Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics
Abstract
1. Introduction
2. Materials and Methods
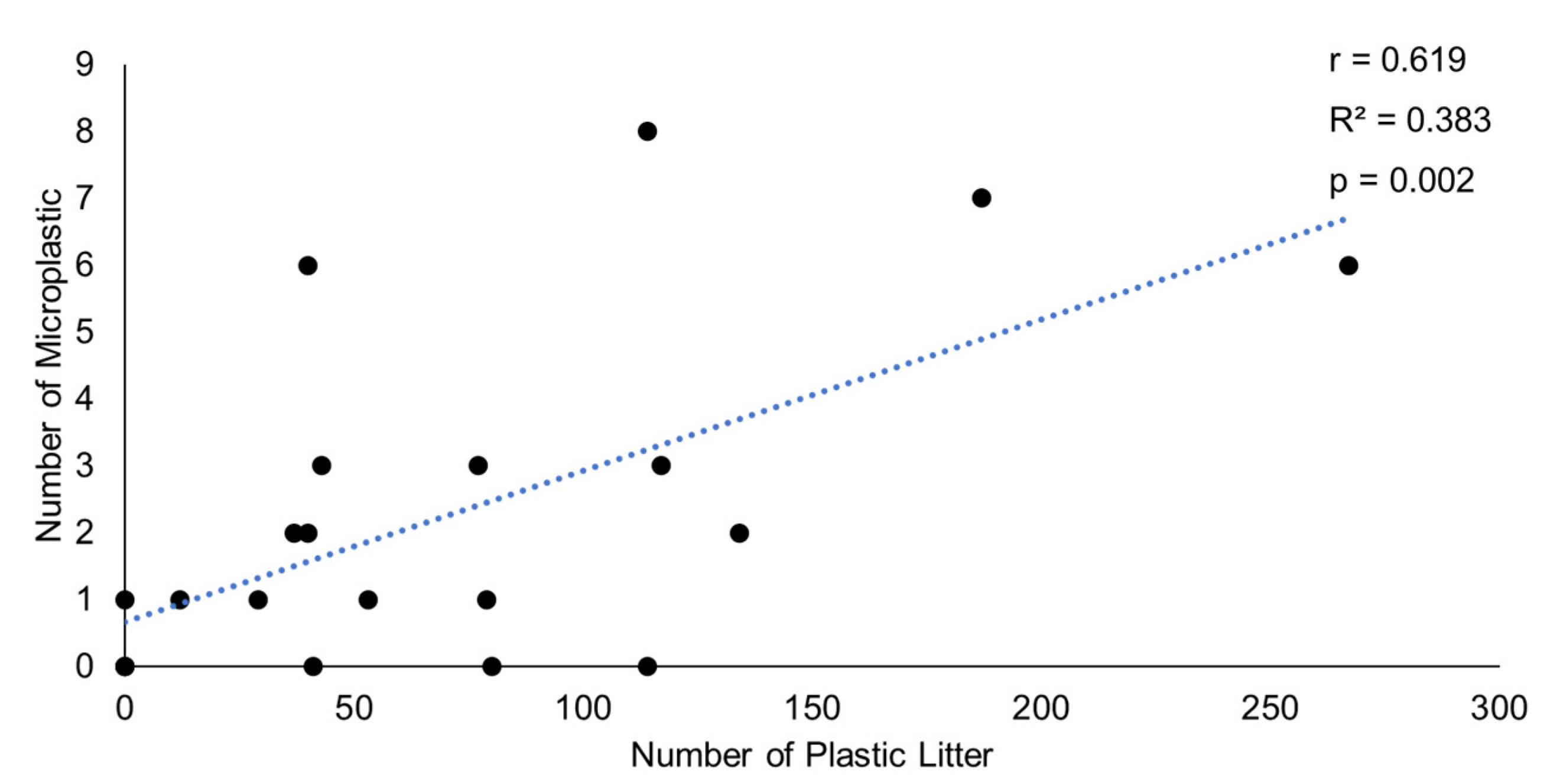
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abreo, N.A.S. Marine plastics in the Philippines: A call for research. Phil. Sci. Letters 2018, 11, 20. [Google Scholar]
- Bangun, A.P.; Wahyuningsih, H.; Muhtadi, A. Impacts of macro—And microplastic on macrozoobenthos abundance in intertidal zone. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Malang, Indonesia, 20–21 September 2018; Volume 122. [Google Scholar] [CrossRef]
- Cheang, C.C.; Ma, Y.; Fok, L. Occurrence and composition of microplastics in the seabed sediments of the coral communities in proximity of a metropolitan area. Int. J. Envi. Res. Pub. Health 2018, 15, 2270. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. NOAA Technical Memorandum NOS-OR&R-30. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Washington, DC, USA, 9–11 September 2008. [Google Scholar]
- GESAMP Sources, fate and effects of microplastics in the marine environment: Part two of a global assessment. In IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/ UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine En; Kershaw, P.J., Rochman, C.M., Eds.; International Maritime Organization: London, UK, 2016. [Google Scholar]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 6, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Obbard, J.P. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761–767. [Google Scholar] [CrossRef]
- Bevan, R.J.; Harrison, P.; Weeks, J.; Marsden, P.; Coulon, F.; Hassard, F.; Jarvis, P.; Jefferson, B.; Campo, P.; Bevan, A.R.; et al. Exposure of humans to microplastics in the environment and potential risks to health: An overview. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P. KEPC Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C. 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Reports 2016, 6, 34351. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab carcinus maenas. Envi. