Screening of Rhizosphere Microbes of Salt-Tolerant Plants and Developed Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Beneficial Microorganisms in the Rhizosphere of Salt-Tolerant Plants
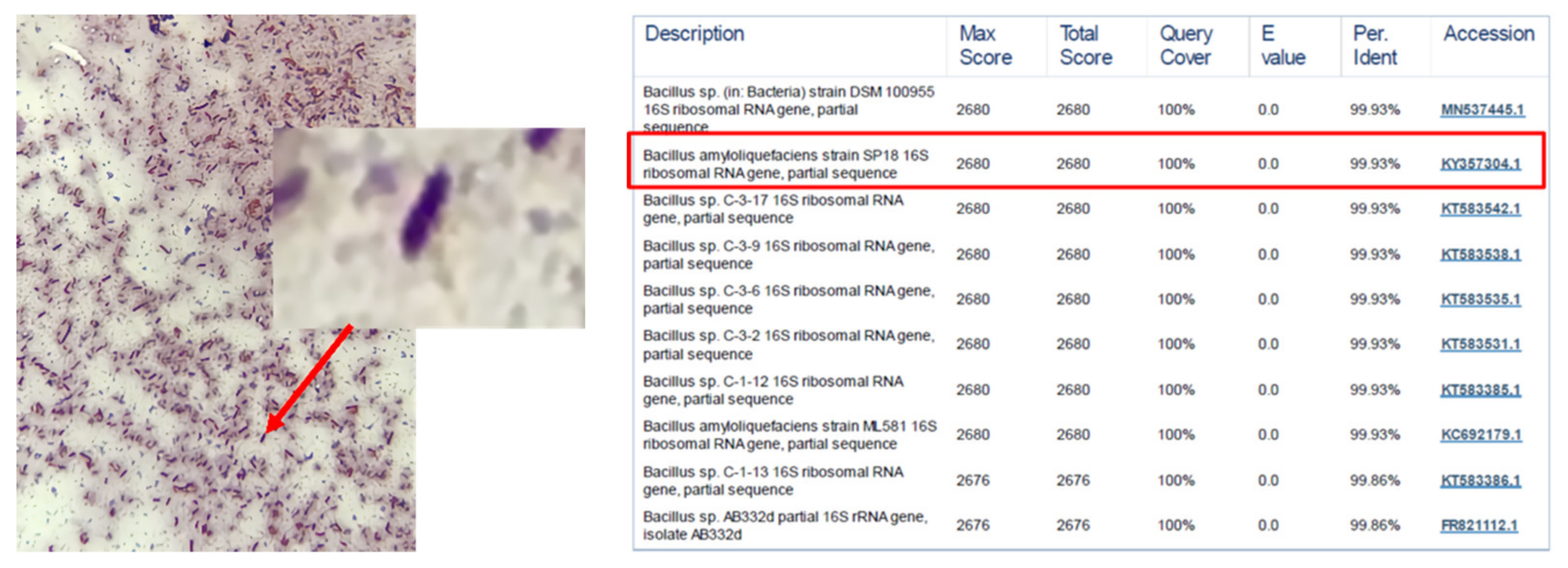
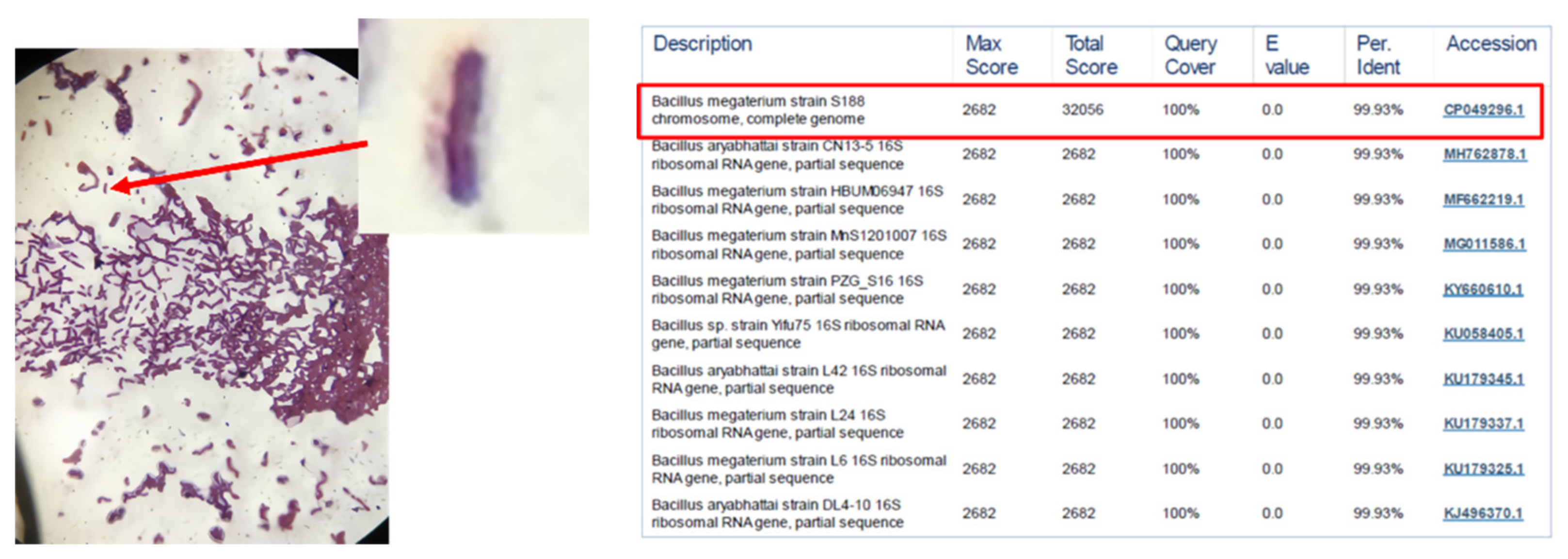
2.2. Identification of Bacterial Species
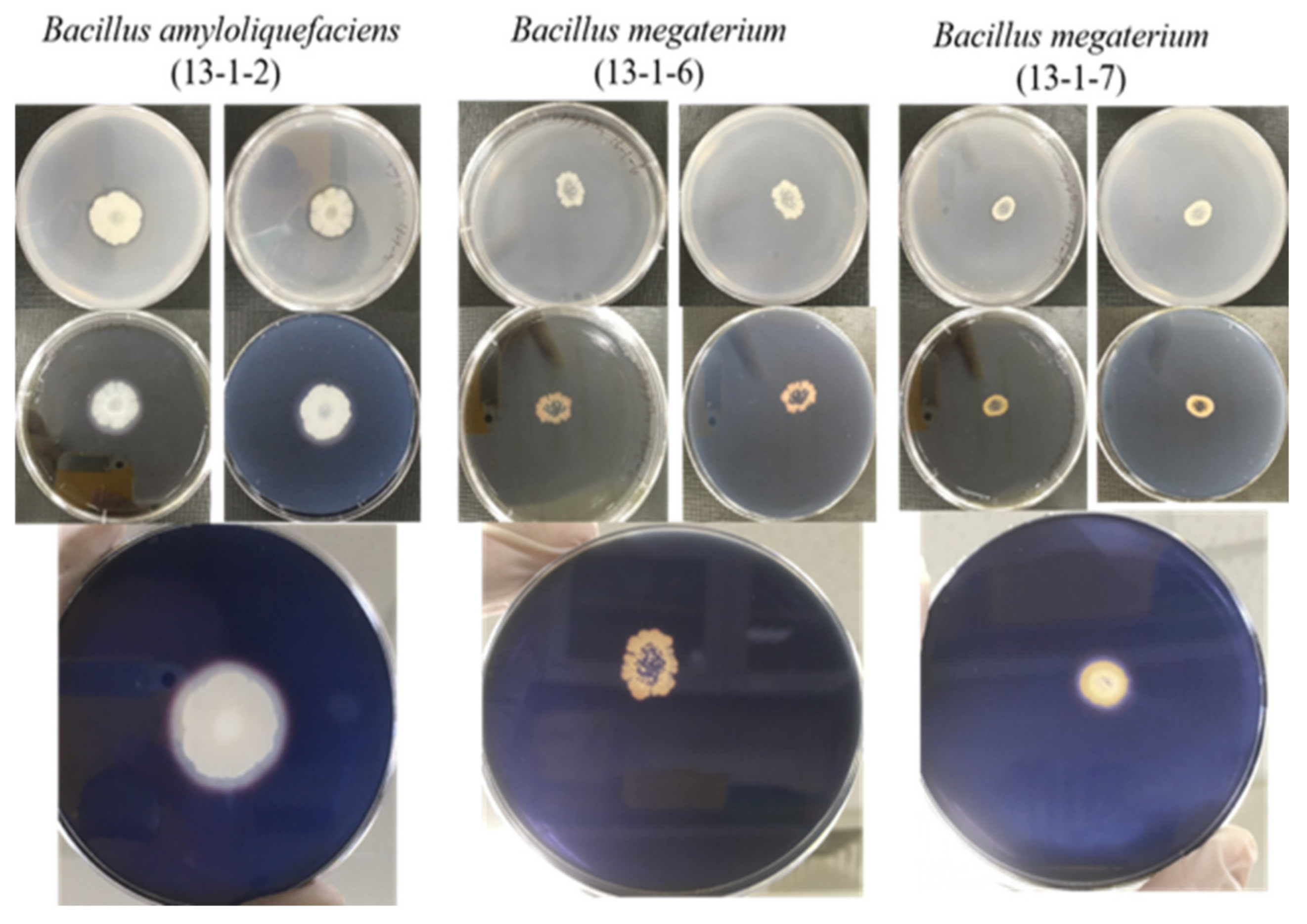
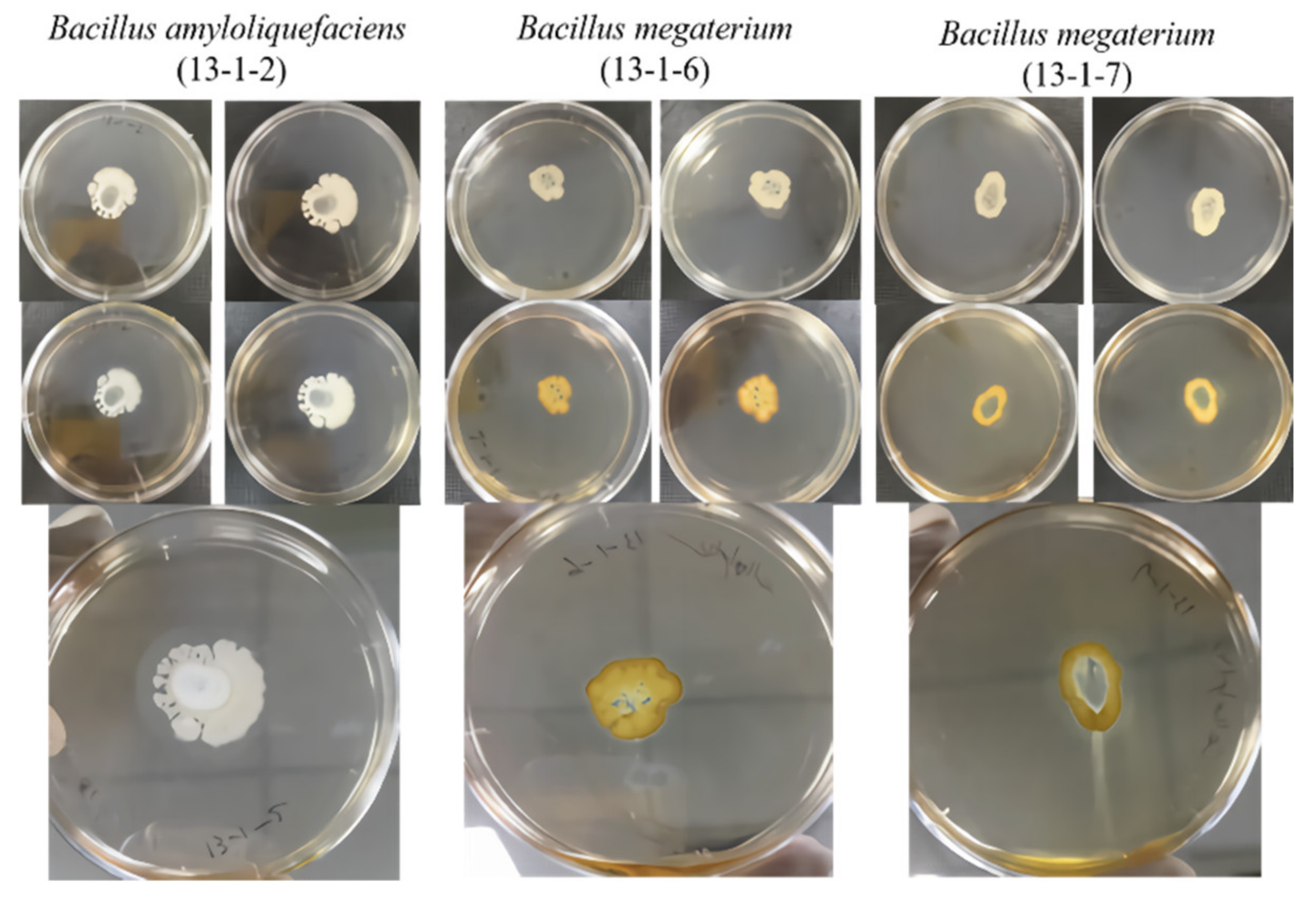
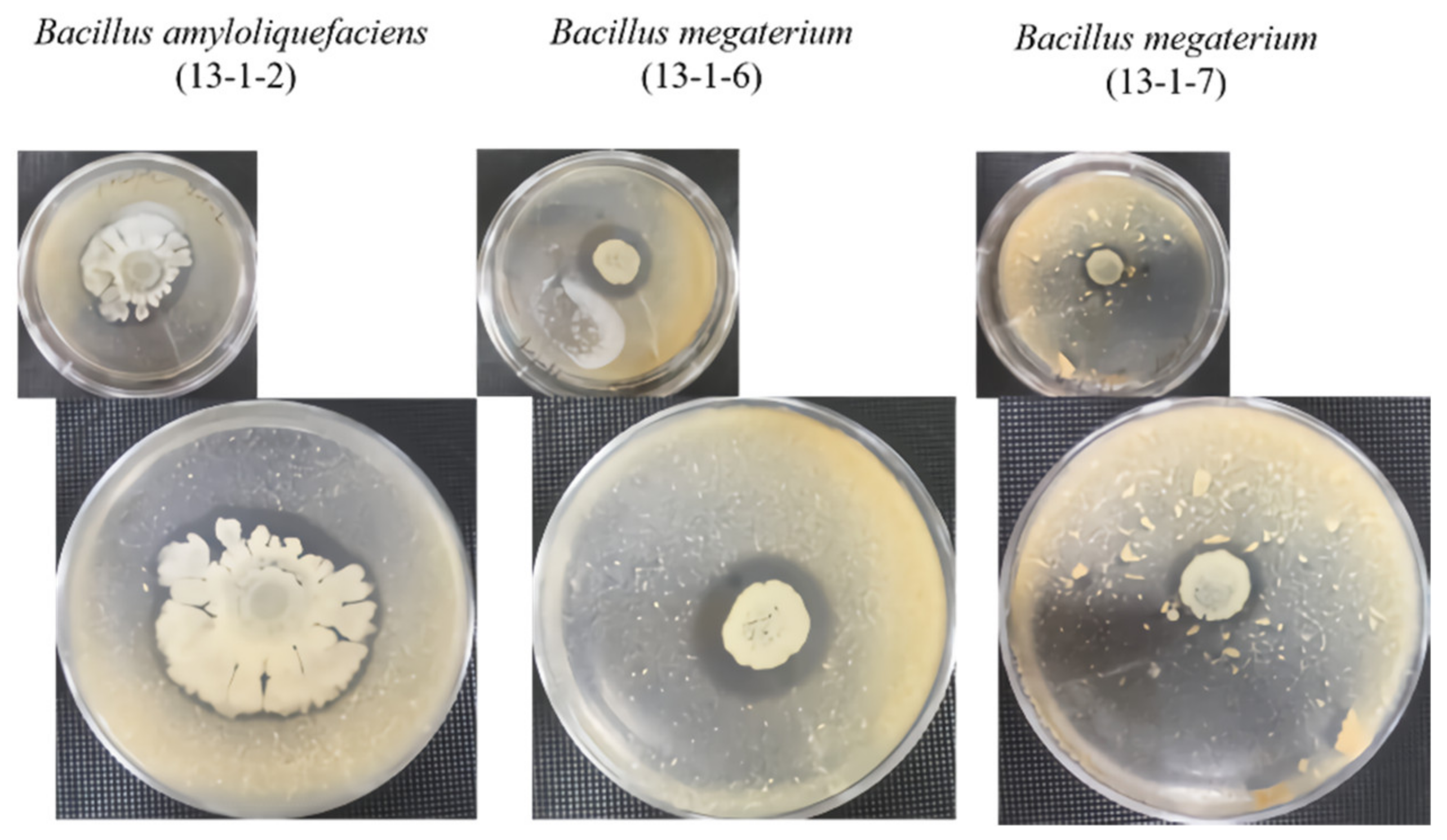
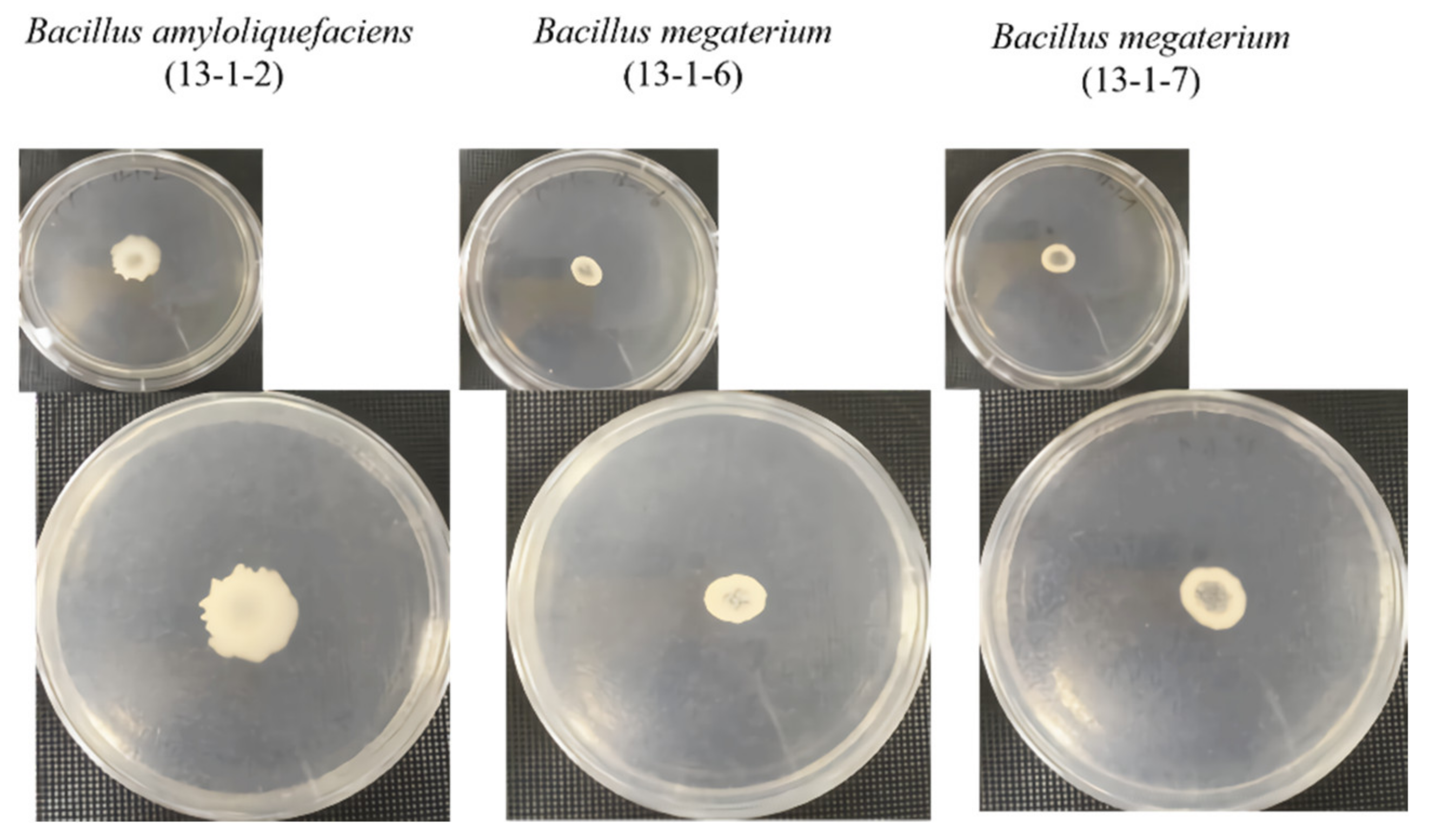
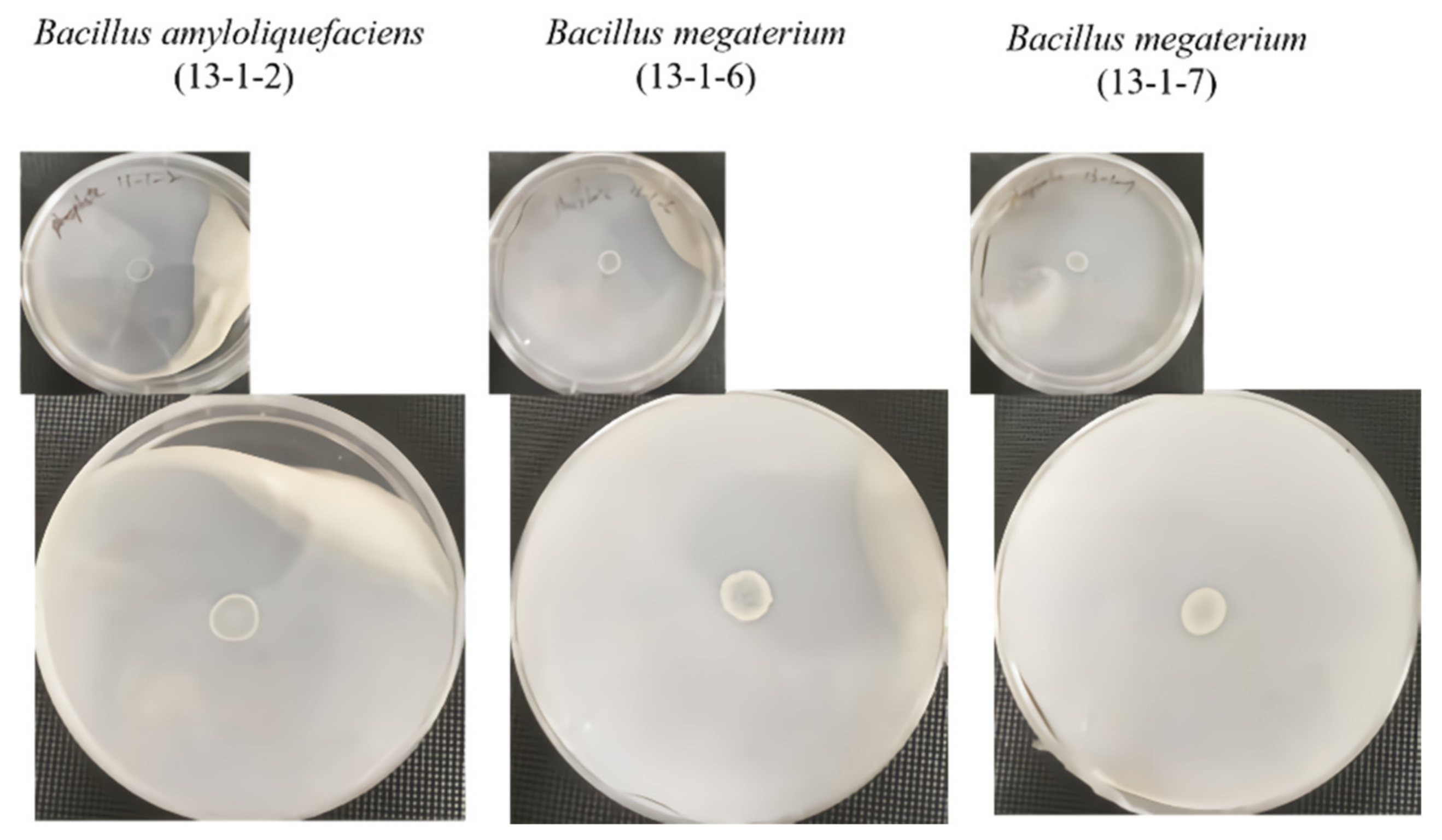
2.3. Amylolytic Activity Assay
2.4. Proteolytic Activity Assay
2.5. Cellulytic Activity Assay
2.6. Lipolytic Activity Assay
2.7. Phosphate Solubilization
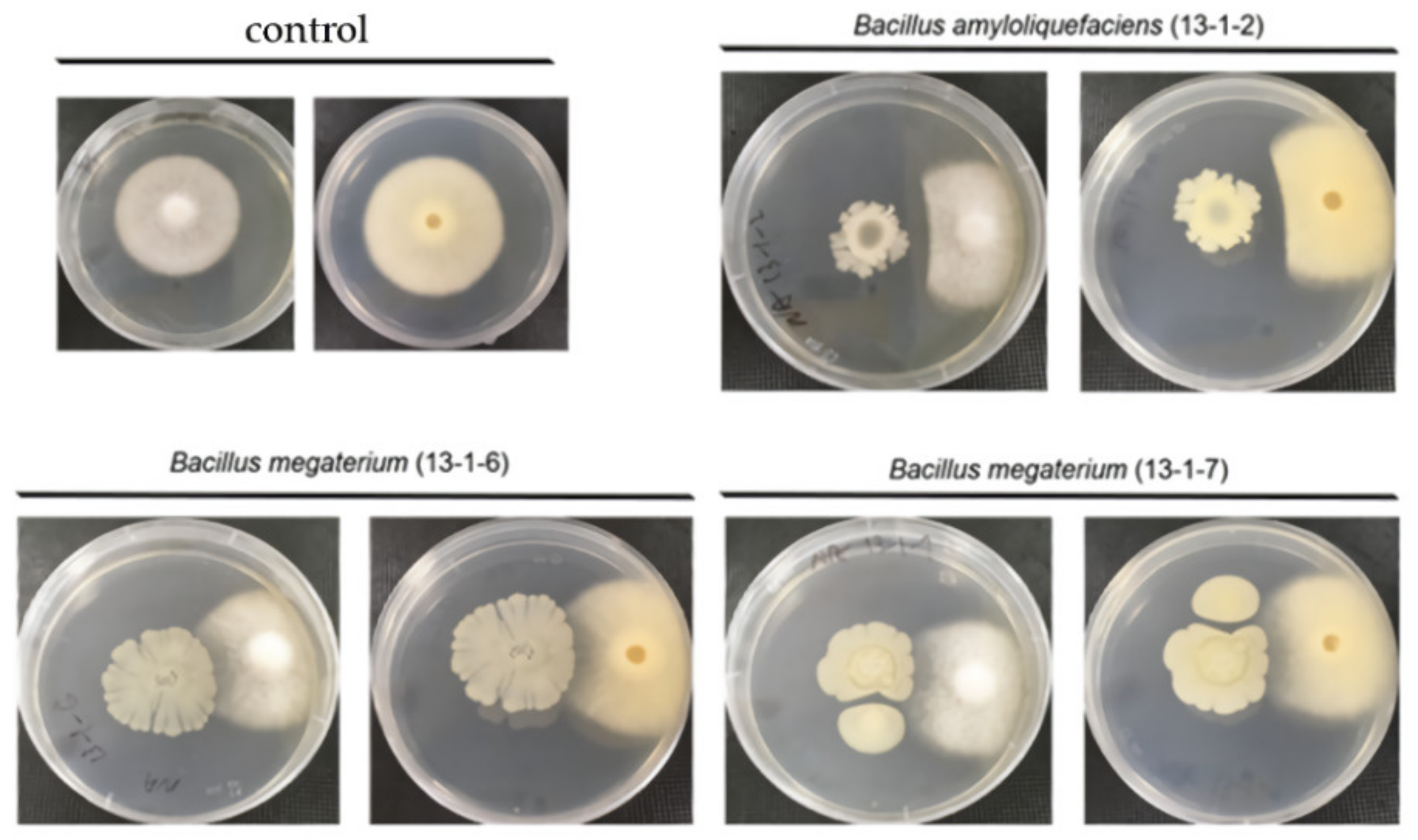
2.8. Pathogenic Microbial Inhibition Test
2.9. Biochar Process
2.10. The Process of Embedded Beneficial Microorganisms with Biochar
2.11. Scanning Electron Microscope (SEM)
2.12. Pot Experiment of Tomato Wilt Fungus
3. Results and Discussion
3.1. Beneficial Microorganisms
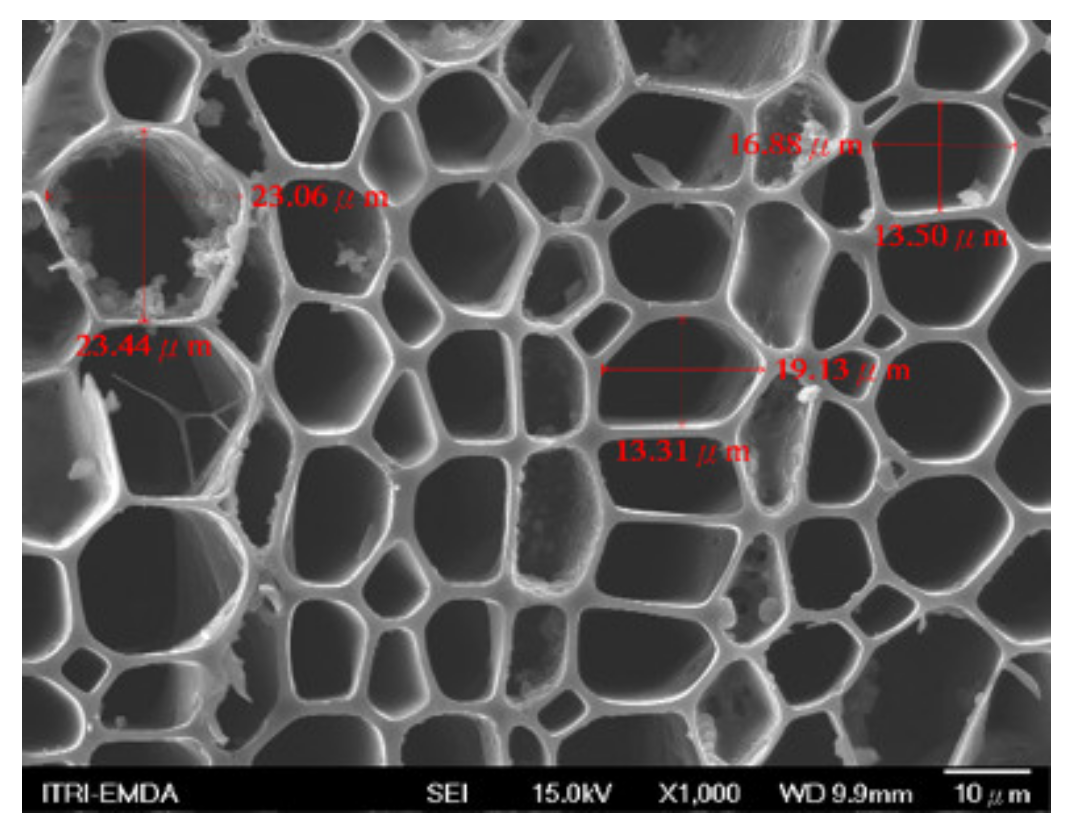
