1. Introduction
Rabbit production is one of the fast-growing projects in Egypt, as rabbits are quiet and highly prolific animals with a short gestation period between 31 and 33 days, fast growth, efficient feed utilization, and high fecundity [
1]. They also provide high-quality protein and healthy meat, which is in high demand [
2]. Egypt is one of the 10 leading rabbit meat producing countries; it produces 56 metric tons and contributes 3.8% to the global production of rabbit meat [
3]. Thus, successful rabbit projects have been recorded in developing countries such as Egypt, which contribute to food security and income [
4]. However, rabbits are highly sensitive to extreme environmental conditions, particularly temperature. Thermoregulation in rabbits is somewhat poor, as they have few functional sweat glands [
5]. The optimal temperature range of rabbits is 15–25 °C, and the optimal humidity is 55–65%. Heat stress (HS) occurs when the ambient temperature is >30 °C. When the temperature is >35 °C, rabbits cannot regulate their body temperature, resulting in heat failure and heat-induced physiological stress [
6]. Recently, a serious hazard to livestock production, including rabbits, has occurred due to global climate change, especially the expected increase in surface temperature [
7].
In tropical and subtropical regions such as Egypt, HS is intensified with high relative humidity (RH), which is normally >85% during the day and can reach 100% during hot months [
5]. HS has multiple unfavorable effects on rabbit health and production performance. It causes a 20–25% reduction in daily weight gain, an 8–15% decrease in feed conversion ratio, and a 9–12% increase in mortality rate. Moreover, the reproductive performance decreases by 6–10% and negatively influences meat quality and carcass traits [
8]. Thus, HS causes a great challenge for the rabbit industry [
5]. In addition, HS conditions caused drastic changes in the biological functions of exposed rabbits, including depression of immune responses, enzymatic activities, hormonal secretions, and blood metabolites, consequently impairing productive and reproductive efficiency [
9]. Furthermore, HS markedly increases reactive oxygen species and free radicals (FR), reduces cellular antioxidants [
10], increases corticosteroid levels, and decreases luteinizing hormone (LH) and follicle-stimulating hormone secretion, affecting ovary development and the rate of ovulation [
11], and having adverse effects on DNA, proteins, and lipids [
12]. Moreover, Villalobos et al. [
13] reported that HS conditions prevent rearing rabbits in high density up to 18 rabbits m
−2, leading to a severe decline in rabbit production.
Amelioration of the drastic effects of HS on rabbits has been attempted using different procedures, including offering cool drinking water [
14], using a short lighting regime [
15], and providing vitamins in the diet [
16] or drinking water [
17]. Many medicinal plants can be used as promising growth promoters for improving the productive and reproductive performances of rabbits [
18], especially under HS conditions, where herbs contain phytoestrogens as plant chemicals similar to sex hormones [
19]. Additionally, medicinal plants have high antioxidant activity, and antioxidants play important roles in protecting animals against HS conditions. Thus, some medicinal plants have been used as protector agents against HS situations in rabbits, such as curcumin [
20], a combination of royal jelly (RJ) and green tea (GT) [
21],
Emblica officinalis [
22],
Moringa oleifera extract [
23],
M. oleifera leaf powder [
24], or ginger root powder [
25].
Maca (
Lepidium meyenii) is traditionally traded as capsules or powder worldwide due to its potential medicinal and nutritional activities [
26]. These include its capacity to improve female [
27] and male fertility and sexual performance [
28], antioxidation [
29], immunomodulatory activities [
30], and cell protection [
31]. Most certainly, these biological and pharmacological activities are mainly related to the chemical components of
L. meyenii [
32]. The main bioactive compounds in
L. meyenii are categorized into six classes: glucosinolates and isothiocyanates, thiohydantoins, macaene and macamides, alkaloids, polysaccharides, and fatty acids [
33]. There is a lack of sufficient research regarding the alleviative effects of the maca plant or its extracts on highly HS-sensitive farm animals such as rabbits, and especially females. Therefore, this study was designed to evaluate the ameliorative effects of black maca (
L. meyenii) hydroalcoholic extract (BMHE) on the productive and reproductive performance of V-line rabbit does reared under HS conditions.
