Abstract
Treatment of acid mine drainage (AMD) was successfully demonstrated using tobacco waste (dust and stem) as a metal cation adsorbent, pH modifier and carbon source for sulphate-reducing bacteria (SRB). Synthetic and industrial AMD wastewaters were used in batch adsorption and SRB facilitated bioremediation experiments. Up to absorbent loading of 80 g/L, metal removal increased. However, increases above 160 g/L did not offer a proportional increase. At an adsorbent loading of 80 g/L, the highest metal removals of 38, 41, 31 and 43% for iron, nickel, copper and zinc respectively were achieved. The iron data fitted well to the Langmuir adsorption isotherm while the Sips adsorption isotherm better-described nickel, copper and zinc adsorption to tobacco waste. SRBs used were able to use tobacco waste as a carbon source while reducing sulphates to metal sulphides in acid mine drainage. In the presence of SRBs, metal removals by both adsorption and sulphide precipitation were 95, 97, 70 and 93% for iron, nickel, copper and zinc, respectively. Copper, however, demonstrated lower removal yields in both adsorption and bioremediation. Bioremediation improved acid mine drainage pH by 2.05 units. The exponential decay function could model both the metal and sulphate removal perfectly. It was concluded that tobacco waste can be confidently used as an adsorbent and carbon source for sulphate-reducing bacteria while facilitating AMD biological treatment.
1. Introduction
Acid mine drainage (AMD) forms when sulphide-bearing material is oxidized in aqueous environments generating low-pH effluent rich in sulphates and metal ions [1]. The low pH and presence of toxic heavy metals in AMD effluent threaten the survival of flora, fauna and human beings. Treatment of AMD is therefore pertinent and a legal mandate in other jurisdictions. Different approaches grouped as either biotic (biological) or abiotic [2] can be used to treat AMD. These approaches can be further classified into active and passive systems [3]. Active systems are common in high-volume applications and at point-of-source AMD treatment facilities, especially for operating mines. Active systems are more costly, and energy and chemical-intensive compared to passive ones [4]. Passive treatment systems provide a controlled environment in which natural and biological reactions that help in the treatment of AMD can occur. Established commercial passive treatment systems are normally used in old dump sites where they employ sulphate-reducing bacteria (SRB) or limestone or both to facilitate neutralization and precipitation of metals in AMD. An example of an SRB is Desulphotomaculum nigrificans (D. nigrificans) [5] which under a conducive environment reduces sulphates to sulphides or elemental sulphur. A suitable organic carbon source for energy and growth must be provided to the SRBs for them to achieve the bioremediation goal [6]. Because of being environmentally friendly and cheaper than abiotic systems [7], there is increasing research focus and application of passive biotic systems. A lot of studies aimed at identifying suitable, readily available, underutilised or cheaper organic carbon sources for the SRBs have been conducted [8,9,10,11,12,13].
The last grouping of studies all showed that involving biomass wastes is a potential way of including organic substances as sources of carbon for microbial remediation of AMD. It has also been proven that in general organic materials have good adsorption capacities for heavy metals [6] and rice husks have been used with heavy metal concentrations ranging from 20–60 mg/L in wastewater [14]. Waste biomass would be the preferred carbon source as it means two waste streams (AMD and waste biomass) are both being eliminated in a single process. Recently, South Africa legislated against landfilling of high calorific value (>20 MJ/kg) organic materials [15], so diverting such wastes towards AMD treatment could be a viable option. Despite the numerous studies mentioned above regarding the use of waste biomass in AMD treatment and the challenges associated with biomass disposal, no AMD treatment studies have been conducted using tobacco waste. Tobacco waste has not attracted a lot of research attention [16] possibly because researchers get overshadowed by the economic benefits that this crop brings to those cultivating it. Tobacco processing waste products (stems and dust), like any other biomass, contain fibres, sugars, lignin, cellulose and proteins [17]. This implies that this waste could potentially be used in AMD treatment either as an adsorbent or as a carbon source for SRBs. The industrial processing of tobacco leaves generates significant waste (95% is tobacco dust and 5% is tobacco stems) which has no commercial value in factories [18]. Landfilled or dumped tobacco wastes leach out toxins such as nicotine and when it rains, these toxins are washed into public water bodies presenting health problems to humans and animals [19].
