1. Introduction
Plastics are materials from petroleum-based polymers widely used due to their easy moldability, low cost, and high mechanical resistance [
1,
2,
3]. According to their moldability, plastics can be used as thermoplastics and thermosets [
4]. However, in 2018 there was a large-scale manufacture of thermoplastics with low-cost production (around 360 million tons) [
5]. Unfortunately, the enormous plastic output worldwide has led to environmental accumulation, with more than 6 billion tons of plastic waste produced in the last six decades [
6,
7].
Microplastics (MPs) are tiny plastic particles with sizes smaller than 5 mm, usually produced from the mechanical, chemical, or physical degradation of macroplastics or manufactured with those dimensions for different industrial purposes. Inherent to their tiny size, MPs indiscriminately spread to the environment at the end of their life cycle [
8]. Scientifics consider microplastics as plastic particles between 1 µm and 5 mm, whereas plastics smaller than these are considered nanoplastics (NPs) [
9,
10]. Typically, MPs/NPs comprise polyethylene (PE), polypropylene (PP), polyamide (PA), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET) [
11].
Microplastics are ubiquitous, with presence in the air [
12], land [
13], sea [
14], rivers [
15], and even in the sediment of polar freshwater lakes [
16]. One of the most concerning aspects of the uncontrolled spreading of MPs and NPs is that they are vehicles for several pollutants, such as polycyclic hydrocarbons [
17], heavy metals [
18], and polybrominated ethers [
19], and pharmaceutical and cosmetic products worsen global contamination [
20,
21]. MPs’ recalcitrant capacity relies on their lower density, facilitating accumulation and spreading to different areas. These particles eventually enter the human body by inhalation or ingestion [
22]. The physical–chemical characteristics of MPs/NPs facilitate bioretention in several organs and tissues. As a result, microplastic accumulation in the human body leads to diseases due to chronic toxicity [
23].
It is well known that MPs can be fragmented into NPs by the action of UV light, the mechanical and shear forces of waves, and wind, which also cause chemical changes in their structure. These reductions in size and chemical changes facilitate their spread and contact with aquatic and terrestrial organisms [
24]. At such a small size, these particles move through body barriers [
25,
26] and internalized cells. For example, the internalization of nanoplastics in vascular plants introduces phytotoxicity, including the adverse effects of photosynthesis and oxidative stress [
27]. In addition, these particles can be inhaled, entering the pulmonary tract. Once present in the pulmonary or gastrointestinal tracts, they accumulate and distribute to various organs and tissues, showing teratogenic, carcinogenic, mutagenic, and toxic effects with long-term impacts on the organism [
28]. However, despite the rising literature on the effects of MP/NP ingestion, there is a lack of detailed knowledge of the effects of MPs/NPs on marine and human health, mainly due to an underestimation of the effect related to the analytical and sampling methods for MP/NP extraction [
29]. MPs/NPs can alter microbial diversity, biogeochemical cycles, and the restoration of healthy soils [
22,
30,
31].
A microorganism is an organism with a large surface-area-to-volume ratio. A microorganism is easy to cultivate and has a high biomass growth capacity [
32]. These tiny organisms are vital in restoring the balance of planet life through various biogeochemical cycles. Microorganisms mineralize organic compounds and help distribute nutrients to other life forms, such as plants and higher organisms [
33,
34,
35]. Many microorganisms are essential to superior organisms, as is the case for intestinal microbiota, which facilitate tissue differentiation, stimulate the immune response, and protect the host from pathogens [
36,
37]. Therefore, the microbiome is fundamental for organisms through the digestion and absorption of nutrients and the prevention of host diseases [
36,
38].
However, the interaction of microorganisms with MPs (and, even worse, with NPs) is still not well understood. Many studies have focused on analyzing the interaction of MPs with terrestrial and aquatic microorganisms, mainly concerning the ecological effects in those environments, but very little is known about the interaction with atmospheric MPs [
39,
40,
41].
Initially, microbes adsorb on MPs’ surfaces, creating a habitat similar to their living environment [
22], as in the case of the “plastisphere” in aquatic environments [
42]. Once microbial colonization occurs on the surface, the microorganism secretes enzymes to degrade the constituent polymers through hydrolysis or oxidation–reduction reactions [
43,
44,
45]. Enzyme secretion generates oligomers, dimers, and monomers, which enter microbial cells for energy conservation through metabolic pathways producing CO
2, H
2O, or CH
4 [
43].
This study presents the most relevant information about microorganisms’ biodegradation of MPs/NPs as a green approach to mitigate the negative impact on the environment. Due to the relevant information generated through the study, the purpose was to perform a bibliometric analysis of 719 documents published from 2010 to 2021 on the research landscape of MPs’ and NPs’ interactions with microorganisms. The information might be suitable for environmental agencies, research institutes, and the general community as a tool analyzing the most recent literature. This study presents the most relevant information about microorganisms’ biodegradation of MPs/NPs as a green approach to mitigate their negative impact on the environment.
2. Methodology
In the present study, information from the citations and abstracts of scientific journals extracted from the Scopus database of Elsevier, created in 2004, was comprehensively reviewed [
46]. Elsevier’s Scopus is one of the most representative databases for scientific information due to the significant content of publishers and journals [
47]. According to the information provided by the publisher, the Scopus database contains more than 25,100 titles from more than 5000 international publishers, including broad research production in different areas of science, technology, medicine, arts, and humanities [
46]. In addition, Scopus has several analysis functions supporting bibliometric analysis [
48]. The analysis functions included by Scopus and extracted here are the journal’s name, the type of document, the year of publication, the authors and their affiliations, the number of citations, and the index
h-metrics for papers, imported in this research as a .csv file and a Microsoft Excel file (
Supplementary Materials) for further analysis. The journal impact factor (IF) of the extracted articles was extracted from the Journal Citation Report (JCR) 2021, while the Cite Score 2021 retrieval was obtained from Scopus.
The search strategy in this article consisted of using the following search term: (TS) = TITLE-ABS-KEY (((microplastic * OR micro-plastic * OR nanoplastic * OR nano-plastic *) AND (microorganism * OR bacteria * OR fungi * OR algae * OR virus *))). This search retrieved 935 documents (10 March 2022). Subsequently, we performed a refined search by limiting the search to the document types “article”, “review”, and “conference paper,” excluding the year 2022 since it was endless, and limiting the language only to English: (TITLE-ABS-KEY (microplastic * OR micro-plastic * OR nanoplastic * OR nano-plastic *) AND TITLE-ABS-KEY (microorganism * OR bacteria * OR fungi * OR algae * OR virus *)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “cp”)) AND (EXCLUDE (PUBYEAR, 2022)) AND (LIMIT-TO (LANGUAGE, “English”)). With this search, we retrieved 719 results. However, to obtain an idea of the publication trend, we completed a separate analysis, including the year 2022, even though the year was endless, and 1152 documents were obtained (30 October 2022). This information helped the separate the trend analysis for the 2022 publications, but we did not use them for mapping since the year still is running and several data might change at the end of the year. Despite no restrictions in the period covered for the search, the first publication retrieved was from 2010. The search was carried out on 12 August 2022, and included a bibliography published up to December 2021.
VOSviewer (
www.vosviewer.com; Van Eck and Waltman, 2009–2022, version 1.6.18, Leiden University, Leiden, The Netherlands) is a free-access software to create network maps of institutions, countries, keywords, and citations per article [
49]. We accessed VOSviewer on 12 August 2022.
