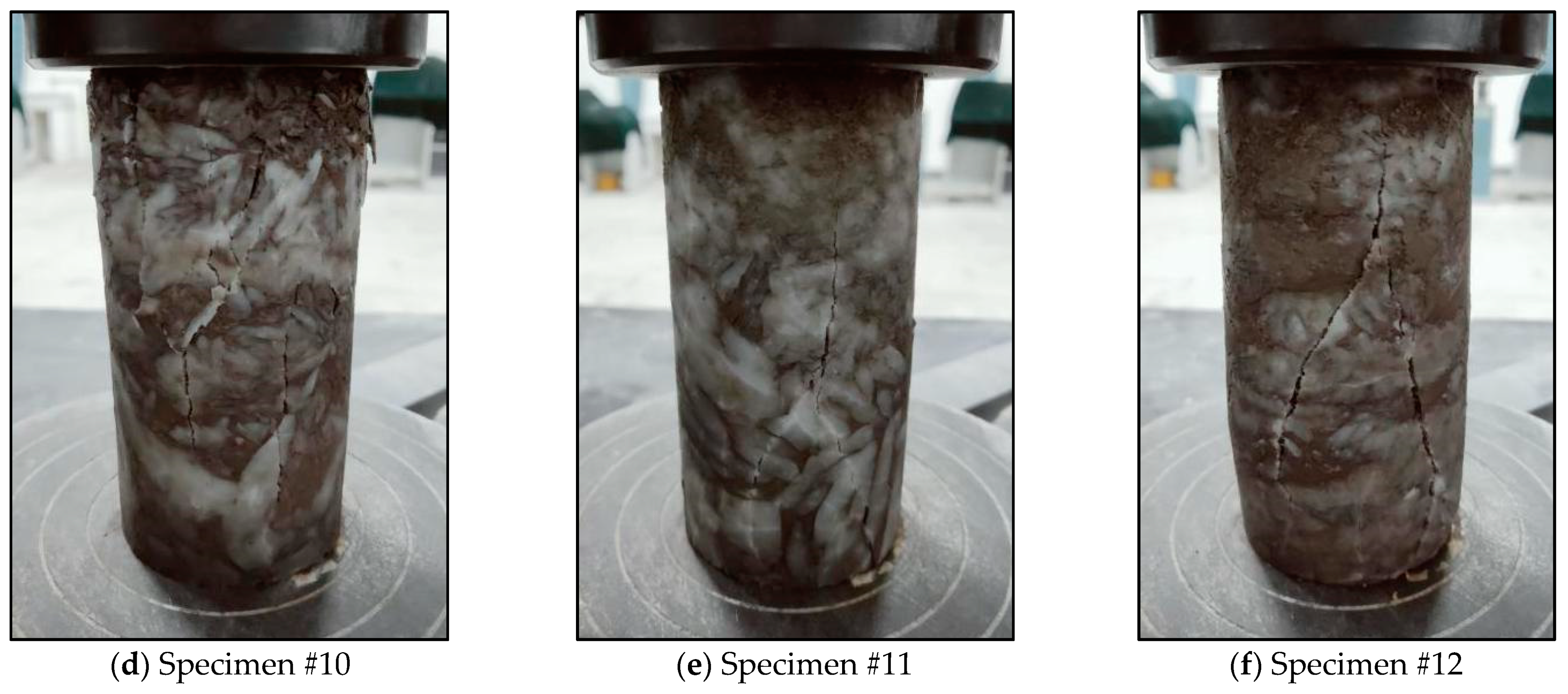
3.3.2. Mechanical Properties after Dissolution
4. Discussion
4.1. Dissolution Process Mechanism of Glauberite Rock under Hydrostatic Conditions
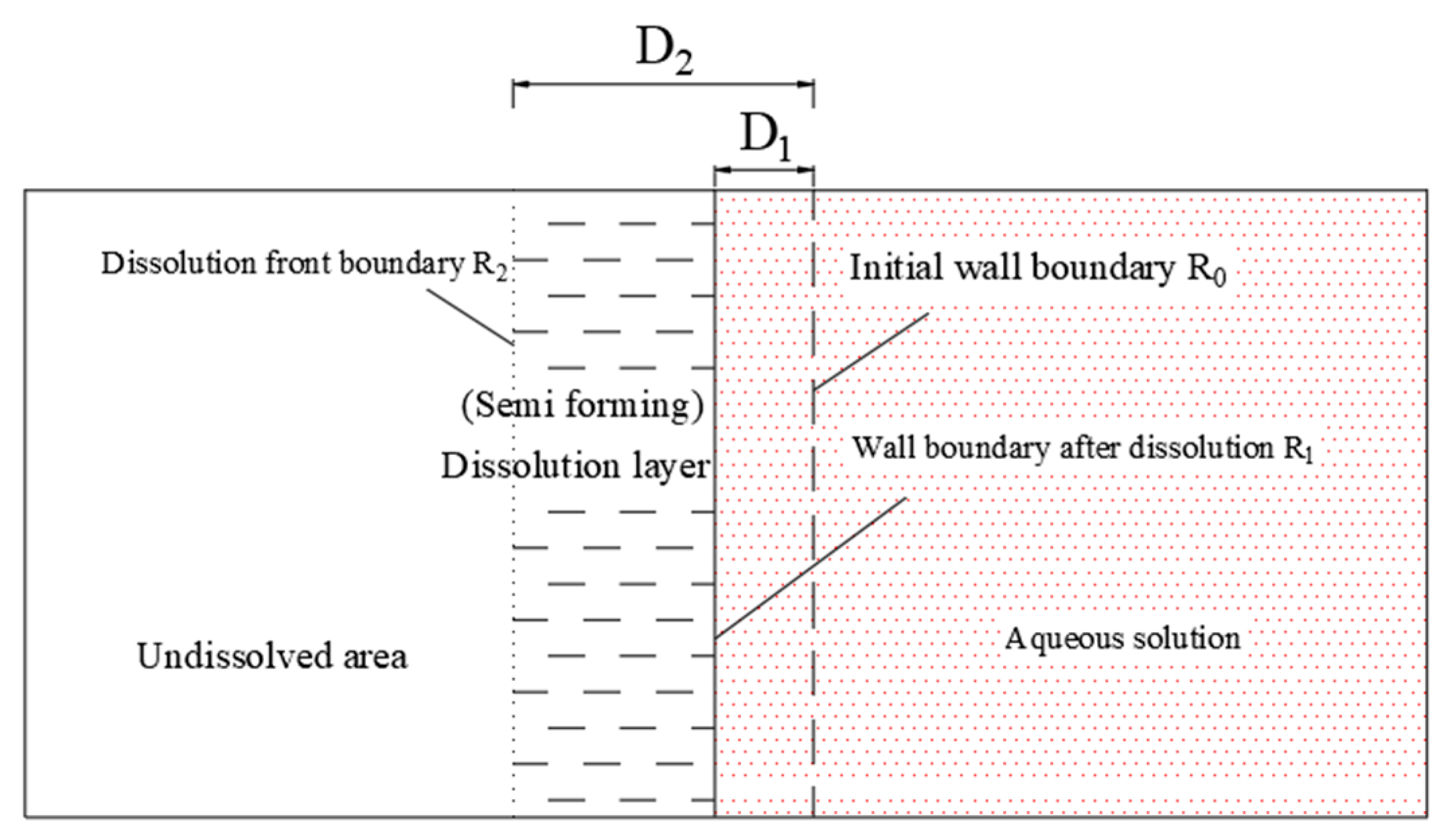
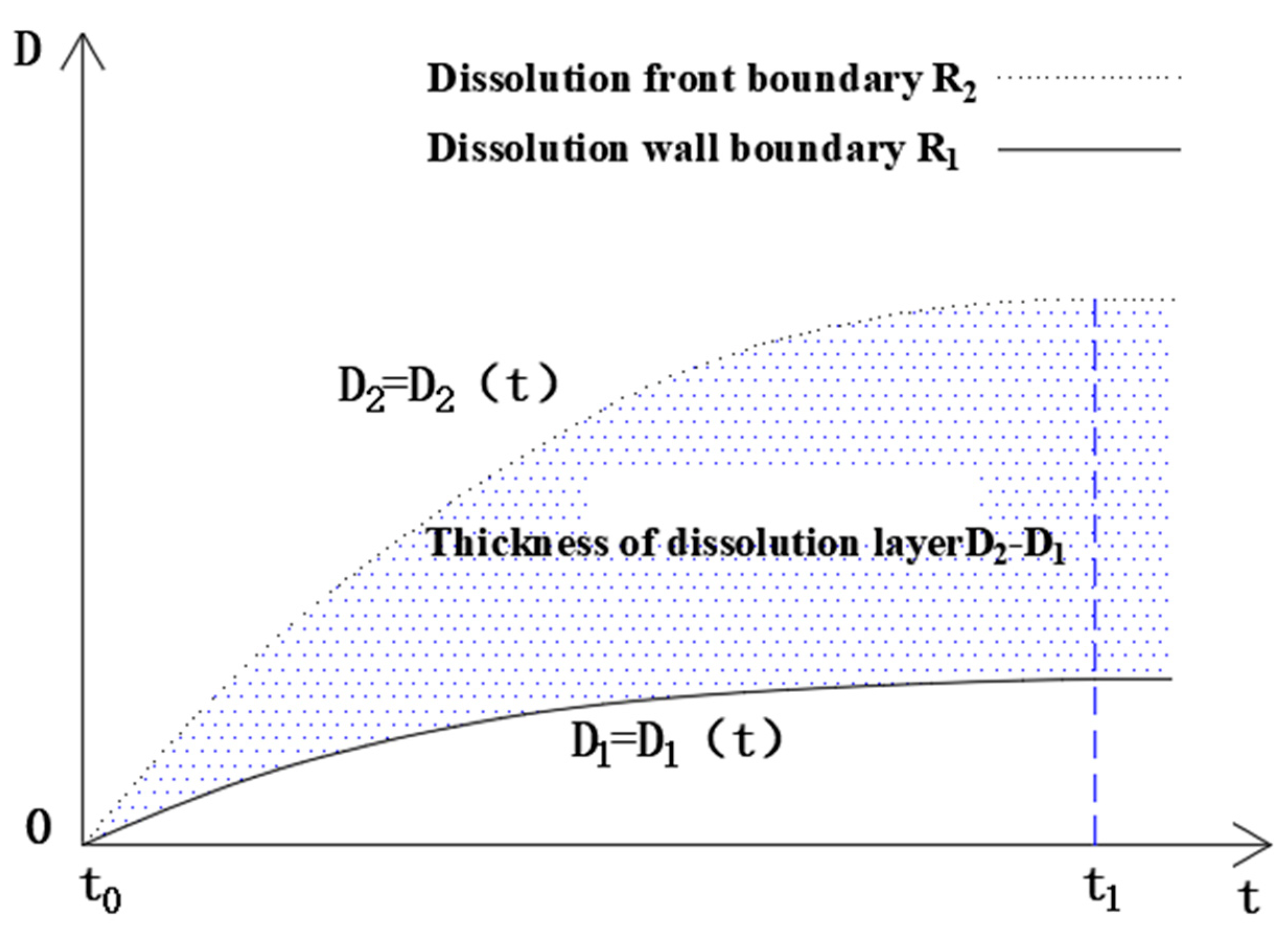
4.2. Dissolution Boundary of Glauberite Rock
4.3. Impacts
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiers, C.J.; Lister, G.S.; Zwart, H.J. The influence of fluid-rock interaction on the rheology of salt rock and on ionic transport in the salt. In Technical Session on Rock Mechanics Advance in Laboratory Sample Testing; Commission European Communities: Luxembourg, 1984; pp. 268–280. [Google Scholar]
- Spiers, C.J.; Urai, J.L.; Lister, G.S. The effect of brine (inherent of added) on rheology and deformation mechanisms in salt rock. In The Mechanical Behavior of Salt; Conference Paper; Trans Tech publ: Clausthal-Zellerfeld, Germany, 1988; Volume 2, pp. 89–102. [Google Scholar]
- Peach, C.J.; Spiers, C.J. Influence of crystal plastic deformation on dilatancy and permeability development in synthetic salt rock. Tectonophysics 1996, 256, 101–128. [Google Scholar] [CrossRef]