Having highlighted the environmental challenges associated with tobacco waste, it is prudent to find alternative and environmentally sustainable ways of disposing of this waste. Tobacco waste usage in AMD treatment is an option being investigated in this study. There is no publicly available knowledge regarding the use of tobacco waste as a carbon source in the AMD passive biotic treatment approach. Tobacco waste has higher alkalinity (pH of 5.1–6.5) [18] compared to that of AMD (pH of 2–3). When these two are mixed, the combined higher pH should potentially increase the reaction rate of converting ferrous iron to ferric iron as this rate is several orders of magnitude faster at near-neutral pH. The tobacco waste could also address the energy needs of the SRBs.
2. Materials and Methods
2.1. Materials and Chemicals
The chemicals for this study were sourced from Sigma Aldrich, South Africa and were of analytical grade. The organic substrate (tobacco dust, 95% and stems, 5%) was collected from Limbe Leaf Tobacco Company in Malawi coordinates 15°48′46.8″ S 35°04′08.8″ E. The flue-cured tobacco waste samples were collected ‘as is’ from the factory, while the factory was running, and used within 3 days from the date of collection. Synthetic acid mine wastewater was prepared according to the literature [13] with some modifications on the amounts of salt dosages used. In this study lower sulphate (3000 mg/L versus 6000 mg/L) and iron (500 mg/L versus 2000 mg/L) levels were used to mimic average concentrations in typical real South African AMD. The synthetic AMD solution was prepared by dissolving 12.4 g of analytical grade ferrous sulphate salt (FeSO4·7H2O) in 5000 mL of distilled water. Sulphuric acid (10.9 g of 99% purity) was added to this solution and caustic soda was added to adjust the pH to 3. Industrial AMD wastewater was collected from a South African old mine dump located in Dobsonville, Johannesburg coordinates 26°12′38.5″ S 27°46′47.0″ E. SRBs that had been previously isolated from mine dumps were supplied by the University of Witwatersrand, Johannesburg, School of Chemical and Metallurgical Engineering laboratory. The SRB culture (5 mL) was added to 100 mL growth media and then allowed to grow under anaerobic conditions at 30 °C in a shaker incubator for 14 days before its use in the bioremediation trials with tobacco waste as substrate [6].
2.2. Methods
The procedures outlined by Zhang et al. [6] were adopted in characterising the tobacco waste for moisture, organic matter content, bound nitrogen (Nb), pH, chemical oxygen demand (COD) and dissolved organic carbon (DOC). Easily available substances (EAS) were taken from the literature [17]. Metal ions in slurries were quantified using standard Atomic Absorption Spectroscopic methods (Perkin Elmer Atomic Absorption Spectrometer PinAAcle 900H) [20]. The detection limit for the various metals was in the range of 0.01 to 0.06 mg·L−1 at a 98% confidence level. Dissolved COD measurements in effluents, were carried out using the Merck COD kit and the Hach Spectrophotometer following the manufacturers’ instructions. The methods of Sheridan and Rimla [13] were used to measure sulphates in the reacting mixtures. A handheld pH meter (FiveGoa F2) was used for pH measurements.