Finally, to analyze the most recent published information related to the biodegradation/bioremediation of MPs and NPs by microorganisms, we reviewed the literature and extracted the most relevant information (including the literature of 2022). All the data were analyzed and are discussed in
Section 3.6: Review of the interaction of microplastics and nanoplastics with microorganisms to understand MPs’/NPs’ biodegradation with bacteria, fungi, microalgae, biofilms, and periphytic films. Since viruses do not contribute to biodegradation, we discarded them in the final selection of the literature.
3. Results and Discussion
Bibliometric analysis involves obtaining and analyzing data on a topic to map academic literature and quantitatively evaluate and predict the patterns and trends of topics of research [
50,
51]. The bibliometric analysis (719 documents obtained) shows that MPs/NPs and their interaction with microorganisms is an area of knowledge with high impact and continuous growth. Recently, several investigations have focused on determining the effects of introducing MPs/NPs in terrestrial and marine habitats [
22,
52,
53,
54,
55].
3.1. How Many Publications Are Produced Per Year on the Topic?
The first publication on microplastics with microorganisms was published in 1972 [
56]. Since then, the topic of MPs/NPs and their interaction with microorganisms has been the focus of few literature reports. The 719 documents obtained from Scopus published between the years 2010 and 2021 on the subject investigated are distributed among 584 (81.2%) scientific articles, 124 (17.2%) review articles, and 11 (1.5%) conference articles.
According to our analysis, between 2010 and 2015, only 12 documents were published (2010 (1), 2011 (1), 2012 (1), 2013 (2), 2014 (4), and 2015 (3)). However, since 2016, there has been exponential growth in the number of publications per year (
Figure 1A). The publications up to 30 October 2022 were included to analyze the rapid increase in 2022 and the general trend, despite the year not being finished yet. As can be seen in
Figure 1B, the number of publications in 2022 (up to 30 October 2022) is 475, demonstrating the rapid increase in publications in the “MPs/NPS and their interaction with microorganisms” topic.
This dramatic increase relates to a primary significant concern about the effects derived from the interaction of MPs/NPs with aquatic, terrestrial, and atmospheric microorganisms, consequently affecting their growth and development [
39,
57] or causing their degradation [
43,
44,
45]. The high number of documents and the high citation rate reflects these issues’ importance. Other studies of MPs’ interaction with food [
28] and marine ecosystems [
24,
29,
58] close to the subject of this study also exhibited this trend.
3.2. What Is the Collaboration between Countries on This Issue?
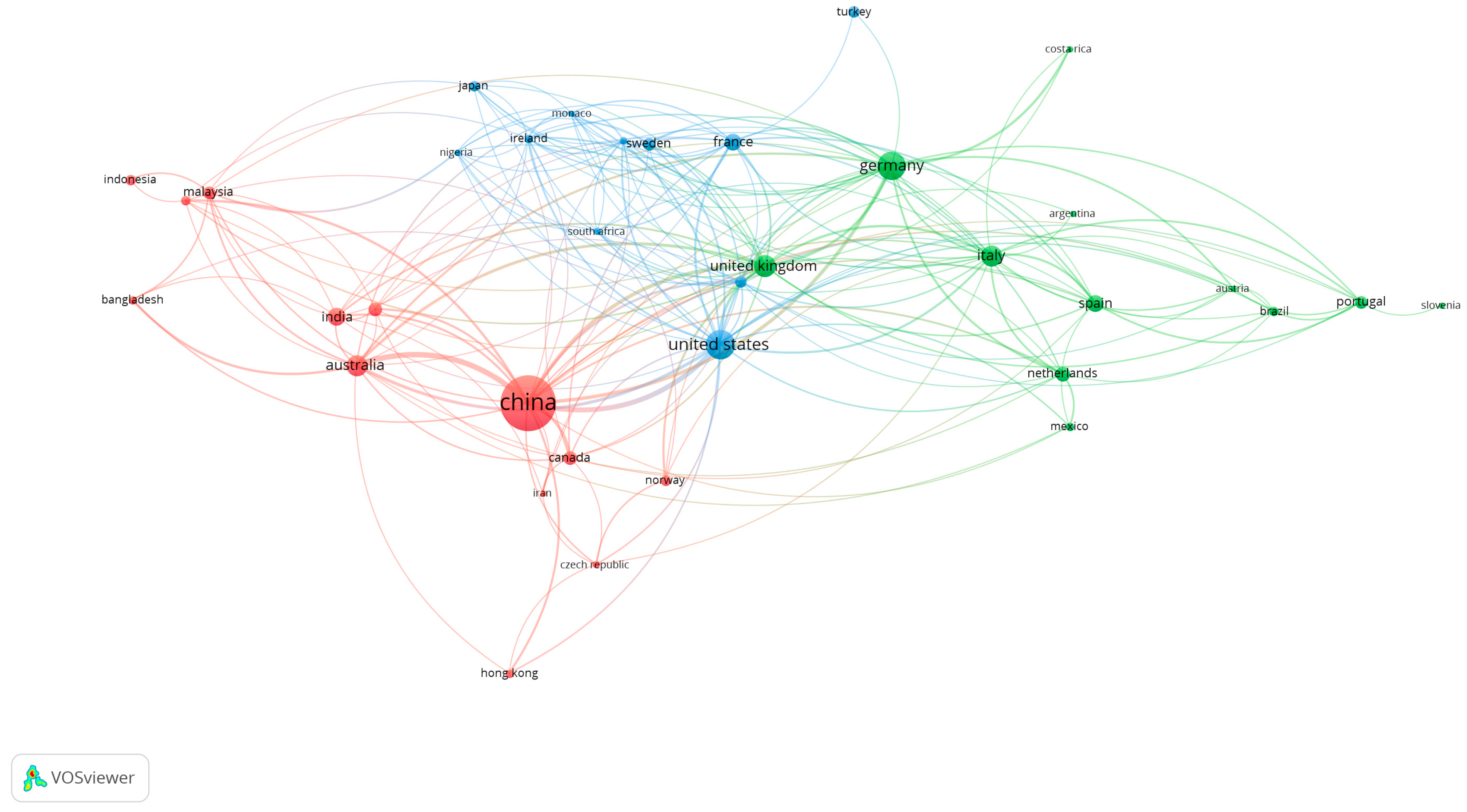
The top 10 countries (
Table 1) with publications on the topic are led by China, with 288 documents, 40.1% of the total retrieved in the literature review. The following countries are the United States with 94 documents, Germany with 72, and Portugal in 10th place with 18 documents. However, the number of documents and distribution does not reflect the collaboration between countries and institutions. As seen in
Figure 2, China, the United States, the United Kingdom, and Germany are the countries with the highest collaboration globally, represented by the proximity and size of the nodes. This result demonstrates the growing concern about the impact of MPs/NPs and their interaction with microorganisms since pathogens spread to environments where they are generally not present and move to other levels of the trophic chain, affecting the health of animals and man. The figure exhibits three clusters. The first cluster is red-colored, led by China, with 299 documents, a total link strength of 118, and 23 links.
The proximity and strength of the link reflect the power of the cooperation between these countries. China’s collaboration with Australia stands out with a link strength of 25, followed by a partnership with the United States with a link strength of 21. The other significant cluster is the United States, which has a total link strength of 83, with 85 documents and 26 links. This country reflects the most effective cooperation worldwide due first to its language, technology, and tradition in many techniques used to analyze microplastics worldwide, despite not having as many documents as China.
