Corrosion Inhibition of Rumex vesicarius Mediated Chitosan-AgNPs Composite for C1018 CS in CO2-Saturated 3.5% NaCl Medium under Static and Hydrodynamic Conditions
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation and Characterization of Rumex vesicarius Mediated CHT-Ag Composite
2.3. Corrosion Inhibition Studies
2.4. Surface Analysis
3. Results and Discussion
3.1. Corrosion under Static Conditions
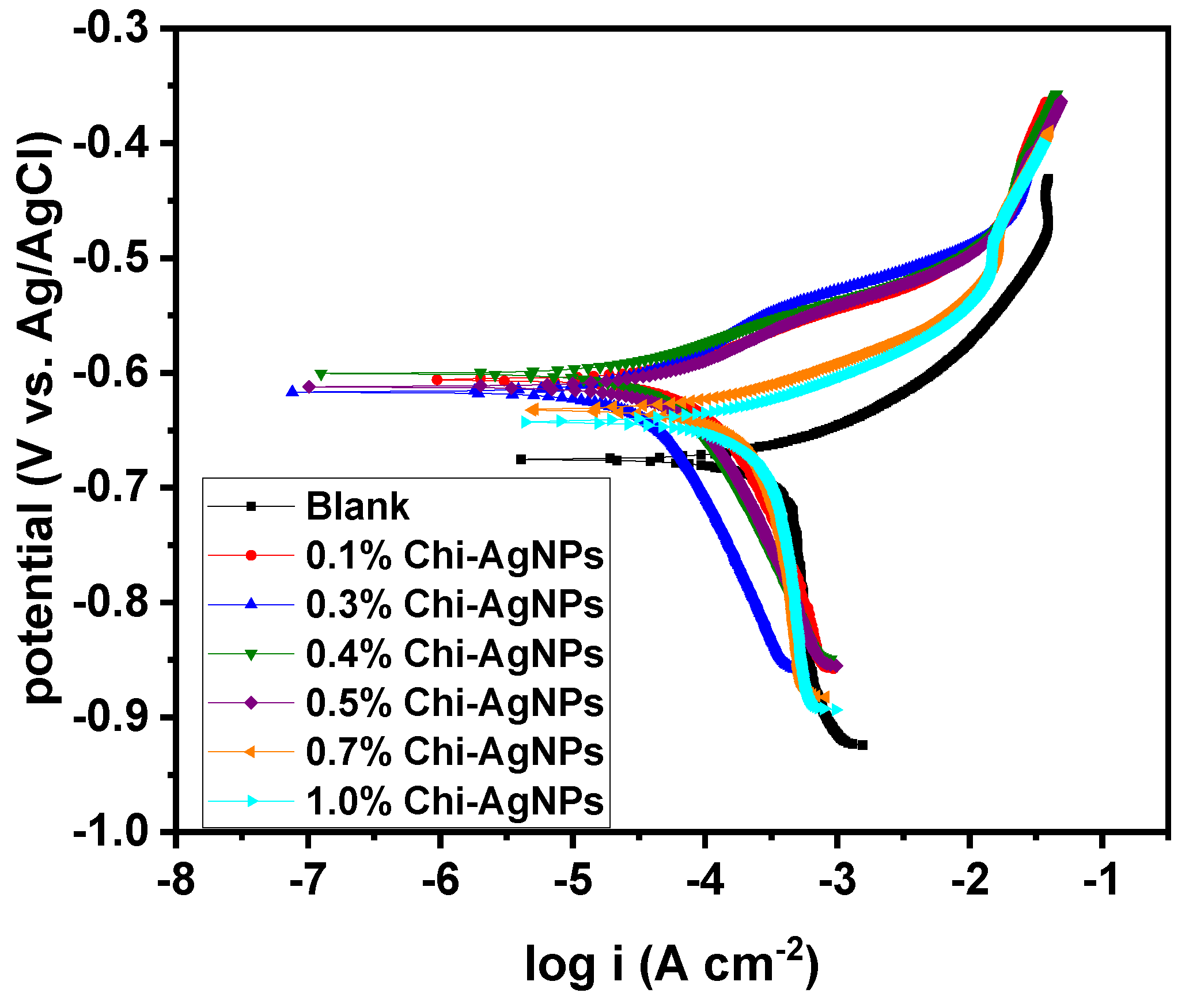
3.1.1. Open Circuit Potential
3.1.2. Effect of Concentration
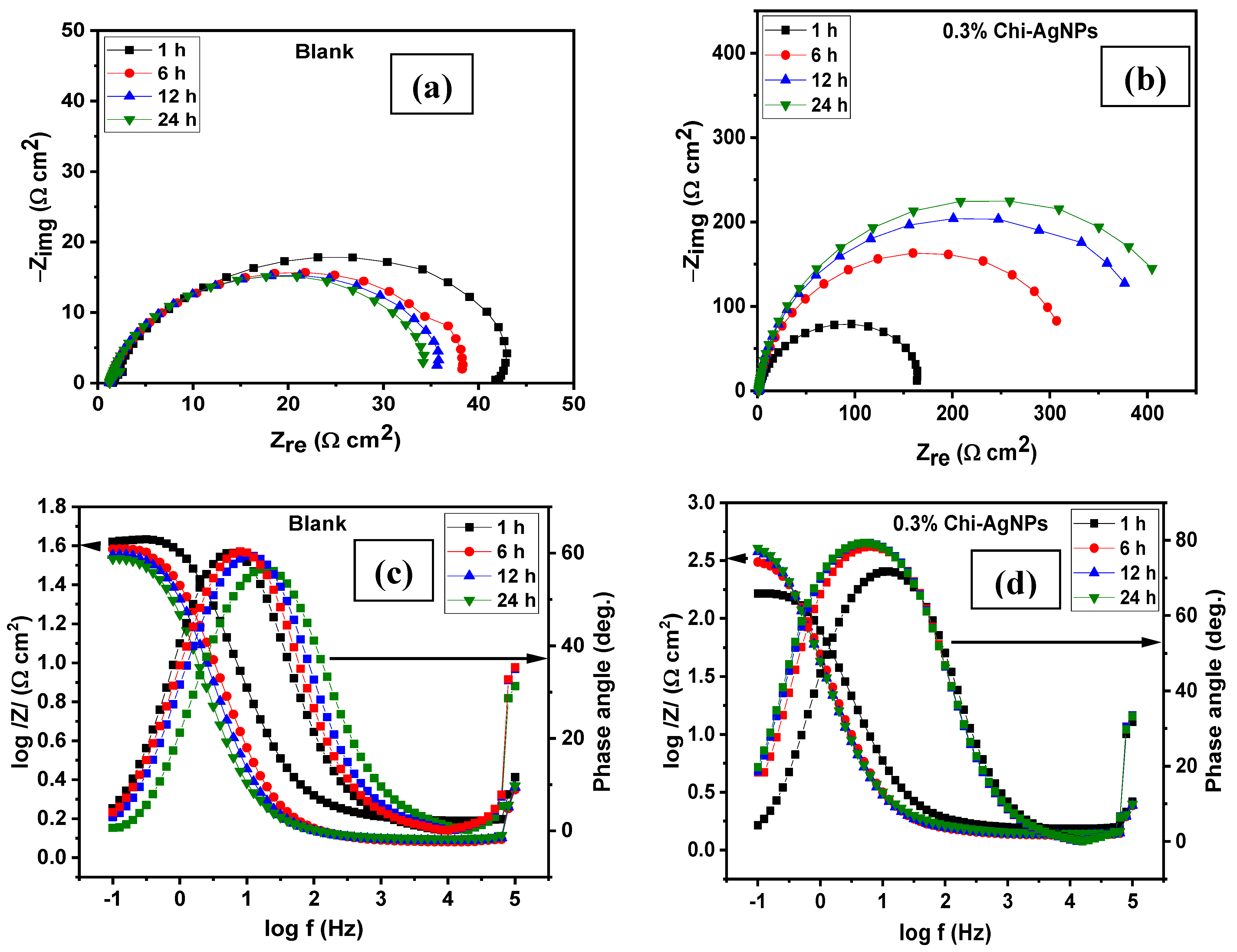
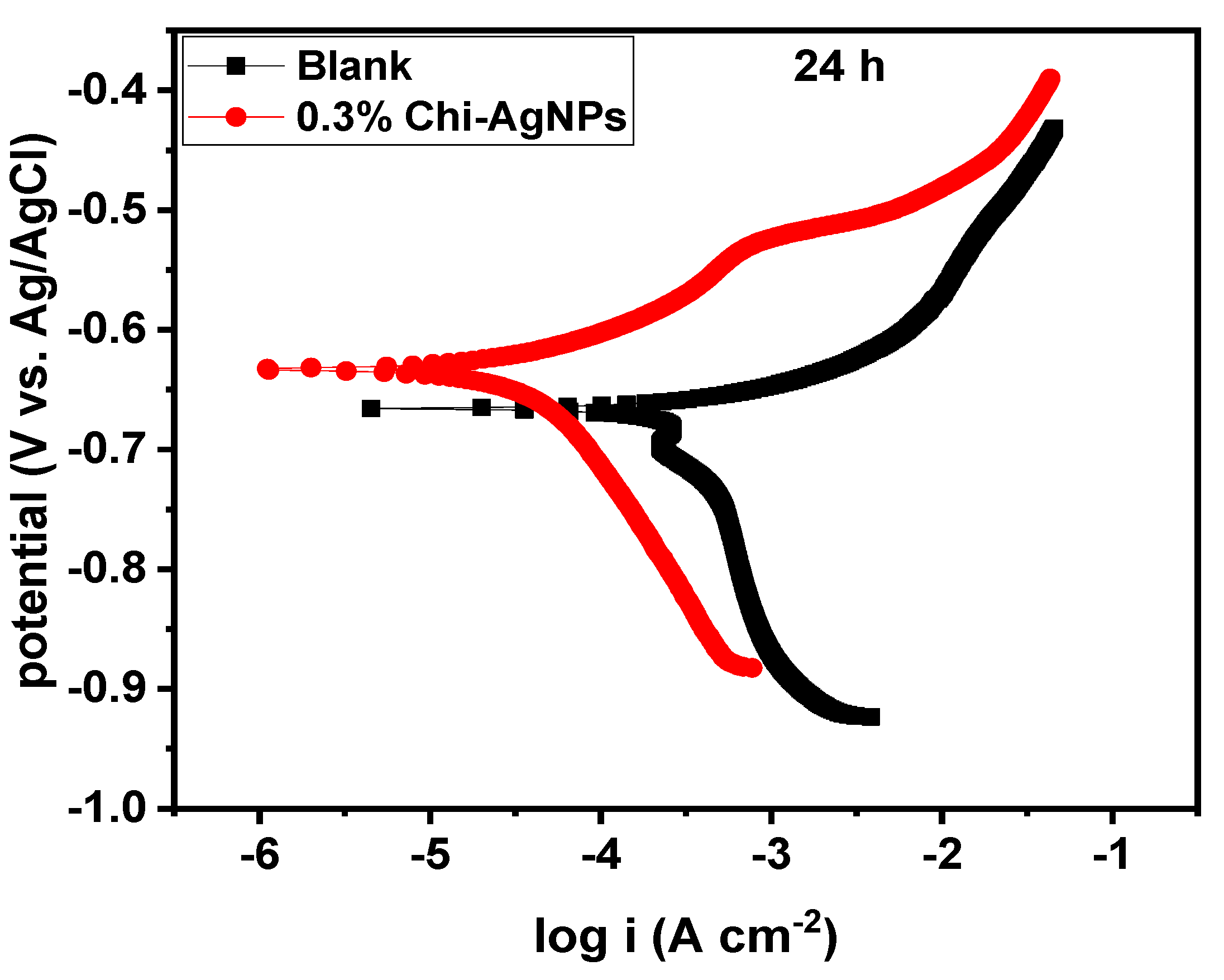
3.1.3. Effect of Immersion Time
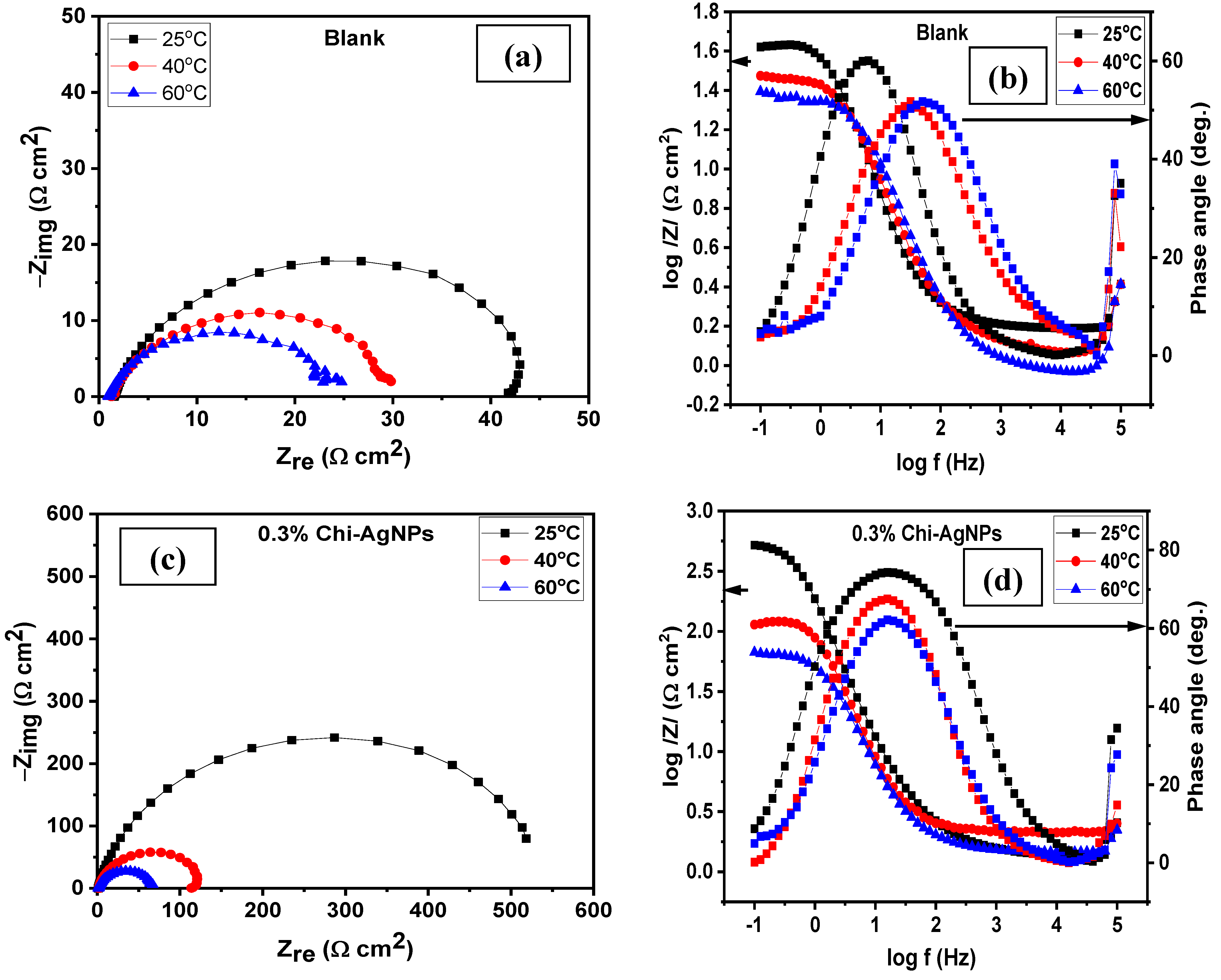
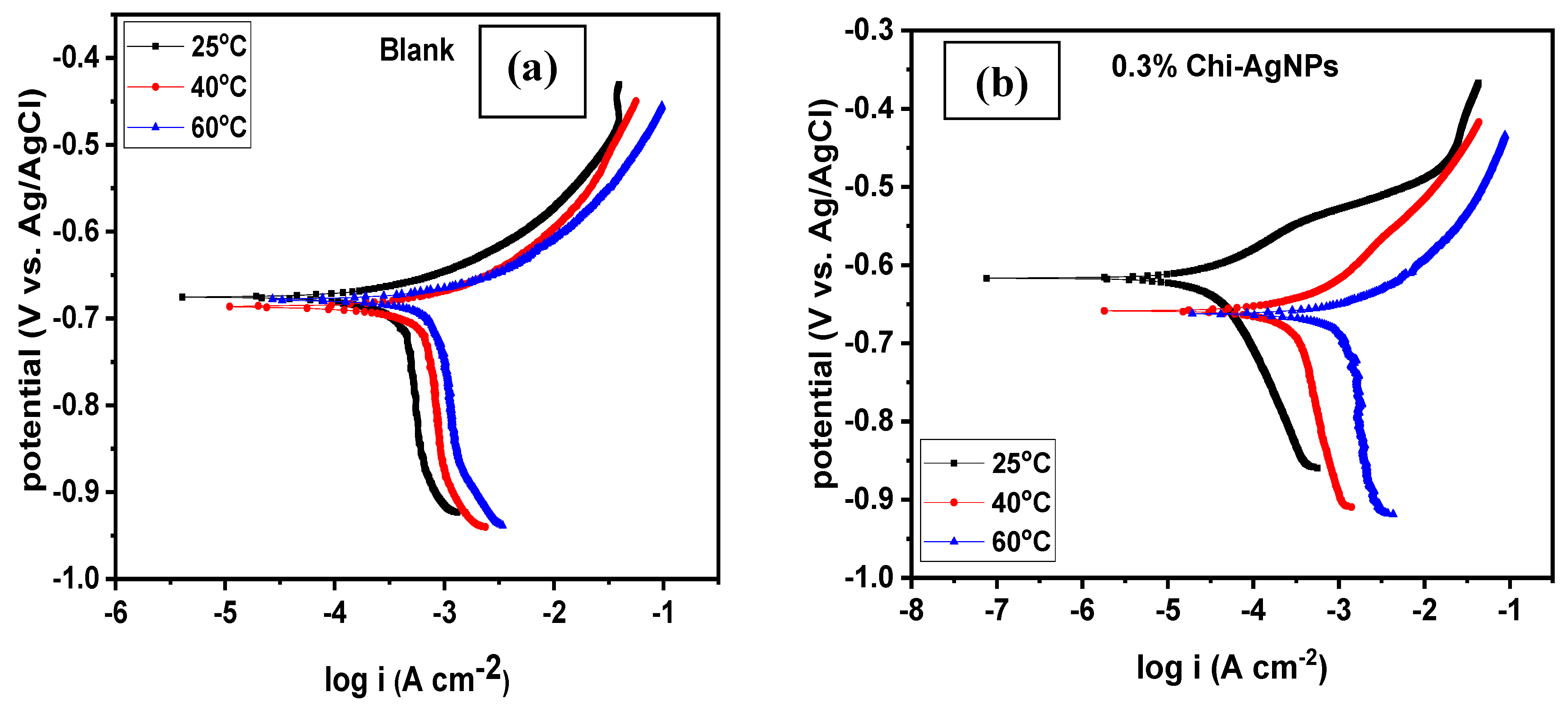
3.1.4. Effect of Temperature
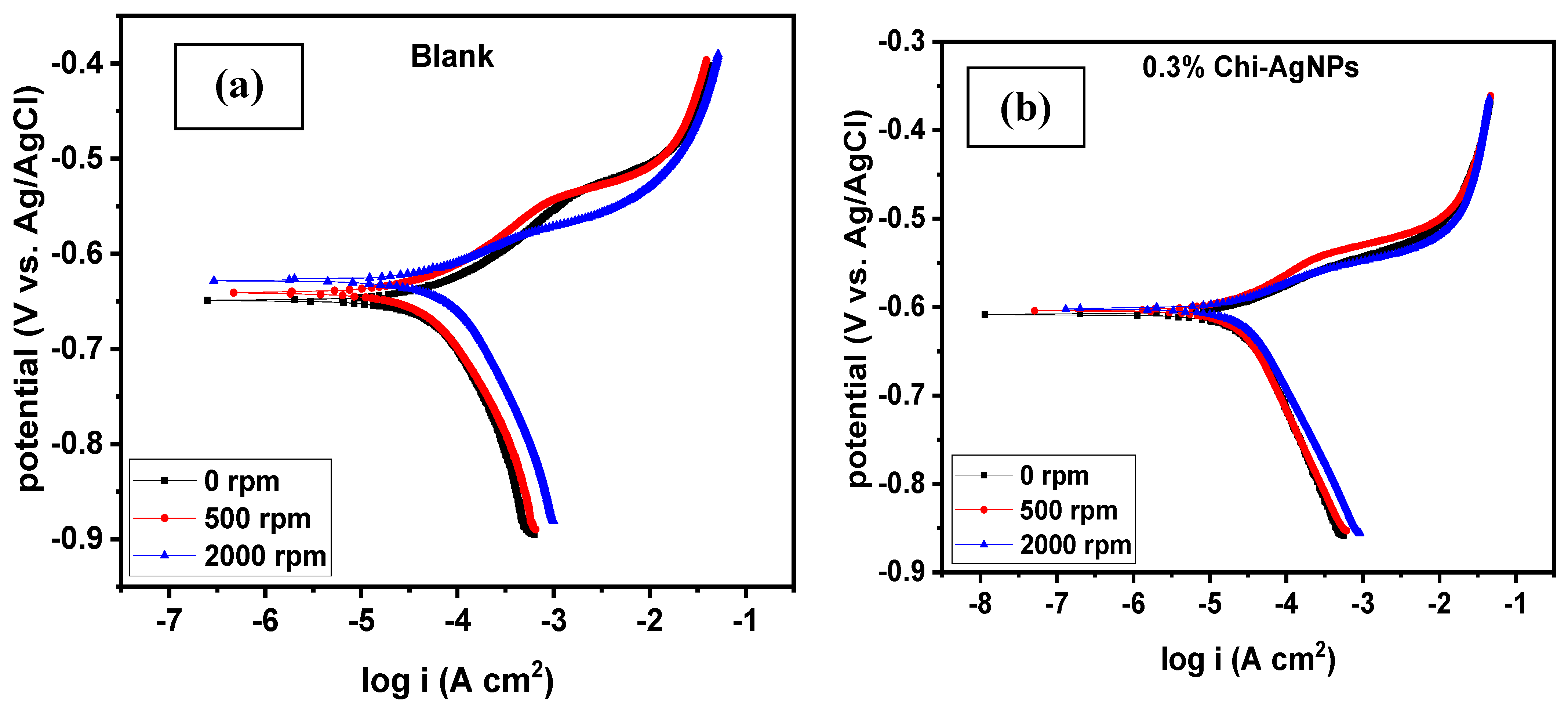
3.2. Corrosion under Hydrodynamic Condition
| PDP Measurements | LPR Measurements | |||||||
|---|---|---|---|---|---|---|---|---|
| System/ Concentration | Rotation Speed (rpm) | –Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | Corrosion Rate (mpy) | IE (%) | Rp (Ω cm2) | IE (%) |
| Blank | ||||||||
| 0 | 649.62 | 74.82 | 80.79 | 10.88 | - | 283.32 | - | |
| 500 | 621.24 | 59.83 | 73.42 | 8.60 | - | 386.23 | - | |
| 2000 | 628.04 | 91.84 | 66.44 | 13.35 | - | 254.48 | - | |
| 0.3% composite | ||||||||
| 0 | 608.39 | 28.50 | 51.93 | 4.14 | 61.91 | 561.80 | 49.57 | |
| 500 | 604.24 | 26.45 | 62.05 | 3.85 | 55.79 | 672.30 | 42.55 | |
| 2000 | 603.07 | 29.81 | 47.80 | 4.33 | 67.54 | 497.18 | 48.81 | |
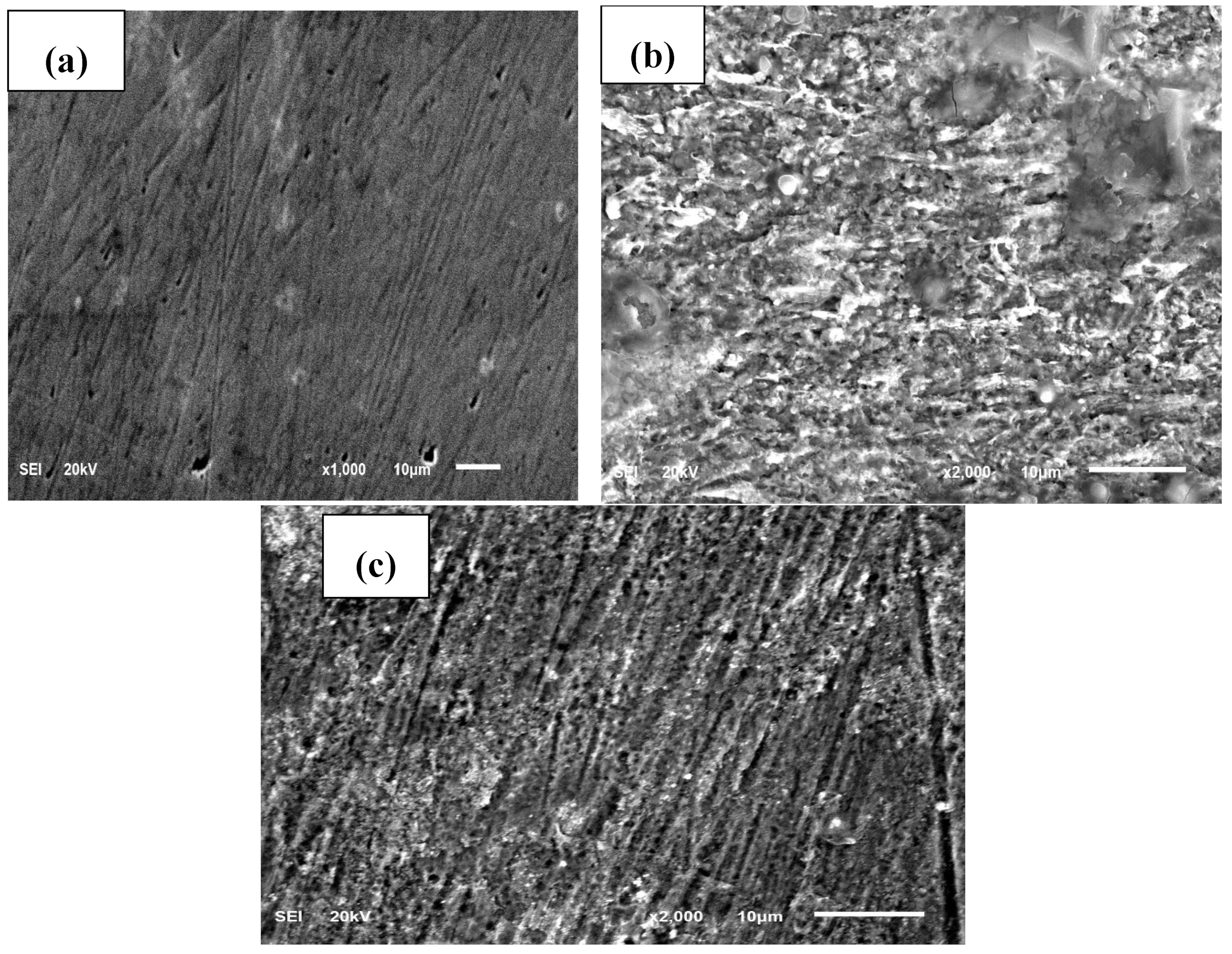
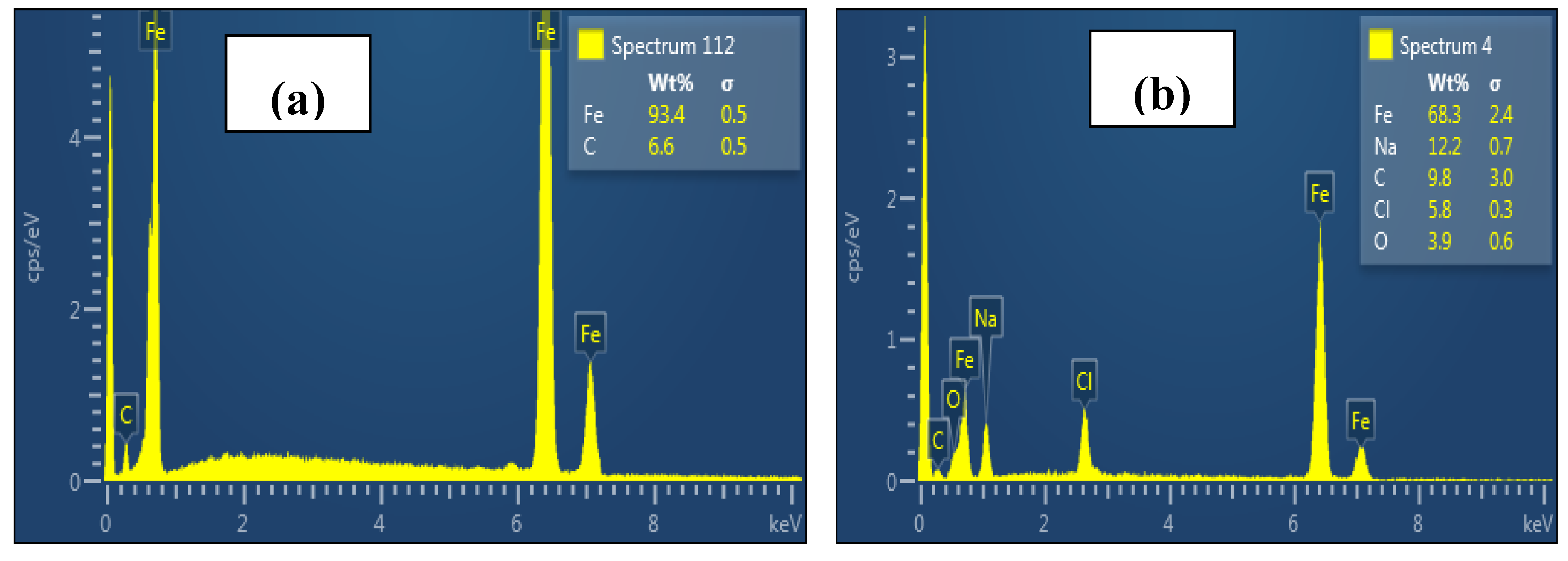
3.3. Surface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kermani, M.B.; Morshed, A. Carbon dioxide corrosion in oil and gas production—A compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Crolet, J.L.; Bonis, M.R. pH measurements in aqueous CO2 solutions under high pressure and temperature. Corrosion 1983, 39, 39–46. [Google Scholar] [CrossRef]
- De Waard, C.; Milliams, D.E. Carbonic acid corrosion of steel. Corrosion 1975, 31, 177–181. [Google Scholar] [CrossRef]
- Papavinasam, S.; Revie, R.W.; Bartos, M. Testing methods and standards for oilfield corrosion inhibitors. In Proceedings of the CORROSION, New Orleans, LA, USA, 26–30 March 2004. [Google Scholar]