4. Discussion
In this study, the calculated THI ranged between 29.69 and 31.70 from June to August, indicating that experimental rabbits were exposed to severe and very severe HS during the experimental period, according to the classification of THI by [
14]. Moreover, Marai et al. [
47] suggested that the optimal THI for rabbit husbandry is 27.8. The thermal stress on rabbits can be estimated by THI, which combines the effects of ambient air temperature and RH, and is used to assess the severity of HS. Thus, Marai et al. [
48] stated that THI is the most used index stress to evaluate the effects of climatic conditions and assess the effects of HS on livestock production.
Litter size at birth and weaning is an important economic trait which determines the number of kits for future breeding programs and lifetime production [
49]. In this study, V-line rabbit does and their litter traits were adversely affected by exposure to severe and very severe HS conditions from June to August. Similarly, Marco Jiménez et al. [
50] reported that litter size, litter weight, and kit birth weight in rabbits were reduced in those whose dams were exposed to HS conditions during gestation compared to unstressed does. In a recent study, Mady et al. [
51] reported differences in birth and weaning weights in rabbits between the winter and summer seasons. Rabbits had a heavier weight at birth (53.38 g) and weaning (374.44 g) during winter than in summer (37.56 and 304.78 g, respectively). The present study showed that the oral administration of BMHE at level 400 mg kg
−1 BW of doe day
−1 significantly (
p ≤ 0.05) increased the rabbit doe’s weight after the weaning period, as well as increased the litter size and litter weight measured at 7, 14, 21, and 28 days after the birth of heat stressed rabbits at June, July and August, compared to other treatments. This effect seems to be due to an effect favoring the survival of embryos. Thus, it can be noted that oral administration of BMHE at level 400 mg kg
−1 BW of doe day
−1 seriously alleviated the drastic effects of HS on rabbit does and their litters. The positive effects of maca were similarly observed in mice [
27], rats [
52], and murine [
53]. These positive effects may be due to maca’s sterol bioactive components, such as campesterol (27.3%), ergosterol (13.6%), brassicasterol (9.1%),
Δ7,22-ergostadienol (4.5%), and sitosterol (45.5%), having phytoestrogen activity [
54]. Recently, El-Sheikh et al. [
55] reported that oral treatment of maca capsules in rabbit does insignificantly affected the number of matings per conception, conception rate, and gestation length, and had no significant effects on the litter weight of maca-treated rabbit does at 7, 21, and 28 days; however, those at 14 days were insignificantly lower than the control group [
49]. The disparities between this study and others may be due to the differences in the experimental animals’ age, maca extract and its levels, exposure time, and experimental management. The litter mortality rate significantly reduced throughout the experimental periods in treated rabbits with BMHE at level 400 mg kg
−1 BW of doe day
−1. This may be due to the improvement of some hematological and biochemical parameters in this group. The 100% mortality with the supplement at 600 mg kg
−1 BW of doe day
−1 (T
4) in July resulted because the rabbit does in T
4 showed a sudden nervous state as a result of exposure to heat stress, thus they killed the whole litter that reared with them in the same cage.
Rabbits are susceptible to HS, which is associated with reductions in growth performance, feed intake, and feed efficiency parameters [
56]. Thus, this study detected negative effects on growth performance and carcass quality parameters in HS-exposed rabbits. In the same line, a significant reduction in slaughter, carcass, and organ weight was noticed in rabbits subjected to HS [
57]. Thus, many studies suggested that the decrease in growth performance of experimental animals caused by thermal stress may be a consequence of damage to the intestinal mucosa and the increase in the hydrolysis of muscle proteins caused by temperature-induced FR [
13,
58]. Moreover, Obled et al. [
59] reported that stress conditions could increase the demand for some amino acids due to the synthesis of specific proteins, selective catabolism, or use in the synthesis of specific molecules. In this study, the improved growth and carcass quality traits of maca-treated rabbit does could be attributed to the properties of the tested maca extract, which acts as an antibacterial, antiprotozoal, antifungal, antioxidant, or immunostimulant agent [
60].