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizuka, K.; Fukuwa, M.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thomson, R.C.; Galloway, T.S.; Tamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Bucol, L.A.; Romano, E.F.; Cabcaban, S.M.; Siplon, L.M.D.; Madrid, G.C.; Bucol, A.A.; Polidoro, B. Microplastics in marine sediments and rabbitfish (Siganus fuscescens) from selected coastal areas of Negros Oriental, Philippines. Mar. Pollut. Bull. 2020, 150, 110685. [Google Scholar] [CrossRef] [PubMed]
- Esquiñas, G.G.M.S.; Mantala, A.P.; Atilano, M.G.; Apugan, R.P.; Galarpe, V.R.K.R. Physical characterization of litter and microplastic along the urban coast of Cagayan de Oro in Macajalar Bay, Philippines. Mar. Pollut. Bull. 2020, 154, 111083. [Google Scholar] [CrossRef] [PubMed]
- Kalnasa, M.L.; Lantaca, S.M.O.; Boter, L.C.; Flores, G.J.T.; Galarpe, V.R.K.R. Occurrence of surface sand microplastic and litter in Macajalar Bay, Philippines. Mar. Pollut. Bull. 2019, 149, 110521. [Google Scholar] [CrossRef] [PubMed]
- Paler, M.K.O.; Malenab, M.C.T.; Maralit, J.R.; Nacorda, H.M. Plastic waste occurrence on a beach off southwestern Luzon, Philippines. Mar. Pollut. Bull. 2019, 141, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Galarpe, V.R.K.R.; Jaraula, C.M.B.; Paler, M.K.O. The nexus of macroplastic and microplastic research and plastic regulation policies in the Philippines marine coastal environments. Mar. Pollut. Bull. 2021, 167, 112343. [Google Scholar] [CrossRef]
- [UNESCO] United Nations Educational, Scientific and Cultural Organization. Palawan Biosphere Reserve, Philippines. Available online: https://en.unesco.org/biosphere/aspac/palawan (accessed on 23 September 2022).
- [PSA] Philippines Statistics Authority. Palawan Recorded the Highest Increase in the Value of Production in Agriculture and Fisheries at 1.0 Percent in 2020 (At Constant 2018 Prices). Available online: https://psa.gov.ph/content/palawan-recorded-highest-increase-value-production-agriculture-and-fisheries-10-percent-2020 (accessed on 23 September 2022).
