Abstract
The mass production and consumption of plastics have serious effects on the environment, human health, and livelihood. Hence, global efforts to reduce plastic generation must be realized. This study aimed to determine the prevalence of microplastics in mangrove sediments of Cabadbaran, Buenavista, and Nasipit in Butuan Bay, Philippines. Seventy-two (72) microplastic particles were extracted from mangrove sediments dominated by fibrous type (71%) and blue (35%) as the most common color. Attenuated total reflectance–Fourier transform infrared (ATR–FTIR) spectroscopy was used to assess the polymer type of microplastics. Results reveal a total of six polymer types including high-density polyethylene, low-density polyethylene, polyethylene terephthalate, ethylene-vinyl acetate, polyamide, and polypropylene, with the latter comprising 39% of samples, the highest among the extracted particles. Overall, Nasipit (71.1/kg) obtained the highest microplastic density followed by Buenavista (48.9/kg) and Cabadbaran (40.0/kg). These data will serve as a piece of baseline information in crafting important environmental policies to address plastic pollution issues in the area. Long-term studies are recommended to better understand, monitor, and prevent further microplastic pollution in Butuan Bay.
1. Introduction
Plastics are synthetic organic polymers derived from the polymerization of monomers extracted from oil or gas [1,2,3]. Since the first modern plastic invention in 1907, various low-cost manufacturing techniques have been refined, allowing for the mass production of a wide range of lightweight, durable, inert, and corrosion-resistant polymers [4]. Plastics have been increasingly crucial to all nations over the last 70 years, with a yearly growth rate output of 8.4 percent between 1950 and 2015 [5]. Estimates of floating plastic in the ocean are as high as 236,000 metric tons [6]. Jambeck et al. [7] suggested that 4.8 to 12.7 million tons of plastic debris per year enter the ocean and projected cumulative inputs will increase tenfold by 2025. The Philippines is the third biggest contributor of plastic litter into the ocean and the number one emitter of plastic debris from riverine sources [7,8]. Recent studies in the country revealed that plastic litters are prevalent in estuaries [9], seagrass beds [10], and beaches [11,12,13].
Through accidental release and indiscriminate discards, plastic waste has accumulated in the environment at an uncontrollable rate. When these wastes enter the ocean, their rate of breakdown accelerates. Plastics’ persistence varies depending on the polymer, shape, density, and other factors [9,12,14,15]. Moreover, plastics will disintegrate into microplastics when exposed to natural forces such as wind, exposure to sunlight, wave action, weathering, temperature, irradiation, pH [16], other harsh environmental conditions, and physical stress [17]. This degradation can also create chemical hazards for marine wildlife. As ultraviolet radiation breaks down plastics, additives that make plastic more durable are caused to leach out into the environment [18]. These small plastic debris are argued to have a potential threat to marine wildlife, from the gene to community levels [19,20,21,22,23]. Some authors [24,25,26] state that because of the high intake of seafood, there would be a potential effect of microplastics on human health.
Occurring between land and ocean, mangrove ecosystems, to some extent, act as traps for marine plastic debris [27]. The combination of being a tropical country that hosts a wide variety of mangrove species, poor plastic waste collections, and the absence of research programs on basic plastic problem research has resulted in a limited understanding of its implications [28]. These factors also are thought to contribute mainly to the Philippines’ mangrove forests as hot spots for microplastic accumulation in the future. Despite these compelling factors, the amount of microplastics in mangrove ecosystems in the country has not received much attention.
Butuan Bay is situated in Agusan Del Norte, in the Philippines’ northeastern part of Mindanao. The Bohol Sea, also known as the Mindanao Sea, connects it to the north and is known for its severe southwest flow of surface currents from the Pacific Ocean [29]. Several tributaries—notably, the Agusan River, the country’s third-longest river—run straight into the sea, transporting water from interconnected rivers, canals, and lakes [30]. These tributaries can transport plastics from different communities from the slopes of Davao Oriental and Agusan del Sur down to Butuan Bay. With the premises mentioned above, this paper sought to assess the prevalence of microplastics in the bay given the amount of plastic litter generated by the capital city of the Caraga Region.
This study was the first to ever document microplastics’ prevalence in the Philippines’ mangrove sediments. The extracted microplastics were counted and grouped according to particle shape, color, and polymer type. The different polymer types were identified using attenuated total reflectance–Fourier transform infrared (ATR–FTIR) spectroscopy. The results of this will serve as a guide in crafting important environmental policies to address the plastic pollution problem in the region and a baseline for conducting further research in these areas.