The assessment of hematological parameters could be useful in determining the rabbits’ health status [
61]. It is worth to be mentioned that all the hematological measurements of the experimental rabbits in the current study were within the normal range for healthy rabbits according to [
62,
63]. Similarly, these findings recorded great perturbation in hematological and serum biochemical parameters in New Zealand White (NZW) rabbits exposed to hot temperatures [
64]. RBCs and PCV were lowered by an ambient temperature of 36 °C over 1 month in adult NZW rabbits [
65], lower than the values obtained under the thermoneutral zone (18–21 °C) of rabbits. In general, animals exposed to various stresses have shown an increase in heterophils (H) and a decrease in lymphocytes (L), leading to an increase in the H/L ratio [
66]. In this regard, Ismaiel [
67] explained that the decrease in the H/L ratio in tropical conditions might be due to the requirements of extra amino acids, probably reflecting the synthesis of proteins or other specific compounds, such as hormones and heat shock proteins 70 that can ameliorate the negative effects of HS. Regarding the mitigation of negative effects of HS, the current findings indicated that rabbits orally treated with 400 and 600 mg BMHE kg
−1 BW of doe day
−1 (
p ≤ 0.05) showed a significant increase in hematological parameters compared to other treatments. These positive effects of maca extract on the hematological parameters of heat stressed rabbits in the present study may justify the positive relationship between nutrients and the health biomarkers of farm animals, including rabbits. Furthermore, maca has some phytochemical components, such as polyphenolic, alkaloids, and flavonoids, which have positive impacts on different hematological measurements that could be attached to the cellular plasma membranes to protect the body cells from the produced FR, and/or activate the antioxidant enzymes [
68]. In the same trend as this study, El-Ratel et al. [