- Kettel, D. The dynamics of gas flow through rock salt in the scope of time. In Norwegian Petroleum Society Special Publications; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7, pp. 175–185. [Google Scholar]
- Lux, K.H.; Heusermann, S. Creep tests on rock salt with changing load as a basis for the verification of theoretical material laws. In Proceedings of the 6th International Symposium on Salt Symposium, Salt Institute, Portland, ME, USA, 12–14 October 1983; Volume 1, pp. 417–435. [Google Scholar]
- Senseny, P.E. Determination of a Constitutive Law for Salt at Elevated Temperature and Pressure; ASTM International: West Conshohocken, PA, USA, 1985; pp. 55–71. [Google Scholar]
- Cristescu, N.D. A general constitutive equation for transient and stationary creep of rock salt. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1993, 30, 125–140. [Google Scholar] [CrossRef]
- Cristescu, N.D.; Paraschiv, I. Creep, damage and failure around large rectangular-like caverns and galleries. Mech. Cohesive Frict. Mater. Int. J. Exp. Model. Comput. Mater. Struct. 1996, 1, 165–197. [Google Scholar] [CrossRef]
- Hampel, A.; Hunsche, U.; Weidinger, P.; Blum, W. Description of the Creep of Rock Salt with the Composite Model-II. Steady-State Creep; Series on rock and soil mechanics; Trans Tech publ: Clausthal-Zellerfeld, Germany, 1998; pp. 287–299. [Google Scholar]
- Zhang, C.; Liang, W.; Li, Z.; Xu, S.; Zhao, Y. Observations of acoustic emission of three salt rocks under uniaxial compression. Int. J. Rock Mech. Min. Sci. 2015, 77, 19–26. [Google Scholar] [CrossRef]
- Jiang, T.; Shao, J.F.; Xu, W.Y.; Zhou, C.B. Experimental investigation and micromechanical analysis of damage and permeability variation in brittle rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 703–713. [Google Scholar] [CrossRef]
- Liang, W.G.; Zhao, Y.S.; Xu, S.G.; Dusseault, M.B. Effect of strain rate on the mechanical properties of salt rock. Int. J. Rock Mech. Min. Sci. 2011, 48, 161–167. [Google Scholar] [CrossRef]
- Yu, Y.M.; Liang, W.G.; Liu, J.S. Influence of solution concentration and temperature on the dissolution process and internal structure of glauberite. Int. J. Min. Met. Mater. 2018, 25, 1246–1255. [Google Scholar] [CrossRef]