2. Materials and Methods
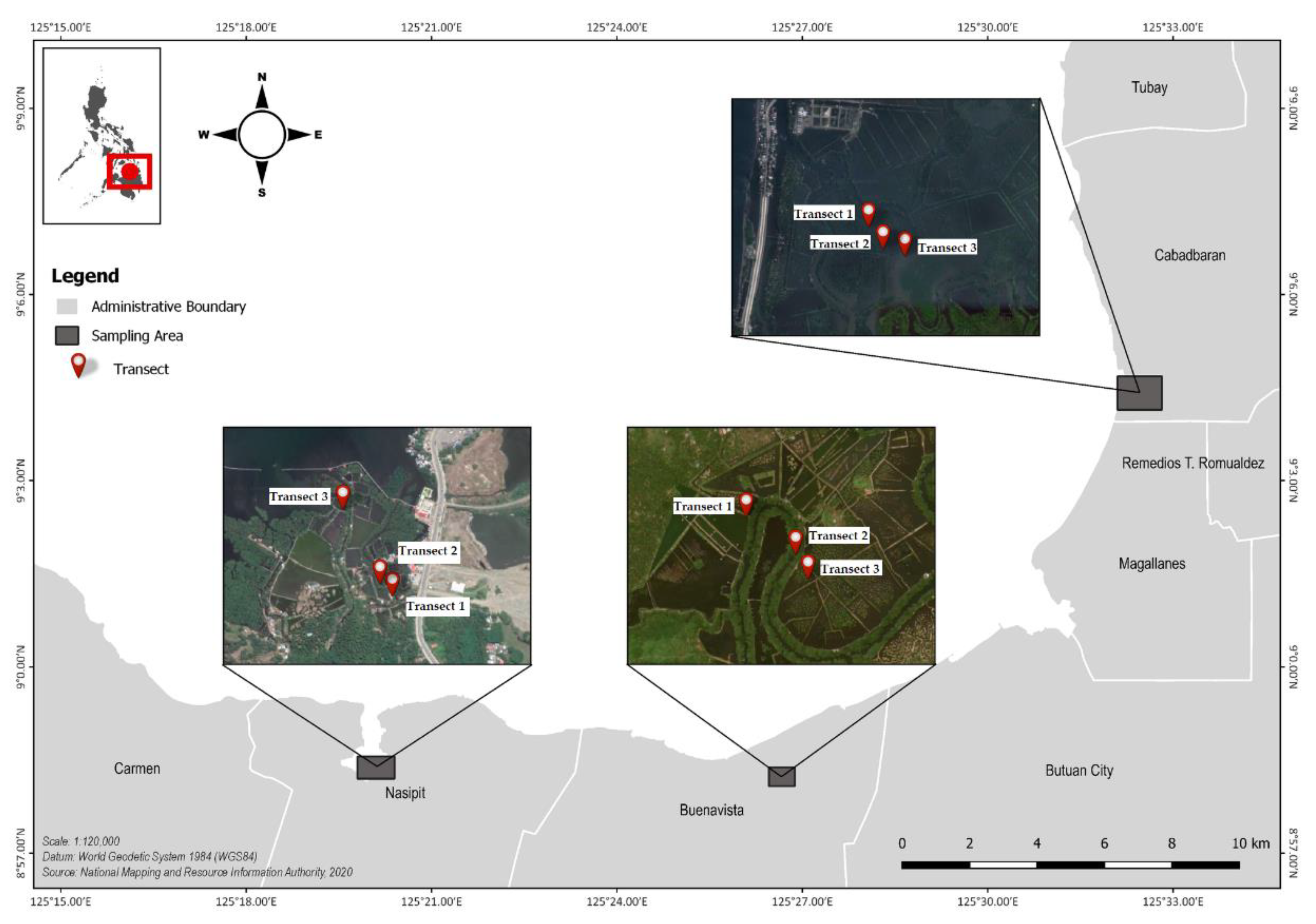
The study was conducted in Butuan Bay located in northeastern Mindanao. To the north, its shoreline connects with the Bohol Sea, also known as the Mindanao Sea. Strong southwest surface currents flowing from the Pacific Ocean are prominent in this sea. A number of river tributaries—notably, the Agusan River, the third-longest river in the Philippines—transport water from connected rivers, canals, and lakes directly into the Bay. Specifically, the study was conducted in three sampling stations, namely, Cabadbaran, Buenavista, and Nasipit (Figure 1).
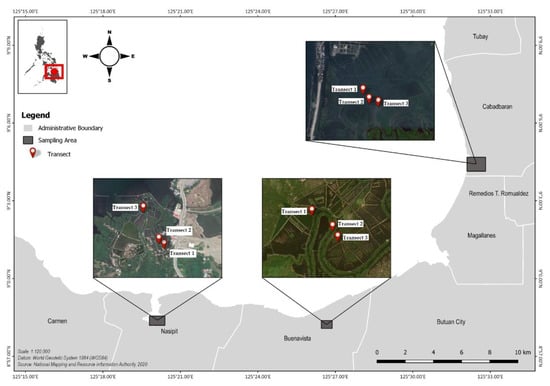
Figure 1.
Location of the three sampling stations for microplastic assessment of mangrove sediments along Butuan Bay (Cabadbaran, Buenavista, and Nasipit).
2.1. Establishment of the Sampling Sites and Sediment Collection
Three forty-meter transect lines were established with three 10 m × 10 m quadrats in each sampling station. Using a metal spoon with little disturbance, two cm of the topmost sediment was collected and stored in a glass jar. Fifty grams of dried sediments obtained from each quadrat were collected for a total of 150 g per transect line. Samples from each transect line were homogenized as representative samples from each sampling station. All the jars were sealed with aluminum foil before closing the lids to avoid exposure to light and prevent contamination from other sources.
2.2. Microplastic Extraction and Identification
Before proceeding with the laboratory works, glassware were washed and cleaned for microplastic decontamination through intensive upside-down rinsing with ultrapure water. Non-plastic materials were used to minimize microplastic contamination. All the samples were kept in a drying oven at 90 °C for 24 h, then weighed in a preweighed beaker. The procedures by Karami et al. [31] were followed with modifications to remove organic matter. A total of 150 g of sediments was soaked with 300 mL of 10% KOH solution and heated in an oven for 24 h at 60 °C. After the samples were retrieved from the oven, vacuum filtration was performed using a Millipore set and 40 mm diameter GF/C glass filters. Each glass filter was washed with distilled water and oven-dried for 24 h [32]. A clean Petri dish housed the filter paper for optical microscopy analysis using 40× magnification. The remaining samples were subjected to floatation using 30% NaCl solution to allow the settled microplastic particles to float. Using a clean needle, all suspected microplastic particles were mounted in glass slides. Microplastics were described as fiber, film, or fragments based on the classification technique used by Su et al. [33]. A fiber was defined as a microplastic with a long, slender appearance, whereas a film was described as a small and thin particle from large plastic debris. When a microplastic could not be identified as a fiber, pellet, or film, the categorization of the fragment was employed. Microplastics were then subjected to ATR–FTIR analysis (PerkinElmer Spectrum Two FT-IR Spectrometer, PerkinElmer Inc., Waltham, MA, USA) to classify the polymer type further. This is a well-recognized, rapid, and reliable method in identifying polymer types of different MPs by comparing the resulting FTIR spectra with known plastic polymers in the spectral library [34]. All laboratory works were conducted at the Chemistry Laboratory of Caraga State University—Main Campus.
2.3. Quality Control
A control group was established using ultrapure water to ensure the data’s quality. For the laboratory works, primarily during the preparation and treatment of samples, the researchers wore medical masks and laboratory gowns. On the other hand, in conducting microscopy and mounting suspected microplastics, wearing of personal protective equipment (PPE) instead of a lab gown was strictly followed to prevent microplastic contamination from the clothes that the researchers wore.
2.4. Density of Plastic Litter
The density of microplastics was computed by the total number of items divided by the total mass of the sediment samples. This method was modified based on the studies of Abreo et al. [35], Espiritu et al. [36], Rahim et al. [37], and Egessa et al. [38].
2.5. Statistical Analysis
The differences among the densities of microplastics in transect lines were analyzed using the Kruskal–Wallis Test.