Three sets of experiments were each performed in triplicate. The adsorbent or carbon source (tobacco waste) was added to AMD and the mixture was placed in a shaking incubator (100 rpm). In the first experiment metal ion adsorption on tobacco waste and the effect of adsorbent: AMD ratio was investigated. Four reactors containing different amounts (2-, 4-, 8- and 16 g, respectively) of tobacco waste and equal amounts (100 mL) of the synthetic AMD were incubated for 15 h at 30 °C temperature. In the fifth reactor, tobacco waste was excluded (control reactor). Metal ion concentration in the mixture was measured at 2-h intervals throughout the incubation period. The second experiment investigated tobacco waste as a carbon source for SRB during AMD bioremediation. Reactors had 8 g of carbon source (tobacco waste) and 100 mL AMD. To each reactor 5 mL of SRB inoculum previously grown on the modified Postgate media without lactate, yeast extract and sodium citrate, (initial pH 5.0–5.5) were added. The control reactor contained AMD and inoculum only but no tobacco waste. All the reactors were incubated as in the previous experiment. To assess remediation efficiency over the 50-day incubation period, sulphate reduction levels, metal ion concentration, COD, and pH changes were measured at 4-day intervals. Observations were also made around the formation of a black precipitate (FeS) and the detection of H2S odour. Experiment 3 was a duplication of experiment 2 with the substitution of synthetic AMD with industrial AMD.
2.3. Results Analysis
MS-Excel software was used in calculating mean values from triplicate experimental results, reported with standard deviations (SD). The common adsorption isotherms for describing heterogeneous systems involving metals are the Langmuir, Freundlich and Sips. These were fitted to the adsorption data using the non-linear regression modelling solver tool of MS Excel. Kinetic modelling, putting into consideration the common kinetic models of pseudo-first-order and pseudo-second-order [21] was also evaluated. Equations used in modelling are depicted in Table S1. For kinetics, only, the results at an adsorbent dosage of 80 g/L were used since this dosage had proven to be optimal during the adsorption studies.
For Langmuir adsorption, the separation factor (RL), which is defined by Equation (1), indicates whether adsorption is favourable or not [22].
The process is irreversible where RL = 0; favourable where 0 < RL < 1; unfavourable where RL > 1 and becomes linear where RL = 1.
For Freundlich isotherm, the adsorption is favourable when n < 1, unfavourable when n > 1 and becomes linear when n = 1.
Metal cation and sulphate concentration in AMD during bioremediation were tested for adherence to exponential decay models as had been previously established by other researchers in separate studies [23,24].
3. Results and Discussion
3.1. Characterisation of AMD
The synthetic AMD assayed 500 mg/L for iron (Fe3+), and 3000 mg/L for sulphate (SO42−) with a pH of 3. The industrial AMD had more (Fe3+) at 420.23 mg/L compared to other cations that were 20.32 mg/L (Ni2+), 38.21 mg/L (Cu2+) and 5.73 mg/L (Zn2+). For the SO42− concentration and pH, industrial AMD was at 3318.23 mg/L and 2.7 respectively. These results were in close agreement with those reported from other AMD samples collected from this region of South Africa by other researchers [25]. However, it is important to note that AMD from different sources may differ significantly [8,26] in terms of composition.
3.2. Characterisation of Tobacco Wastes
Table 1 summarizes the key characteristics that make tobacco waste attractive for SRB-mediated AMD bioremediation. The high organic matter content (77%) may imply that enough carbon for microorganisms and adsorption will be available. The C/N ratio of 35 in tobacco waste has been established as optimal for the growth and catalytic activity of most microbial species [27].

Table 1.
Composition of tobacco waste used in this study.
However, the possibility of some of these organics being locked up in lignin is high given the crude fibre content (7%) whose main component could be lignocellulosic elements.
3.3. Tobacco Waste Metal Cation Adsorption Capabilities
3.3.1. Adsorbent Loading Rate Effects
Metal cations removal improved with adsorbent loading between 20–160 g/L. The 80 g/L adsorbent loading was close to the optimal loading rates because at this point the percentage of metal removals started to plateau as shown in Figure 1. The same trends of an initial increase in metal removals with an increase in adsorbent loading were also observed by Hegazi in their AMD treatment experiments using rice husks and fly ash [14]. The plateau may be attributed to reduced adsorbent-cation contact in thicker slurries caused by poor mixing. This plateauing phenomenon implies that to operationalise tobacco waste used as a metal adsorbent in AMD, an optimal dosage rate that balances out the cost of procuring the tobacco waste and process efficiency must be sought out.