- Guan, H.; Jenkins, A. Testing equipment and procedures suitable to evaluate deepwater scale and corrosion inhibitors. In Proceedings of the CORROSION, Houston, TX, USA, 10–14 March 2011. [Google Scholar]
- Ramachandran, S.; Tsai, B.L.; Blanco, M.; Chen, H.; Tang, Y.; Goddard, W.A. Self-assembled monolayer mechanism for corrosion inhibition of iron by imidazolines. Langmuir 1996, 12, 6419–6428. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, Y.B.; Tomoe, Y.; Bando, K.; Guo, X.P. Interaction of inhibitors with corrosion scale formed on N80 steel in CO2-saturated NaCl solution. Mater. Corros. 2011, 62, 1153–1158. [Google Scholar] [CrossRef]
- Öztürk, S.; Yıldırım, A.; Çetin, M.; Tavaslı, M. Synthesis of quaternary, long-chain n-alkyl amides and their corrosion inhibition in acidic media. J. Surfactants Deterg. 2014, 17, 471–481. [Google Scholar] [CrossRef]
- Rihan, R.; Shawabkeh, R.; Al-Bakr, N. The effect of two amine-based corrosion inhibitors in improving the corrosion resistance of carbon steel in sea water. J. Mater. Eng. Perform. 2014, 23, 693–699. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Recent developments on the use of polymers as corrosion inhibitors-a review. Open Mater. Sci. J. 2014, 8, 39–54. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl cellulose/silver nanoparticles composite: Synthesis, characterization and application as a benign corrosion inhibitor for St37 steel in 15% H2SO4 medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A.; Gerengi, H. Exploration of dextran for application as corrosion inhibitor for steel in strong acid environment: Effect of molecular weight, modification, and temperature on efficiency. ACS Appl. Mater. Interfaces 2018, 10, 28112–28129. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Umoren, S.A. Enhanced corrosion inhibition effect of chitosan for St37 in 15% H2SO4 environment by silver nanoparticles. Int. J. Biol. Macromol. 2017, 104, 638–649. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A.; Essien, N.B.; Essien, U.B.; Kaya, E. Gum Arabic-silver nanoparticles composite as a green anticorrosive formulation for steel corrosion in strong acid media. Carbohydr. Polym. 2018, 181, 43–55. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Israel, A.U.; Ebenso, E.E. Polypropylene glycol-silver nanoparticle composites: A novel anticorrosion material for aluminium in acid medium. J. Mater. Eng. Perform. 2015, 24, 4206–4218. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Abai, E.J. Poly(methacrylic acid)/silver nanoparticles composites: In-situ preparation, characterization and anticorrosion property for mild steel in H2SO4 solution. J. Mol. Liq. 2015, 212, 340–351. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A. Performance assessment of poly (methacrylic acid)/silver nanoparticles composite as corrosion inhibitor for aluminium in acidic environment. J. Adhes. Sci. Technol. 2015, 29, 2311–2333. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Albokheet, W.A.; Gouda, M. Carboxymethyl cellulose/metal (Fe, Cu and Ni) nanocomposites as non-precious inhibitors of C-steel corrosion in HCl solutions: Synthesis, characterization, electrochemical and surface morphology studies. Cellulose 2020, 27, 8039–8057. [Google Scholar] [CrossRef]
- John, S.; Joseph, A.; Jose, A.J.; Narayana, B. Enhancement of corrosion protection of mild steel by chitosan/ZnO nanoparticle composite membranes. Prog. Org. Coat. 2015, 84, 28–34. [Google Scholar] [CrossRef]
- Umoren, P.S.; Kavaz, D.; Umoren, S.A. Corrosion inhibition evaluation of chitosan–CuO nanocomposite for carbon steel in 5% HCl solution and effect of KI addition. Sustainability 2022, 14, 7981. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.K.; Nikhil; Ji, G.; Prakash, R. Chitosan based new nanocomposites for corrosion protection of mild steel in aggressive chloride media. Int. J. Biol. Macromol. 2019, 140, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fetouh, H.A.; Hefnawy, A.; Attia, A.M.; Ali, E. Facile and low-cost green synthesis of eco-friendly chitosan-silver nanocomposite as novel and promising corrosion inhibitor for mild steel in chilled water circuits. J. Mol. Liq. 2020, 319, 114355. [Google Scholar] [CrossRef]
- Mobin, M.; Ahmad, I.; Aslam, R.; Basik, M. Characterization and application of almond gum-silver nanocomposite as an environmentally benign corrosion inhibitor for mild steel in 1 M HCl. Mater. Chem. Phys. 2022, 289, 126491. [Google Scholar] [CrossRef]
