Potential Allelopathic Effect of Wheat Straw Aqueous Extract on Bermudagrass Noxious Weed
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Wheat Straw Aqueous Extract
2.2. HPLC Analysis of Wheat Extract
2.3. Greenhouse Bioassay
2.4. Determination of Morphological Parameters
2.4.1. Germination Percentage (G%)
2.4.2. Germination Index (GI)
2.4.3. The Fresh and Dry Weights of Shoots and Roots
2.4.4. Shoot and Root Lengths
2.4.5. Inhibition Percentages
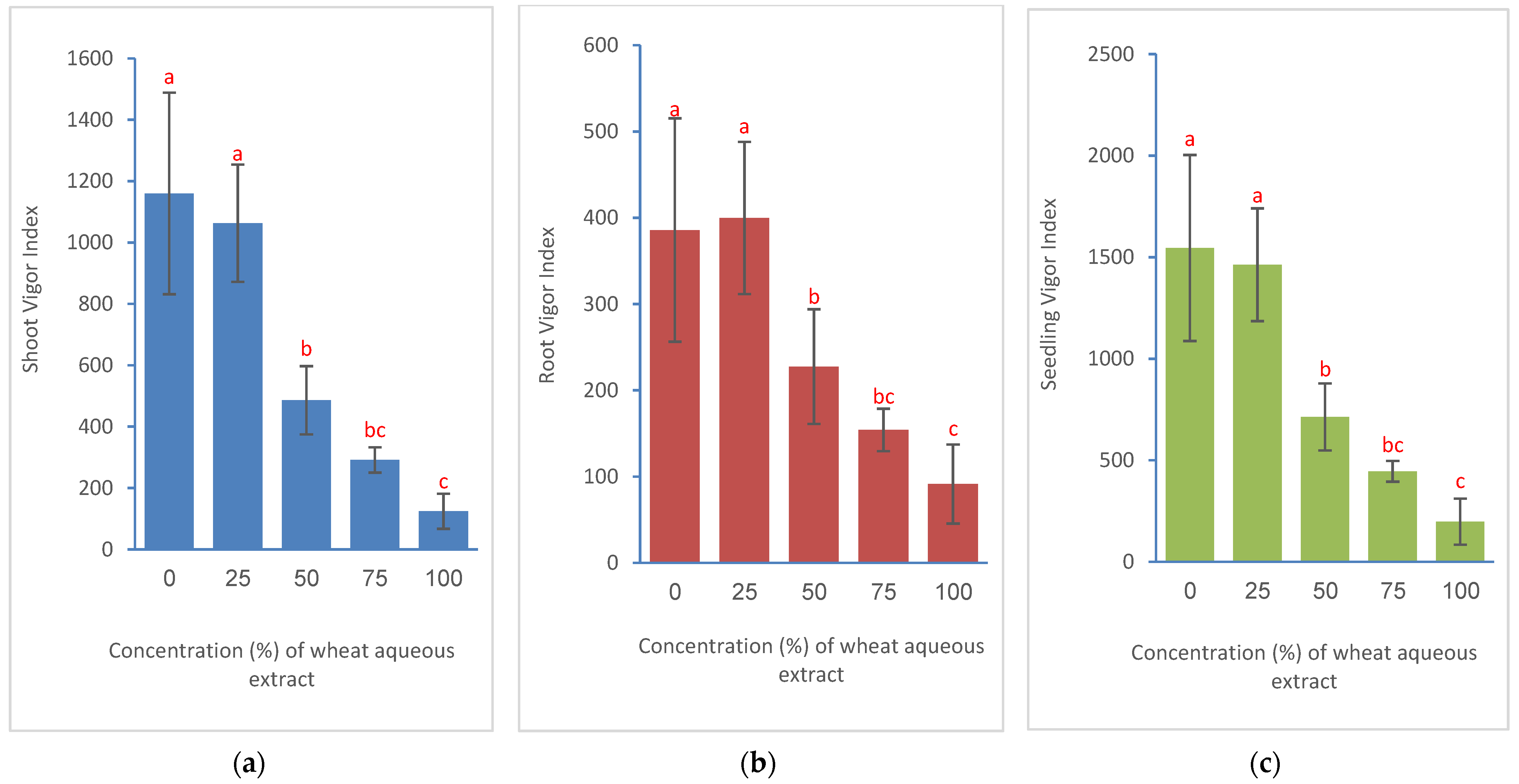
2.4.6. Vigor Indices
2.5. Determination of Physiological and Biochemical Parameters
2.5.1. Chlorophyll and Carotenoid Contents
2.5.2. Total Soluble Carbohydrates
2.5.3. Total Soluble Proteins
2.5.4. Total Free Amino Acids
2.5.5. Antioxidant Enzymes
2.6. Statistical Analysis
3. Results
3.1. HPLC Analysis of Wheat Straw Aqueous Extract
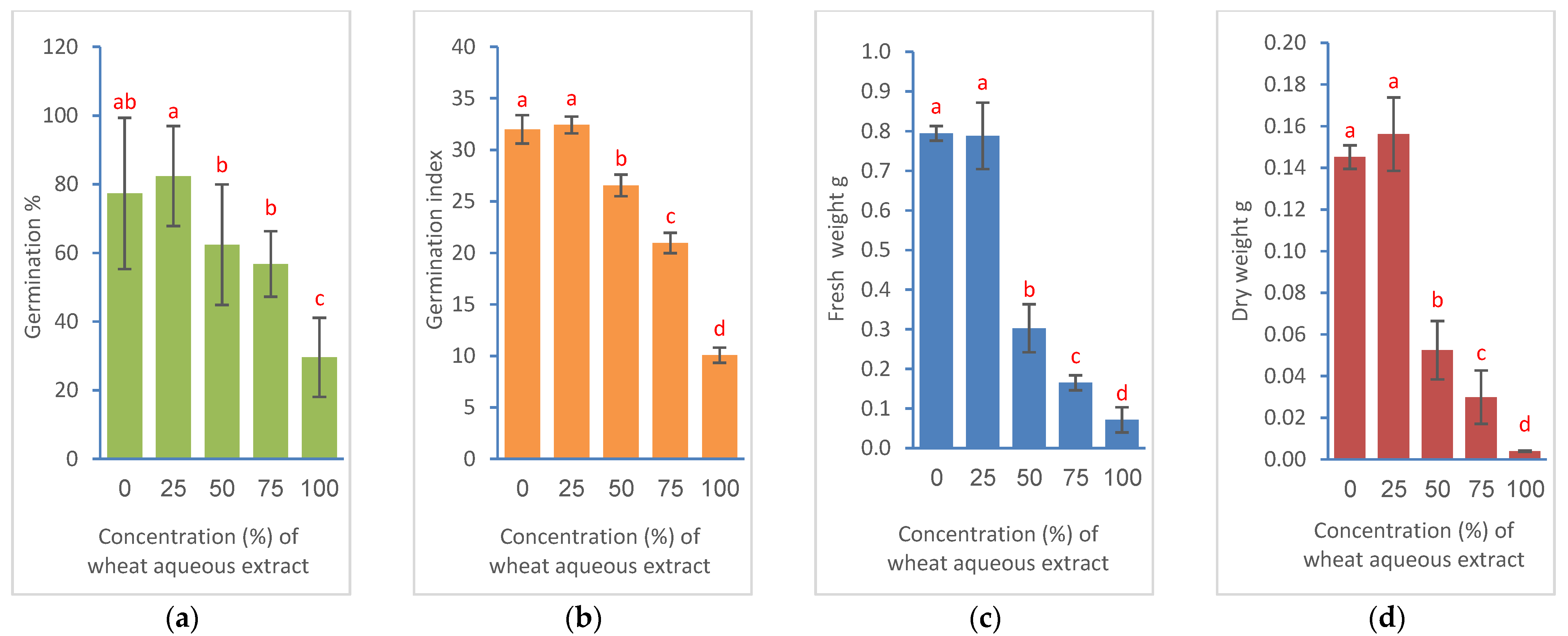
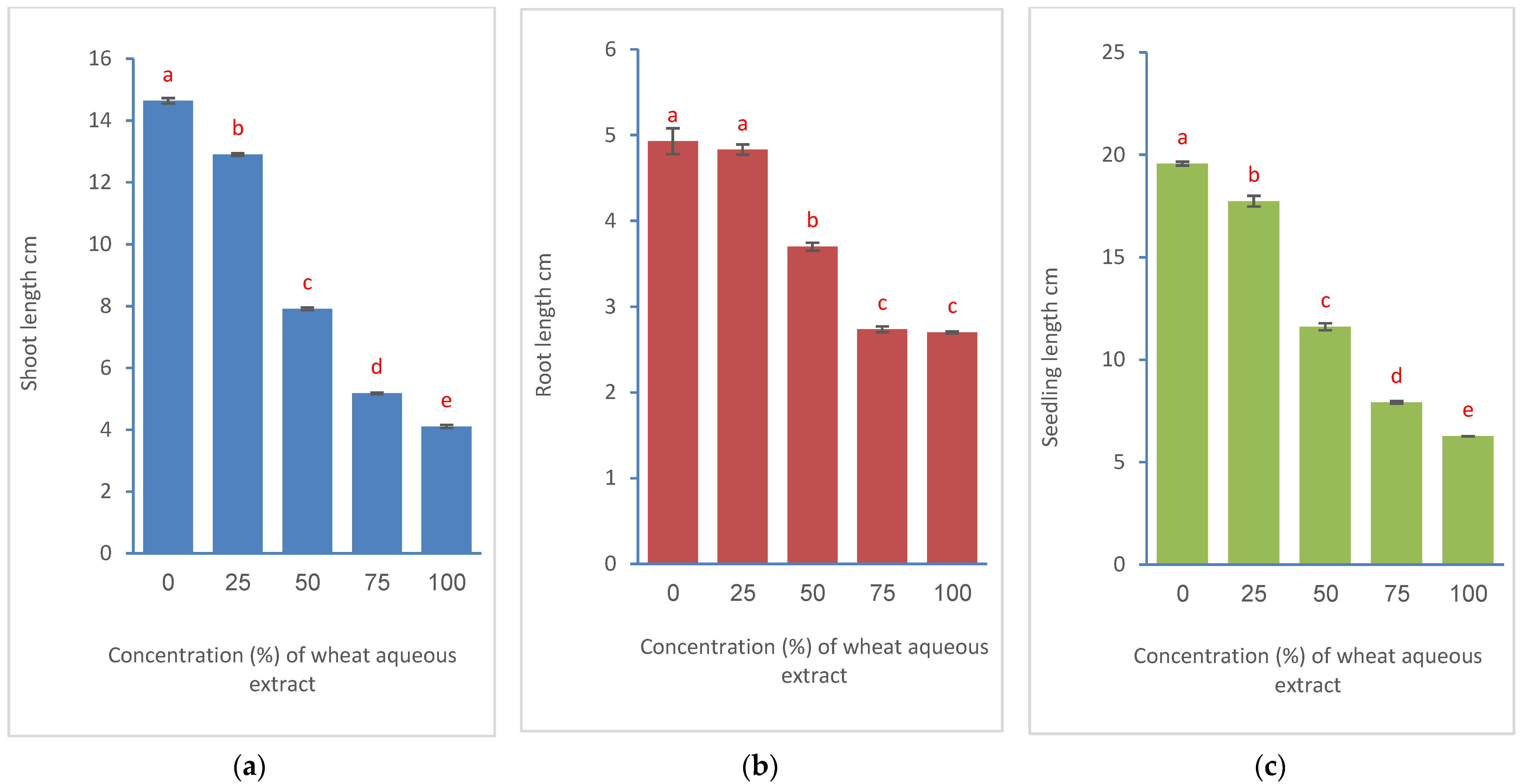
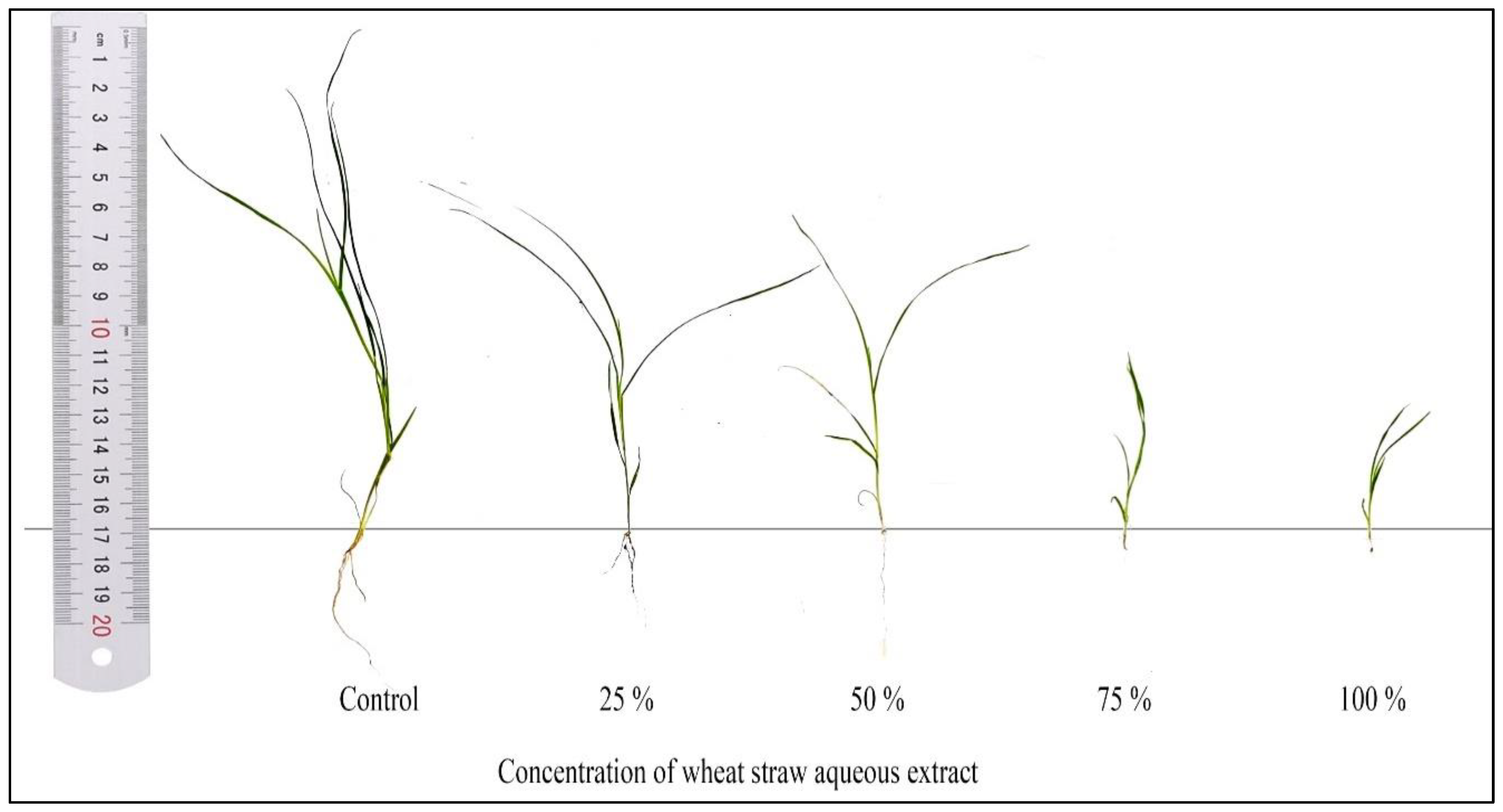
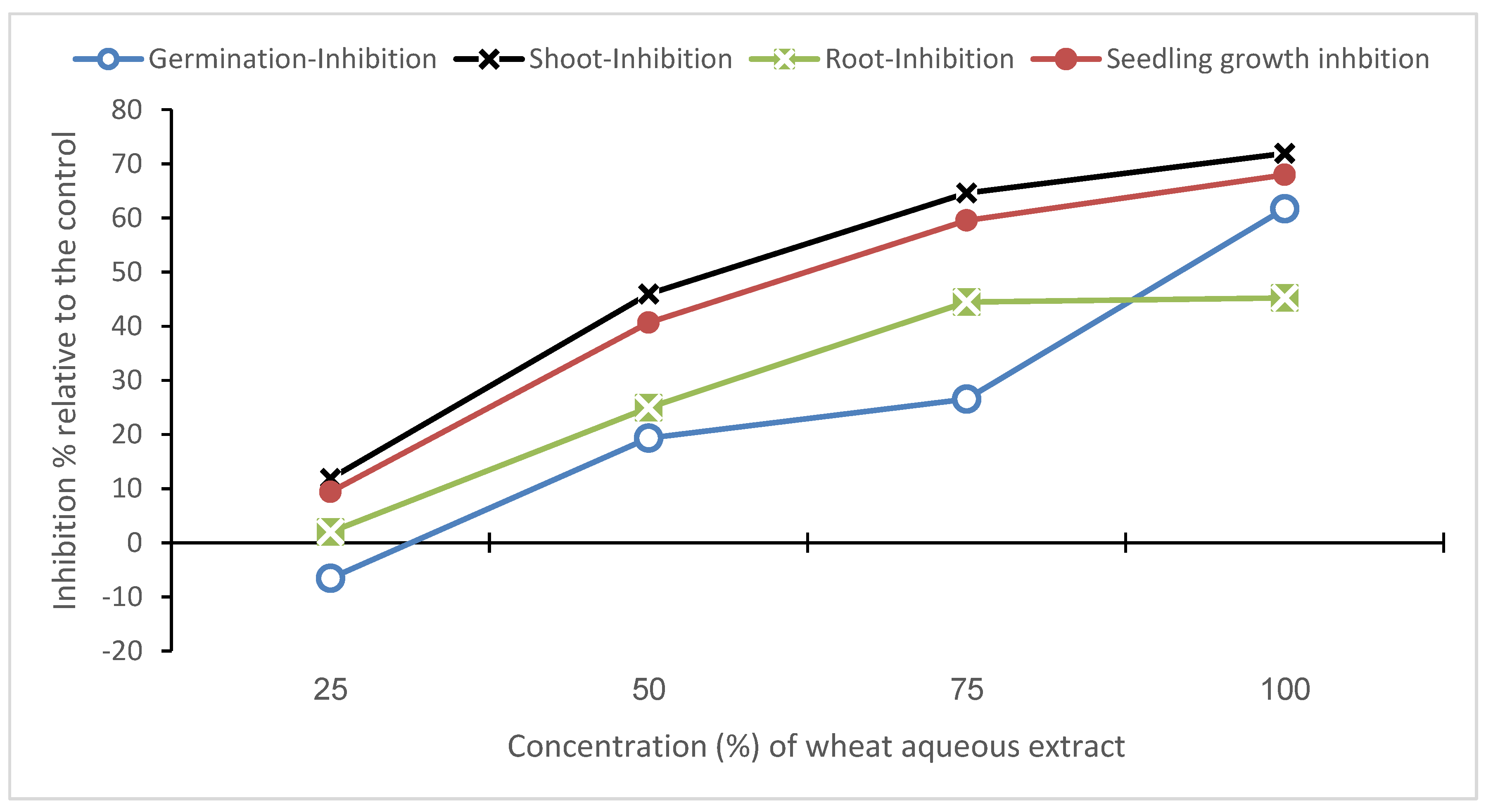
3.2. Effects on Seed Germination and Seedling Growth
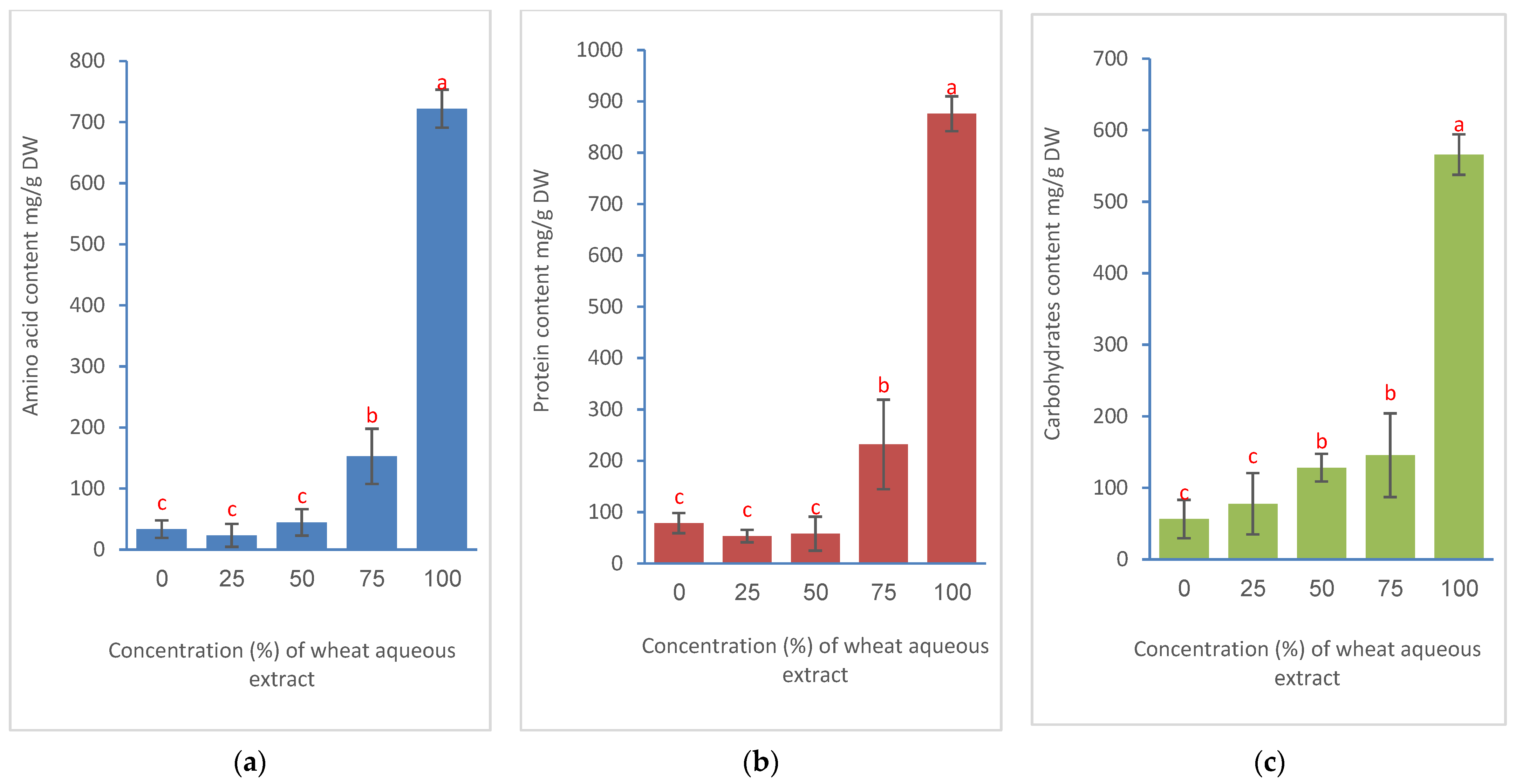
3.3. Effects on Physiological and Biochemical Traits of Bermudagrass
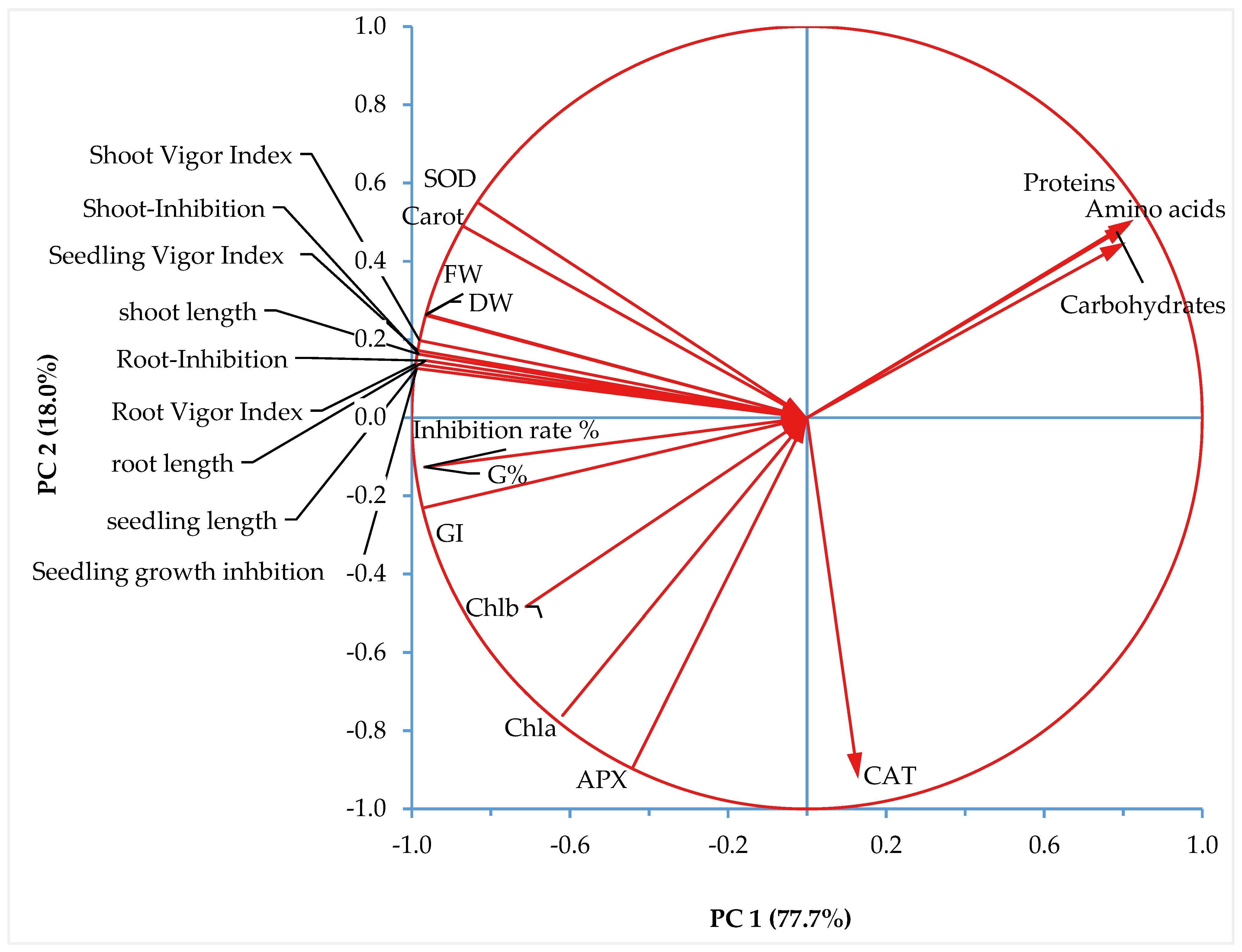
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, Y.; Sze, C.W.; Tong, Y.; Ng, T.B.; Tang, S.C.W.; Ho, J.C.M.; Xiang, Q.; Lin, X.; Zhang, Y. Research on the allelopathic potential of wheat. Agric. Sci. 2012, 3, 979–985. [Google Scholar] [CrossRef]
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]
- Eisler, R. Handbook of Chemical Risk Assessment: Health Hazards to Humans, Plants, and Animals, Three Volume Set; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Macías, F.A.; Varela, R.M.; Torres, A.; Oliva, R.M.; Molinillo, J.G. Bioactive norsesquiterpenes from Helianthus annuus with potential allelopathic activity. Phytochemistry 1998, 48, 631–636. [Google Scholar] [CrossRef]
- Uremis, I.; Arslan, M.; Uludag, A.; Sangun, M. Allelopathic potentials of residues of 6 brassica species on johnsongrass [Sorghum halepense (L.) Pers.]. Afr. J. Biotechnol. 2009, 8, 3497–3501. [Google Scholar]
- Alsaadawi, I.S.; Hadwan, H.A.; Malih, H.M. Weed Management in Cowpea through Combined Application of Allelopathic Sorghum Residues and Less Herbicide. J. Adv. Agric. Technol. 2019, 6, 205–2011. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: London, UK, 1984. [Google Scholar]
- Lemerle, D.; Verbeek, B.; Cousens, R.; Coombes, N. The potential for selecting wheat varieties strongly competitive against weeds. Weed Res. 1996, 36, 505–513. [Google Scholar] [CrossRef]