3. Results
Microplastics were present in all nine sediment samples collected from three mangrove habitats in Butuan Bay (Table 1). A total of 141 suspected microplastics were extracted from the sediment samples, 72 of which were confirmed and identified as MPs based on the FTIR results. There was a 48.94% (69 particles) decrease in the number of confirmed plastics after conducting the confirmatory test, mainly because of three reasons: (1) some suspected microplastics were too small for the machine to detect its polymer type; (2) due to the samples’ minute size, some were lost while transferring them from the glass slides to the FTIR machine; (3) degraded samples due to different environmental factors can affect the similarity score of the samples based on the data stored in the computer’s library.

Table 1.
Sampling area coordinates and incidence of microplastics in mangrove sediments of Cabadbaran, Buenavista, and Nasipit along Butuan Bay.
Before and after conducting the FTIR analysis, the highest number of microplastics was found in Nasipit with 62 and 32 particles, respectively (Table 1), followed by Buenavista (42, 22) and Cabadbaran (37, 18). There is also variation in the number of microplastics per transect. For example, the confirmed microplastics in Cabadbaran Transect 1 was nine, while there were only four microplastics in Transect 3. However, statistical analysis revealed that there is no significant difference among the sites and transects (p > 0.05). Calculating the density of microplastics per kilogram of dried sediment samples showed that Nasipit was the highest (71.1/kg), followed by Buenavista (48.9/kg) and Cabadbaran (40.0/kg). The highest concentration of microplastics was found in Nasipit since it was located near human settlements, and high amounts of plastic debris were observed during the sampling such as fishing nets, plastic detergent containers, plastic bottles, and food wrappers.
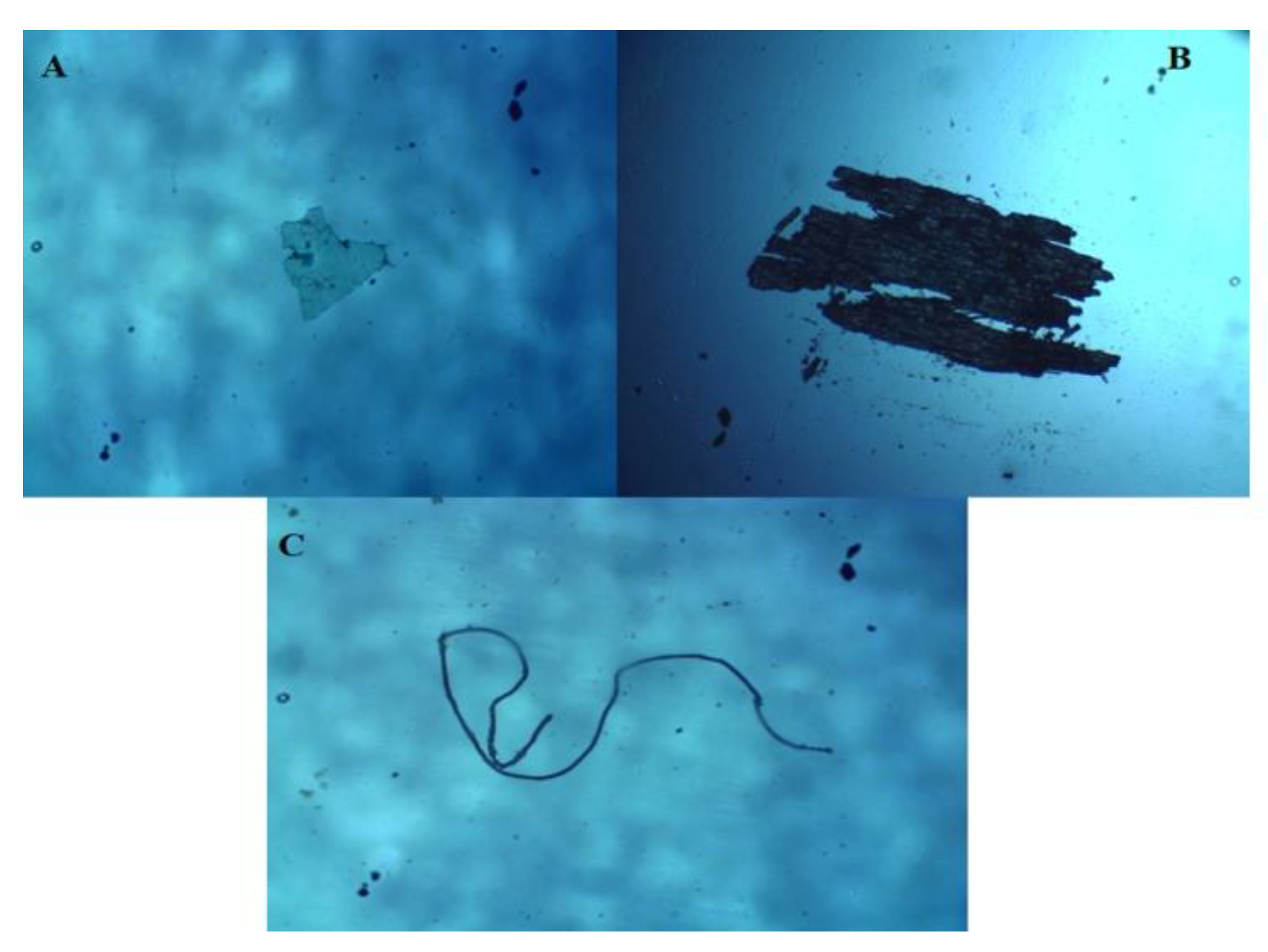
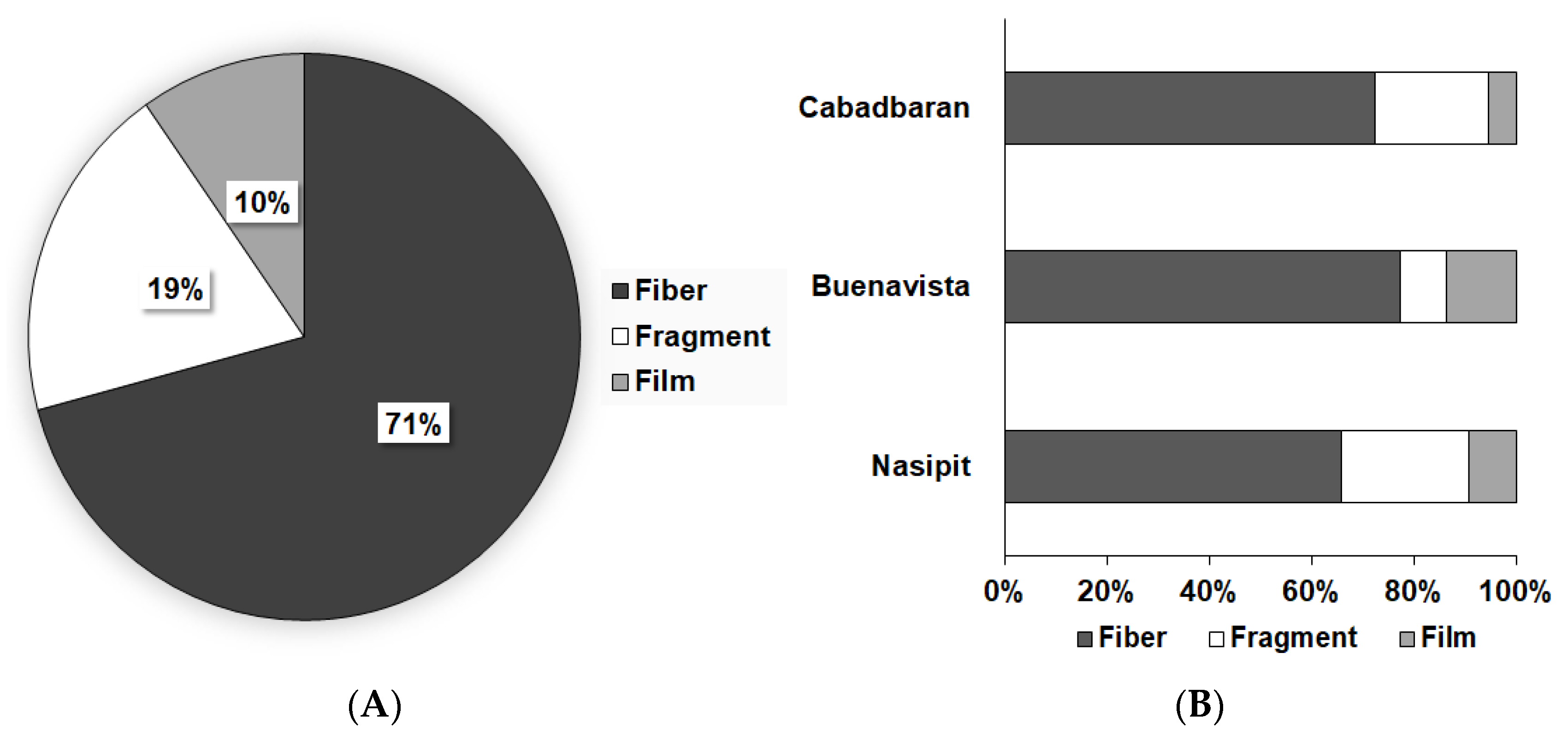
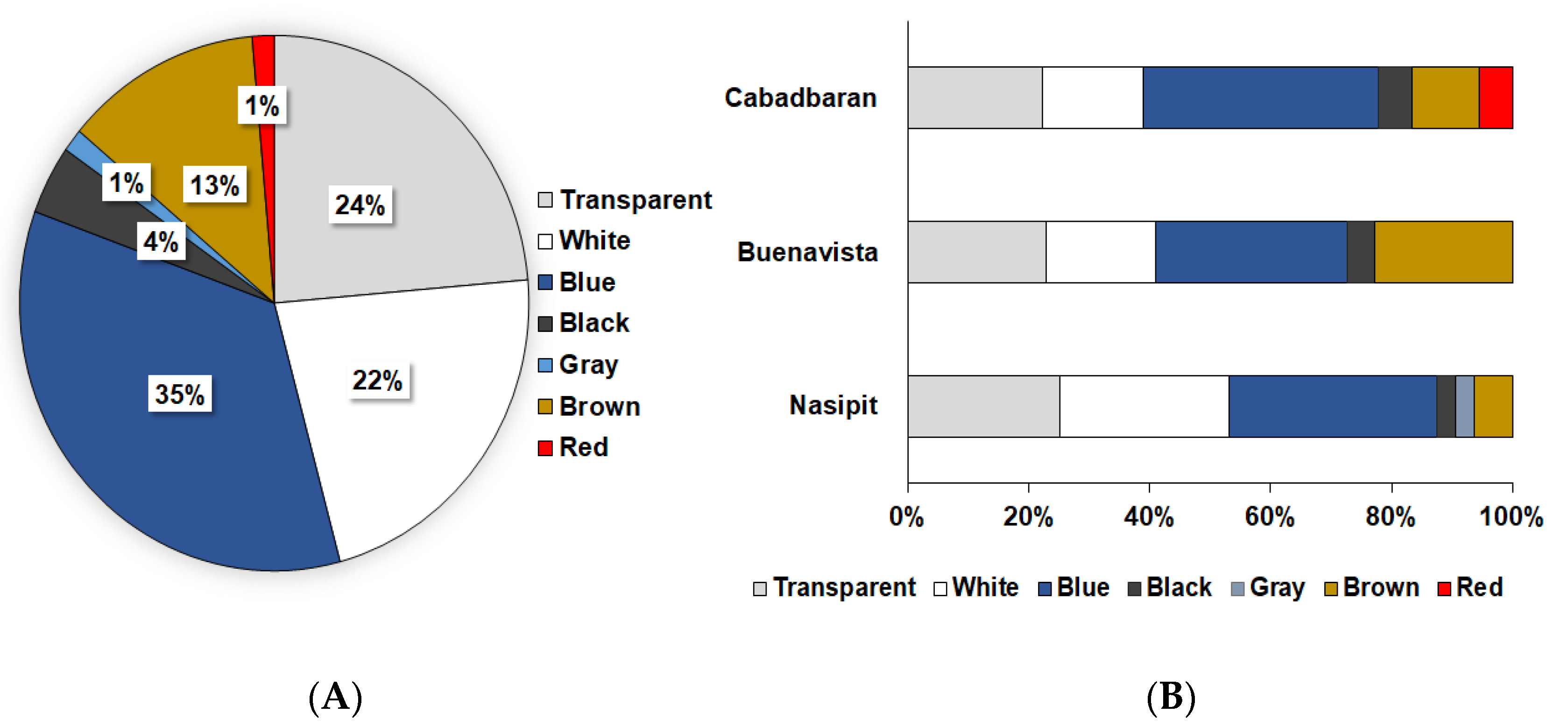
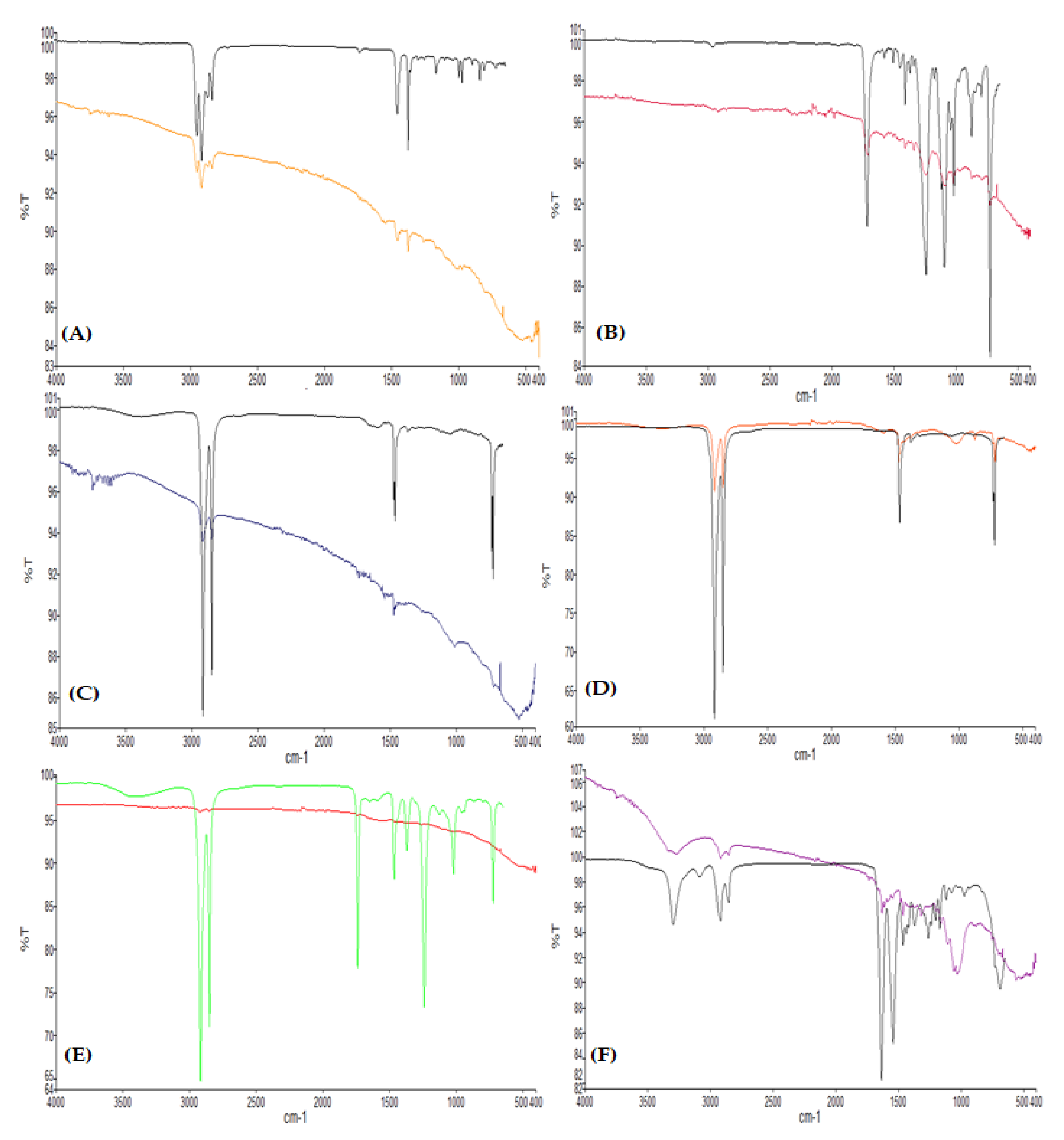
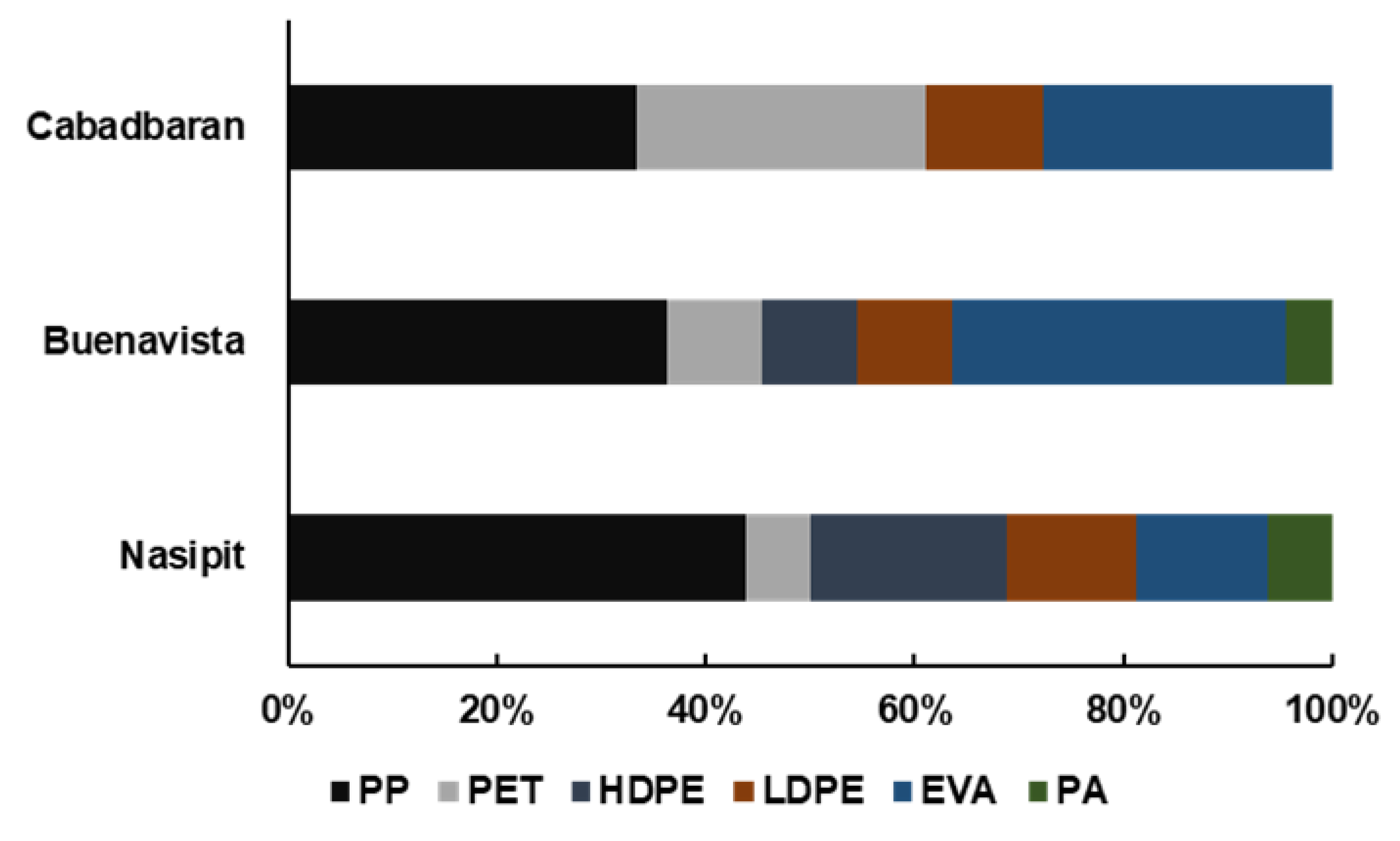
During microscopy, three shapes of microplastics were identified: fiber, fragments, and films (Figure 2). Fibers (71%) were the most abundant type of particles found in this study, followed by fragments and films, which cover 19% and 10% of the samples, respectively (Figure 3). Moreover, seven colors were observed (Figure 4), with blue as the most prevalent, comprising 35% of the isolated and identified microplastic