- Obot, I.B.; Ul-Haq, M.I.; Sorour, A.A.; Alanazi, N.M.; Al-Abeedi, T.M.; Ali, S.A.; Al-Muallem, H.A. Modified-polyaspartic acid derivatives as effective corrosion inhibitor for C1018 steel in 3.5% NaCl saturated CO2 brine solution. J. Taiwan Instig. Chem. Eng. 2022, 135, 104393. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Nzila, A.; Obot, I.B. Preparation of silver/chitosan nanofluids using selected plant extracts: Characterization and antimicrobial studies against gram-positive and gram-negative bacteria. Materials 2020, 13, 1629. [Google Scholar] [CrossRef]
- Khaled, K.F.; Babić-Samardžija, K.; Hackerman, N. Cobalt(III) complexes of macrocyclic-bidentate type as a new group of corrosion inhibitors for iron in perchloric acid. Corros. Sci. 2006, 48, 3014–3034. [Google Scholar] [CrossRef]
- Umoren, S.A.; AlAhmary, A.A.; Gasem, Z.M.; Solomon, M.M. Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel. Int. J. Biol. Macromol. 2018, 117, 1017–1028. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Venkatachari, G. Influence of halide ions on the adsorption of diphenylamine on iron in 0.5 M H2SO4 solutions. Electrochim. Acta 2006, 51, 4080–4088. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Valenti, R.V.; Ruiz Hidalgo, J.; Brandán, S.A.; Gervasi, C.A. Improved electrochemical strategy to characterize adsorption and corrosion inhibition related to biomolecules from plant extracts: The case of Annona cherimola. Results Chem. 2022, 4, 100233. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A. Alternative corrosion inhibitor formulation for carbon steel in CO2-saturated brine solution under high turbulent flow condition for use in oil and gas transportation pipelines. Corros. Sci. 2019, 159, 108140. [Google Scholar] [CrossRef]
- Palimi, M.J.; Tang, Y.; Alvarez, V.; Kuru, E.; Li, D.Y. Green corrosion inhibitors for drilling operation: New derivatives of fatty acid-based inhibitors in drilling fluids for 1018 carbon steel in CO2-saturated KCl environments. Mater. Chem. Phys. 2022, 288, 126406. [Google Scholar] [CrossRef]
- Loganathan, K.T.; Thimmakondu, V.S.; Nagarajan, S.; Natarajan, R. Corrosion inhibitive evaluation and DFT studies of 2-(Furan-2-yl)-4,5-diphenyl-1H-imidazole on mild steel at 1.0 M HCl. J. Indian Chem. Soc. 2021, 98, 100121. [Google Scholar]