- Olofsdotter, M.; Navarez, D.; Rebulanan, M. Rice Allelopathy-Where Are We and How Far Can We Get? British Crop Protection Council: Farnham, UK, 1997; Volume 1. [Google Scholar]
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Tursun, N.; Işık, D.; Demir, Z.; Jabran, K. Use of living, mowed, and soil-incorporated cover crops for weed control in apricot orchards. Agronomy 2018, 8, 150. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Pethö, M. Occurence and physiological role of benzoxazinones and their derivates. IV: Isolation of hydroxamic acids from wheat and rye root secretions. Acta Agron. Hung. 1992, 41, 167–175. [Google Scholar]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for Weed Management. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 505–519. [Google Scholar]
- Rao, V.S. Principles of Weed Science, 2nd ed.; CRC Press: New Yourk, NY, USA, 2000. [Google Scholar]
- Juraimi, A.S.; Drennan, S.H.D.; Anuar, N. Competitive effect of Cynodon dactylon (L.) Pers. on four crop species, soybean [Glycine max (L.) Merr.], maize (Zea mays), spring wheat (Triticum aestivum) and faba bean [Vicia faba (L.)]. Asian J. Plant Sci. 2005, 4, 90–94. [Google Scholar] [CrossRef][Green Version]
- Korav, S.; Dhaka, A.K.; Singh, R.; Premaradhya, N.; Reddy, G.C. A study on crop weed competition in field crops. J. Pharmacogn. Phytochem. 2018, 7, 3235–3240. [Google Scholar]
- Narwal, S.; Sarmah, M.; Nandal, D. Allelopathic effects of wheat residues on growth and yield of fodder crops. Allelopath. J. 1997, 4, 111–120. [Google Scholar]
- Copaja, S.V.; Nicol, D.; Wratten, S.D. Accumulation of hydroxamic acids during wheat germination. Phytochemistry 1999, 50, 17–24. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T.; Verbeek, B. Differential allelopathic potential among wheat accessions to annual ryegrass. In Proceedings of the 9th Australian Agronomy Conference, Wagga Wagga, Australia, 20–23 July 1998; pp. 567–571. [Google Scholar]
- Wu, H.; Pratley, J.; Haig, T. Phytotoxic effects of wheat extracts on a herbicide-resistant biotype of annual ryegrass (Lolium rigidum). J. Agric. Food Chem. 2003, 51, 4610–4616. [Google Scholar] [CrossRef]
- Macias, F.A. Allelopathy in the search for natural herbicide models. In Allelopathy. Organisms, Processes and Applications; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; ACS Publications: Cambridge, MA, USA, 1995; pp. 310–329. [Google Scholar]