The third cluster is Germany, with 77 documents, a total link force of 59, and 24 links, while the United Kingdom presents 48 papers, with a total link force of 70, and 24 links. The United Kingdom, the United States, China, and Germany present cooperation, reflected by their closeness and link strength. It is also important to highlight that European countries, in addition to the United States, Canada, and Australia, cooperate the most with these group leaders. However, there is evidence of the presence of a few African (South Africa, Nigeria), Asian (Japan, Hong Kong, Malaysia, Indonesia, Bangladesh, India, South Korea, and Iran), and American (Brazil, Argentina, Mexico, Canada, and Costa Rica) countries, demonstrating the lack of information and research in these continents due to the lack of resources and technology for that purpose. Therefore, research needs more government and institutional investment programs.
3.3. What Are the Journals That Publish the Most in This Area?
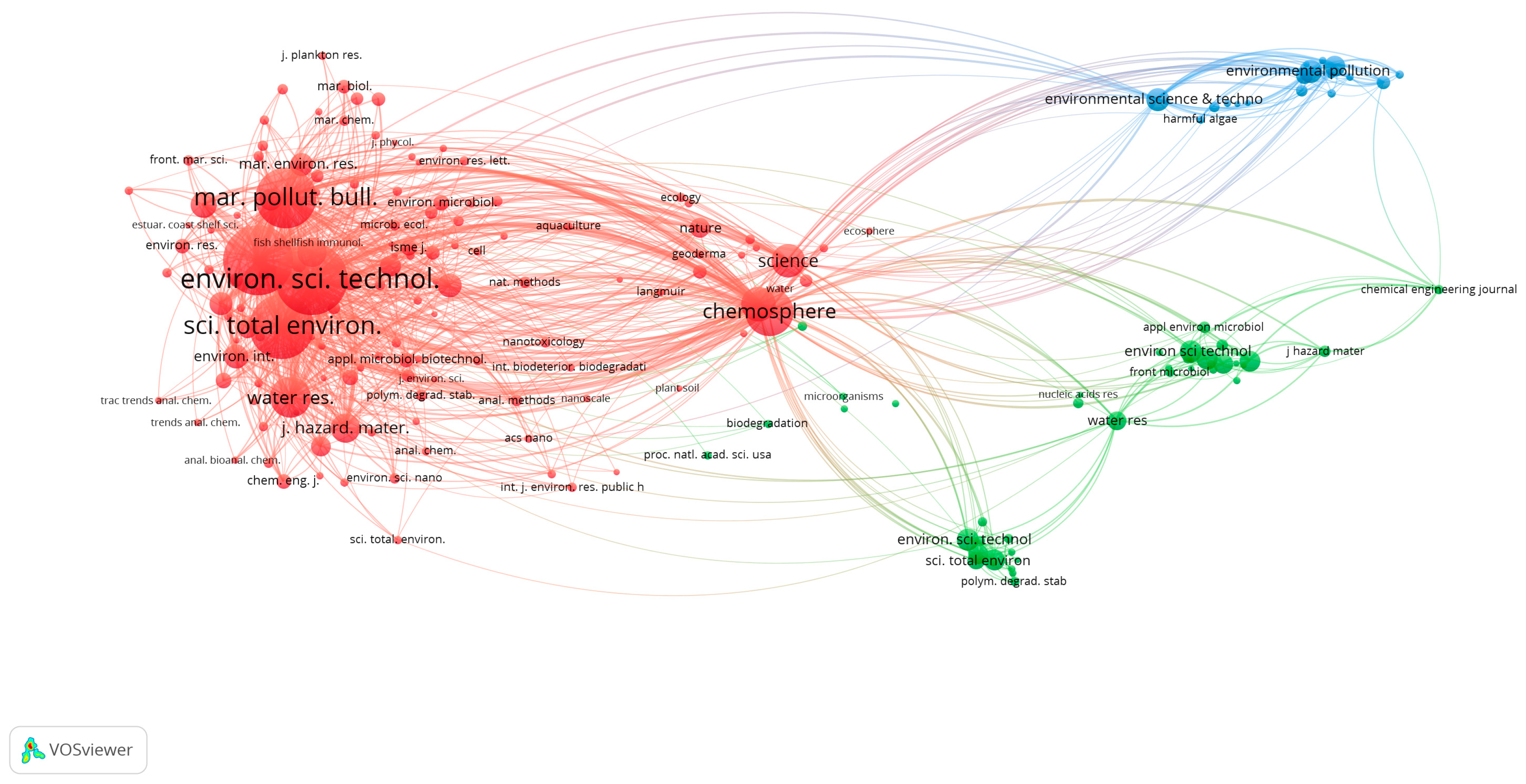
Table 2 shows ten journals with the highest number of published documents (with at least twelve or more publications). The papers published in the ten journals add up to 59.81% of the total publications investigated in Scopus, of which the first three represent 33.52%. According to the Clarivate Journal Citation Report, those ten journals cover at least one of the following areas: environmental sciences; marine and freshwater biology; water resources; environmental engineering; toxicology. In all of these areas, except for the journal
Bulletin of Environmental Contamination and Toxicology, journals are Q1 in the topics mentioned above, demonstrating the impact generated with the topic: “interaction of MPs/NPs with microorganisms and viruses.” A relevant aspect to highlight is that all the Journal Impact Factors consulted for 2021 in Clarivate’s Journal Citation Reports increased in the last few years, as did Cite Score 2021 consulted via Scopus for all these journals, confirming a high index of consultation and citation of these journals and a high relevance of the topic globally.
Figure 3, constructed using VOSviewer, exhibits the map of co-citations between the journals. The line thickness that joins two nodes (journals) determines the co-citation between two journals. The node size represents the journal’s total binding strength, while the thickness of the line joining two nodes represents the intensity of co-citation between them. As can be seen, the journal
Science of The Total Environment and the journal
Environmental Pollution have very strong co-citations, in addition to being very close to each other, indicating that they are closely related to the themes. A similar case is also seen between
Marine Pollution Bulletin and
Environmental Science and Technology, with a strong collaboration with the two previously described journals. Likewise, the
Journal of Hazardous Materials and
Chemosphere are heavily co-cited.
Similarly,
Chemosphere and
Science are very close. The strength of these co-citations is that the journals have topics that affect environmental sciences in their scope and the interaction of MPs/NPs with microorganisms and viruses, affecting the environment’s stability. MPs and NPs are also composed of additives such as flame retardants, antioxidants, plasticizers, and pigments that, after the partial or total degradation of MPs/NPs, can be released into the environment and affect ecosystems drastically [
59]. In addition, as stated above, they also affect the quality of the soil, plants, and water, affecting microbial diversity.