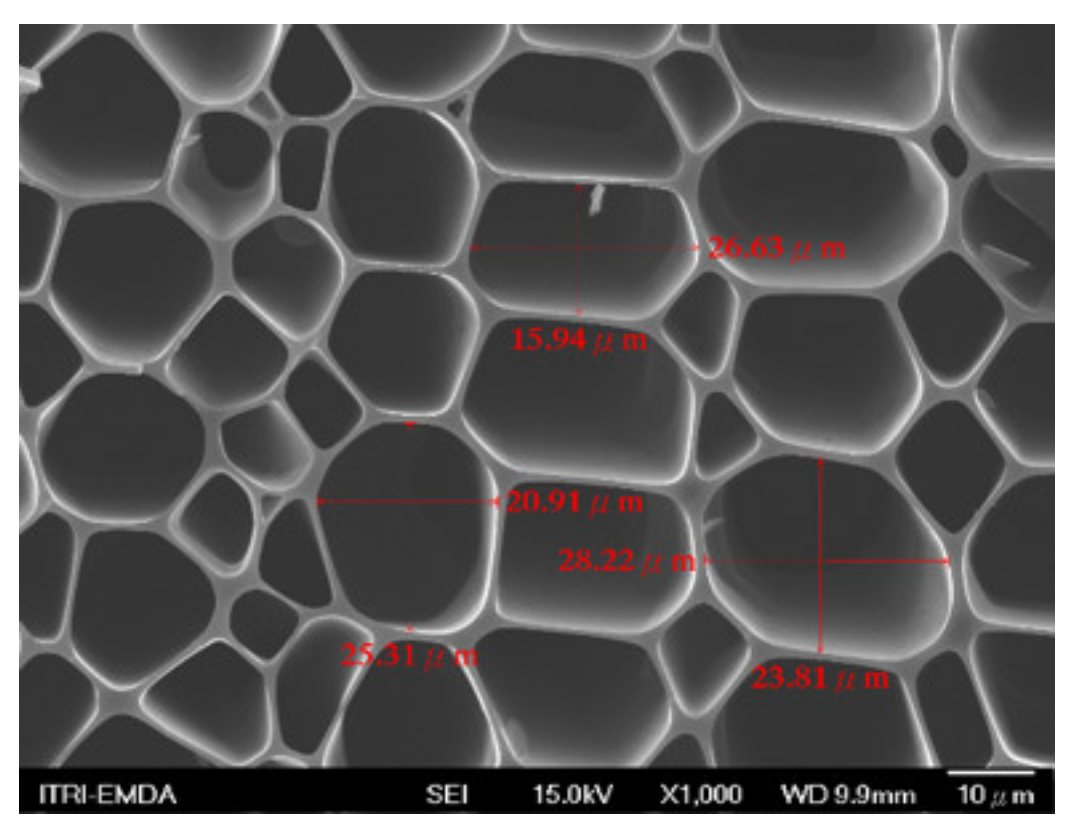
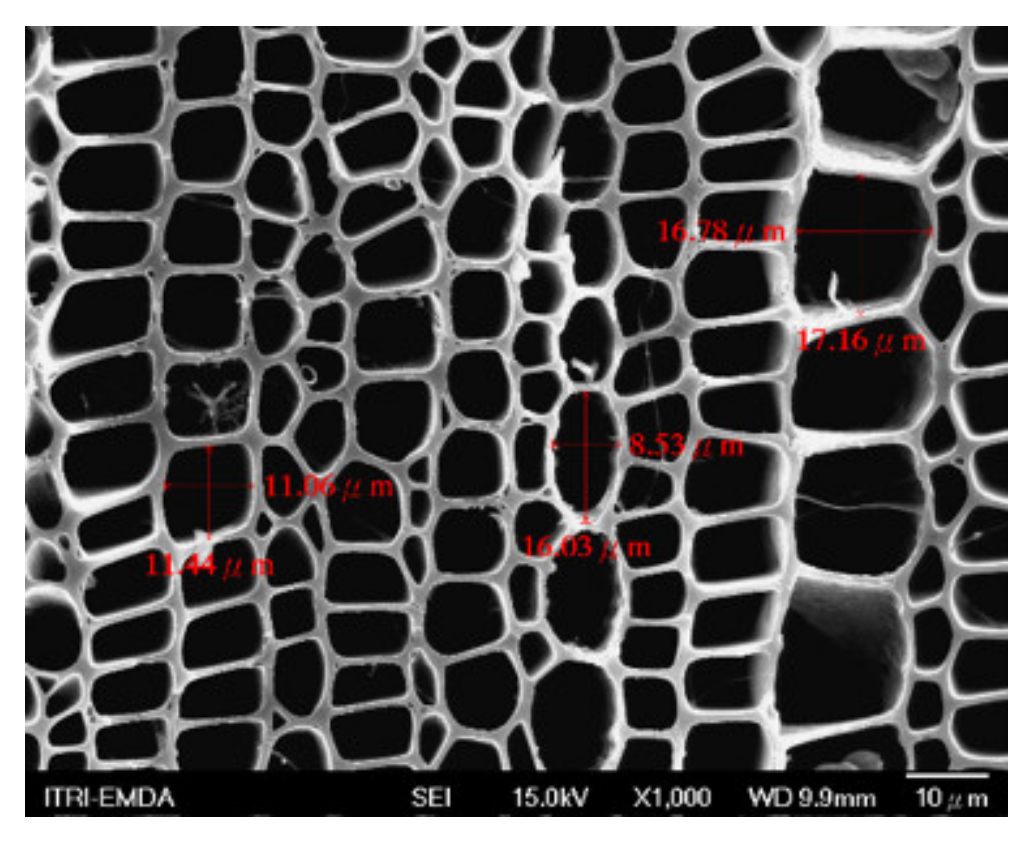
3.2. Biochar Preparation
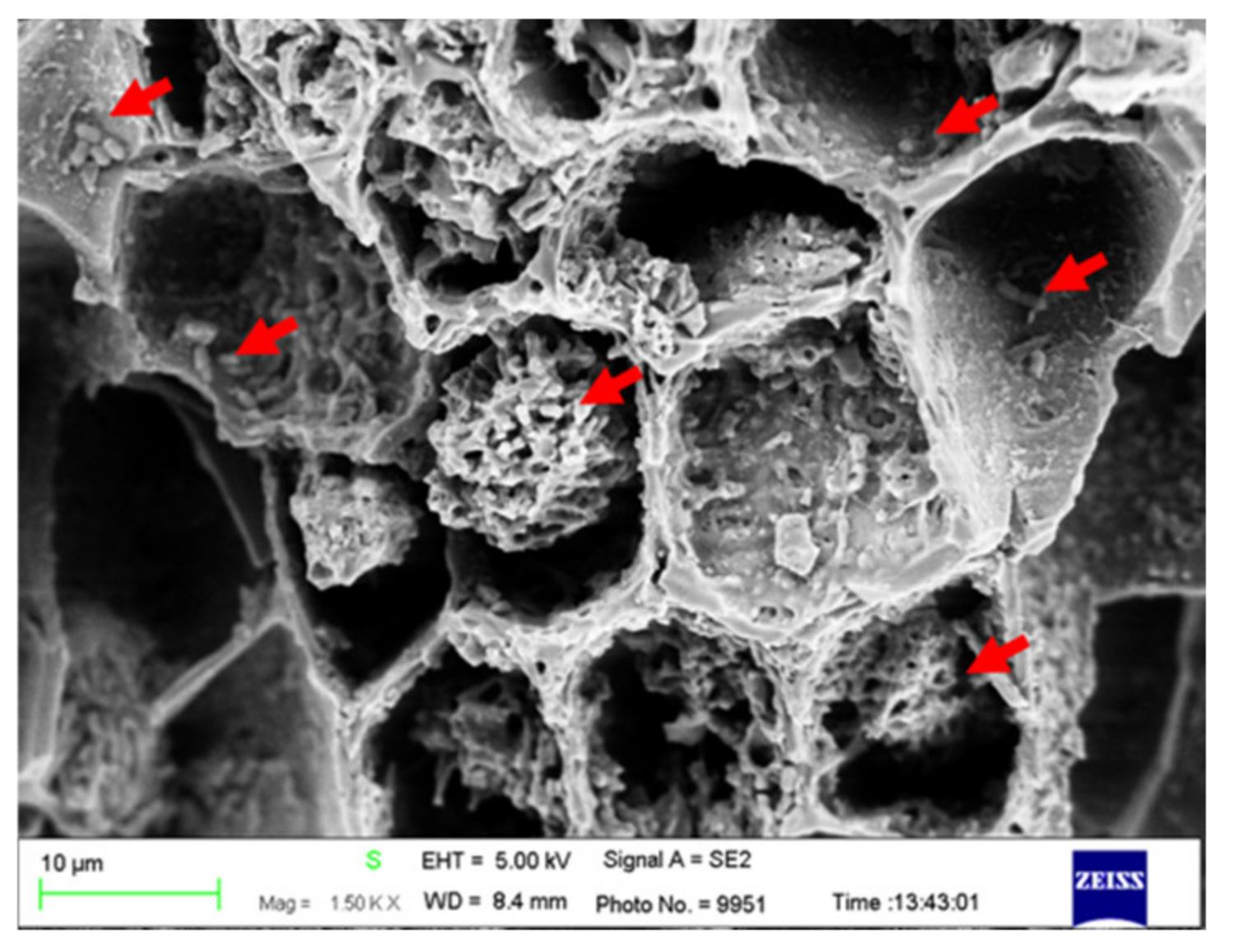
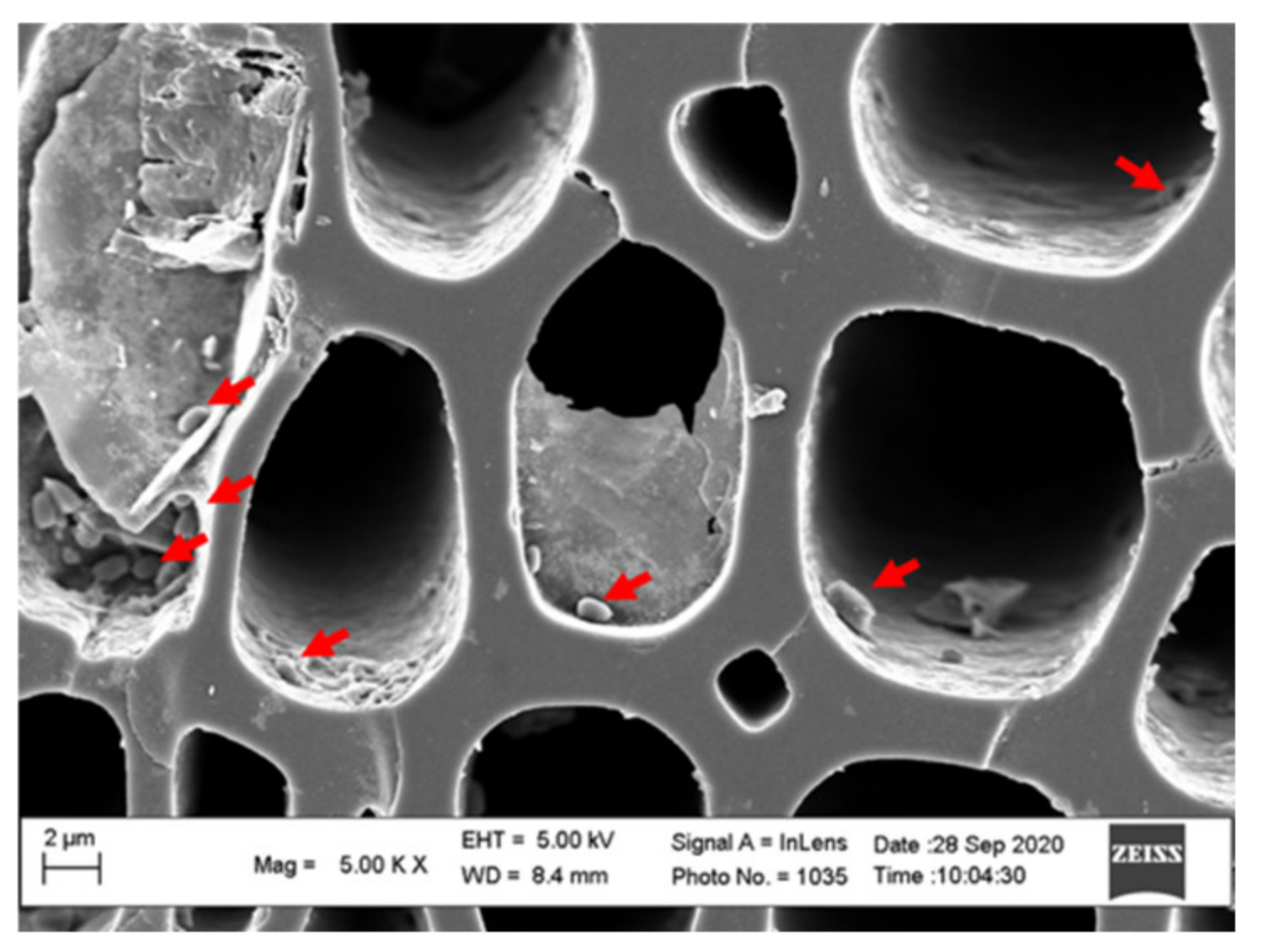
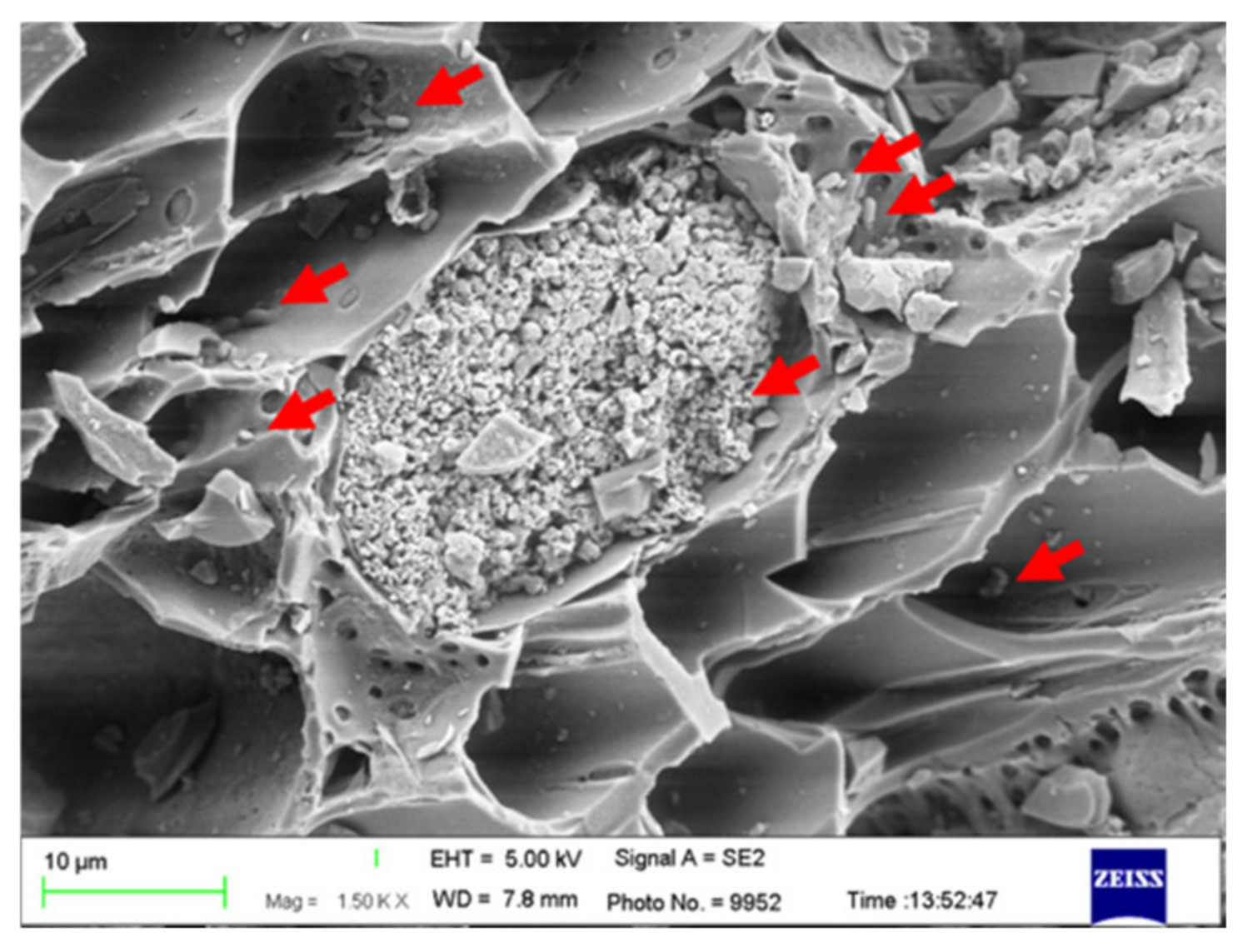
3.3. Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms
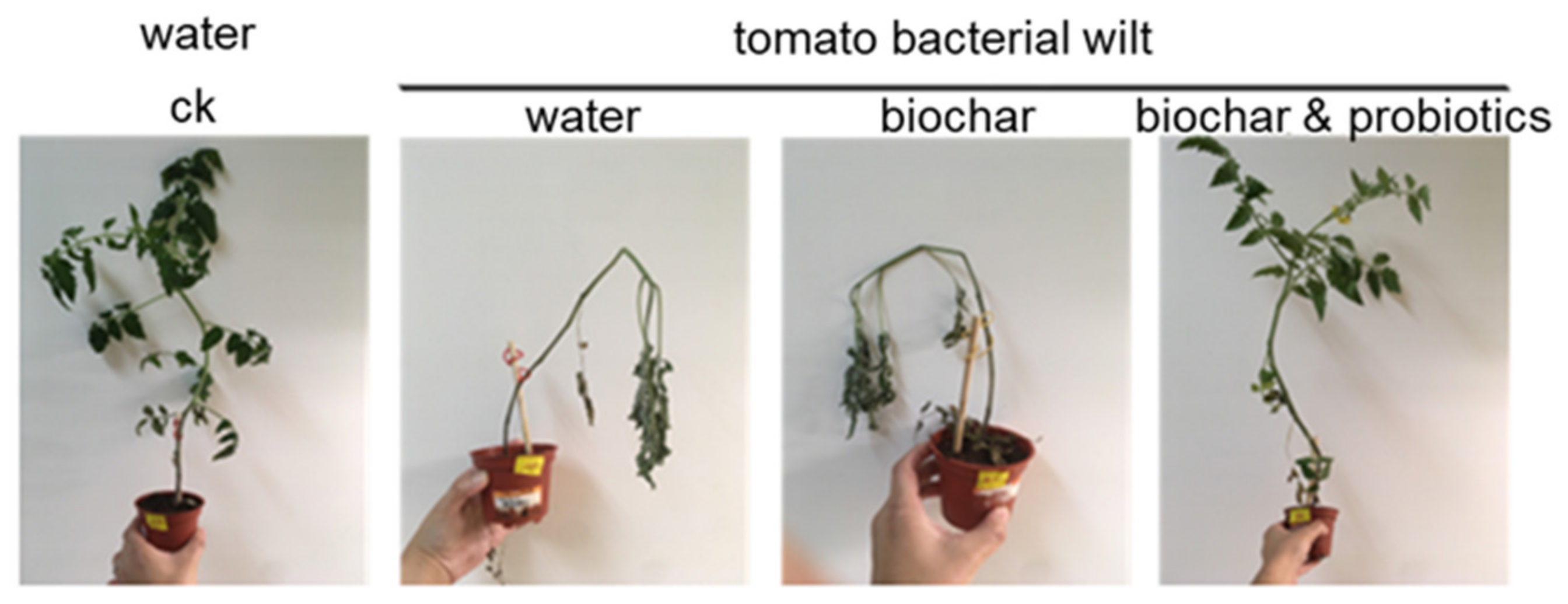
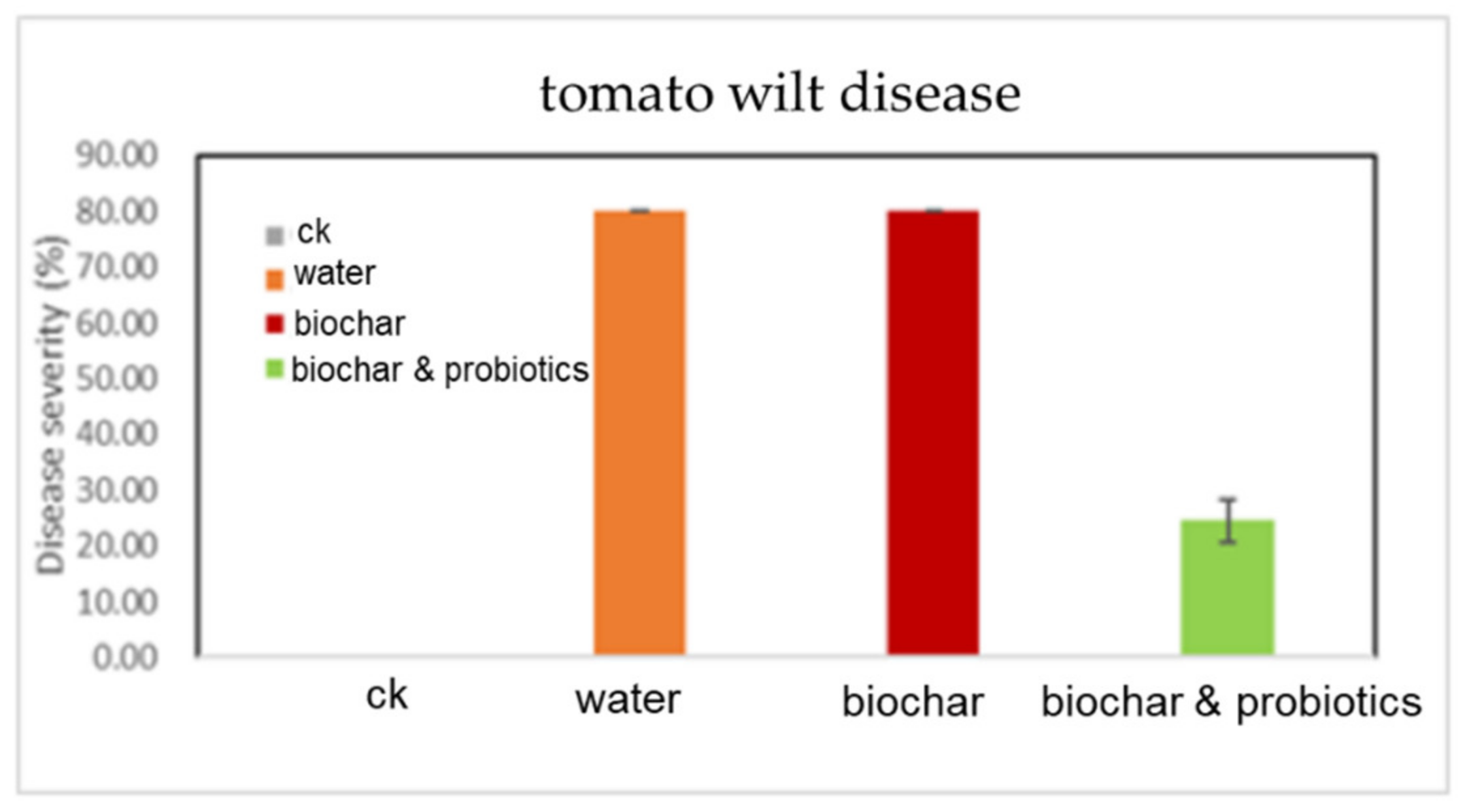
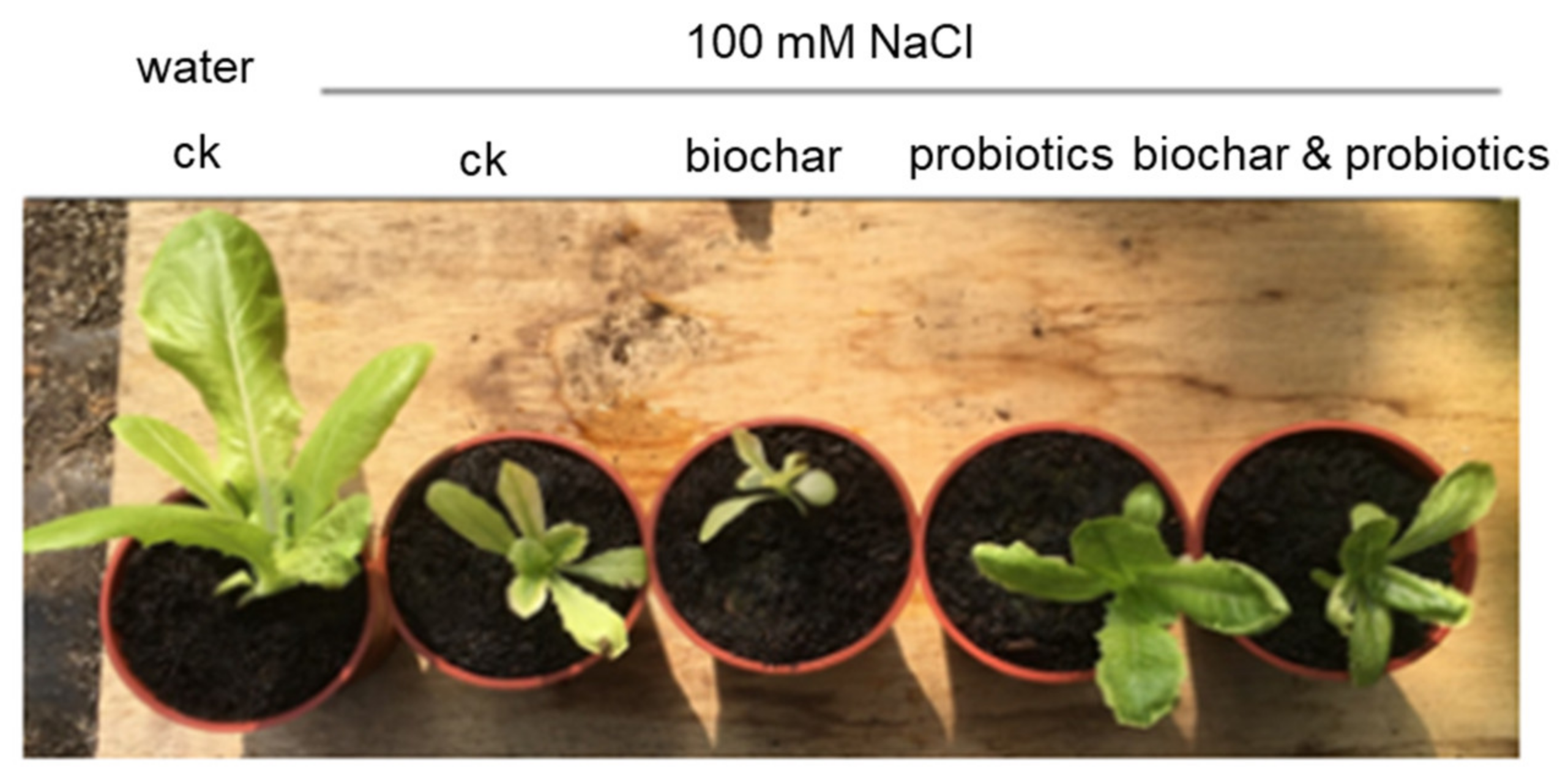
3.4. Pot Experiment of Tomato Wilt Fungus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A. Soil saninity: A global threat to sustainable development. Soil Use Manag. 2021, 38, 39–67. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Toler, H.; Lee, J.; Ownley, B.; Stutz, J.C.; Moore, J.; Augé, R.M. Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 2006, 163, 517–528. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldan, A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregates stabilization and promotion of biological properties in rhizosphere soil of lettuce plants under field conditions. Soil Use Manag. 