- Madarcos, J.R.; Alcantara-Creencia, L.; Roberts, B.; White, M.; Nayoan, J.; Morrissey, K.; Fleming, L. Understanding Local Perceptions of the Drivers/Pressures on the Coastal Marine Environment in Palawan, Philippines. Front. Mar. Sci. 2021, 8, 1122. [Google Scholar] [CrossRef]
- Sajorne, R.E.; Bacosa, H.P.; Cayabo, G.D.B.; Ardines Jr, L.B.; Sumeldan, J.D.C.; Omar, J.M.; Creencia, L.A. Plastic litter pollution along sandy beaches in Puerto Princesa, Palawan Island, Philippines. Mar. Pollut. Bull. 2021, 169, 112520. [Google Scholar] [CrossRef]
- Sajorne, R.E.; Cayabo, G.D.B.; Madarcos, J.R.V.; Madarcos, K.G.; Omar, D.M., Jr.; Ardines, L.B.; Mabuhay-Mar, J.A.; Cheung, V.T.; Creencia, L.A.; Bacosa, H.P. Occurrence of COVID-19 personal protective equipment (PPE) litters along the eastern coast of Palawan Island, Philippines. Mar. Pollut. Bull. 2022, 182, 113934. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Annals Int. Med. 2019, 171, 453. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Envi. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Worldwide: Sources and Sinks. Envi. Sci. Tech. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Balisco, R.A.T.; Tahajudjin, C.J.D.; Vigonte, A.C.M. Fishing gears and their common catch in two coastal areas of Palawan, Philippines: Implications to fisheries management. Intl. J. Fish. Aq. Stud. 2019, 7, 216–222. [Google Scholar]
- Zhao, S.; Zhu, L.; Li, D. Characterization of small plastic debris on tourism beaches around the South China Sea. Reg. Stud. Mar. Sci. 2015, 1, 55–62. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc B. 2009, 364, 19851998. [Google Scholar] [CrossRef]