- Petracchini, L.; Antonellini, M.; Billib, A.; Scrocca, D.; Trippetta, F.; Mollo, S. Pressure solution inhibition in a limestone-chert composite multilayer: Implications for the seismic cycle and fluid flow. Tectonophysics 2015, 646, 96–105. [Google Scholar] [CrossRef]
- Meer, S.D.; Spiers, C.J.; Peach, C.J. Kinetics of precipitation of gypsum and implication for pressure-solution creep. J. Geol. Soc. Lond. 2000, 157, 269–281. [Google Scholar] [CrossRef]
- Schenk, O.; Urai, J.L. Microstructural evolution and grain boundary structure during static rescystallization in synthetic polycrystals of sodium chloride containing saturated brine. Contrib. Miner. Petrol. 2004, 146, 671–682. [Google Scholar] [CrossRef]
- Roded, R.; Paredes, X.; Holtzman, R. Reactive transport under stress: Permeability evolution in deformable porous media. Earth Planet. Sci. Lett. 2018, 493, 198–207. [Google Scholar] [CrossRef]
- Urai, J.L.; Spiers, C.J.; Zwart, H.J.; Lister, G.S. Weakening of rock salt by water during long-term creep. Nature 1986, 324, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.D. Calcite Dissolution under Turbulent Flow Condit ions: A Remaining Conundrum. Acta Carsologica 2014, 43, 195–202. [Google Scholar] [CrossRef]