2006, 22, 298–304. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Nia, S.H.; Zarea, M.J.; Rejali, F.; Varma, A. Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil. J. Saudi Soc. Agric. Sci. 2012, 11, 113–121. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2011, 14, 605–611. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halo-tolerant bacteria isolated from saline habitats. Springer Plus 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of bi-ochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.D.; Rutherford, D.W.; Sparrevik, M.; Hale, S.E.; Obia, A.; Mulder, J. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef]
- Lee, S.S.; Shah, H.S.; Awad, Y.M.; Kumar, S.; Ok, Y.S. Synergy effects of biochar and polyacrylamide on plants growth and soil erosion control. Environ. Earth Sci. 2015, 74, 2463–2473. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.-W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.-H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Law, J.F. Method for purification of bacterial endospores from soils: UV resistance of natural Sonoran desert soil populations of Bacillus spp. with reference to B. subtilis strain 168. J. Microbiol. Methods 1999, 35, 13–21. [Google Scholar] [CrossRef]
- Selvam, A.; Tsai, S.-H.; Liu, C.-P.; Chen, I.-C.; Chang, C.-H.; Yang, S.-S. Microbial communities and bacterial diversity of spruce, hemlock and grassland soils of Tatachia Forest, Taiwan. J. Environ. Sci. Health Part B 2010, 45, 386–398. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kitamura, Y.; Chien, C.-C.; Cheng, C.-S.; Cheng, J.-H.; Tsai, S.-H.; Hsieh, C.-C. Development of Meso- and Macro-Pore Carbonization Technology from Biochar in Treating the Stumps of Representative Trees in Taiwan. Sustainability 2022, 14, 14792. [Google Scholar] [CrossRef]
- Shanmugam, V.; Kanoujia, N. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f. sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol. Control 2011, 57, 85–93. [Google Scholar] [CrossRef]