3.4. What Are the Most Relevant Keywords to MPs/NPs’ Interactions with Microorganisms and Viruses?
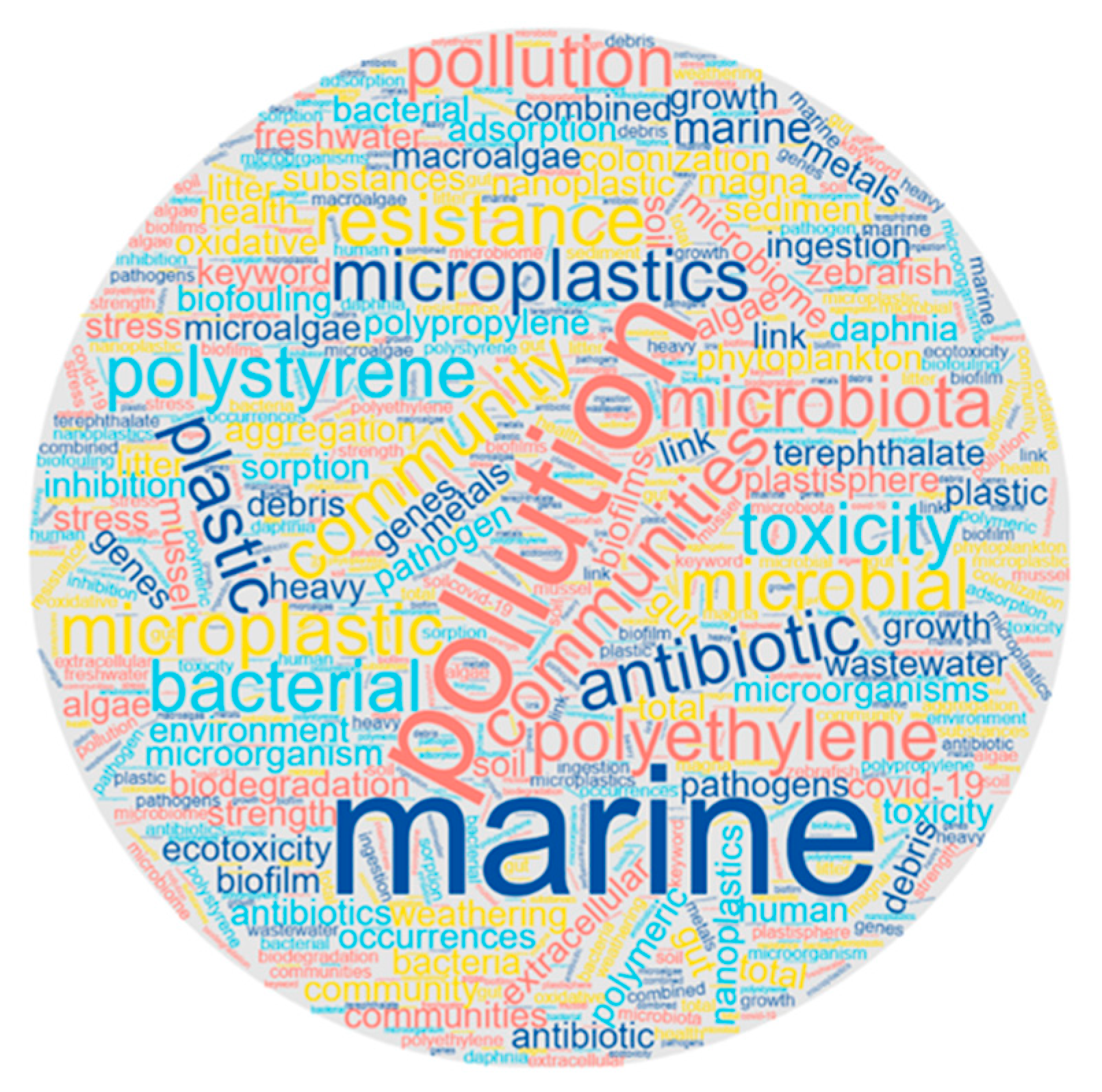
According to the method reported by Salgado-Cruz et al. (2021), we analyzed the importance of keywords with a word cloud as a first qualitative approach to determine the most relevant words of all the articles reviewed in the area of “microplastics and nanoplastics and their interaction with microorganisms and viruses” keywords [
60]. We discovered that the essential words in the topic are “marine”, “pollution”, “microplastics”, “microbiota”, “toxicity”, “polyethylene” and “plastics”, among other related words. The importance is related to microorganisms’ (many belonging to the microbiota of marine animals) interactions with MPs/NPs.
Figure 4 shows the cloud of the most critical keywords (determined by the size of the words).
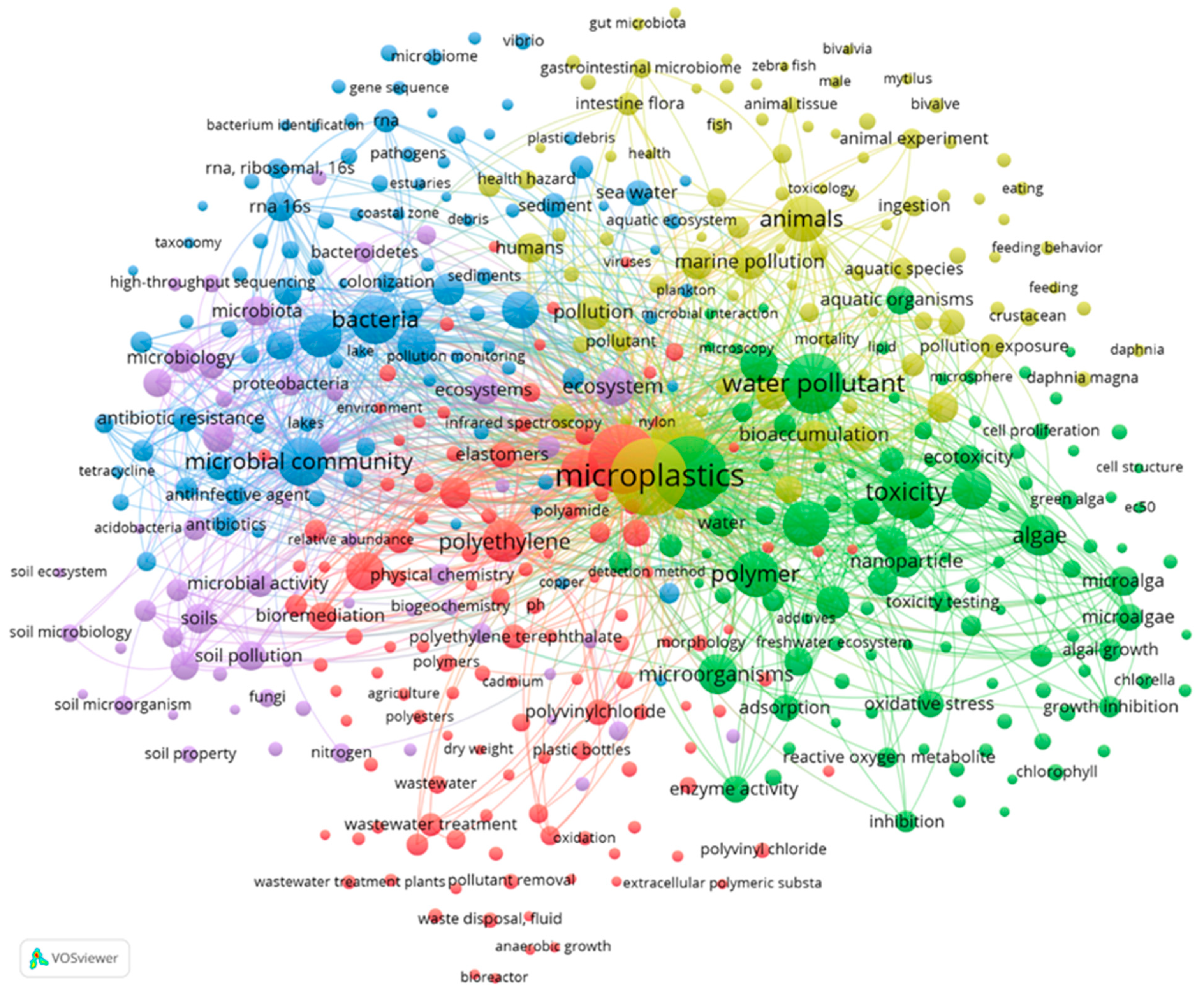
VOSviewer software allows a more detailed analysis of the interactions between the keywords and the authors of those works (
Figure 5), reflecting the importance of a word with the node’s size and the interaction with other works’ terms by the thickness of the link line.