- Durie, R.W.; Jessen, F.W. The laminar boundary layer in the free convection dissolution of salt. In Proceedings of the Paper in the 2nd Symposium on Salt, Cleveland, OH, USA, May 1962. [Google Scholar]
- De Las Cuevas, C. Pore structure characterization in rock salt. Eng. Geol. 1997, 47, 17–30. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Wang, J.; Li, D.; Li, P.; Yang, Z. A dynamic dissolution model of rock salt under gravity for different flow rates. Arab. J. Geosci. 2016, 9, 226. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Xu, S.; Dusseault, M.B. Dissolution and seepage coupling effect on transport and mechanical properties of glauberite salt rock. Transp. Porous Media 2008, 74, 185–199. [Google Scholar] [CrossRef]
- Rong, K.; Wu, A.; Yin, S.; Li, H. Mineral CO2 Sequestration by Carbonation of Glauberite. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 032092. [Google Scholar] [CrossRef]
- Schulze, O. Investigations on damage and healing of rock salt. In The Mechanical Behavior of Salt–Understanding of THMC Processes in Salt; CRC Press: Boca Raton, FL, USA, 2017; pp. 33–43. [Google Scholar]
- Minkley, W.; Mühlbauer, J. Constitutive models to describe the mechanical behavior of salt rocks and the imbedded weakness planes. In The Mechanical Behavior of Salt–Understanding of THMC Processes in Salt; CRC Press: Boca Raton, FL, USA, 2017; pp. 119–127. [Google Scholar]
- Cui, S.; Pei, X.; Jiang, Y.; Wang, G.; Fan, X.; Yang, Q.; Huang, R. Liquefaction within a bedding fault: Understanding the initiation and movement of the Daguangbao landslide triggered by the 2008 Wenchuan Earthquake (Ms = 8.0). Eng. Geol. 2021, 295, 106455. [Google Scholar] [CrossRef]
- Hampel, A.; Schulze, O. The composite dilatancy model: A constitutive model for the mechanical behavior of rock salt. In The Mechanical Behavior of Salt–Understanding of THMC Processes in Salt; CRC Press: Boca Raton, FL, USA, 2017; pp. 99–107. [Google Scholar]
- Noiriel, C. Resolving time-dependent evolution of pore-scale structure, permeability and reactivity using X-ray microtomography. Rev. Mineral. Geochem. 2015, 80, 247–285. [Google Scholar] [CrossRef]