- The European Biochar Certificate (EBC). Available online: https://www.european-biochar.org/en (accessed on 10 January 2022).
- International Biochar Initiative. Available online: https://biochar-international.org/ (accessed on 30 September 2013).
- Wei, Z.; Gu, Y.; Friman, V.-P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhang, Z.; Liu, Y.; Wei, Y. Tomato Endophytic Bacteria Composition and Mechanism of Suppres-siveness of Wilt Disease (Fusarium oxysporum). Front. Microbiol. 2021, 12, 731764. [Google Scholar] [CrossRef]

| Location | Soil pH Value | Soil EC Value (dS/m) |
|---|---|---|
| Site 1 | 7.3 | 4.3 |
| Site 2 | 7.6 | 1.2 |
| Site 3 | 8.1 | 0.2 |
| Site 4 | 8.4 | 0.4 |
| Location | Treatment A | Treatment B | Total |
|---|---|---|---|
| Site 1 | 5 | 3 | 8 |
| Site 2 | 13 | 25 | 38 |
| Site 3 | 7 | 2 | 9 |
| Site 4 | 10 | 13 | 23 |
| Activity | Bacillus amyloliquefaciens | Bacillus megaterium A | Bacillus megaterium B |
|---|---|---|---|
| Amylolytic | + | − | + |
| Cellulolytic | + | + | + |