particles followed by transparent (24%), white (22%), brown (13%), black (4%), gray (1%), and red (1%). Furthermore, all suspected microplastics were analyzed using ATR–FTIR spectroscopy, the most frequently used method in identifying microplastic polymers and diagnosing chemical compositions of unknown plastic pieces. Six polymer types (Figure 5) were identified in the current study, namely, high-density polyethylene (HDPE) and low-density polyethylene (LDPE), polyethylene terephthalate (PET), ethylene-vinyl acetate (EVA), polyamide (PA), and polypropylene (PP). PP comprises 39% of the identified microplastics and is the most dominant among the identified polymer types, followed by EVA (22%), PET (13%), HDPE (11%), LDPE (11%), and PA (4%) (Figure 6).

Figure 2.
Types of microplastics based on shape: (A) film from Nasipit, (B) fragment from Buenavista, and (C) fiber from Cabadbaran.
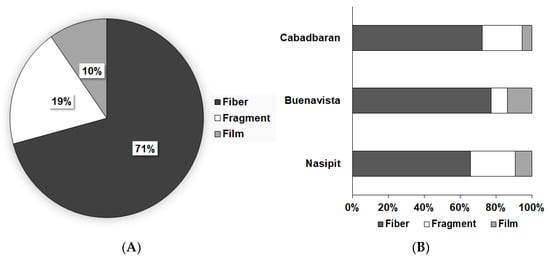
Figure 3.
(A) Mean relative abundance of microplastics based on shape and (B) shape relative abundance in different sampling areas.

Figure 4.
(A) Mean relative abundance of microplastics based on color and (B) color relative abundance in different sampling areas.
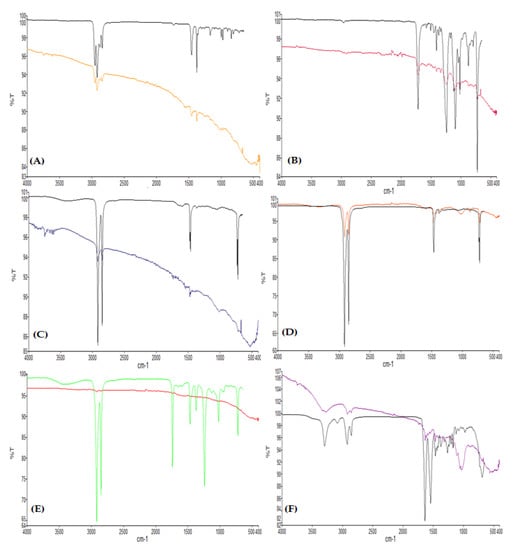
Figure 5.