- Piñon-Colin, T.; Rodriguez-Jimenez, R.; Pastran-Corral, M.A.; Rogel-Hernandez, E.; Wkaida, F.T. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018, 131, 63–71. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ. 2018, 636, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Martin, C.; Cózar, A.; Duarte, C.M. Low Abundance of Plastic Fragments in the Surface Waters of the Red Sea. Front. Mar. Sci. 2017, 4, 333. [Google Scholar] [CrossRef]
- Akkajit, P.; Tipmanee, D.; Cherdsukjai, P.; Suteerasak, T.; Thongnonghin, S. Occurrence and distribution of microplastics in beach sediments along Phuket coastline. Mar. Pollut. Bull. 2021, 169, 112496. [Google Scholar] [CrossRef] [PubMed]
- Bissen, R.; Chawchai, S. Microplastics on beaches along the eastern Gulf of Thailand A preliminary study. Mar. Pollut. Bull. 2020, 157, 111345. [Google Scholar] [CrossRef]
- Stolte, A.; Forster, S.; Gerdts, G.; Schubert, H. Microplastic concentrations in beach sediments along the German Baltic coast. Mar. Pollut. Bull. 2015, 99, 216–229. [Google Scholar] [CrossRef]
- Navarro, C.K.P.; Arcadio, C.G.L.A.; Similatan, K.M.; Inocente, S.A.T.; Banda, M.H.T.; Capangpangan, R.Y.; Torres, A.G.; Bacosa, H.P. Unraveling Microplastic Pollution in Mangrove Sediments of Butuan Bay, Philippines. Sustainability 2022, 14, 14469. [Google Scholar] [CrossRef]
- Arcadio, C.G.L.A.; Navarro, C.K.P.; Similatan, K.M.; Inocenter, S.A.T.; Ancla, S.M.B.; Banda, M.H.T.; Capangpangan, R.Y.; Bacosa, H.P.B. Microplastics in surface water of Laguna de Bay: First documented evidence on the largest lake in the Philippines. Environ. Sci. Pollut. Res. 2022. revised. [Google Scholar] [CrossRef]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dolega, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea-a preliminary study. Environ. Sci. Pollut. Res. Int. 2017, 24, 7650–7661. [Google Scholar] [CrossRef]