- Yang, X.; He, Y.; Song, X.E.; Yuan, X.; Li, Y.; Sun, D. Reduced growth responses of mesosulfuron-methyl-resistant blackgrass to allelopathic wheat are driven by underground chemical interaction. Plant Soil 2020, 448, 369–381. [Google Scholar] [CrossRef]
- Mahmood, K.; Khaliq, A.; Cheema, Z.A.; Arshad, M. Allelopathic activity of Pakistani wheat genotypes against wild oat (Avena fatua L.). Pak. J. Agri. Sci 2013, 50, 169–176. [Google Scholar]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Kaur, G.J.; Arora, D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 30. [Google Scholar] [CrossRef]
- Schmer, M.R.; Xue, Q.; Hendrickson, J.R. Salinity effects on perennial, warm-season (C4) grass germination adapted to the northern Great Plains. Can. J. Plant Sci. 2012, 92, 873–881. [Google Scholar] [CrossRef][Green Version]
- Kitis, Y.E.; Tekin, M.; Taner, A. The Allelopathic Effects of Turkish Hulled Wheat Lines on Germination of Amaranthus retroflexus L. and Lolium perenne L. Seeds. Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2021, 7, 56–62. [Google Scholar] [CrossRef]
- Biswas, K.; Das, A.P. Allelopathic effects of teak (Tectona grandis Lf) on germination and seedling growth of Plumbago zeylanica L. Pleione 2016, 10, 262–268. [Google Scholar]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Chang, K.-W.; Yin, M.-H.; Huang, H.-M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Braniša, J.; Jenisová, Z.; Porubská, M.; Jomová, K.; Valko, M. Spectrophotometric determination of chlorophylls and carotenoids. An effect of sonication and sample processing. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 61–64. [Google Scholar]
- Fales, F.W. The Assimilation and Degradation of Carbohydrates of Yeast Cells. J. Biol. Chem. 1951, 193, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar]
- Rizvi, S.; Haque, H.; Singh, V.; Rizvi, V. A discipline called allelopathy. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 1992; pp. 1–10. [Google Scholar]
- Harper, J.R.; Balke, N.E. Characterization of the Inhibition of K+ Absorption in Oat Roots by Salicylic Acid. Plant Physiol. 1981, 68, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–824. [Google Scholar] [CrossRef]
- Kruse, M.; Strandberg, M.; Strandberg, B. Ecological Effects of Allelopathic Plants—A Review; NERI Technical Report No. 315; National Environmental Research Institute: Silkeborg, Denmark, 2000. [Google Scholar]