69] recently reported that dietary administration of curcumin or nano-curcumin significantly improved the hematological parameters of HS-exposed rabbits.
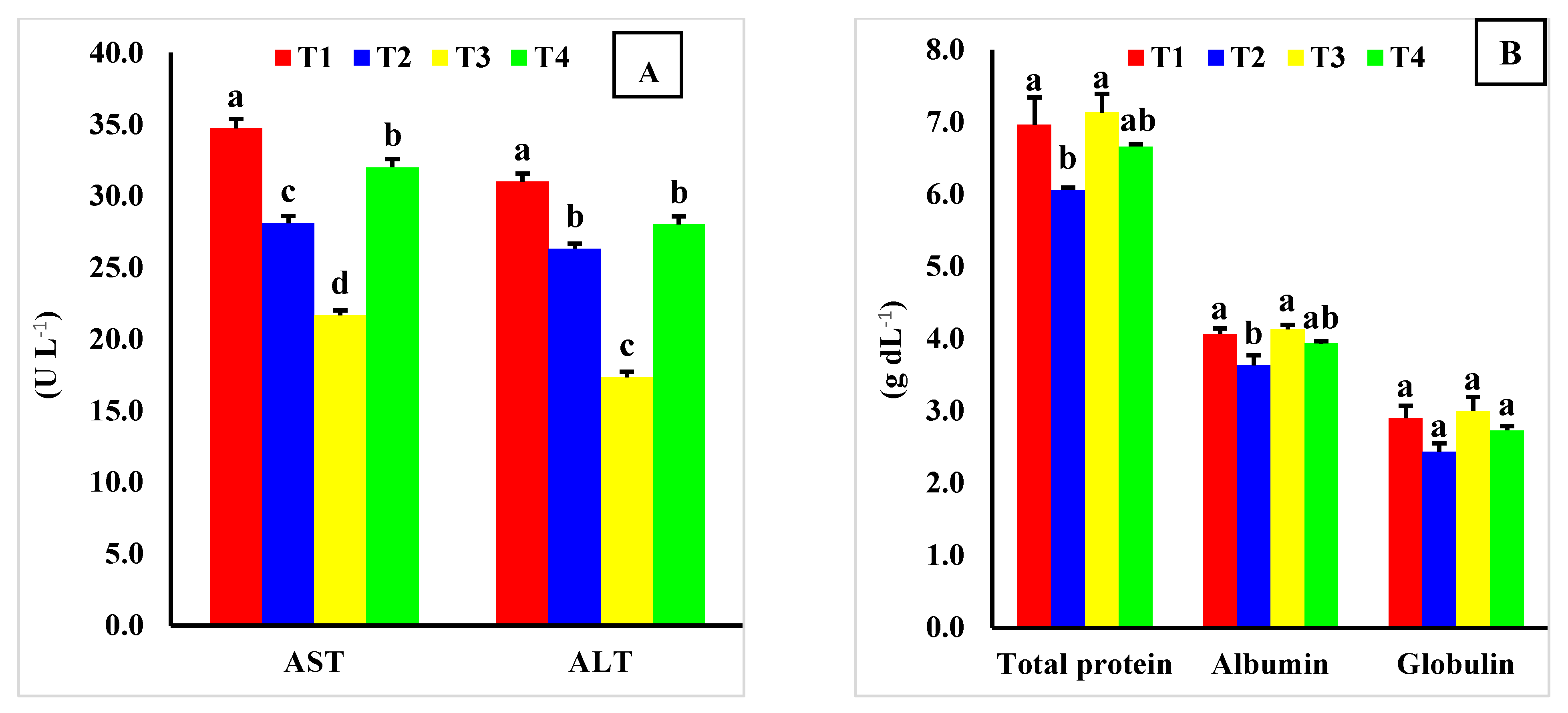
In this study, severe and very severe HS conditions led to harmful effects on all serum tested parameters of exposed rabbits, reflecting the dysfunctions of hepatic and renal organs, as well as impaired immune responses and antioxidant biomarkers in HS rabbits [
70]. Similar to this study, Gabr et al. [
21] stated that liver enzyme activities (AST and ALT) significantly increased in NZW weaned rabbits exposed to HS, suggesting some liver damage in mammals and birds, as cited by Faisal et al. [
71]. Liang et al. [
6] recently reported that blood biochemical parameters play critical roles in reflecting the metabolic changes and organ damage of rabbits under HS situations. Thus, total protein, glucose, and triglyceride concentrations in the blood decrease, whereas TC concentrations are markedly increased in rabbits exposed to HS [
6]. Continuous exposure of rabbits to HS causes severe disruptions in homeostatic mechanisms, damages various organs [
72], makes rabbits vulnerable to pathogens, and consequently brings serious losses to rabbit production [
73]. In the present study, orally treated BMHE at level 400 mg kg
−1 BW of doe day
−1 significantly improved the tested serum biochemical parameters of HS-exposed rabbits. Reduced serum AST and ALT activities and increased total protein and albumin in orally treated BMHE groups reflect the beneficial effects of maca extract on liver function, alleviating the drastic effects of HS on exposed rabbits by increasing oxygen flow to the liver [
74]. According to Gabr et al. [
21], a combination of RJ and GT reduced the serum AST activity and increased the thyroid hormones of NZW weaned rabbits reared during a hot summer season in Egypt. According to [
58], the high dose of dried Xinjiang maca powder (1200 mg kg
−1 BW) has harmful effects on the serum parameters of treated mice. The different results may be related to the different planting sources, types, dosages, and chemical composition.