- Qiu, Q.; Peng, J.; Yu, X.; Chen, F.; Wang, J.; Dong, F. Occurrence of microplastics in the coastal marine environment: First observation on sediment of China. Mar. Pollut. Bull. 2015, 98, 274e280. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms based on multi-technology. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tarnminga, M. Microplastic pollution in lakes and lake shoreline sediments- a case study of Lake Bolsena and lake Chiusi (Central Italy). Environ Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Abreo, N.A.S.; Macusi, E.D.; Blatchley, D.D.; Cuenca, G.C. Ingestion of marine plastic debris by green turtle (Chelonia mydas) in Davao Gulf, Mindanao. Philippines. Philipp. J. Sci. 2016, 145, 17–23. [Google Scholar]
- Abreo, N.A.S.; Macusi, E.D.; Blatchey, D.; Cuenca, G. First Evidence of Plastic Ingestion by the Rare Deraniyagala’s Beaked Whale (Mesoplodon hotaula). IAMURE Int. J. Ecol. Conserv. 2016, 19, 16–36. [Google Scholar]
- Zhang, W.; Ma, X.; Zhang, Z.; Wang, Y.; Wang, J.; Wang, J.; Ma, D. Persistent organic pollutants carried on plastic resin pellets from two beaches in China. Mar. Pollut. Bull. 2015, 99, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Comm. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc. R. Soc. B Bio. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russel, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 354–364. [Google Scholar] [CrossRef]
- Fok, L.; Cheung, P.K. Hong Kong at the Pearl River Estuary: A hotspot of microplastic pollution. Mar. Pollut. Bull. 2015, 99, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.S.; Xu, X.; Wong, C.Y.; Cheung, S.G. Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environ. Pollut. 2018, 236, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Peng, J.; Wang, J.; Wang, K.; Bao, S. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea. Environ. Pollut. 2016, 214, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Doyen, P.; Hermabessiere, L.; Dehaut, A.; Himber, C.; Decodts, M.; Degraeve, T.; Delord, L.; Gaboriaud, M.; Sacco, J.; Tavernier, E.; et al. Occurrence and identification of microplastics in beach sediments from Hauts-de-France region. Envi. Sci. Poll. Res. 2019, 26, 28010–28021. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, X.; Peng, C.; Qiao, S.; Teng, W.; Wenwen, Y.; Khokiattiwong, S.; Kornkanitnan, N. Occurrence and distribution of microplastics in surface sediments from the Gulf of Thailand. Mar. Pollut. Bull. 2020, 152, 110916. [Google Scholar] [CrossRef]
- Prarat, P.; Thanayotmethi, T.; Sriboonyapirat, T.; Donsomchit, P.; Hongsawat, P.; Chouychai, B. Preliminary study of abundance and characteristics of microplastics on beach sediment along the coast of Rayong Province, Thailand. In Proceedings of the 10th International Conference on Future Environment and Energy, Kyoto, Japan, 7–9 January 2020. [Google Scholar] [CrossRef]
- Hengstmann, E.; Tamminga, M.; Vom Bruch, C.; Fischer, E.K. Microplastic in beach sediments of the Isle of Rugen (Baltic Sea)—Implementing a novel glass elutriation column. Mar. Pollut. Bull. 2018, 126, 263–274. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
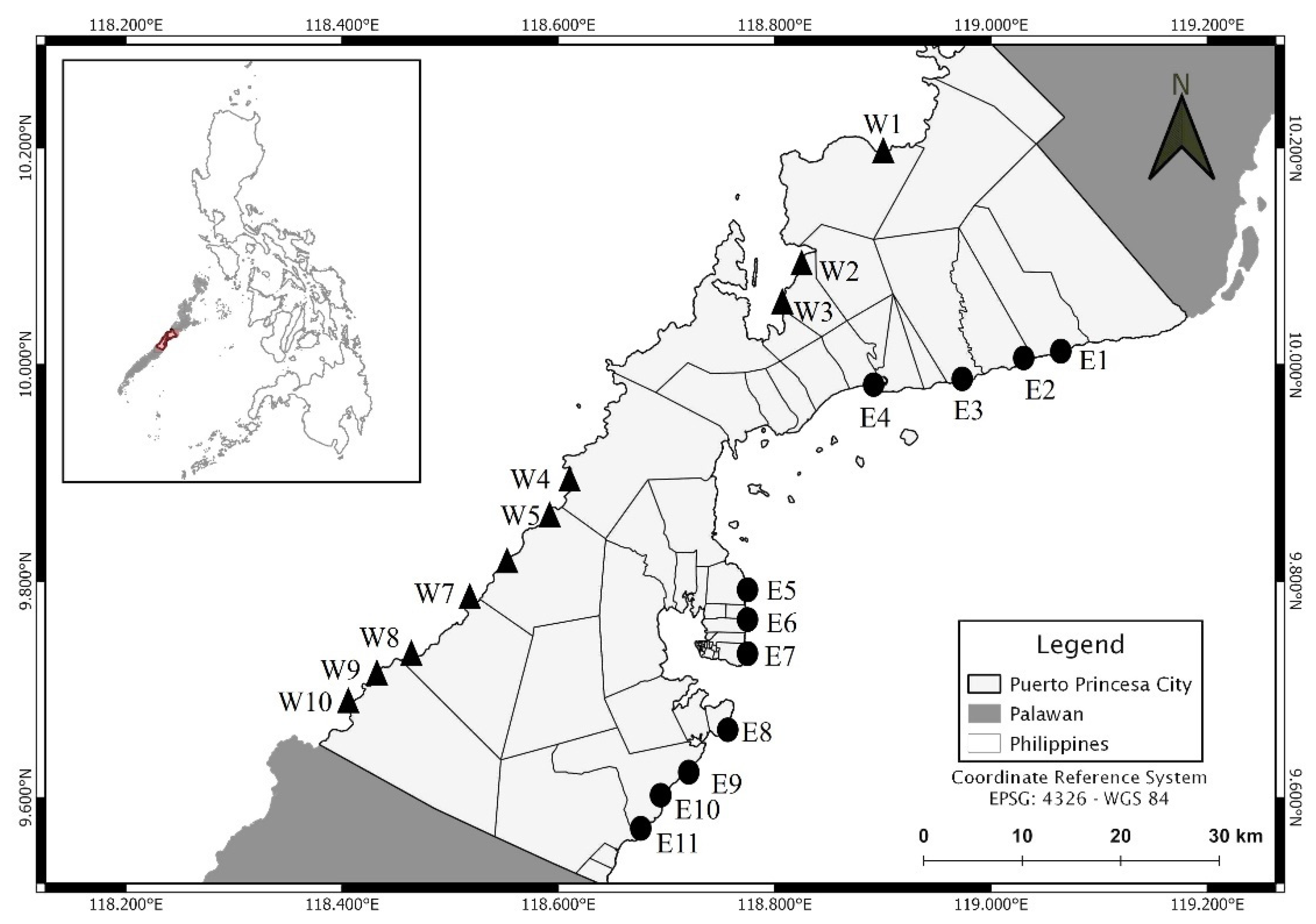
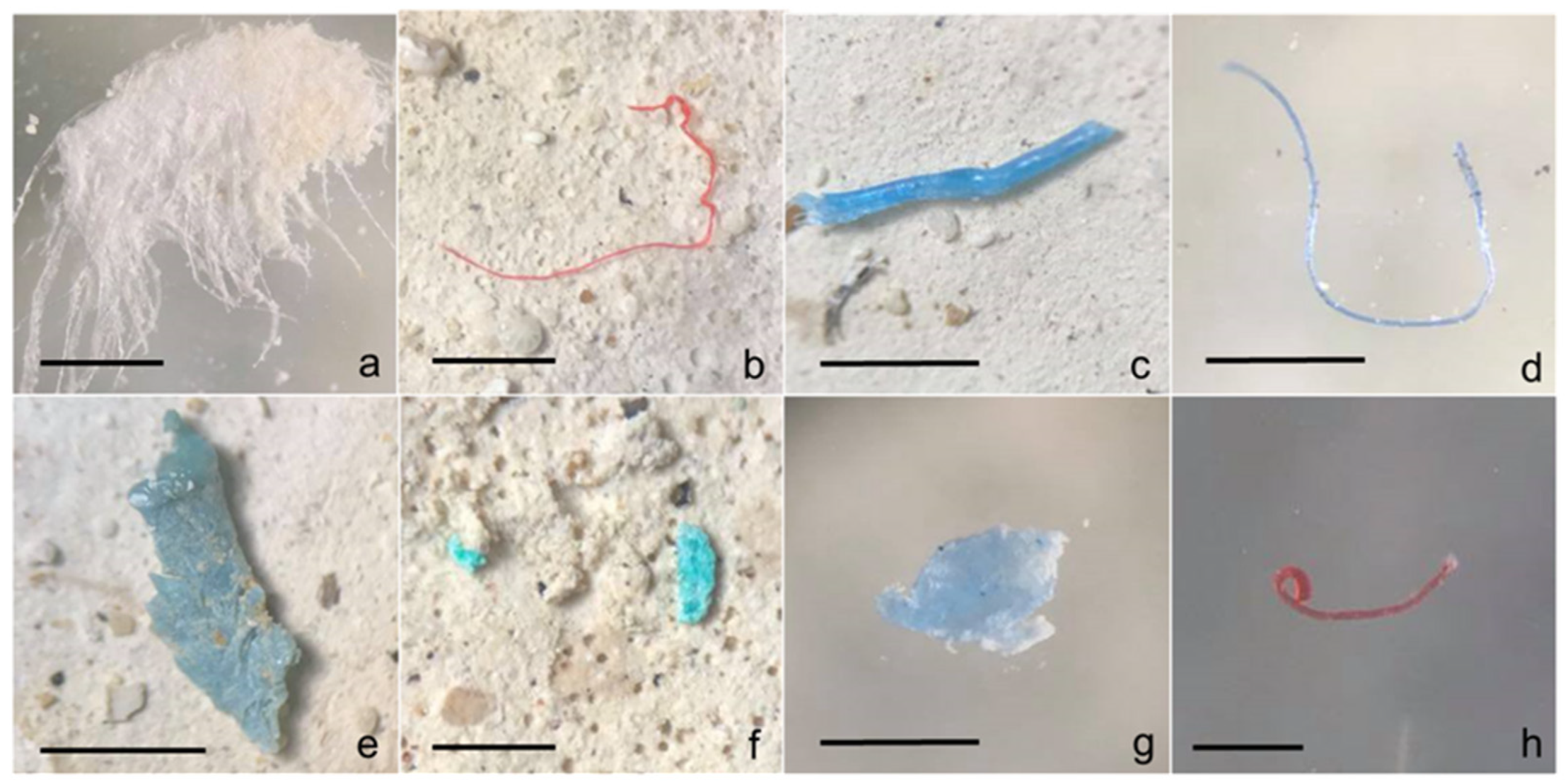

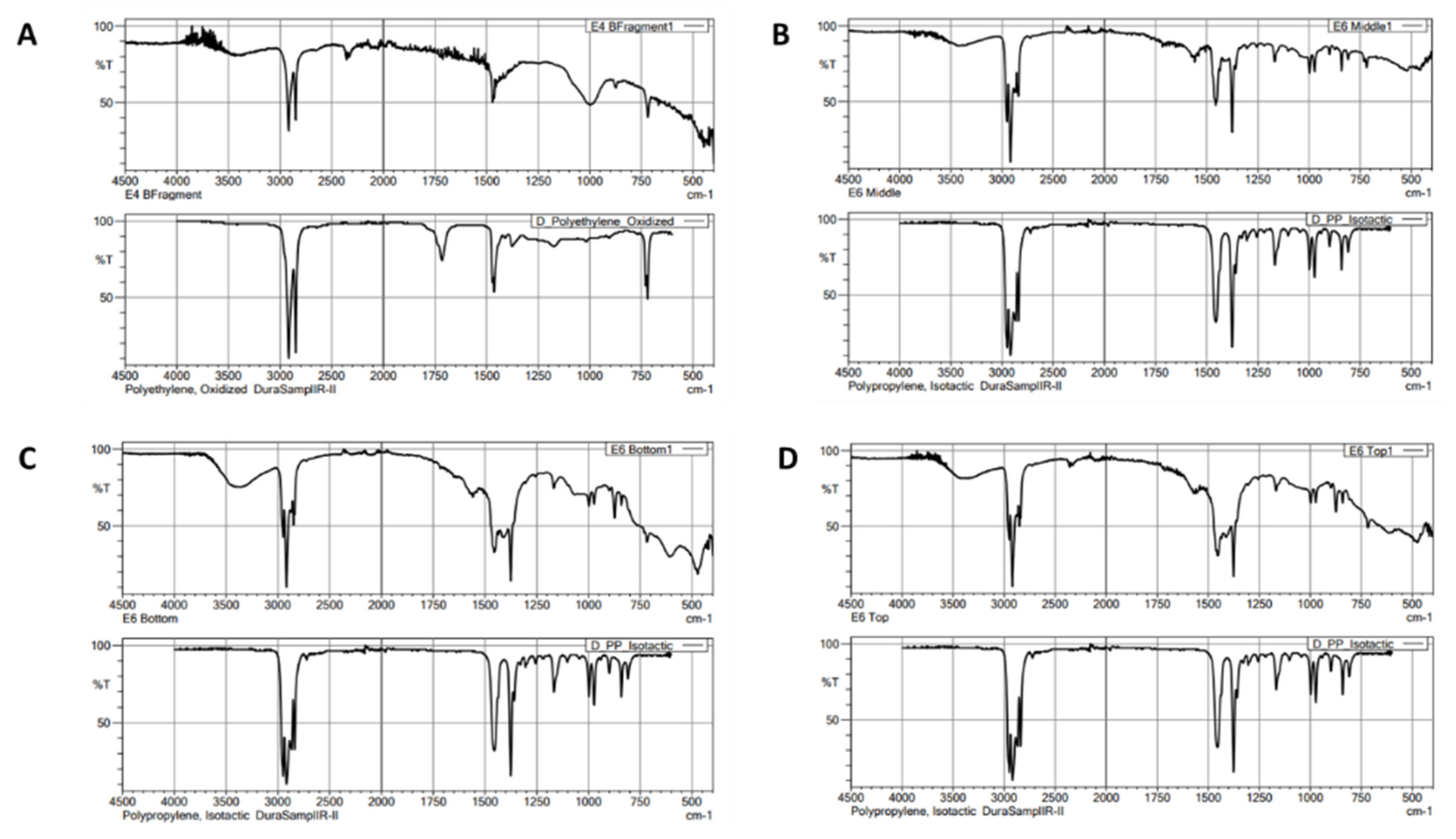
| Station Code | Sampling Site | Latitude | Longitude | Classification | Number of MPs | MP Count (Particle/g) |
|---|---|---|---|---|---|---|
| E1 | Binduyan | 10°1′5.22″ N | 119°4′37.02″ E | Residential | 3 | 0.020 |
| E2 | Binduyan | 10°0′15.06″ N | 119°1′48.54″ E | Non-residential | 3 | 0.020 |