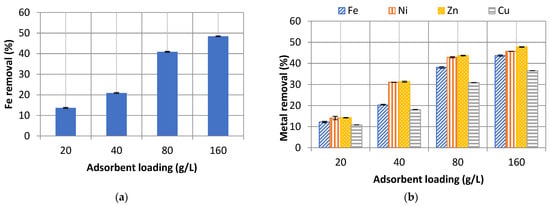
Figure 1.
Overall metal ion removals in AMD during adsorption. (a) Synthetic. (b) Industrial. The vertical error bars are the SD of triplicate sample values.
Experiments with industrial AMD demonstrated a reduction in overall efficiencies for iron cation at each loading rate when compared to synthetic AMD runs. Possibly this observation may be attributed to competition for active sites with other ions. Overall metal removal efficiencies reflect that nickel adsorbs strongly and more competitively than iron onto tobacco wastes which are relatively higher for iron at all adsorbent loading rates. However, copper exhibits poorer adsorption characteristics than zinc and iron which portray near-similar adsorption patterns.
3.3.2. Adsorption Isotherms Modelling
Using the formulae in Table 1 and least squares regression methods, the adsorption isotherms model fitting parameters for the different setups were determined and reported in Table 2. The adsorption model fitting was better at a higher initial iron concentration in AMD than if the initial iron concentration was lower. This could be due to the purity of the solutions. At a higher initial concentration, the iron solution was purer and maybe could not suffer from interaction effects from other metals as exhibited in the industrial sample which has a lower initial iron concentration. However, in both cases of higher and lower initial iron concentration, all three isotherm models studied, based on the R2 values from Table 2, were equally suitable in modelling the dynamics of iron adsorption to tobacco waste.

Table 2.
Adsorption isotherms model fitting parameters.
In all the other metals found in the industrial AMD and the study conditions, the Sips isotherm fitted the data much better than the other two isotherms. The general goodness of fit was in the order Sips > Langmuir > Freundlich for copper, nickel and zinc ions. The Sips and Langmuir model’s strength compared to the Freundlich in describing AMD metals adsorption to lignocellulosic substrates was also observed in other independent studies [5,28,29]. The Langmuir has also proved to supersede the Freundlich even in studies involving non-metals and inorganics adsorption [30]. The performance demonstrated by the Sips, in better describing the adsorption of many metals, compared to Langmuir and Freundlich isotherms was expected since the Sips model was developed to address the adsorbate concentration-related weaknesses of both Freundlich and Langmuir models [31]. The isotherms that best fitted the experimental adsorption data for the various systems studied are plotted in Figure S1.
3.3.3. Adsorption Kinetics Modelling
Adsorption was rapid with 50% of the overall metal removals being recorded in the first 2 h of the total 15-h incubation period. This helps in quickly reducing metal toxicity to the bioremediation microorganisms when adsorption is coupled with SRBs. Fitting the adsorption experimental data to pseudo-first-order and pseudo-second-order models which are the most popular kinetic models for adsorption revealed better iron adsorption represented by the pseudo-first-order model than the second-order model. The rest of the metals were better described by second-order kinetics than by the first-order model. The superiority of the second-order model in describing metal-adsorption systems has also been reported elsewhere [32,33]. The reason why iron was an exception to this trend could not be immediately established. The adsorption kinetics parameters for this study are reported in Table 3 while the plots are displayed in Figure S2.

Table 3.
Kinetic modelling parameters for AMD metal adsorption onto tobacco waste.