ATR–FTIR spectra showing (A) polypropylene, (B) polyethylene terephthalate, (C) high-density polyethylene, (D) low-density polyethylene, (E) ethylene-vinyl acetate, and (F) polyamide.
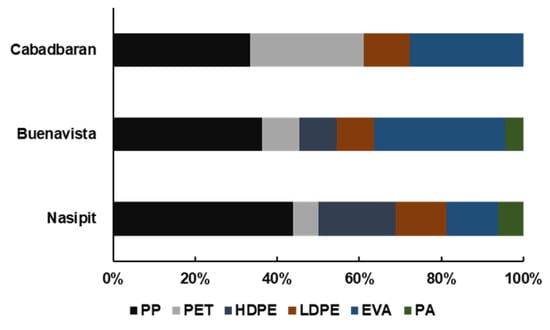
Figure 6.
Composition of microplastics based on polymer type: PP—polypropylene, PET—polyethylene terephthalate, HDPE—high-density polyethylene, LDPE—low-density polyethylene, EVA—ethylene-vinyl acetate, and PA—polyamide.
4. Discussion
Higher microplastic concentrations were observed in sites where elevated amounts of marine debris were seen during the sampling. Of the three sampling areas, Nasipit exhibited the highest mean density value of 71.1/kg (Table 2), which can be linked to the level of marine plastic debris in the area. The mean density of the three study areas in the present study was relatively higher compared with that of Changi in Singapore [39] and significantly lower than a similar study conducted in Fujian, China [40]. This difference in the density of microplastics, as seen among different mangrove ecosystems from different regions of Asia, show evidence of regional variations in environmental conditions and activities, which have an impact on microplastic concentrations.

Table 2.
Comparison of microplastic density in different mangrove areas in Asia.