| E3 | Lucbuan | 9°59′4.44″ N | 118°57′6.9″ E | Residential | 0 | 0.000 |
| E4 | Lucbuan | 9°58′51.6″ N | 118°53′8.16″ E | Non-residential | 3 | 0.020 |
| E5 | San Manuel | 9°45′3.24″ N | 118°46′20.34″ E | Residential | 6 | 0.040 |
| E6 | San Miguel | 9°45′3.24″ N | 118°46′20.34″ E | Residential | 8 | 0.053 |
| E7 | Bancao-Bancao | 9°45′3.54″ N | 118°46′20.52″ E | Residential | 7 | 0.047 |
| E8 | Mangingisda | 9°39′37.56″ N | 118°44′24.9″ E | Non-residential | 0 | 0.000 |
| E9 | Inagawan | 9°36′24.36″ N | 118°42′5.7″ E | Residential | 6 | 0.040 |
| E10 | Inagawan | 9°34′17.94″ N | 118°40′49.98″ E | Non-residential | 0 | 0.000 |
| E11 | Inagawan | 9°32′39.9″ N | 118°39′12.54″ E | Residential | 2 | 0.013 |
| W1 | Cabayugan | 10°11′51.78″ N | 118°54′10.08″ E | Non-residential | 1 | 0.007 |
| W2 | Buenavista | 10°4′32.28″ N | 118°49′5.58″ E | Residential | 2 | 0.013 |
| W3 | Buenavista | 10°4′23.82″ N | 118°48′23.82″ E | Residential | 1 | 0.007 |
| W4 | Bacungan | 9°52′26.94″ N | 118°36′28.5″ E | Non-residential | 1 | 0.007 |
| W5 | Bacungan | 9°52′26.76″ N | 118°36′28.5″ E | Non-residential | 1 | 0.007 |
| W6 | Simpocan | 9°48′12.66″ N | 118°32′26.88″ E | Non-residential | 0 | 0.000 |
| W7 | Simpocan | 9°45′32.94″ N | 118°30′34.14″ E | Residential | 1 | 0.007 |
| W8 | Napsan | 9°43′50.94″ N | 118°28′18.66″ E | Non-residential | 0 | 0.000 |
| W9 | Napsan | 9°43′40.56″ N | 118°27′0.66″ E | Residential | 2 | 0.013 |
| W10 | Napsan | 9°43′36.12″ N | 118°27′1.28″ E | Non-residential | 0 | 0.000 |
| City/Country | Mean Count of MPs (Particle/g) | Reference |
|---|---|---|
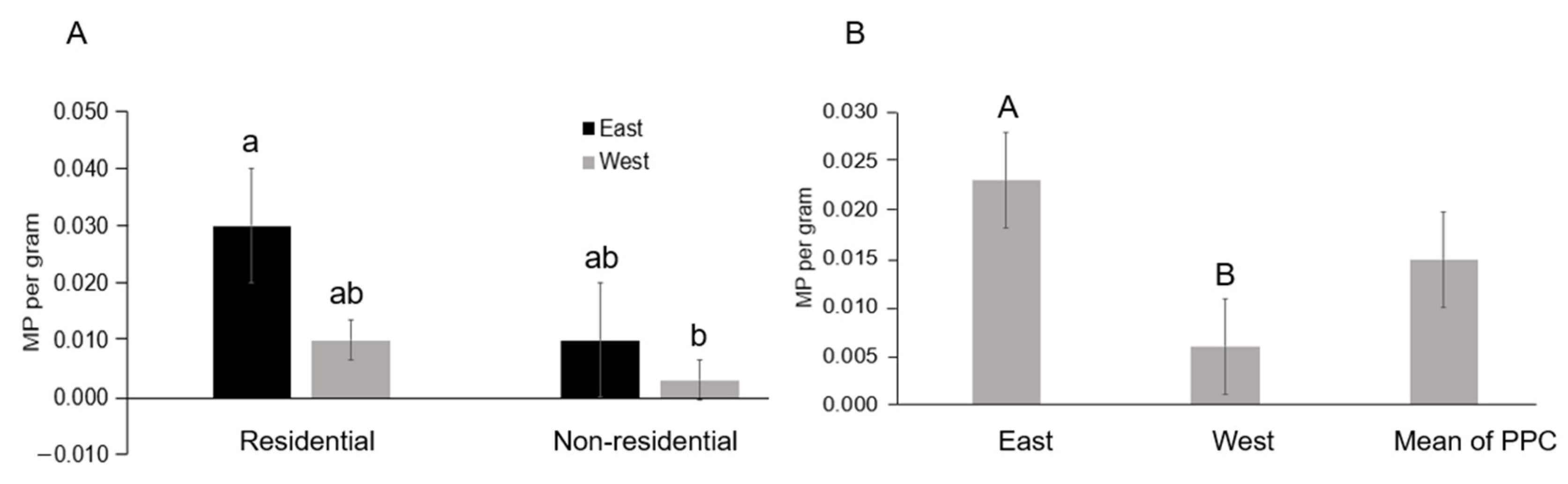
| East coast of Puerto Princesa, Palawan, Philippines | 0.023 | This study |
| West coast of Puerto Princesa, Palawan, Philippines | 0.006 | This study |