- Scavo, A.; Pandino, G.; Restuccia, A.; Caruso, P.; Lombardo, S.; Mauromicale, G. Allelopathy in Durum Wheat Landraces as Affected by Genotype and Plant Part. Plants 2022, 11, 1021. [Google Scholar] [CrossRef]
- Shan-lin, L.I.; Zhen-guo, Y.O.U.; Sun-rong, L.I. Allelopathy of wheat extraction to the growth of two weeds. Chin. J. Biol. Control 1996, 12, 168–170. [Google Scholar]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Allelopathy in wheat (Triticum aestivum). Ann. Appl. Biol. 2001, 139, 1–9. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Aslam, F.; Mushtaq, M.N.; Khan, M.B. Toxic action of aqueous wheat straw extract on horse purslane. Planta Daninha 2012, 30, 269–278. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Riaz, Y. Bio-economic and qualitative impact of reduced herbicide use in direct seeded fine rice through multipurpose tree water extracts. Chil. J. Agric. Res. 2012, 72, 350–357. [Google Scholar] [CrossRef]
- Ma, Y. Allelopathic studies of common wheat (Triticum aestivum L.). Weed Biol. Manag. 2005, 5, 93–104. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, Y.M. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agron. Crop Sci. 2004, 190, 145–150. [Google Scholar] [CrossRef]
- Hashem, A.; Adkins, S.W. Introduction of Allelopathy to Wheat: Effect of Triticum speltoides on Two Weeds of Wheat. In Proceedings of the Eleventh Australian Weeds Conference, Melbourne, Australia, 30 September–3 October 1996; pp. 45–48. [Google Scholar]
- Spruell, J.A. Allelopathic Potential of Wheat Accessions; The University of Oklahoma: Norman, OK, USA, 1984. [Google Scholar]
- Wynne, K.; Adams, C.; Neely, C.; DeLaune, P.; Kimura, E.; Thapa, S. Canola Emergence and Early Growth Were Not Affected by Allelopathic Properties of Wheat Residue. Agrosyst. Geosci. Environ. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Variation of phenolic acids in shoot tissues. J. Chem. Ecol. 2001, 27, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, A.C.; Theander, O.; Aman, P. Quantitative determination by GLC of phenolic acids as ethyl derivatives in cereal straws. J. Agric. Food Chem. 1978, 26, 830–835. [Google Scholar] [CrossRef]
- Lodhi, M.A.K.; Bilal, R.; Malik, K.A. Allelopathy in agroecosystems: Wheat phytotoxicity and its possible roles in crop rotation. J. Chem. Ecol. 1987, 13, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hammouda, M.; Kremer, R.J.; Minor, H.C.; Sarwar, M. A chemical basis for differential allelopathic potential of sorghum hybrids on wheat. J. Chem. Ecol. 1995, 21, 775–786. [Google Scholar] [CrossRef]