- Desimone, M.P.; Gordillo, G.; Simison, S.N. The effect of temperature and concentration on the corrosion inhibition mechanism of an amphiphilic amido-amine in CO2 saturated solution. Corros. Sci. 2011, 53, 4033–4043. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, X.; Zheng, Y.G. Corrosion inhibition of mild steel by ethylamino imidazoline derivative in CO2-saturated solution. Corros. Sci. 2009, 51, 761–768. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; He, Y.; Du, Y. Study of the inhibition mechanism of imidazoline amide on CO2 corrosion of Armco iron. Corros. Sci. 2001, 43, 1417–1431. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2, 5-bis (n-thienyl)-1, 3, 4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Poulson, B. Electrochemical measurements in flowing solutions. Corros. Sci. 1983, 23, 391–430. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Adesina, A.Y. Green corrosion inhibitor for oilfield application II: The time–evolution effect on the sweet corrosion of API X60 steel in synthetic brine and the inhibition performance of 2-(2-pyridyl) benzimidazole under turbulent hydrodynamics. Corros. Sci. 2020, 168, 108589. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef]
- Branzoi, V.; Branzoi, F.; Baibarac, M. The inhibition of the corrosion of Armco iron in HCl solutions in the presence of surfactants of the type of N-alkyl quaternary ammonium salts. Mater. Chem. Phys. 2000, 65, 288–297. [Google Scholar] [CrossRef]
- Iravani, D.; Esmaeili, N.; Berisha, A.; Akbarinezhad, E.; Aliabadi, M.H. The quaternary ammonium salts as corrosion inhibitors for X65 carbon steel under sour environment in NACE 1D182 solution: Experimental and computational studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130544. [Google Scholar] [CrossRef]
- ISO 4287:1997. Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. ISO. Available online: https://www.iso.org/standard/10132.html (accessed on 11 October 2022).

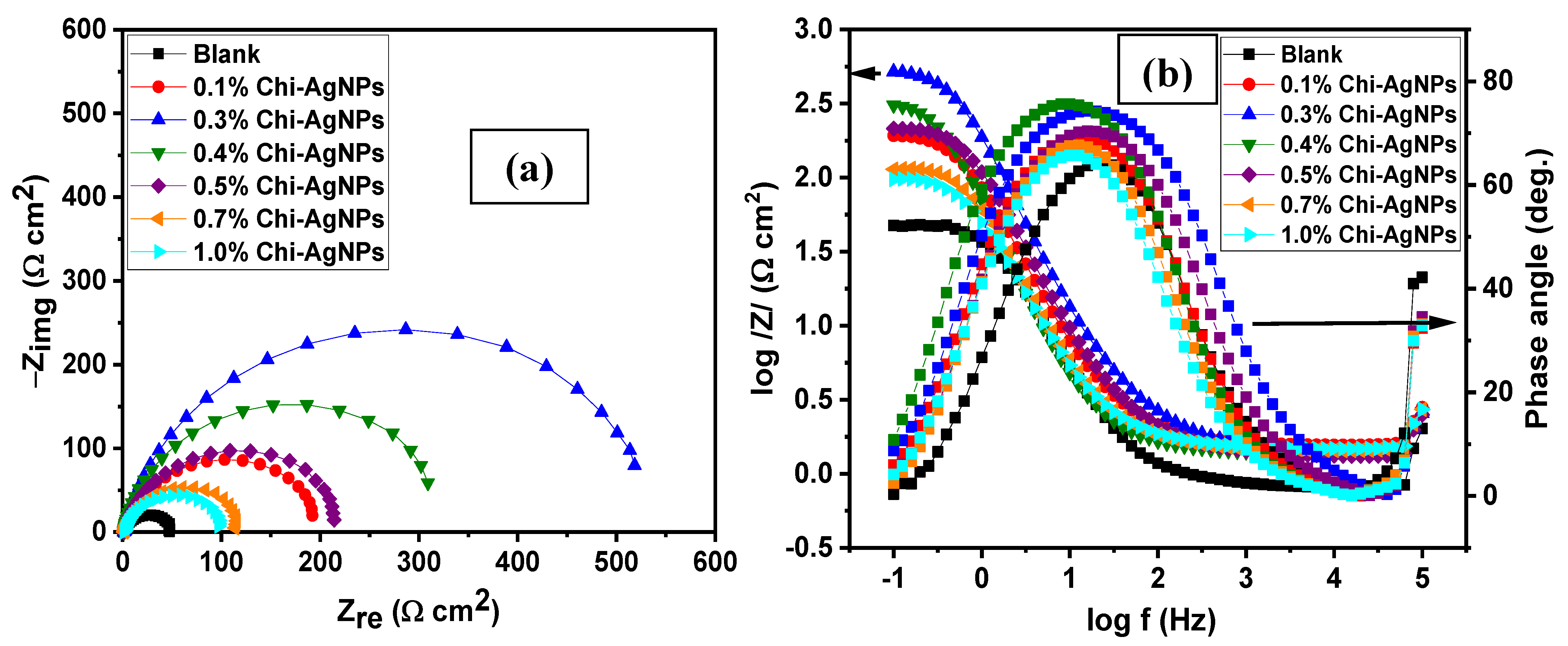
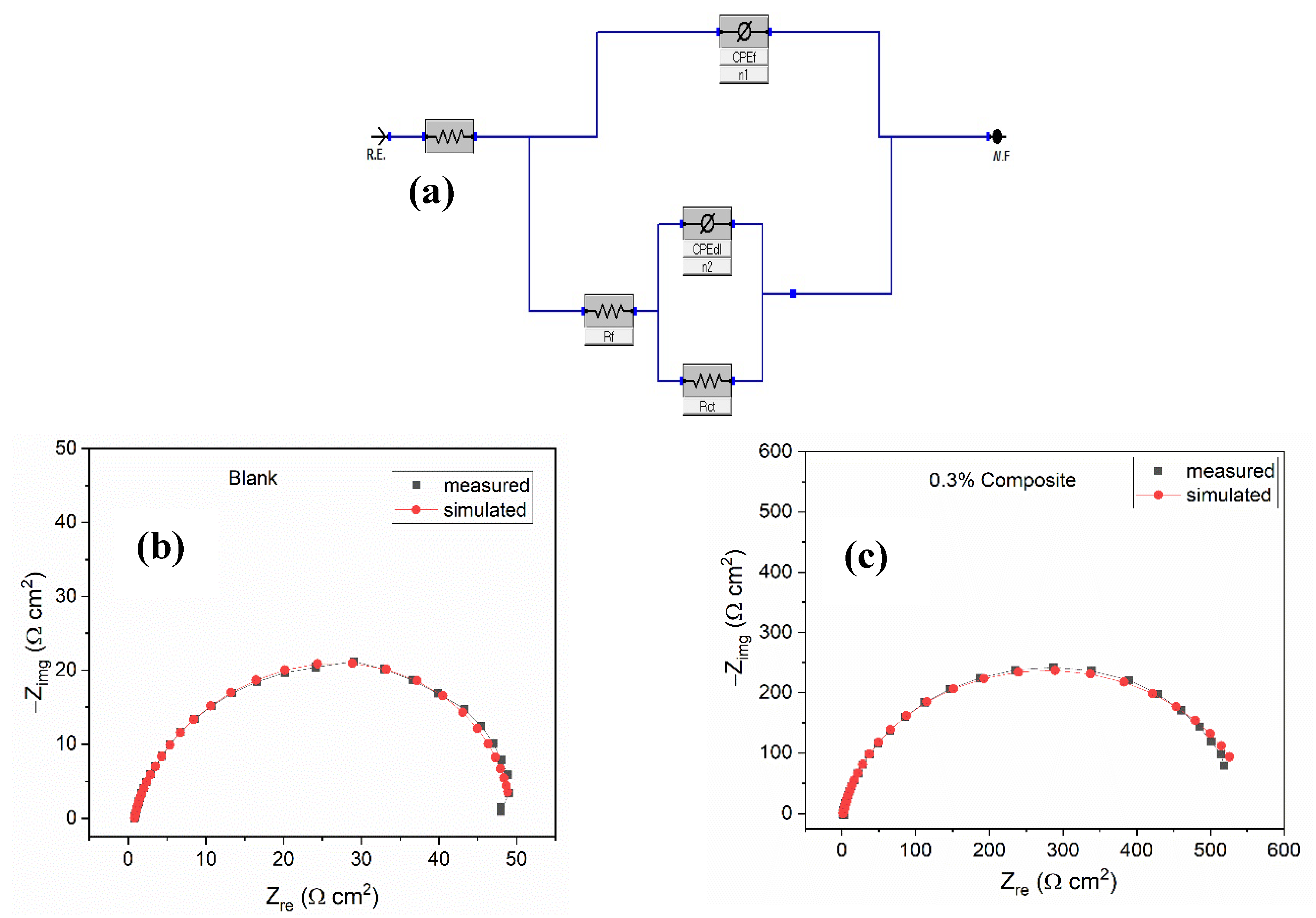
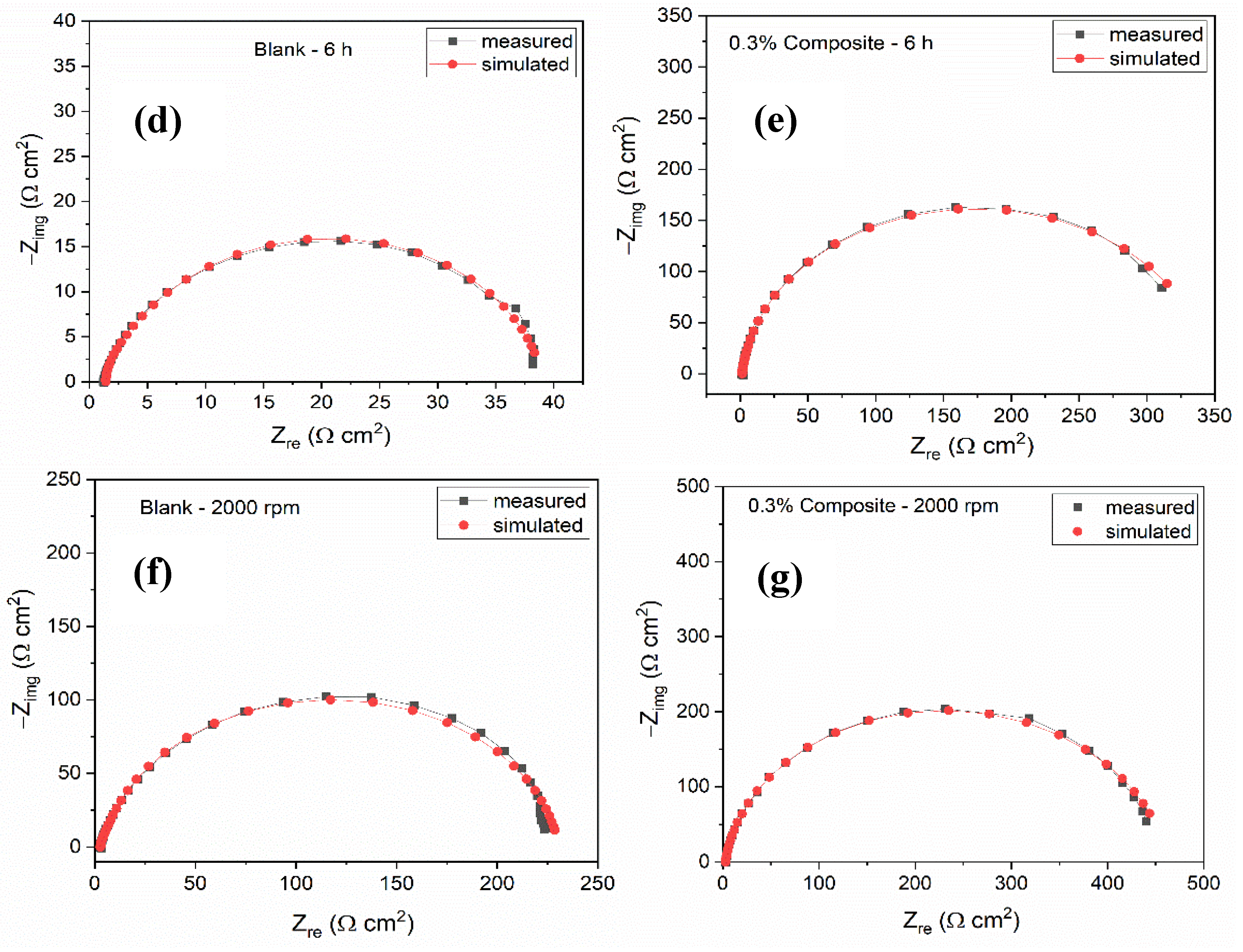
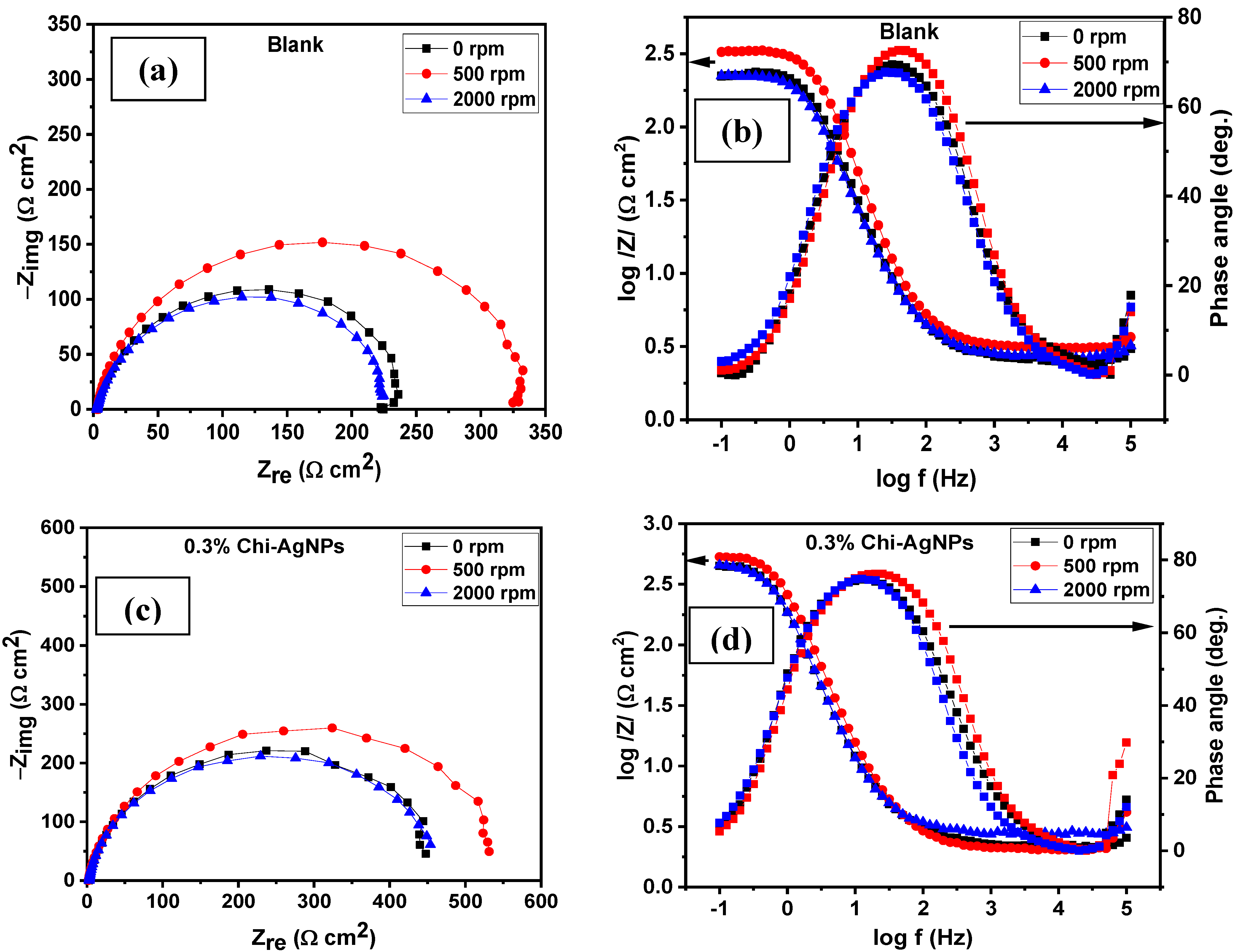
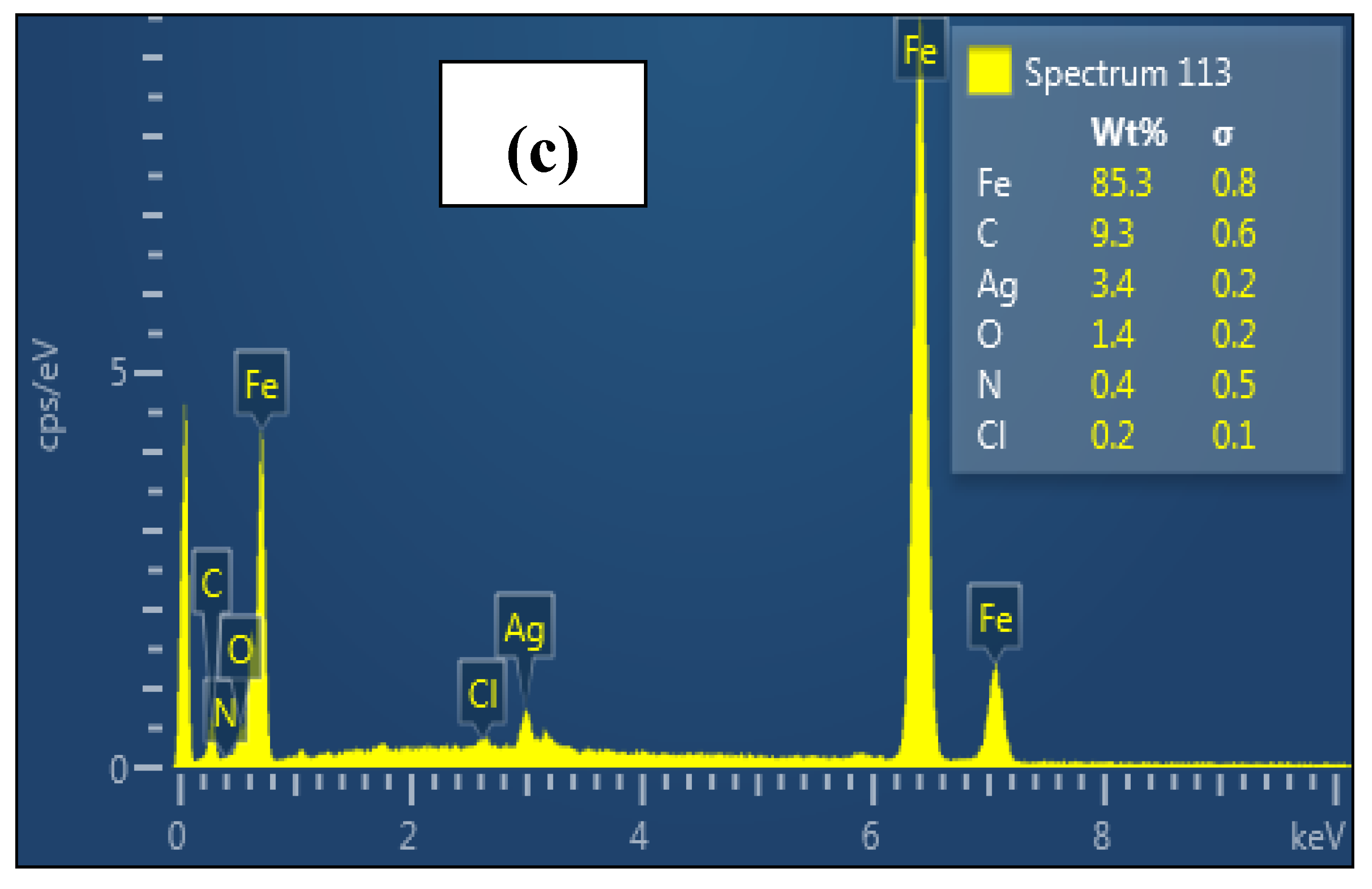
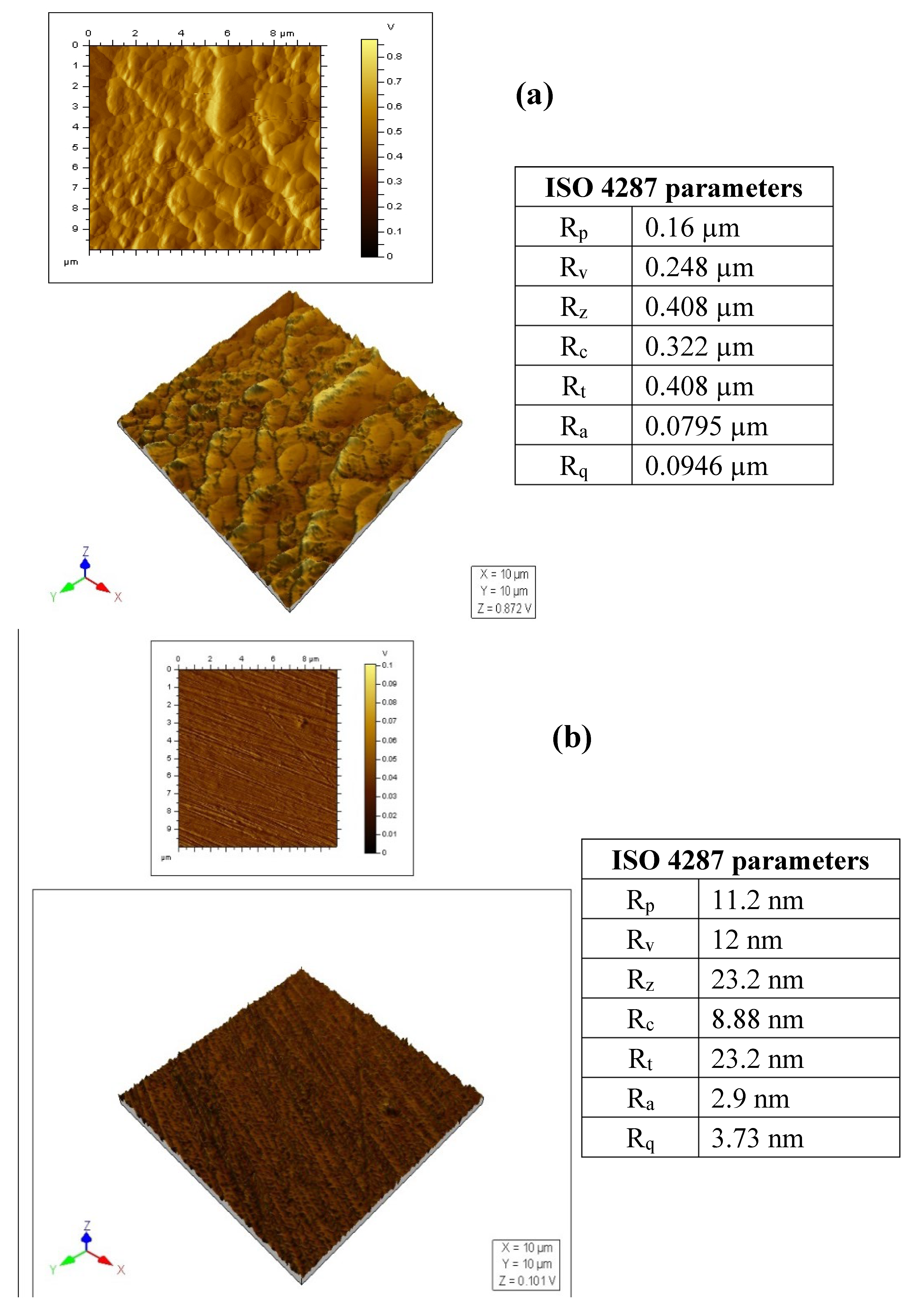