The keywords network builded from those cited at least ten times, distributed in 467 words grouped in 5 clusters with a total link strength of 189,827. This number of words is the result of eliminating too generic keywords such as “human”, “non-human”, or terms indicating the same thing (microplastic or microplastics). The first cluster (red color) is related to polymers derived from petroleum that cause significant pollution. Terms such as “polypropylene”, “polyethylene”, “elastomers” and “microplastic pollution” stand out as members of this cluster, whose primary term is “plastics” with 466 links, a total bond strength of 9120, and 368 occurrences.
The second cluster (green) is related to contamination in freshwater caused by MPs and NPs, which cause toxicity to microbial species. In this cluster, terms such as “water pollutant” (458 links, a total binding force of 7026 and 277 occurrences) and “toxicity” (449 links, a total binding force of 5031 and 203 occurrences) stand out, highlighting the importance of recognizing these terms within the most co-cited articles around the world, as a growing concern about the effect of MPs/NPs on microorganisms.
The third cluster (blue) is more related to microorganisms that may be using MPs as propagation vectors, including terms such as “antibiotic resistance”, “bacteria” and “microbial community”, which reflect another problem inherent to the recalcitrance of this type of materials serving as vehicles for pathogenic microorganisms. In this cluster, words related to identifying and analyzing the DNA and rRNA of many microorganisms for identification purposes and the effects of their interaction are evident. One of the most prominent terms is “microplastics” present in cluster four (yellow) with 466 links, 10,108 total link strength, and 460 occurrences.
Another interesting word in this cluster is “plastic waste”, with 463 links, 5823 total link strength, and 227 occurrences. In this cluster, many terms are not related to other words due to the recent publication dates. Still, that accounts for the growing concern and interest in research to establish the relationship between microorganisms, marine organisms, and MPs/NPs.
The last cluster (violet) accounts for the concern about the effect of MPs/NPs on soil and terrestrial microorganisms. Words such as “soil pollution”, “soil microbiology”, “microbiota” and “soil property” reflect this aspect.
3.5. What Are the Most Cited Articles?
These studies are interested in the impact of incorporating these particles into the food chain, which considerably affects the stability of ecosystems and represents a threat to human health. Of the total number of articles extracted, 183 (25.4%) contain 50 or more citations. The three most cited articles included in
Table 3 correspond to ‘Microplastic Ingestion by Zooplankton’ with 1292 citations [
57], ‘The distribution and Importance of Microplastics in the Marine environment: A review of the Sources, Fate, Effects and Potential Solutions’ with 963 citations [
61], and ‘Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River’ with 683 citations [
62].
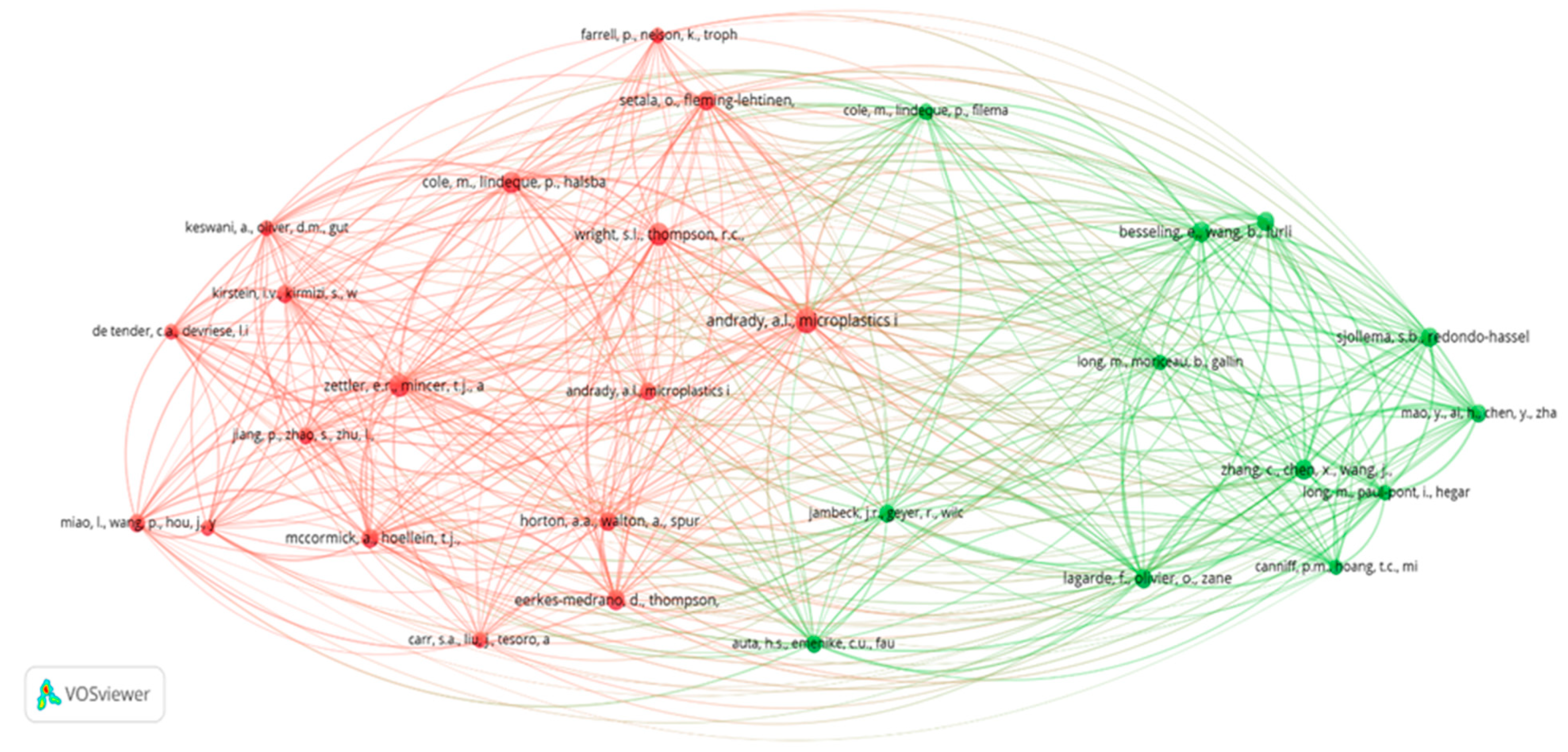
Figure 6 shows the map of co-citations of the sources with more than ten citations. This map shows a high relevance of the MP/NP interactions with microorganisms and viruses, with a high rate of co-citation reflected in the thickness of the links and their proximity. The last observation results from a global need for more standardized methodologies and techniques to analyze MPs/NPs and determine their direct effects on the interaction with microorganisms.
From the first cluster (red), Cole et al. [
57] publication stands out with 28 links, a total link strength of 205, and 59 citations. The publication by McCormick et al. [
62] follows, with 28 links, a total link strength of 211, and 51 citations. This cluster reveals an analysis of microplastics’ impact on marine animals.
In the second cluster (green), the interaction of microorganisms with MPs/NPs stands out more. The study by Besseling et al. [
66] stands out which is related to the affectation of microorganism growth by the effect of NPs. This reference has 26 links, with a total link strength of 233 and 54 citations.
Some studies reveal more than ten citations. For example, there is a pioneer study with 27 links, 256 total link strength, and 80 citations, demonstrating that it is a study that many researchers take as a reference due to the impact of MPs in the marine environment and the early stage of publication [
79].