- Fatholahi, S.; Karimmojeni, H.; Ehsanzadeh, P. Phenolic compounds and allelopathic activities of ancient emmer wheats: Perspective for non-chemical weed control scenarios. Acta Physiol. Plant. 2020, 42, 135. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Crop cultivars with allelopathic capability. Weed Res.-Oxf. 1999, 39, 171–180. [Google Scholar] [CrossRef]
- Batish, D.; Tung, P.; Singh, H.; Kohli, R. Phytotoxicity of sunflower residues against some summer season crops. J. Agron. Crop Sci. 2002, 188, 19–24. [Google Scholar] [CrossRef]
- Đorđević, T.; Sarić-Krsmanović, M.; Gajić Umiljendić, J. Phenolic compounds and allelopathic potential of fermented and unfermented wheat and corn straw extracts. Chem. Biodivers 2019, 16, e1800420. [Google Scholar] [CrossRef]
- McClure, J.W. Physiology and functions of flavonoids. In The Flavonoids; Springer: Berlin/Heidelberg, Germany, 1975; pp. 970–1055. [Google Scholar]
- Chung, I.M.; Kim, K.H.; Ahn, J.K.; Chun, S.C.; Kim, C.S.; Kim, J.T.; Kim, S.H. Screening of allelochemicals on barnyardgrass (Echinochloa crus-galli) and identification of potentially allelopathic compounds from rice (Oryza sativa) variety hull extracts. Crop Prot. 2002, 21, 913–920. [Google Scholar] [CrossRef]
- Avelar Amado, P.; Fonsêca Castro, A.H.; Samúdio Santos Zanuncio, V.; Stein, V.C.; Brentan da Silva, D.; Alves Rodrigues dos Santos Lima, L. Assessment of allelopathic, cytotoxic, genotoxic and antigenotoxic potential of Smilax brasiliensis Sprengel leaves. Ecotoxicol. Environ. Saf. 2020, 192, 110310. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant Growth Inhibiting Flavonoids in Exudate of Cistus ladanifer and in Associated Soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.; de Carvalho, M.G.; de Moraes, M.d.L.L.; Rossiello, R.O.P.; de Oliveira, D.R.; de Amorim, R.M.Q.; Junior, E.B. Chemical screening of Urochloa humidicola: Methods for characterizing secondary metabolites and allelopathic activity on forage legumes. Am. J. Plant Sci. 2018, 9, 1260–1278. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 2008, 165, 297–305. [Google Scholar] [CrossRef]
- Guan, Y.; Lin, H.; Ma, L.; Yang, Y.; Hu, X. Nitric oxide and hydrogen peroxide are important signals mediating the allelopathic response of Arabidopsis to p-hydroxybenzoic acid. Physiol. Plant. 2014, 152, 275–285. [Google Scholar] [CrossRef]