| Conc. | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (µFcm−2) | χ2 (×10−3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm−2 sn−1) | nf | Ydl (µF cm−2 sn−1) | ndl | ||||||||
| Blank | 1.22 | 895.0 | 1.00 | 16.00 | 1990.0 | 0.91 | 32.1 | 48.10 | 1069.0 | 84.60 | – |
| 0.1% | 1.82 | 519.6 | 1.00 | 18.68 | 773.0 | 0.79 | 184.6 | 203.28 | 293.0 | 41.42 | 76.34 |
| 0.3% | 1.68 | 217.0 | 1.00 | 18.59 | 319.4 | 0.80 | 541.8 | 560.39 | 114.0 | 47.77 | 92.67 |
| 0.4% | 1.63 | 684.3 | 1.00 | 29.73 | 588.1 | 0.81 | 305.8 | 335.48 | 309.0 | 52.76 | 85.66 |
| 0.5% | 1.62 | 385.3 | 1.00 | 16.77 | 598.3 | 0.82 | 221.1 | 237.82 | 243.0 | 51.00 | 79.77 |
| 0.7% | 1.69 | 791.9 | 1.00 | 19.64 | 971.9 | 0.86 | 99.3 | 118.94 | 540.0 | 50.16 | 59.56 |
| 1.0% | 1.74 | 931.0 | 1.00 | 13.62 | 1400.0 | 0.80 | 88.3 | 101.93 | 584.0 | 45.51 | 52.80 |
| PDP Measurements | LPR Measurements | ||||||
|---|---|---|---|---|---|---|---|
| System/ Concentration | –Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | Corrosion Rate (mpy) | IE (%) | Rp (Ω cm2) | IE (%) |
| Blank | 675.36 | 545.53 | 62.77 | 47.63 | - | 54.46 | - |
| 0.1% composite | 606.25 | 102.75 | 75.39 | 8.97 | 81.16 | 219.97 | 75.24 |
| 0.3% composite | 616.97 | 52.25 | 87.28 | 4.56 | 90.42 | 573.97 | 90.51 |
| 0.4% composite | 600.49 | 55.56 | 60.95 | 4.85 | 89.82 | 372.61 | 85.38 |
| 0.5% composite | 612.47 | 88.97 | 78.66 | 7.77 | 83.69 | 281.00 | 80.62 |
| 0.7% composite | 634.64 | 411.11 | 105.66 | 35.89 | 24.64 | 121.25 | 55.08 |
| 1.0% composite | 644.18 | 454.14 | 94.84 | 39.65 | 16.75 | 101.86 | 46.53 |
| System/ Time | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (mFcm−2) | χ2 (×10−3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm−2 sn−1) | nf | Ydl (µF cm−2 sn−1) | ndl | ||||||||
| Blank | |||||||||||
| 1 h | 1.78 | 818.0 | 1.00 | 9.29 | 1698.0 | 0.85 | 32.70 | 41.99 | 764.0 | 33.53 | - |
| 6 h | 1.42 | 1860.0 | 1.00 | 11.18 | 3658.0 | 0.82 | 26.55 | 37.73 | 1589.0 | 53.09 | - |
| 12 h | 1.54 | 2504.0 | 1.00 | 11.97 | 4456.0 | 0.80 | 26.24 | 38.21 | 1938.0 | 42.42 | - |
| 24 h | 1.46 | 3368.0 | 1.00 | 10.88 | 4281.5 | 0.87 | 22.74 | 33.62 | 2659.0 | 53.89 | - |
| 0.3% Composite | |||||||||||
| 1 h | 1.74 | 684.1 | 1.00 | 25.23 | 597.5 | 0.85 | 144.3 | 169.53 | 369.0 | 36.37 | 75.23 |
| 6 h | 1.56 | 975.6 | 1.00 | 45.42 | 477.3 | 0.84 | 297.4 | 342.82 | 403.0 | 45.50 | 88.99 |