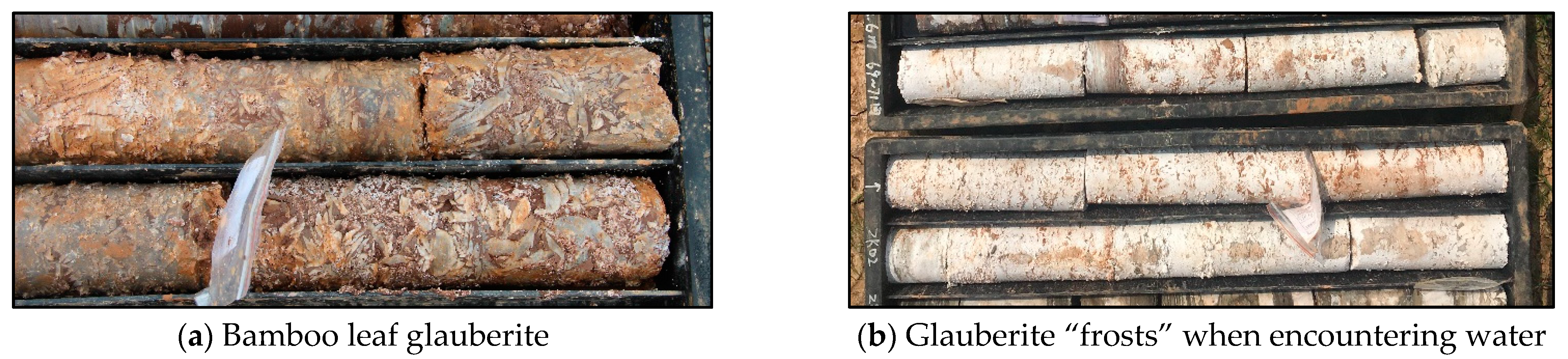
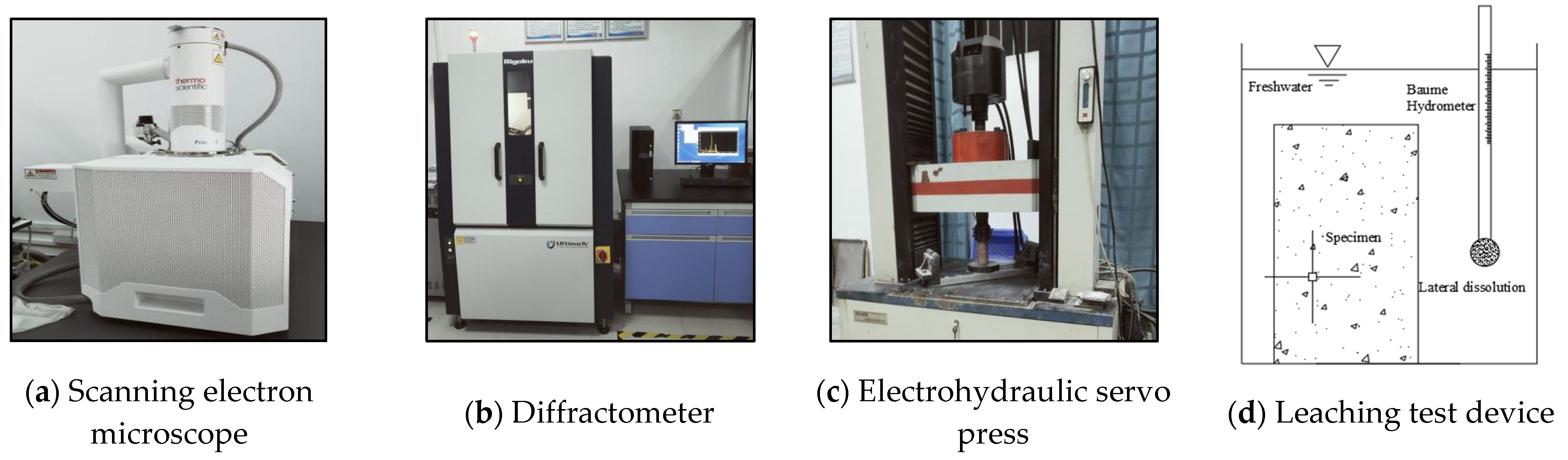
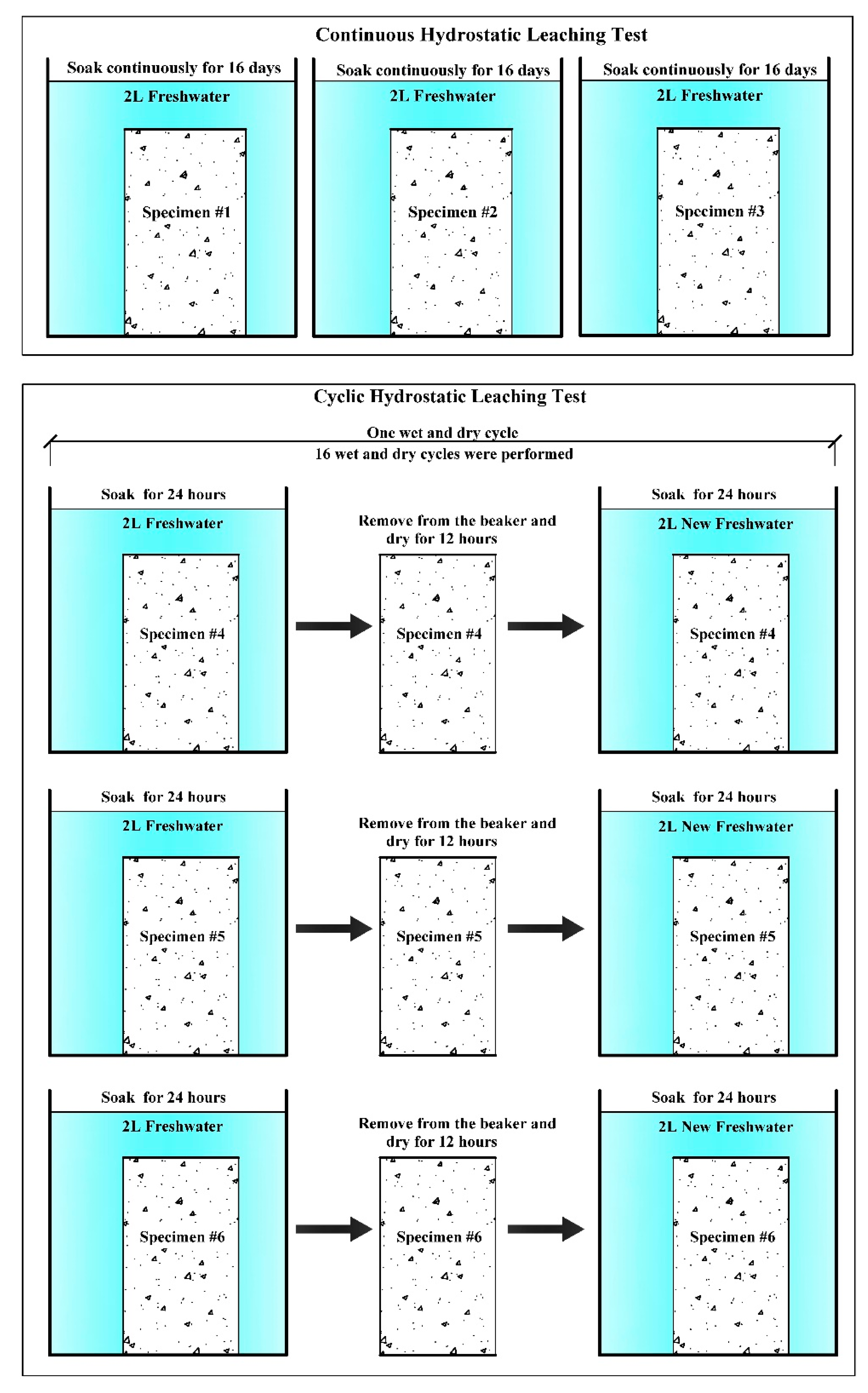

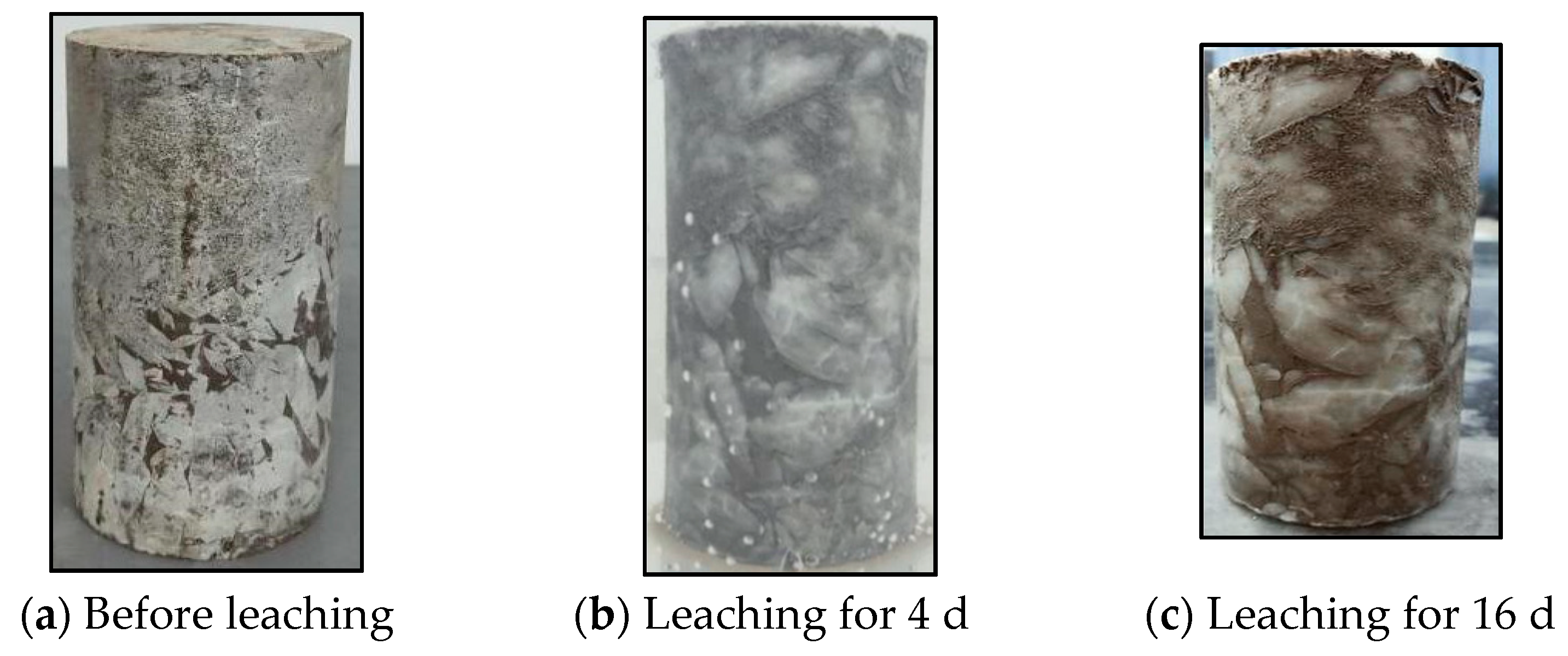
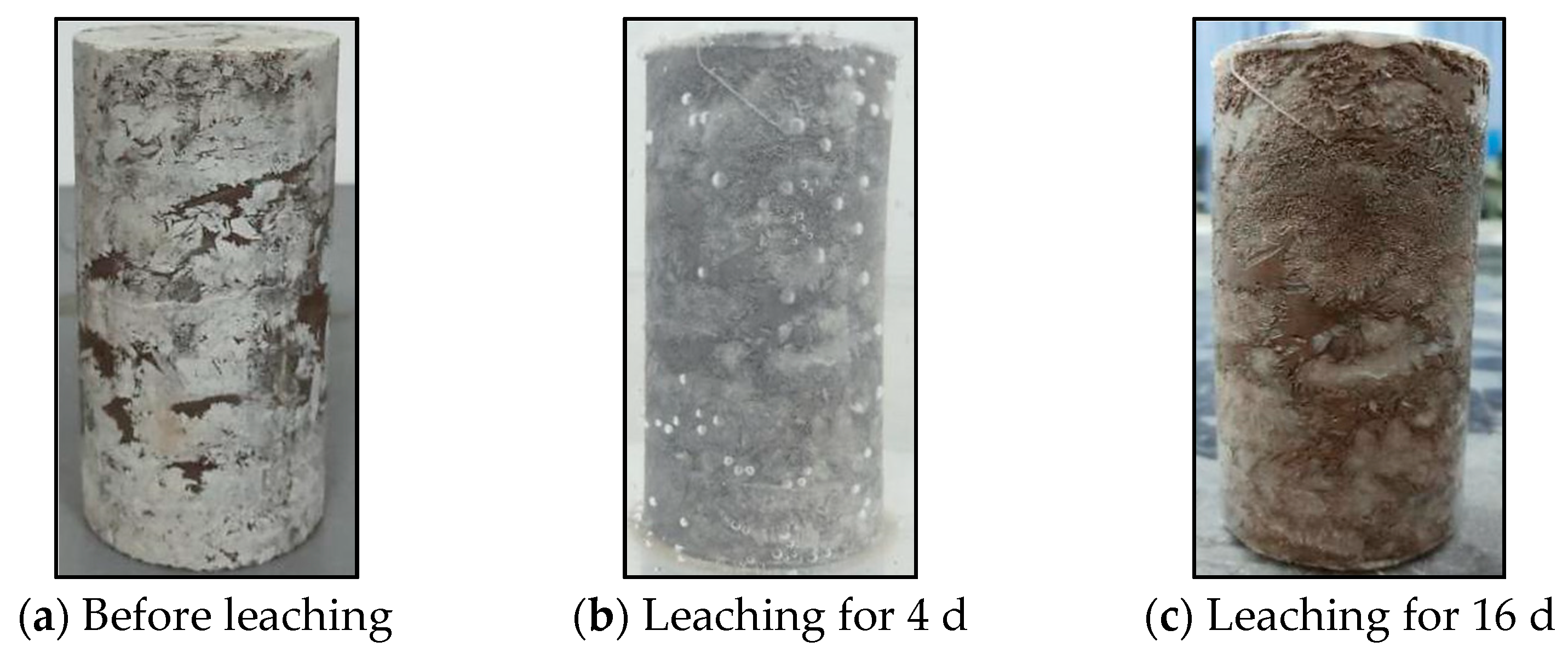
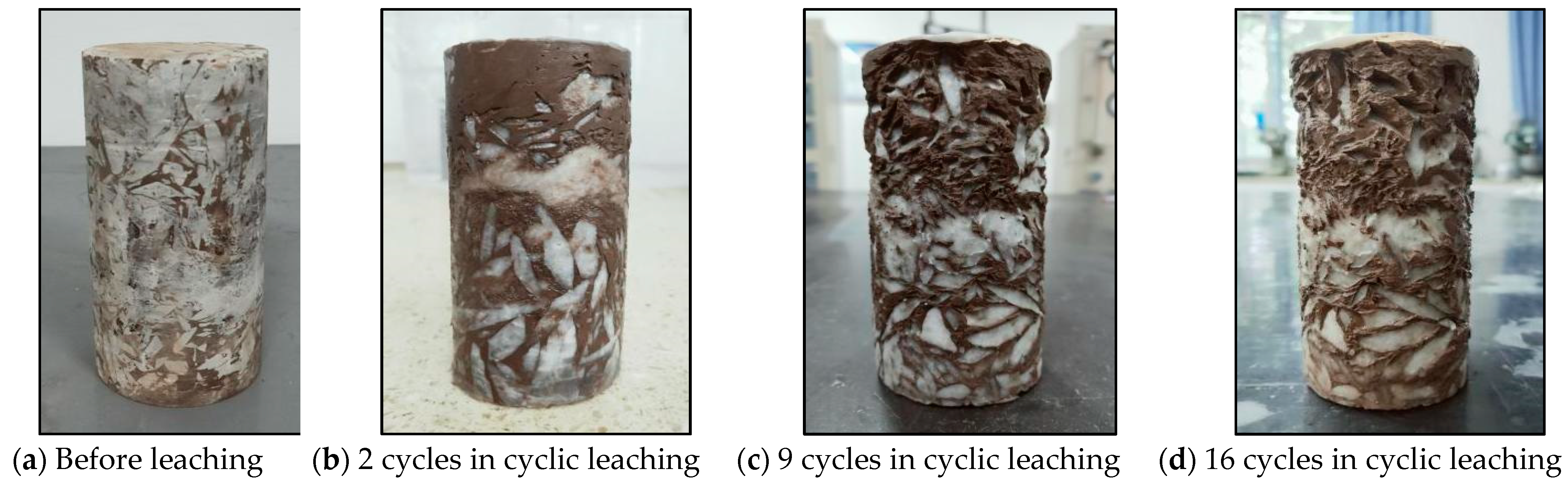
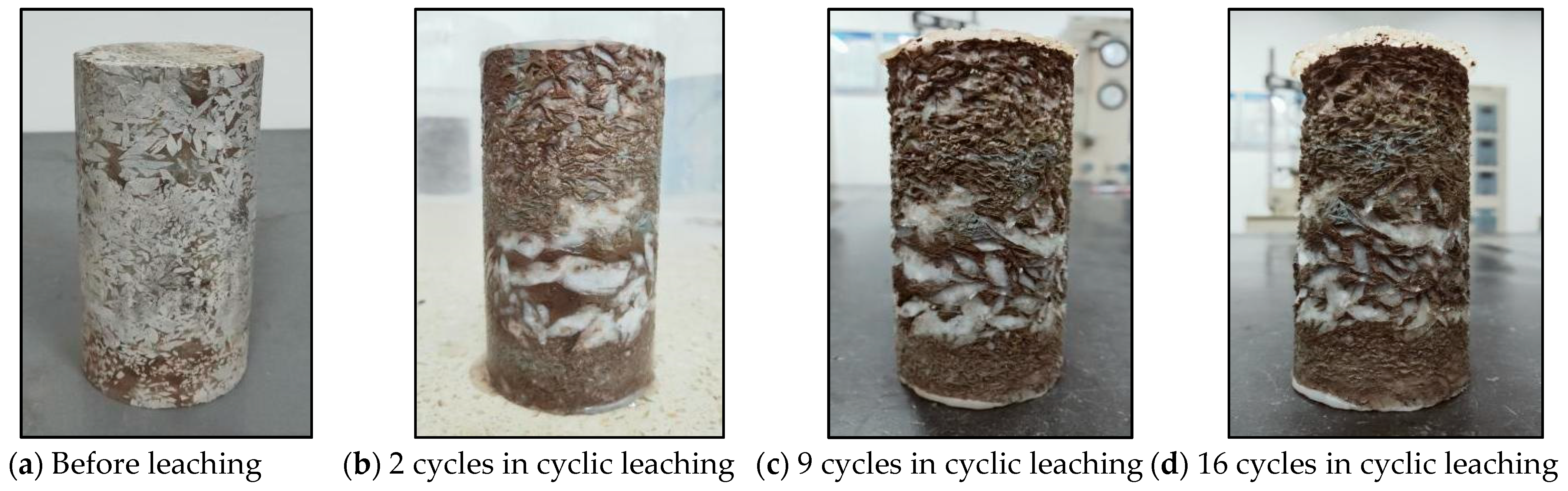
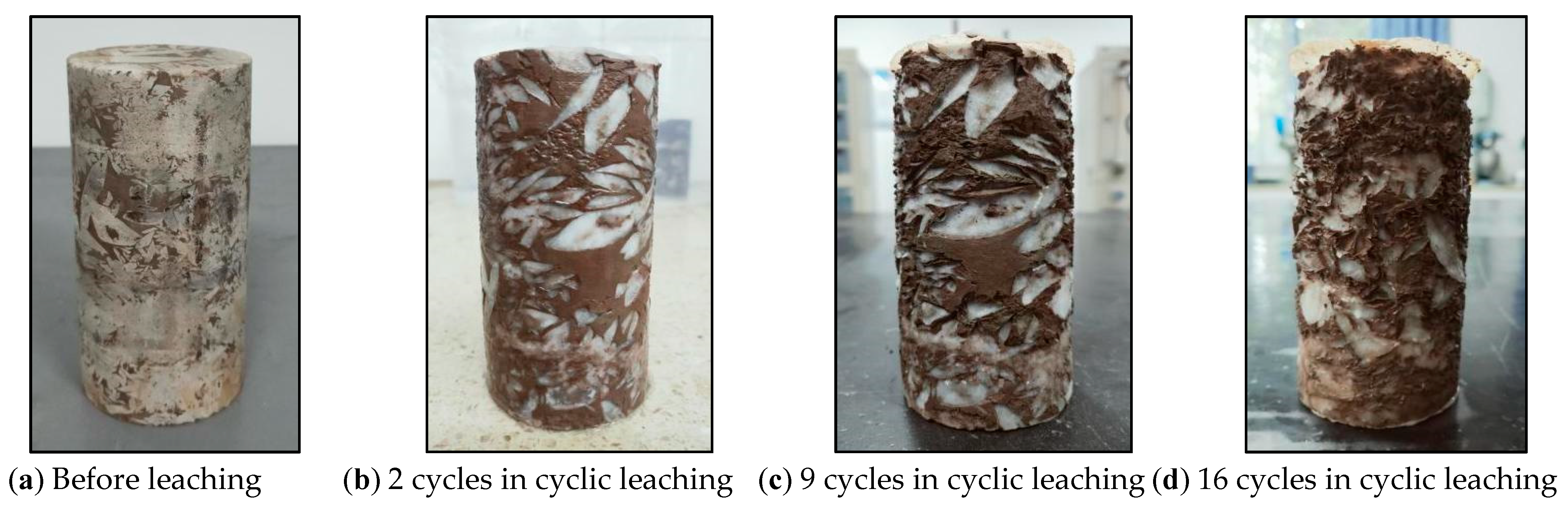



| Number | Test Name | Specimen | Description | Environment |
|---|---|---|---|---|
| 1 | Continuous hydrostatic leaching test | #1, #2, #3 | Leaching for 16 d | Water PH = 7.2 temperature 24 °C (±0.2 °C), laboratory temperature 25.3 °C (±0.7 °C) |
| 2 | Cyclic hydrostatic leaching test | #4, #5, #6 | Soaking for 24 h + natural drying for 12 h in a dry–wet cycle, and 16 dry and wet cycles are carried out, with a total duration of 16 d | |
| 3 | Solution concentration monitoring test | #1, #2, #3, #4, #5, #6 | Monitor the solution concentration with a hydrometer | |
| 4 | Uniaxial compression test | #7, #8, #9 | Uniaxial compression test in natural state | |