3.4. Tobacco Waste in SRB-Mediated AMD Bioremediation
The SRB grew on tobacco waste as an energy source and these microorganisms facilitated sulphide precipitation by reducing sulphates in AMD. This precipitation had a triple effect of metal cation removal, sulphates removal and AMD pH improvement from being too acidic to near neutral. Additional metal precipitations occurred as hydroxides and carbonates were triggered mainly by the pH changes. The tobacco waste also acted as a support matrix for the SRB microorganisms.
3.4.1. Metal Removal Efficiencies
Metal removal efficiencies were more than 70% when bacterial intervention was coupled to the adsorption runs. However, since microbial processes are generally slow the overall retention time of bioremediation had to be extended to 50 days. In the presence of SRBs, the nickel removal matched that of iron and zinc, though from adsorption studies it had earlier been demonstrated that much of the iron and zinc is depleted through adsorption as compared to nickel. This might imply that microorganisms use relatively more nickel than other metals in their metabolic activities. With copper, the poor removals previously exhibited in adsorption trials persisted even in the presence of SRBs. The reasons for poor copper adsorption performance were not immediately obvious, but the poor removals by SRBs could be attributed to the toxicity effects which has been associated with copper on many anaerobes and have been observed in other separate studies [34,35,36]. Metal ion and sulphate ion concentrations during remediation were fitted well to the exponential decay function [29] with the constants for the various metals (Table S2) and the plots for experimental and curve fittings shown in Figure 2.
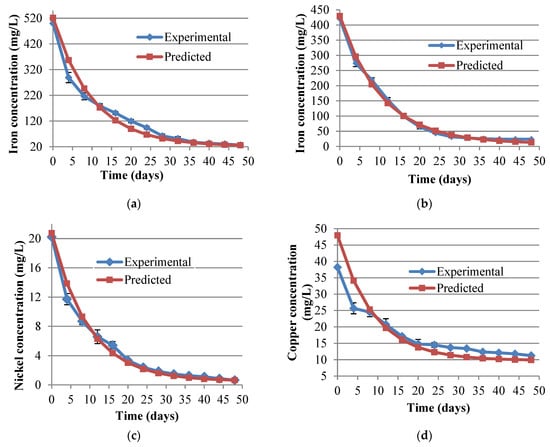
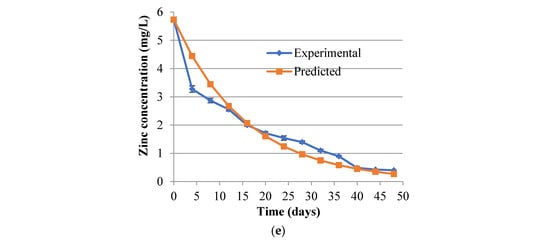
Figure 2.
(a–e) Metal concentration in aqueous media during bioremediation at 80 g/L of tobacco waste loading. The vertical error bars are the SD of triplicate sample values. (a) is for Fe with an initial concentration of 500 mg/L while (b) is for an initial concentration of 420 mg/L.
3.4.2. Sulphate Reduction in Biological AMD Treatment
Significant sulphate ions were removed during remediation. The removal was sluggish at the beginning picking up at around day 10–15 after which it increased rapidly before plateauing on day 20 onwards. Overall sulphate removal yields were 25 g SO42−/g·tobacco waste for the synthetic AMD and 29 g SO42−/g·tobacco waste for the industrial AMD. These are very high yields when compared to those obtained using grass cutting as an energy source by Greben et al. [37] though these researchers used a different AMD to that used in the current study in terms of composition. Possibly tobacco waste outperformed grass cuttings may because of the improved surface areas in the finer dust of tobacco as compared to the coarser grass cuttings in the other research. Another contributing factor to this difference could be the higher content of easily available substrates (EAS) of tobacco which is opposed to the highly lignocellulosic [10,38] grass cuttings. Sulphate levels in AMD were fitted to exponential decay models as depicted in Figure 3.