| 12 h | 1.57 | 969.1 | 1.00 | 34.87 | 400.5 | 0.84 | 400.5 | 435.32 | 378.0 | 45.00 | 91.22 |
| 24 h | 1.61 | 943.3 | 0.99 | 49.22 | 379.6 | 0.87 | 408.8 | 458.02 | 395.0 | 43.12 | 92.67 |
| PDP Measurements | LPR Measurements | |||||||
|---|---|---|---|---|---|---|---|---|
| System/ Concentration | Immersion Time (h) | –Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | Corrosion Rate (mpy) | IE (%) | Rp (Ω cm2) | IE (%) |
| 1 | - | - | - | - | - | 55.08 | - | |
| Blank | 6 | - | - | - | - | - | 41.23 | - |
| 12 | - | - | - | - | - | 37.78 | - | |
| 24 | 682.29 | 438.50 | 77.99 | 38.28 | - | 34.94 | - | |
| 1 | - | - | - | - | - | 186.12 | 70.41 | |
| 0.3% composite | 6 | - | - | - | - | - | 365.18 | 88.71 |
| 12 | - | - | - | - | - | 474.26 | 92.03 | |
| 24 | 633.49 | 49.14 | 71.59 | 4.29 | 88.79 | 519.63 | 93.28 | |
| System/ Temperature | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (mFcm−2) | χ2 (×10−3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm−2 sn−1) | nf | Ydl (µF cm−2 sn−1) | ndl | ||||||||
| Blank | |||||||||||
| 25 °C | 1.22 | 895.0 | 1.00 | 16.00 | 1990.0 | 0.91 | 32.10 | 48.10 | 1069.0 | 84.60 | - |
| 40 °C | 1.20 | 2624.0 | 0.89 | 0.85 | 2399.0 | 0.74 | 29.07 | 29.92 | 667.0 | 1.58 | - |
| 60 °C | 0.90 | 2000.0 | 0.80 | 0.20 | 1085.0 | 0.81 | 23.30 | 23.50 | 311.0 | 1.42 | - |
| 0.3% Composite | |||||||||||
| 25 °C | 1.68 | 217.0 | 1.00 | 18.59 | 319.4 | 0.80 | 541.8 | 560.39 | 114.0 | 47.77 | 92.67 |
| 40 °C | 1.60 | 626.5 | 1.00 | 5.35 | 130.45 | 0.78 | 60.77 | 66.12 | 151.0 | 21.95 | 55.49 |
| 60 °C | 0.92 | 2416.0 | 0.84 | 7.35 | 1495.0 | 0.96 | 6.46 | 13.81 | 962.0 | 0.68 | −0.70 |
| PDP Measurements | LPR Measurements | |||||||
|---|---|---|---|---|---|---|---|---|
| System/ Concentration | Temperature (°C) | –Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | Corrosion Rate (mpy) | IE (%) | Rp (Ω cm2) | IE (%) |
| 25 | 675.36 | 545.53 | 62.77 | 47.63 | - | 54.46 | - | |
| Blank | 40 | 686.92 | 743.06 | 48.44 | 64.87 | - | 32.31 | - |
| 60 | 678.77 | 828.27 | 40.17 | 72.31 | - | 26.85 | - | |
| 25 | 616.97 | 52.25 | 87.28 | 4.56 | 90.42 | 573.97 | 90.51 | |
| 0.3% composite | 40 | 658.19 | 358.22 | 62.22 | 31.27 | 51.79 | 75.94 | 57.45 |