Finally, the study by Zettler et al. [
42], in which the interaction of MPs with the complex oceanic microbial community that they baptized “plastisphere” [
42], made up of autotrophs, heterotrophs, predators, and symbionts, was analyzed. The study is interesting because it has 28 links, a total link strength of 260, and 65 citations [
42]. Its high relevance and citation correspond to the fact that they were pioneers in introducing the term “plastisphere”, referring to a very complex community, including opportunistic microorganisms of the genus Vibrio.
Generally, these studies with more than ten citations have a high number of citations because their common shared themes serve as reference studies on the subject.
3.6. Review of the Interaction of Microplastics and Nanoplastics with Microorganisms
Studies show that MPs/NPs in different habitats have a negative impact. MPs/NPs alter the apparent density of the soil due to the different densities present in the MPs’ components [
80,
81,
82]. Introducing MPs/NPs into the soil also affects soil porosity [
80,
83]. The latter involves a flow of gases, vital in the decomposition of organic matter; in addition, porosity affects the proper introduction and evaporation of water (affecting the nutrition of plants, animals, and microorganisms) [
80,
84,
85], as well as the natural flow of oxygen. These aspects negatively impact the life of anaerobic or aerobic microorganisms in the soil [
86].
In this way, changes due to the introduction of MPs/NPs into terrestrial and aquatic ecosystems can alter the pore space of the soil, affecting the water cycle, gas flow, and the associated microorganisms [
87].
Other concomitant changes demonstrated by the introduction of MPs/NPs in ecosystems are related to changes in soil nutrients, alterations in pH (due to changes in ion exchange capacity), and the release of toxins (products of adsorption and the release of pesticides, heavy metals, and polycyclic hydrocarbons, among others) [
13,
22,
87,
88,
89]. Changes in the physicochemical parameters of the soil lead to variations in microbial diversity.
Microplastics alter rhizosphere communities (mycorrhizae and rhizobacteria) due to plants’ altered water and nutrient absorption capacities. In turn, this also changes the ability to break down organic matter and the natural cycle of elements, which ultimately affects plants and all levels of the food chain.
On the other hand, the faunas that ingest MPs/NPs and the adsorbed pollutants alter their intestinal microbiota, with severe consequences for animals’ health [
87,
89,
90,
91,
92]. For example, the interaction of vascular plants can introduce phytotoxicity and negatively affect growth.
3.6.1. Enzymatic Degradation of MPs/NPs Mediated by Microorganisms
Studies directed towards different alternatives for the degradation of MPs/NPs are of global interest, given the negative impact they cause both on human health and the environment. There are a high number of studies including relevant aspects such as transport, toxicity, and various chemical additives [
93]. Microorganism-mediated enzymatic degradation involves mixed oxidative and biochemical reactions, which vary depending on the species of microorganisms, the morphology of MPs/NPs, and environmental conditions [
11]. The stress induced by MPs/NPs causes an enzymatic response in microorganisms, allowing them to adapt to these new conditions by regulating various cellular aspects [
94].
The enzymatic response induced by anthropogenic contaminants in microorganisms, in addition to the optimization function in cellular function for their adaptation, plays a critical role in the degradation of these anthropogenic contaminants, such as MPs/NPs.
The enzymes of these pollutants degrade the polymers of the MPs/NPs until they are converted into monomers through biochemical processes or oxidative reactions until they can assimilate and incorporate them into their biochemical cycles for the production of energy as a carbon source [
95]. Microorganisms tend to present two types of MP/NP degradation, depending on the enzymes involved in the process. For example, the structural modification of the ester type is catalyzed by
Fusarium solani pisi cutinases [
96]. Hydrolases are very efficient in the structural transformation of these anthropogenic polymers. These enzymes increase the surface hydrophilicity of MPs/NPs, facilitating their degradation [
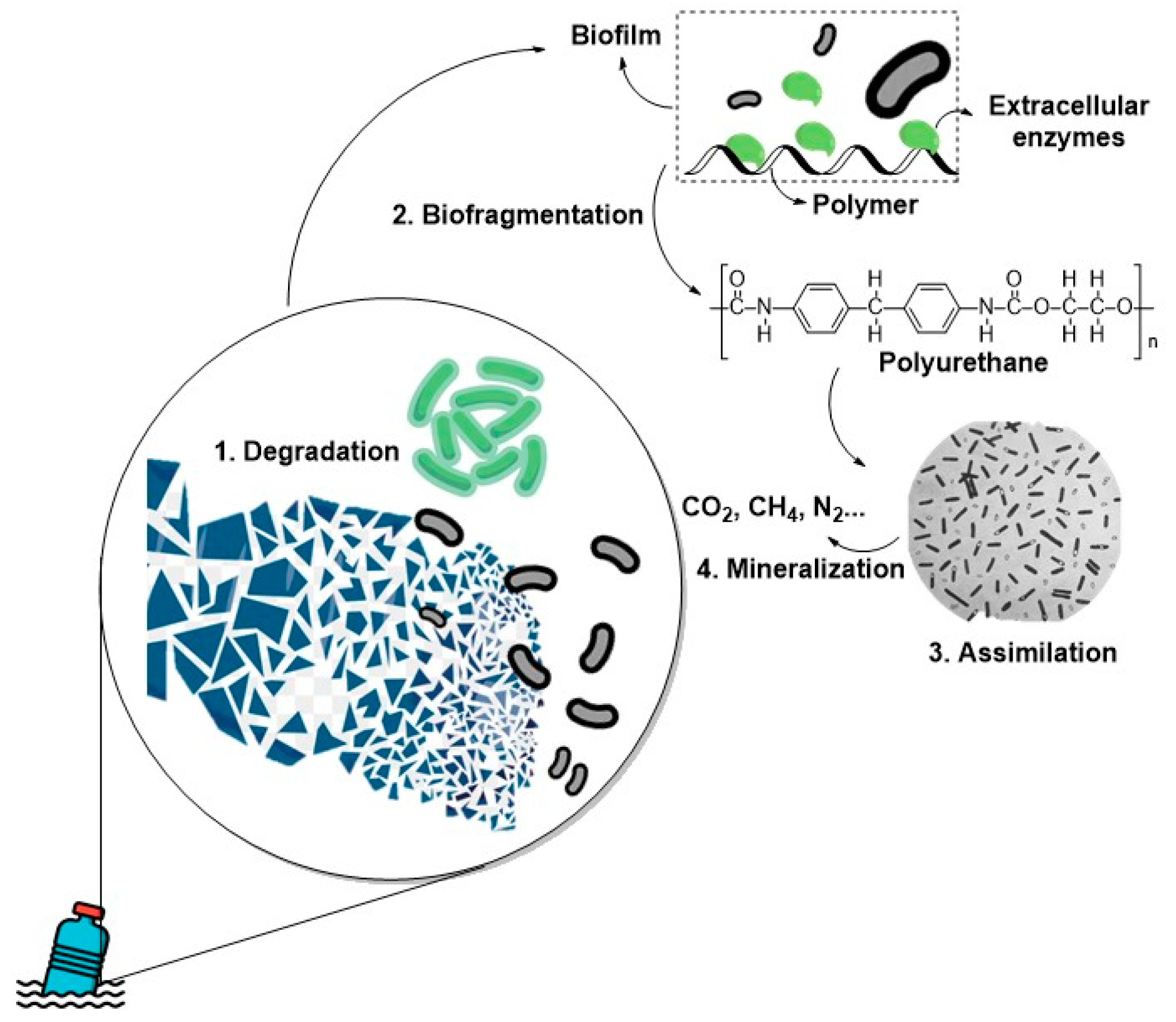
97]. However, more studies are needed to understand the mechanisms of these enzymes fully and for optimization to increase biodegradation efficiency. A common enzyme-mediated mechanism that alters C-O bonds in polymer backbones to their constituent monomers is shown schematically in
Figure 7 [
98].