| Proteolytic | + | + | + |
| Lipolytic | − | − | − |
| Phosphorus solubilizing | + | + | + |
| Elements | N % | P % | K % | Ca ppm | Mg ppm | Mn pm | Fe ppm | Cu ppm | Zn ppm | Na ppm |
|---|---|---|---|---|---|---|---|---|---|---|
| Syzygium samarangense | 1.01 | 0.24 | 1.16 | 25,232 | 3471 | 25.4 | 145.2 | 14.7 | 12.9 | 1162 |
| Leucaena leucocephala | 1.07 | 0.05 | 0.74 | 13,633 | 1838 | 22.3 | 187.3 | 6.4 | 12 | 677 |
| Ziziphus jujuba | 0.87 | 0.29 | 2.05 | 36,618 | 4692 | 72.6 | 188.1 | 23.6 | 32.1 | 1213 |
| Elements | B ppm | Cd ppm | Cr ppm | Ni ppm | Pb ppm | As ppm | Hg ppm | pH (1:10) | Conductivity (1:10) mS/cm | CEC (cmol(+)/kg soil) |
|---|---|---|---|---|---|---|---|---|---|---|
| Syzygium samarangense | 46.8 | <0.009 | 6.52 | 5.7 | <0.027 | <0.005 | 0.52 | 10.6 | 3.13 | 10.4 |
| Leucaena leucocephala | 53 | <0.009 | 32.8 | 20.7 | <0.027 | <0.005 | 0.3 | 10.2 | 1.8 | 6.8 |
| Ziziphus jujuba | 31.8 | <0.009 | 37.4 | 26.9 | <0.027 | <0.005 | 0.52 | 10.9 | 5.33 | 13.3 |