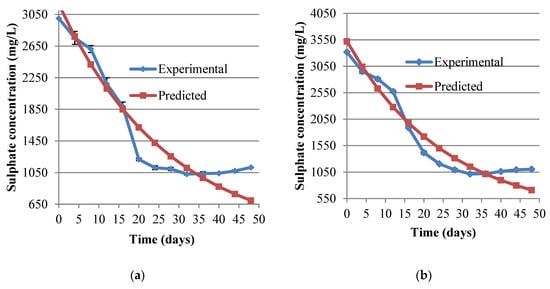
Figure 3.
Sulphate concentration during biotreatment of (a) synthetic AMD and (b) industrial AMD. The vertical error bars are the SD of triplicate sample values.
3.4.3. pH Adjustment
Removal of sulphates in solution and precipitation of metal sulphides had a net effect of increasing the treated AMD pH. Overall, the pH of synthetic AMD improved by 2.05 units from 3.18 to 5.23, while that of industrial AMD shifted by 2.41 units, from an initial value of 2.75 to 5.16 over the incubation period (Figure S3). The implication of this pH change on the absorption was not investigated and should be added to future works.
3.4.4. Process Stability and Other Observations
The ratios of COD to sulphate ion concentration during AMD remediation is used to infer process stability. When this ratio fluctuates between 0.55 and 0.67 [37] the process is said to be stable. Ratios higher than 0.67 reflecting excess COD may point to potential instability arising from microbial inhibition by VFAs. Conversely COD/sulphate ratios lower than 0.67, signal potential instability introduced by microbial activity inhibition induced by excess sulphate concentration. In this study, the process was fairly stable from beginning to end with the ratio ranging from 0.56 to 0.93. This reflects that the degradable components from the tobacco waste are released gradually from the hydrolysis of hemicellulose by the AMD effluent acidity. Physical changes like yellowish precipitations and a ‘rotten egg’ smell of sulphur were also monitored to deduce SRBs’ action on AMD.
4. Conclusions
Tobacco waste is a good adsorbent for metal cations found in AMD. Metal removal in batch adsorption tests ranged between 12–48% for the different metals depending on the tobacco waste loading rate. The adsorption of metals could be modelled by Langmuir isotherms for iron while the other metal cations fitted better to the Sips isotherm. The first-order kinetics could also describe the iron adsorption well and the rest of the metals followed second-order kinetics.
Tobacco waste supported the growth of SRBs which are also helpful in the biological treatment of AMD. The tobacco waste-SRB combination maintained a conducive COD/Sulphate ratio for SRBs throughout the experimental period. AMD treatment was proved by the reduction in sulphate levels, and detection of sulphur smell in reactors. Metal cations reduction and the precipitation of sulphides in reactors also gave evidence of remediation. The exponential decay curve fitting models are capable of predicting the metal cation and sulphate concentrations in AMD during the SRB-tobacco system-mediated bioremediation.
Intensive evaluation of tobacco waste in this application must be performed through column tests that will allow potential microbial toxicity and sustained process stability to be ascertained. It may also be necessary to consider co-substrates [39] that may improve the tobacco-SRB systems’ metal and sulphate yields. Cost–benefit studies aimed at establishing optimal adsorbent loading rates under passive conditions of minimal or no mixing must be conducted as well. It may also be necessary to experiment with the coupling of different systems. This may potentially address the recalcitrance of components such as copper and further improve the removal of other metal cations and sulphates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su142114333/s1, Figure S1: Adsorption isotherms for (a) Fe, (b) Ni, (c) Cu, and (d) Zn on 80 g/L tobacco waste; Figure S2: Adsorption kinetics for (a) Fe (5) and (b) Fe (4), (c) Ni, (d) Cu, (e) Zn on tobacco waste; Figure S3: Variation of pH during AMD bioremediation; Table S1: Equations used in analysing adsorption data; Table S2: Exponential decay parameters for modelling metal ion concentration during bioremediation.