It is known that the degradation of the polymers that constitute MPs/NPs occurs through hydrolyzation reactions, which can act on hydrolyzable polymers and non-hydrolyzable polymers by binding to the MPs’/NPs’ surfaces to catalyze the chemical transformation essential for polymer degradation. The enzymatic degradation processes mediated by microorganisms break the polymeric bonds of the MPs through the action of enzymes such as hydrolases, lipases, and extracellular esterases until oligomers, dimers, or monomers are produced, followed by the assimilation and mineralization to CH4, H2O, and CO2.
PE degradation by
Staphylococcus epidermis [
99] is an example of enzymatic degradation, as well as in species of the Vibrio genus which colonize MPs/NPs, known as the “plastisphere” [
42]. Plastisphere fungi, bacteria, and bryozoans are involved in MP/NP degradation. For example, LDPE is degraded via by
Aspergillus sp. [
100] and PP and LDPE are degraded by
Rhodococcus sp. [
101,
102]. There are also reports of PE degradation by
Zalerion maritimum and PU degradation by
Bacillus subtilis [
103]. In contrast, some reports are related to PS degradation by
Thermus sp.,
Exiguobacterium sp.,
Geobacillus sp., and
Bacillus sp. [
104].
3.6.2. Bacterial Capacity for Biodegradation of MPs/NPs
The adverse effects caused by MP/NP contamination in biota have promoted the study of alternatives for bacterial-mediated degradation, which is of interest given the widespread relevance throughout the world, especially in the oceans, where hundreds of thousands of bacterial species live with degradation potential, evidenced in several reviews of various MP degradation alternatives with several mechanisms [
105].
The efficiency of MP degradation varies depending on the type of bacteria, environmental conditions, MP density, functional groups, and chemical additives such as plasticizers [
43]. The degradation of MPs mediated by bacteria involves a series of biochemical transformations by extracellular hydrolases that induce polymer backbone deterioration in MPs, reducing their size into monomers that are easy to assimilate. The enzymatic digestion also alters the shape and physicochemical properties, allowing the assimilation as a carbon source incorporated in mineralization processes [
106].
The products from bacterial degradation vary depending on the conditions. For example, the enzymatic hydrolysis of polyhydroxy butyrate (PHB) generates 3-hydroxybutyric acid (HBA) as a product. That is, under anaerobic conditions, polyhydroxyalkanoate (PHA) degradation tends to produce methane, unlike aerobic degradations, which generate carbon dioxide and water [
107].
Even with the current mechanistic knowledge regarding the biodegradation of MPs/NPs by fungi and bacteria, there is a need for more studies to understand these processes comprehensively. For example, species such as
Streptomyces,
Pseudomonas,
Arthrobacter,
Corynebacterium,
Rhodococcus, and
Micrococcus have good degradation potential [
108]. In addition,
Polaromonas,
Agreia,
Subtercola,
Cryobacterium,
Leifsonia,
Flavobacterium, and
Cryobacterium with degradation potential due to their production of hydrolases have been isolated [
109].
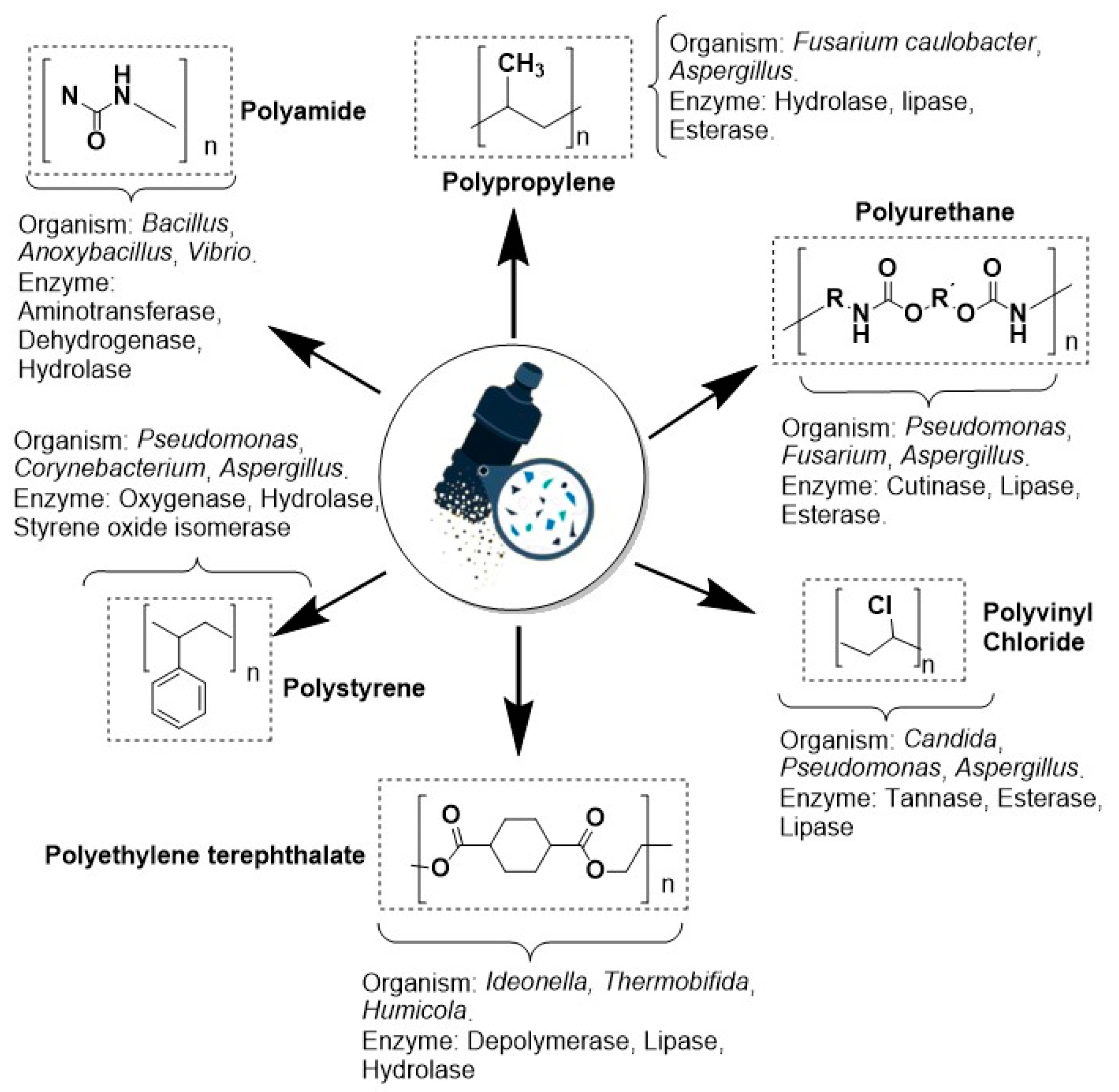
Figure 8 shows the degradation process of plastics due to various synergistic environmental effects, such as biotic and abiotic factors, until sizes less than 5 mm are reached (MPs). Once fragmentation occurs, different bacterial and fungal species interact with these substances, exerting degrading effects on their surface by releasing various bacterial and fungal enzymes such as esterases, cutinases, and lipases. Additionally, oxidative mechanisms in MP degradation involving enzymes such as monooxygenase, dioxygenase, and carboxylesterases have been reported [
110,
111]. However, many of the studies reported are in silico, which do not entirely cover all the natural conditions that allow the optimization of degradation mechanisms in a short time or with greater efficiency.