| 5 | #10, #11, #12 | Uniaxial compression test after continuous hydrostatic immersion for 16 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, M.; Cui, S.; Pei, X.; Sun, D.; Yang, X.; Qin, L.; Liang, Y.; Yang, M. Effects of Hydrostatic Dissolution and Seepage on the Transport and Mechanical Properties of Glauberite. Sustainability 2022, 14, 16739. https://doi.org/10.3390/su142416739
Meng M, Cui S, Pei X, Sun D, Yang X, Qin L, Liang Y, Yang M. Effects of Hydrostatic Dissolution and Seepage on the Transport and Mechanical Properties of Glauberite. Sustainability. 2022; 14(24):16739. https://doi.org/10.3390/su142416739
Chicago/Turabian StyleMeng, Minghui, Shenghua Cui, Xiangjun Pei, Dong Sun, Xuezhi Yang, Liang Qin, Yufei Liang, and Mengjie Yang. 2022. "Effects of Hydrostatic Dissolution and Seepage on the Transport and Mechanical Properties of Glauberite" Sustainability 14, no. 24: 16739. https://doi.org/10.3390/su142416739
APA StyleMeng, M., Cui, S., Pei, X., Sun, D., Yang, X., Qin, L., Liang, Y., & Yang, M. (2022). Effects of Hydrostatic Dissolution and Seepage on the Transport and Mechanical Properties of Glauberite. Sustainability, 14(24), 16739. https://doi.org/10.3390/su142416739