The amount of plastic waste in the mangrove ecosystem may have originated from the community and the marine plastic litter accumulating in the area from different fishing activities, including passenger and cargo ship operations in the municipal port terminal. Previous studies have reported increasing microplastic levels in coastal sediments and seawater near populated areas [32,41,42,43], implying a close association between land-based human activities and microplastic pollution of marine environments. Meanwhile, mangrove sites in Buenavista (48.9/kg) and Cabadbaran (40.0/kg) had lower microplastic mean density with less local human activities associated with these areas.
The dominance of fibers among the three microplastic shapes was similar to different microplastic studies conducted in Singapore [39], the United Kingdom [45], and Belgium [46], which reported that fibers were the most common type of microplastic particle. The morphology of these microplastics suggests that they are likely to be of secondary origin from deteriorated plastic wastes and different fishing equipment such as plastic rope, and ropes used for netting and longline culture that have been in the marine environment for a very long time [39,47]. According to Catarino et al. [48], Leslie et al. [49], and Renzi et al. [50], fibers were the most dominant using visual identification and spectroscopy identification. In addition, Cho et al. [51] reported that more than 80% of microfibers on filter papers were identified as natural fibers such as cotton and paper. Since many synthetic fibers are colorless, and natural fibers might contain colors in synthetic fibers, visual identification cannot correctly discriminate between synthetic and natural fibers, resulting in the overestimation of microfiber concentration.
Blue was the most dominant microplastic color in this study, which is comparable to the study conducted by Peng et al. [52] in the sediments of Changjiang Estuary, China. Blue makes up 32.9% of the 132 microplastics studies, making it the most prevalent microplastic color [53]. The usage of protective masks as an infection control tool, which was widespread in East and South-East Asia at the beginning of the COVID-19 pandemic and eventually around the world in 2020 and 2021, can be linked to the abundance of blue microplastic in this study [11,54]. Additionally, a study conducted by Chen et al. [55] showed that blue microplastics in the form of fibers and fragments were the most predominant and believed to be coming from disposable face masks. Thus, these colored microplastics are probably quickly taken up by many organisms mistaken as food resources [39,56,57], thus causing starvation to death and the loss of biodiversity in mangrove habitats [58].
All nine transect lines established in the three mangrove areas recorded PP, which covers 39% of the total isolated and recorded microplastic particles. This polymer type is the most predominant in mangrove sediments, which is comparable to a study by Jang et al. [59]; it is considered one of the most common polymer types used in clothing and plastic products, is widely used in modern societies, and is popular in the fishery and marine culture [60,61]. Of the six polymer types, polyamide was the most uncommon, comprising only 4% of the samples.
5. Conclusions and Recommendations
To the best of our knowledge, the current study is the first to document and report the presence of microplastics in the Philippines’ mangrove sediments. The present work demonstrated the dominance of polypropylene, which comprises 39% of the total number of isolated microplastics in the form of fibers, fragments, and films. The dominance of polypropylene in all sites dictates that these particles came from several sources such as clothing, plastic cups, and plastic wrappers, which degrade over time due to wave action, UV exposure, and other environmental factors. Overall, Nasipit (71.1/kg) obtained the highest density, followed by Buenavista (48.9/kg) and Cabadbaran (40.0/kg), thus linking the higher concentration of microplastics to both location and different human influences. Further studies shall be conducted to quantify the presence of microplastics in the mangrove biota of the Bay, mainly in filter feeders such as clams and mussels, which are economically important species. The results of this study can be utilized as baseline data for researchers and authorities to craft policies to address issues concerning human and environmental health.
Author Contributions
C.K.P.N.: conceptualization, investigation, formal analysis, writing—original draft. C.G.L.A.A., K.M.S., S.A.T.I. and M.H.T.B.: sorting and analysis of microplastics. R.Y.C.: formal analysis, supervision, review of the manuscript. A.G.T.: formal analysis, supervision, review of the manuscript. H.P.B.: conceptualization, supervision, investigation, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Department of Science and Technology—Accelerated Science and Technology Human Resource Development Program and DOST-NRCP (National Research Council of the Philippines) with Project No. E-255.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed in this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank the Department of Science and Technology—Accelerated Science and Technology Human Resource Development Program (DOST-ASTHDRP) for the financial assistance in the conduct of this study. The authors would also like to thank the Caraga State University and Mindanao State University—Iligan Institute of Technology for allowing us to conduct laboratory experiments and to Lea G. Navidad for her valuable help during the conduct of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Plastics Europe. Plastic waste: Ecological and Human Health Impacts. 2010. Available online: https://ec.europa.eu/environment/integration/research/newsalert/pdf/IR1_en.pdf (accessed on 15 August 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Meijer, L.; Van Emmerik, T.; Lebreton, L. Over 1000 rivers accountable for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Requiron, J.; Bacosa, R. Macroplastic Transport and Deposition in the Environs of Pulauan River. Philipp. J. Sci. 2022, 151, 1211–1220. [Google Scholar] [CrossRef]
- Gaboy, S.M.; Guihawan, J.Q.; Leopardas, V.M.; Bacosa, H.P. Unravelling macroplastic pollution in seagrass beds of Iligan City, Mindanao, Philippines. Mar. Pollut. Bull. 2022, 185, 114233. [Google Scholar] [CrossRef]
- Sajorne, R.E.; Cayabo, G.D.B.; Madarcos, J.R.V.; Madarcos, K.G.; Omar Jr, D.M.; Ardines, L.B.; Bacosa, H.P. Occurrence of COVID-19 personal protective equipment (PPE) litters along the eastern coast of Palawan Island, Philippines. Mar. Pollut. Bull. 2022, 182, 113934. [Google Scholar] [CrossRef]