- Liu, Y.A.N.; Zhang, C.; Wei, S.; Cui, H.; Huang, H. Compounds from the subterranean part of Johnsongrass and their allelopathic potential. Weed Biol. Manag. 2011, 11, 160–166. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, D.; Fang, T.; Zhu, B.; Liu, W.; He, X.; Sun, X.; Duan, S. In vitro allelopathic effects of compounds from Cerbera manghas L. on three Dinophyta species responsible for harmful common red tides. Sci. Total Environ. 2021, 754, 142253. [Google Scholar] [CrossRef]
- Shao, J.; Wu, Z.; Yu, G.; Peng, X.; Li, R. Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): From views of gene expression and antioxidant system. Chemosphere 2009, 75, 924–928. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z.A. Allelopathy in agro-ecosystems: A critical review of wheat allelopathy-concepts and implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Barkosky, R.R.; Einhellig, F.A. Allelopathic interference of plant-water relationships by para-hydroxybenzoic acid. Bot. Bull. Acad. Sin. 2003, 44, 53–58. [Google Scholar]
- Barkosky, R.R.; Einhellig, F.A. Effects of salicylic acid on plant-water relationships. J. Chem. Ecol. 1993, 19, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreiras, A.M.; Weiss, O.A.; Reigosa-Roger, M.J. Allelopathic evidence in the Poaceae. Bot. Rev. 2003, 69, 300–319. [Google Scholar] [CrossRef]
- Barkosky, R.R.; Einhellig, F.A.; Butler, J.L. Caffeic Acid-Induced Changes in Plant–Water Relationships and Photosynthesis in Leafy Spurge Euphorbia esula. J. Chem. Ecol. 2000, 26, 2095–2109. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N.V. Effect of ferulic acid on growth and hydrolytic enzyme activities of germinating maize seeds. J. Chem. Ecol. 1992, 18, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Unal, B.T.; Bayram, M. The Allelopathic Effects of Sunflower and Wheat Root Exudates on Sinapis arvensis and Sinapis alba. Phyton 2019, 88, 413–423. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Alharbi, M.M.; Darwish, D.B.; Gad, D. Allelopathic potential of aqueous leaf extract of Rumex dentatus L. on metabolites and enzyme activities of common purslane leaves. J. Plant Interact. 2022, 17, 267–276. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Darier, S.M. Phytochemistry, allelopathy and anticancer potentiality of Withania somnifera (L.) Dunal (Solanaceae). Braz. J. Biol. 2022, 84, e263815. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Rakszegi, M.; Darko, E.; Lovegrove, A.; Molnár, I.; Lang, L.; Bedő, Z.; Molnár-Láng, M.; Shewry, P. Drought stress affects the protein and dietary fiber content of wholemeal wheat flour in wheat/Aegilops addition lines. PLoS ONE 2019, 14, e0211892. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Protein synthesis by plants under stressful conditions. In Handbook of Plant and Crop Stress, Fourth Edition; CRC Press: Boca Raton, FL, USA, 2019; pp. 405–449. [Google Scholar]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Gulzar, A.; Siddiqui, M.B. Evaluation of allelopathic effect of Eclipta alba (L.) Hassk on biochemical activity of Amaranthus spinosus L., Cassia tora L. and Cassia sophera L. Afr. J. Environ. Sci. Technol. 2014, 8, 1–5. [Google Scholar]
- Abdulghader, K.; Nojavan, M.; Naghshbandi, N. Chemical stress induced by heliotrope (Heliotropium europaeum L.) allelochemicals and increased activity of antioxidant enzymes. Pak. J. Biol. Sci. PJBS 2008, 11, 915–919. [Google Scholar] [CrossRef][Green Version]
- Sahar, A.; El-Khawas, S.M.M. The allelopathic potential of Acacia nilotica and Eucalyptus rostata on monocot (Zea mays L.) and dicot (Phaseolus vulgaris) plants. Biotechnology 2005, 4, 23–34. [Google Scholar]
- Baziramakenga, R.; Leroux, G.D.; Simard, R.R.; Nadeau, P. Allelopathic effects of phenolic acids on nucleic acid and protein levels in soybean seedlings. Can. J. Bot. 1997, 75, 445–450. [Google Scholar] [CrossRef]
- Yang, C.-M.; Lee, C.-N.; Chou, C.-H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot. Bull. Acad. Sin. 2002, 43, 299–304. [Google Scholar]
- Mersie, W.; Singh, M. Phenolic acids affect photosynthesis and protein synthesis by isolated leaf cells of velvet-leaf. J. Chem. Ecol. 1993, 19, 1293–1301. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa, M.J. Phytotoxic effect of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelopath. J 2010, 26, 157–174. [Google Scholar]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass roots chemistry: Meta-Tyrosine, an herbicidal nonprotein amino acid. Proc. Natl. Acad. Sci. USA 2007, 104, 16964–16969. [Google Scholar] [CrossRef] [PubMed]
- Gniazdowska, A.; Dobrzyńska, U.; Babańczyk, T.; Bogatek, R. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 2007, 225, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Kamal, J. Impact of allelopathy of sunflower (Helianthus annuus L.) roots extract on physiology of wheat (Triticum aestivum L.). Afr. J. Biotechnol. 2011, 10, 14465–14477. [Google Scholar]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Chauhan, B.S. Can hormesis of plant-released phytotoxins be used to boost and sustain crop production? Crop Prot. 2017, 93, 69–76. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Y.-G.; Yan, Z.