HS conditions lead to the generation of FR, such as O
2− and HO, which can damage cell membranes by inducing lipid peroxidation of polyunsaturated fatty acids in the cell membrane [
6]. In this study, exposure to HS and oral administration of maca extract in rabbits would have weakened the deleterious heat-induced oxidative stress, with significantly decreased serum TC, triglycerides, LDL, and vLDL, and significantly increased serum HDL. In this respect, Gabr et al. [
21] reported that an oral combination of RJ and GT achieved the best findings of productive performance and physiological response parameters of HS NZW weaned rabbits. As in this study, Elnagar et al. [
75] observed that RJ treatment significantly reduced serum total lipids, TC, triglycerides, creatinine, and uric acid concentrations in growing rabbits or male rabbits [
76] treated with RJ at 50, 100 and 150 mg kg
−1 BW compared to HS control rabbits. Several authors indicated that the bioactive compound content in GT [
77] and RJ [
78] in their studies (or black maca in this study) such as polyphenols, could inhibit the key enzymes involved in the biosynthesis and absorption of lipids, triglycerides, and cholesterol as well as energy expenditure stimulation, fat oxidation, HDL concentrations, and lipid excretion in feces [
32].
This study detected adverse effects on oxidative damage and stress-related biomarkers in HS-exposed rabbits. The current findings also indicated that oral administration of BMHE at level 400 mg kg
−1 BW led to enhance the profile of heat stressed rabbits, which indicates that BMHE can be used to reduce HS-induced oxidative damage and alleviate the drastic effects of HS on rabbits. This level of BMHE significantly increased serum TAC, while significantly decreasing both serum cortisol hormone and glucose in HS-exposed rabbits. These results indicated the useful application of BMHE as an viable protocol for rabbits reared in hot regions such as Egypt. These positive effects may be due to BMHE containing substantial amounts of antioxidant compounds, especially phenols, glucosinolates, alkamides, and polysaccharides [
79]. In a recent study, Tafuri et al. [
80] reported that specific bioactive metabolites in maca extract, such as glucosinolates and macamides, might be responsible for the antioxidant activity of maca. In this respect, black maca also reduced glucose levels, and consumption of this variety is associated with lower blood pressure and an improved health score [
81]. In the same line, Choi et al. [
82] reported that rats treated with a lipid-soluble extract of maca showed the highest levels of catalase in the liver and glutathione antioxidants in muscle and liver than in the control group. They also suggested that this effect depends on the effects of maca extracts on suppressing oxidative stress.
The endocrine system plays an important role in the animal’s response to stress, including HS [
6]. When rabbits are exposed to HS, the hypophysis–pituitary–adrenal axis is activated [
83]; consequently, several changes in blood constituents and reproductive efficiency are detected [
6]. Thus, in this study, HS conditions have severe effects on serum sex hormones in experimental rabbit does. These drastic effects of HS conditions on the reproductive performance or sex hormones of females rabbits have been previously reported [
14,
84]. The female reproductive system is very sensitive to oxidative stress, and a reduction in the production and secretion of gonadotropins is necessary for the vital growth and development of ovarian follicles [
11]. In this study, oral administration of BMHE at level 400 mg kg
−1 BW alleviated the harmful effects of HS on serum sex hormones in treated rabbit does above all other treatments. The progressive effects of maca on the reproductive performance and sex hormones of different experimental animals have been previously reported. Serum P4 levels increased in maca-treated female mice compared to the untreated group [
85]. Similarly, Uchiyama et al. [
86] demonstrated that maca intake enhances serum LH levels in female rats. Recently, El-Sheikh et al. [
55] reported that maca-treated rabbit does have a numerically higher concentration of E2 hormone and a lower concentration of P4 hormone than those in the control group. This and the enhancement of serum LH levels may be the mechanisms that improve fertility [
87]. The positive effects of maca may be due to saponins, which play an important role in sex hormone secretion and the treatment of sexual dysfunction. Generally, one of the maca’s main actions is stimulating the hypothalamus, which regulates the pituitary gland, acting as a tonic for the hormone system. Excess E2 levels can cause P4 levels to become too low, known as E2 dominance [
88]. Administration of maca may help balance the E2/P4 ratio, leading to potential increased female fertility and achieving a healthy pregnancy in treated animals [
89] by eliminating FR and generating an antioxidant function, mainly due to its metabolites (alkaloids and glycosylates) [
90].