| pH | BET (m2/g) | Fixed Carbon (Wt%) | Pore Ratio (%) | |
|---|---|---|---|---|
| Syzygium samarangense | Adjust to 7.3 ± 0.2 | 315.09 | 92.16 | Mesopore (2~50 nm): 27.63 |
| Macropore (>50 nm): 72.37 | ||||
| Leucaena leucocephala | Adjust to 7.3 ± 0.2 | 213.53 | 93.69 | Mesopore (2~50 nm): 2.58 |
| Macropore (>50 nm): 97.42 | ||||
| Ziziphus jujuba | Adjust to 7.3 ± 0.2 | 318.53 | 87.13 | Mesopore (2~50 nm): 2.19 |
| Macropore (>50 nm): 97.81 |
| CK (Water) | 100 mM NaCl | ||||
|---|---|---|---|---|---|
| CK | Biochar | Probiotics | Biochar & Probiotics | ||
| SPAD * | 21.9 | 7.6 | 3.4 | 20.2 | 19 |
| Fresh weight (g) | 7.86 | 0.64 | 0.12 | 2.02 | 0.77 |
| Dry weight (g) | 0.83 | 0.05 | 0.01 | 0.2 | 0.1 |
| Dry weight/Fresh weight (%) | 10.56 | 7.81 | 8.33 | 9.9 | 12.99 |
| EC (dS/m) | 1.5 | 9.5 | 9.6 | 9.9 | 9.8 |
| pH | 7 | 7.3 | 7.1 | 7.2 | 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-C.; Kitamura, Y.; Tsai, S.-H.; Chien, C.-C.; Cheng, C.-S.; Hsieh, C.-C. Screening of Rhizosphere Microbes of Salt-Tolerant Plants and Developed Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms. Sustainability 2022, 14, 16724. https://doi.org/10.3390/su142416724
Lee S-C, Kitamura Y, Tsai S-H, Chien C-C, Cheng C-S, Hsieh C-C. Screening of Rhizosphere Microbes of Salt-Tolerant Plants and Developed Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms. Sustainability. 2022; 14(24):16724. https://doi.org/10.3390/su142416724
Chicago/Turabian StyleLee, Shih-Chi, Yutaka Kitamura, Shu-Hsien Tsai, Chuan-Chi Chien, Chun-Shen Cheng, and Chin-Cheng Hsieh. 2022. "Screening of Rhizosphere Microbes of Salt-Tolerant Plants and Developed Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms" Sustainability 14, no. 24: 16724. https://doi.org/10.3390/su142416724
APA StyleLee, S.-C., Kitamura, Y., Tsai, S.-H., Chien, C.-C., Cheng, C.-S., & Hsieh, C.-C. (2022). Screening of Rhizosphere Microbes of Salt-Tolerant Plants and Developed Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms. Sustainability, 14(24), 16724. https://doi.org/10.3390/su142416724