-L.; Zeng, G.-M.; Liu, S.-B.; Wang, W.-J.; Tan, X.-F.; Deng, J.-Q.; Tang, X.; Wang, Q.-P. Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.-Y.; Xie, X.; Sakoda, A.; Sagehashi, M.; Li, F.-M. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2009, 91, 262–269. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Garrido, I.; Casimiro, I.d.J.; Casero, P.J.; Espinosa, F.; García-Romera, I.; Aranda, E. Defence response of tomato seedlings to oxidative stress induced by phenolic compounds from dry olive mill residue. Chemosphere 2012, 89, 708–716. [Google Scholar] [CrossRef]
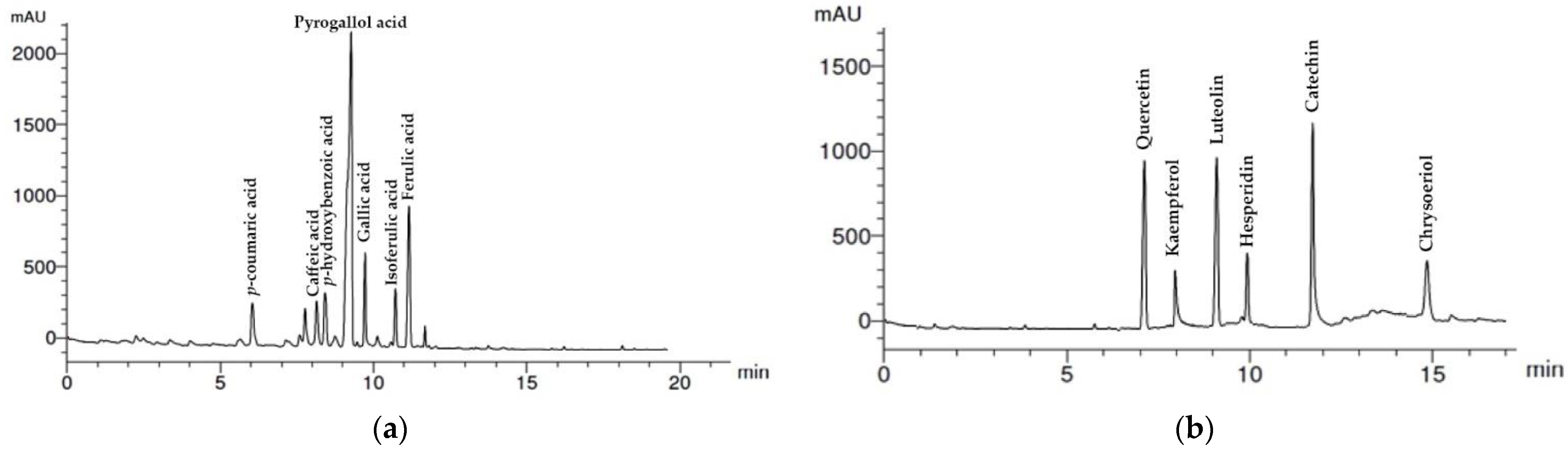


| No | Compound | Retention Time min | Concentration | ||
|---|---|---|---|---|---|
| μg/mL Aqueous Extract | µg/g Dry Extract | μg/g Straw DW | |||
| 1 | p-coumaric acid | 6.0 | 3.66 | 20.91 | 1.99 |
| 2 | Caffeic acid | 8.1 | 2.65 | 15.14 | 1.44 |
| 3 | p-hydroxybenzoic acid | 8.5 | 3.05 | 17.43 | 1.66 |
| 4 | Pyrogallol | 9.0 | 25.33 | 144.74 | 13.75 |
| 5 | Gallic acid | 9.8 | 15.66 | 89.49 | 8.50 |
| 6 | Isoferulic acid | 10.5 | 8.23 | 47.03 | 4.47 |
| 7 | Ferulic acid | 11.0 | 18.09 | 103.37 | 9.82 |
| No | Compound | Retention Time min | Concentration | ||
|---|---|---|---|---|---|
| μg/mL Aqueous Extract | µg/g Dry Extract | μg/g Straw DW | |||
| 1 | Quercetin | 7.0 | 14.25 | 81.43 | 7.74 |
| 2 | Kaempferol | 8.0 | 5.36 | 30.63 | 2.91 |
| 3 | Luteolin | 9.0 | 15.21 | 86.91 | 8.26 |
| 4 | Hesperidin | 10.0 | 7.14 | 40.80 | 3.88 |
| 5 | Catechin | 11.9 | 20.33 | 116.17 | 11.04 |
| 6 | Chrysoeriol | 15.0 | 5.21 | 29.77 | 2.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.A.; Al-Nehmi, A.A.; Ibrahim, O.H.M. Potential Allelopathic Effect of Wheat Straw Aqueous Extract on Bermudagrass Noxious Weed. Sustainability 2022, 14, 15989. https://doi.org/10.3390/su142315989
Alghamdi SA, Al-Nehmi AA, Ibrahim OHM. Potential Allelopathic Effect of Wheat Straw Aqueous Extract on Bermudagrass Noxious Weed. Sustainability. 2022; 14(23):15989. https://doi.org/10.3390/su142315989
Chicago/Turabian StyleAlghamdi, Sameera A., Ashwag A. Al-Nehmi, and Omer H. M. Ibrahim. 2022. "Potential Allelopathic Effect of Wheat Straw Aqueous Extract on Bermudagrass Noxious Weed" Sustainability 14, no. 23: 15989. https://doi.org/10.3390/su142315989
APA StyleAlghamdi, S. A., Al-Nehmi, A. A., & Ibrahim, O. H. M. (2022). Potential Allelopathic Effect of Wheat Straw Aqueous Extract on Bermudagrass Noxious Weed. Sustainability, 14(23), 15989. https://doi.org/10.3390/su142315989