Table 4 reports various bacteria with MP degradation activity, such as PP degradation with efficiencies of up to 4.0% and 6.4% mediated by
Rhodococcus sp. and
Bacillus sp. after 40 days of incubation [
101].
3.6.3. Fungal Capacity for the Biodegradation of MPs/NPs
There are also reports on MP biodegradation using fungi. For example, the evaluation of PE biodegradation responses of the marine fungus
Z. maritimum through quantifiable mass differences in PE microplastic granules exhibited good biodegrading activity [
124]. Given the marine habitat of
Z. maritimum and its great capacity for PE degradation, it is a good bioremediation alternative against the increase in MPs/NPs in the oceans. Like bacteria, fungi can also form biofilms on the MP’s surface and cause deformations in their structure, which promotes the formation of functional groups such as carboxyl and carbonyl, reducing properties such as hydrophobicity [
125]. Fungi can also use MPs as carbon sources through mineralization processes [
126].
Aspergillus niger and
Penicillium pinophillum have shown LDPE biodegradation activities through a weight loss of 0.57% and 0.64%, respectively [
127].
Table 5 reports the biodegrading activity of some species of fungi, as well as the degradation conditions. However, there is a general requirement for more detailed knowledge of the various degradation mechanisms these fungi use, which could facilitate the use of microorganisms as MP degradation alternatives in the oceans.
3.6.4. Biodegradation Capacity of Microplastics Using Microalgae
The biodegradation of emerging pollutants through microalgae and cyanobacteria has been studied in many fields, given the high efficiency of different processes through the efficient use of sunlight. The microalgae
Phaeodactylum trycornutum and
Chlamy domonas reinhardtii have shown great potential in environmental and biotechnological research through the energy of photosynthesis [
135].
Although there are reports on the various mechanisms and microalgae–MP/NP interaction, more studies are still required to understand the various species of microorganisms and mechanisms for using these microorganisms for biodegradation [
136]. A complete understanding of these mechanisms could help accelerate the process or increase the biodegradation efficiency of emerging pollutants such as MPs/NPs, which represent a significant risk for aquatic biota and human health. However, both microalgae and cyanobacteria promise to be exciting alternatives for MP/NP biodegradation and are efficient, sustainable, and environmentally friendly.
Figure 9 schematically shows the three main pathways that microalgae employ during the bioremediation of various emerging contaminants: biouptake, bioadsorption, and biodegradation. Biouptake involves the active transport of emerging contaminants into the cell by binding to intracellular proteins that act on the contaminants. During bioadsorption, emerging contaminant adsorption occurs on the cell wall or extracellular components, and biodegradation involves the transformation of compounds to complex organics (such as MP/NP contaminants) into substances, and into more specific and less toxic compounds through catalytic metabolic degradation processes [
137].
Microalgae can colonize synthetic substrates such as PE surfaces, forming biofilms on the MP/NP surfaces and exerting degradative actions [
138]. In this context, a microalgae known as
Uronema africanum Borge has been reported, which has been identified on surfaces of plastic debris collected in lakes and in domestic waste dumps [
139].
U. africanum Borge exerts a degradative action on the LDPE surface after 30 days.
Table 6 reports the biodegradation activities induced by various species of microalgae which have the potential to be exciting biodegradation alternatives to MPs/NPs that respect the environment.
3.6.5. Biofilms and Periphytic Films
The use of periphytic biofilms is a relatively new method for the biodegradation of MPs/NPs such as PP, PE, and PET. Biodegradations ranged between 18.02% for PP, 14.02% for PE, and 19.72% for PET after 60 days of treatment with
Deinococcus thermus,
Proteobacteria, and
Cyanobacteria as the predominant species in the biofilms [
147].
While the use of biofilms for MP/NP biodegradation in marine environments promises to be a valuable alternative to mitigate the negative impacts of these pollutants on various marine species, water quality, and human health, synergistic collaborative research efforts to understand the mechanistic process of degradation entirely is not enough. Therefore, more research is required to understand the mechanisms by which these biofilms degrade MPs and the different interactions between MPs/NPs and microorganisms to fully understand the effects of these biofilms’ various biodegradative processes.
In this context, there are reports on biofilms’ biodegrading potential in marine environments. The current research on biodegradation processes through biofilms has been reviewed, along with lagoons and future research prospects [
148].
Biofilm formation on the surfaces of MPs, such as PE, usually takes up to a week [
149]. After a week, the biofilms begin to be visible on the surfaces of the MPs, which increase in the following weeks, generating morphological and structural changes in the MPs, evidenced by the registration of weight losses of the MPs and structural and morphological alterations.
Table 7 exhibits some studies on the degradation effects of MPs/NPs mediated by biofilms and periphytic films.
4. Conclusions
The bibliometric analysis retrieved 719 documents from the 2010 to 2021 timeline, demonstrating MP/NP interactions with microorganisms/viruses, an area of knowledge with high impact and continuous growth. This dramatic increase is related to a more significant concern about the effects derived from the interaction of MPs/NPs with aquatic, terrestrial, and atmospheric microorganisms with consequences on their growth and development, facilitating the biodegradation of MPs/NPs. In addition, there is growing concern about the role that MPs play as vectors since pathogens can spread to environments where they are generally not present and move to other levels of the food chain, affecting the health of animals and humans. From the analysis of the source countries, China (288), the United States (94), and Germany (72) are the leading publishing countries in MP/NP interactions with microorganisms and viruses, accounting for more than 454 documents (65.4%) of the total literature retrieved. However, China, the United States, the United Kingdom, and Germany are the countries with the most significant collaboration at a global level, represented by the proximity and size of the nodes. Nevertheless, there is a need to strengthen synergistic efforts regarding the study of MP/NP degradation in some countries of continents such as Africa (South Africa, Nigeria), Asia (Japan, Hong Kong, Malaysia, Indonesia, Bangladesh, India, South Korea), and America (Brazil, Argentina, Mexico, Canada, and Costa Rica), given their low rate of research reports associated with these issues.
The publications on this research topic have occurred in the most prestigious journals in areas related to environmental sciences, marine and freshwater biology, water resources, environmental engineering, and toxicology. The most relevant keywords related to the topic are “marine”, “pollution”, “microplastics”, “microbiota”, “toxicity”, “polyethylene”, and “plastics”, related to microorganisms’ (many belonging to the microbiota of marine animals) interactions with MPs/NPs. The most cited papers are interested in the effect of these particles’ ingestion and incorporation into the food chain, which considerably affects the stability of ecosystems and represents a threat to human health.
With the analysis of this bibliographic review, there was documentation of the biodegradation of MPs/NPs by bacteria, fungi, microalgae, and biofilms, which promises to mitigate the negative impact generated by the ubiquitous presence of anthropogenic pollutants’ MPs/NPs in various environments, such as terrestrial, aquatic and atmospheric environments. However, the study reveals a need to increase cooperative research on the various interactions between microorganisms and MPs/NPs with pilot-scale analyses that allow the expansion of existing biodegradation methodologies. There is still a lack of detailed knowledge of marine and human health effects, mainly due to an underestimated effect related to the analytical and sampling methods for MP/NP extraction. Finally, in-depth study of the degradation mechanisms and stimulation conditions involved in the periphytic films and microalgae of MPs is necessary.