| 60 | 662.44 | 1494.00 | 60.11 | 130.48 | −80.38 | 17.89 | −50.08 | |
| Rotation Speed (rpm) | u (m/s) | Re | τ (Pa) |
|---|---|---|---|
| 500 | 0.366 | 5247 | 0.831 |
| 2000 | 1.465 | 21,002 | 8.780 |
| System/ Rotation Speed | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (mFcm−2) | χ2 (×10−3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm−2 sn−1) | nf | Ydl (µF cm−2 sn−1) | ndl | ||||||||
| Blank | |||||||||||
| 0 rpm | 2.56 | 223.3 | 0.95 | 72.65 | 85.6 | 0.99 | 153.5 | 226.15 | 121.0 | 6.59 | - |
| 500 rpm | 3.63 | 59.4 | 1.00 | 37.32 | 138.2 | 0.81 | 228.5 | 265.77 | 42.9 | 35.89 | - |
| 2000 rpm | 2.77 | 227.2 | 0.96 | 39.25 | 181.9 | 0.86 | 184.1 | 223.35 | 97.5 | 4.13 | - |
| 0.3% Composite | |||||||||||
| 0 rpm | 2.21 | 304.0 | 0.97 | 16.25 | 181.7 | 0.86 | 454.8 | 471.00 | 121.0 | 3.74 | 51.99 |
| 500 rpm | 2.47 | 209.2 | 1.00 | 91.73 | 165.7 | 0.87 | 455.4 | 547.13 | 110.0 | 47.09 | 51.42 |
| 2000 rpm | 2.64 | 297.5 | 0.99 | 22.78 | 232.3 | 0.79 | 446.1 | 468.83 | 108.0 | 2.37 | 52.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umoren, S.A.; Solomon, M.M.; Nzila, A.; Obot, I.B. Corrosion Inhibition of Rumex vesicarius Mediated Chitosan-AgNPs Composite for C1018 CS in CO2-Saturated 3.5% NaCl Medium under Static and Hydrodynamic Conditions. Sustainability 2022, 14, 16142. https://doi.org/10.3390/su142316142
Umoren SA, Solomon MM, Nzila A, Obot IB. Corrosion Inhibition of Rumex vesicarius Mediated Chitosan-AgNPs Composite for C1018 CS in CO2-Saturated 3.5% NaCl Medium under Static and Hydrodynamic Conditions. Sustainability. 2022; 14(23):16142. https://doi.org/10.3390/su142316142
Chicago/Turabian StyleUmoren, Saviour A., Moses M. Solomon, Alexis Nzila, and Ime B. Obot. 2022. "Corrosion Inhibition of Rumex vesicarius Mediated Chitosan-AgNPs Composite for C1018 CS in CO2-Saturated 3.5% NaCl Medium under Static and Hydrodynamic Conditions" Sustainability 14, no. 23: 16142. https://doi.org/10.3390/su142316142
APA StyleUmoren, S. A., Solomon, M. M., Nzila, A., & Obot, I. B. (2022). Corrosion Inhibition of Rumex vesicarius Mediated Chitosan-AgNPs Composite for C1018 CS in CO2-Saturated 3.5% NaCl Medium under Static and Hydrodynamic Conditions. Sustainability, 14(23), 16142. https://doi.org/10.3390/su142316142