| Lian, Batangas, Philippines | 0.260 | [17] |
| Negros Oriental, Philippines | 0.082 | [14] |
| Philippines | 0.025 | [30] |
| Hong Kong | 0.168 | [57] |
| Hong Kong | 0.189 | [3] |
| Bohai Sea, China | 0.103–0.163 | [58] |
| China | 0.015–12.852 | [59] |
| Beibu Gulf, China | 6.87 | [44] |
| Mexico | 0.135 | [35] |
| Hauts-de-France, France | 0.0234–0.0693 | [60] |
| Phuket, Thailand | 1.883 | [38] |
| Thailand | 0.42–200 | [39] |
| Thailand | 0.15 | [61] |
| Rayong, Thailand | 0.568 | [62] |
| Singapore | 0.00032 | [7] |
| Isle of Rügen, Germany | 0.093 | [63] |
| Halifax Harbor, California | 4.5 | [64] |
| Belgian Coast, Belgium | 0.134 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajorne, R.E.; Cayabo, G.D.B.; Gajardo, L.J.A.; Mabuhay-Omar, J.A.; Creencia, L.A.; Bacosa, H.P. Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics. Sustainability 2022, 14, 15303. https://doi.org/10.3390/su142215303
Sajorne RE, Cayabo GDB, Gajardo LJA, Mabuhay-Omar JA, Creencia LA, Bacosa HP. Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics. Sustainability. 2022; 14(22):15303. https://doi.org/10.3390/su142215303
Chicago/Turabian StyleSajorne, Recca E., Genese Divine B. Cayabo, Lea Janine A. Gajardo, Jhonamie A. Mabuhay-Omar, Lota A. Creencia, and Hernando P. Bacosa. 2022. "Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics" Sustainability 14, no. 22: 15303. https://doi.org/10.3390/su142215303
APA StyleSajorne, R. E., Cayabo, G. D. B., Gajardo, L. J. A., Mabuhay-Omar, J. A., Creencia, L. A., & Bacosa, H. P. (2022). Disentangling Microplastic Pollution on Beach Sand of Puerto Princesa, Palawan Island, Philippines: Abundance and Characteristics. Sustainability, 14(22), 15303. https://doi.org/10.3390/su142215303