- Inocente, S.A.T.; Bacosa, H.P. Assessment of macroplastic pollution on selected tourism beaches of Barobo, Surigao Del Sur, Philippines. J. Mar. Isl. Cult. 2022. Available online: https://jmic.online/ (accessed on 26 October 2022). [CrossRef]
- Acot, F.T.; Sajorne, R.E.; Omar, N.A.K.; Suson, P.D.; Rallos, L.E.E.; Bacosa, H.P. Unraveling macroplastic pollution in rural and urban beaches in Sarangani Bay Protected Seascape, Mindanao, Philippines. J. Mar. Sci. Eng. 2022, 10, 1532. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS ONE 2014, 9, 111913. [Google Scholar] [CrossRef] [PubMed]
- Sajorne, R.E.; Bacosa, H.P.; Cayabo, G.D.B.; Ardines, L.B.; Sumeldan, J.D.; Omar, J.M.; Creencia, L.A. Plastic litter pollution along sandy beaches in Puerto 440 Princesa. Mar. Pollut. Bull. 2021, 169, 112520. [Google Scholar] [CrossRef]
- Akbay, İ.K.; Özdemir, T. Monomer migration and degradation of polycarbonate via UV-C irradiation within aquatic and atmospheric environments. J. Macromol. Sci. 2016, 53, 340–345. [Google Scholar] [CrossRef]
- Adhikari, P.L.; Bam, W.; Campbell, P.L.; Oberhaensli, F.; Metian, M.; Besson, M.; Swarzenski, P.W. Evaluating Microplastic Experimental Design and Exposure Studies in Aquatic Organisms. In Microplastic in the Environment: Pattern and Process; Springer: Berlin/Heidelberg, Germany, 2022; pp. 69–85. [Google Scholar]
- Chin, L.W.; Fung, T.H. Plastic in marine litter. In Plastics and the Environment; Hester, R.E., Harrison, R.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 21–59. [Google Scholar]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Gimenez, B.C.G. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Cordova, M.R.; Riani, E.; Shiomoto, A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 2020, 161, 111763. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [PubMed]
- Purwiyanto, A.I.S.; Suteja, Y.; Ningrum, P.S.; Putri, W.A.E.; Agustriani, F.; Cordova, M.R.; Koropitan, A.F. Concentration and adsorption of Pb and Cu in microplastics: Case study in aquatic environment. Mar. Pollut. Bull. 2020, 158, 111380. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef]
- Cordova, M.R.; Ulumuddin, Y.I.; Purbonegoro, T.; Shiomoto, A. Characterization of microplastics in mangrove sediment of Muara Angke Wildlife Reserve, Indonesia. Mar. Pollut. Bull. 2021, 163, 112012. [Google Scholar] [CrossRef]
- Onda, D.F.L.; Gomez, N.C.F.; Purganan, D.J.E.; Tolentino, M.P.S.; Bitalac, J.M.S.; Calpito, J.V.M.; Viernes, A.C.A. Marine microbes and plastic debris: Research status and opportunities in the Philippines. Philipp. J. Sci. 2020, 149, 71–82. [Google Scholar]
- Cabrera, O.C.; Villanoy, C.L.; David, L.T.; Gordon, A.L. Barrier layer control of entrainment and upwelling in the Bohol Sea, Philippines. Oceanography 2011, 24, 130–141. [Google Scholar] [CrossRef]
- Primavera, J.; Tumanda Jr, M.I. The Agusan Marsh: A situationer with focus on scientific aspects. In Proceedings of the 1st Scientific Conference on the Agusan Marsh, Butuan City, Agusan del Norte, Philippines, 21–23 May 2007; UNESCO Jakarta Office, Philippine Council for Aquatic and Marine Research and Development: Los Baños, Philippines, 2008; pp. 5–14. [Google Scholar]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, 548 China. Env. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Vethamony, P. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2681–2743. [Google Scholar] [CrossRef]
- Abreo, N.A.S.; Blatchley, D.; Superio, M.D. Stranded whale shark (Rhincodon typus) reveals the vulnerability of filter-feeding elasmobranchs to marine litter in the Philippines. Mar. Pollut. Bull. 2019, 141, 79–83. [Google Scholar] [CrossRef]
- Espiritu, E.Q.; Dayrit, S.A.S.; Coronel, A.S.O.; Paz, N.S.C.; Ronquillo, P.I.L.; Castillo, V.C.G.; Enriquez, E.P. Assessment of quantity and quality of microplastics in the sediments, waters, oysters, and selected fish species in key sites along the Bombong estuary and the coastal waters of Ticalan in San Juan, Batangas. Philipp. J. Sci. 2019, 148, 789–801. [Google Scholar]
- Rahim, S.; Widayati, W.; Analuddin, K.; Saleh, F.; Sahar, S. Spatial distribution of marine debris pollution in mangrove-estuaries ecosystem of Kendari Bay. IOP Conf. Ser. Earth Environ. Sci. 2020, 412, 012006. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic pollution in surface water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar]
- Zhou, Q.; Tu, C.; Fu, C.; Li, Y.; Zhang, H.; Xiong, K.; Luo, Y. Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 2020, 703, 134807. [Google Scholar]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and vertical distribution of microplastics in Korean coastal waters. Environ. Sci. Technol. 2018, 52, 12188–12197. [Google Scholar] [CrossRef]
- Naji, A.; Nuri, M.; Amiri, P.; Niyogi, S. Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 2019, 146, 305–311. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Kazmiruk, T.N.; Kazmiruk, V.D.; Bendell, L.I. Abundance and distribution of microplastics within surface sediments of a key shellfish growing region of Canada. PLoS ONE 2018, 13, 0196005. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals. Environ. Int. 2017, 101, 133–142. [Google Scholar]
- Renzi, M.; Guerranti, C.; Blašković, A. Microplastic contents from maricultured and natural mussels. Mar. Pollut. Bull. 2018, 131, 248–251. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Worby, C.J.; Chang, H.H. Face mask use in the general population and optimal resource allocation during the COVID-19 pandemic. Nat. Commun. 2020, 11, 1–9. [Google Scholar]
- Chen, X.; Chen, X.; Liu, Q.; Zhao, Q.; Xiong, X.; Wu, C. Used disposable face masks are significant sources of microplastics to environment. Environ. Pollut. 2021, 285, 117485. [Google Scholar] [CrossRef]
- Setälä, O.; Lehtiniemi, M.; Coppock, R.; Cole, M. Microplastics in marine food webs. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 339–363. [Google Scholar]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017. [Google Scholar]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef]
- Chowdhury, M.J.; Nasrin, S.; Al Faruque, M.A. Significance of agro-textiles and future prospects in Bangladesh. Eur. Sci. J. 2017, 13, 10–19. [Google Scholar] [CrossRef]
- Poeta, G.; Fanelli, G.; Pietrelli, L.; Acosta, A.T.; Battisti, C. Plastisphere in action: Evidence for an interaction between expanded polystyrene and dunal plants. Environ. Sci. Pollut. Res. 2017, 24, 11856–11859. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).