Author Contributions
Conceptualization, K.H.; Data curation, H.D.; Formal analysis, H.D.; Funding acquisition, K.H.; Investigation, H.D.; Project administration, K.H.; Resources, K.H.; Validation, K.H.; Writing—original draft, H.D.; Writing—review & editing, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; Mcdonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, E.; Subramanian, S. Studies on removal of metal ions and sulphate reduction using rice husk and Desulfotomaculum nigrificans with reference to remediation of acid mine drainage. Chemosphere 2006, 62, 699–708. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Organic wastes as carbon sources to promote sulfate reducing bacterial activity for biological remediation of acid mine drainage. Miner. Eng. 2014, 69, 81–90. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage_ Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Burman, N.W.; Harding, K.G.; Sheridan, C.M. Use of Acidic Mine Drainage for the Pre-treatment of Lignocellulosic Biomass. In Proceedings of the International Water Association World Water Congress, Tokyo, Japan, 16–21 September 2018. [Google Scholar]
- Burman, N.W.; Harding, K.G.; Sheridan, C.M.; Van Dyk, L. Evaluation of a combined lignocellulosic/waste water bio-refinery for the simultaneous production of valuable biochemical products and the remediation of acid mine drainage. Biofuels Bioprod. Biorefining 2018, 12, 649–664. [Google Scholar] [CrossRef]
- Burman, N.W.; Sheridan, C.M.; Harding, K.G. Lignocellulosic bioethanol production from grasses pre-treated with acid mine drainage: Modeling and comparison of SHF and SSF. Bioresour. Technol. Rep. 2019, 7, 100299. [Google Scholar] [CrossRef]
- Burman, N.W.; Sheridan, C.M.; Harding, K.G. Feasibility assessment of the production of bioethanol from lignocellulosic biomass pretreated with acid mine drainage (AMD). Renew. Energy 2020, 157, 1148–1155. [Google Scholar] [CrossRef]
- Westensee, D.K.; Rumbold, K.; Harding, K.G.; Sheridan, C.M.; van Dyk, L.D.L.D.; Simate, G.; Postma, F. The availability of second generation feedstocks for the treatment of acid mine drainage and to improve South Africa’s bio-based economy. Sci. Total Environ. 2018, 637–638, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ramla, B.; Sheridan, C. The potential utilisation of indigenous South African grasses for acid mine drainage remediation. Water SA 2015, 41, 247–252. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. Hous. Build. Natl. Res. Cent. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Molewa, E. National Norms and Standards for Disposal of Waste to Landfill; South African Government: Cape Town, South Africa, 2013.
- Novotny, T.E.; Bialous, S.A.; Burt, L.; Curtis, C.; Costa, V.L.D.; Iqtda, S.U.; Liu, Y.; Espaignet, E.T. The environmental and health impacts of tobacco agriculture, cigarette manufacture and consumption. Bull. World Health Organ. 2015, 93, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell, J. Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types. In Tobacco: Production, Chemistry and Technology; Nielson, M.T., Layten Davis, D., Eds.; Blackwell Science: Hoboken, NJ, USA, 1999; pp. 266–284. [Google Scholar]
- Tedesco, M.J.; Bortolon, L.; Henrique, I.C.; Clesio, G.; Marcio Henrique, L. Land disposal potential of tobacco processing residues. Ciência Rural 2011, 41, 236–241. [Google Scholar] [CrossRef]
- Cosic, I.; Marija, V.; Nina, K.; Dajana, K.; Felicita, B. Environmental Management. In Proceedings of the 3rd International Symposium on Environmental Management: Towards Sustainable Technologies, Zagreb, Croatia, 26–28 October 2011. [Google Scholar]
- Jawad, M.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of fl oating wetlands for the treatment of polluted water of river Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar]
- Simonin, J. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Jorgensen, B.B.; Weber, A.; Zopfi, J. SRBs in sea beds. Deap-Sea Res. 2001, 48, 2097–2120. [Google Scholar]
- Westrich, J.T.; Berner, R.A. The role of sedimentary organic matter in bacterial sulfate reduction: The G model tested. Limnol. Oceanogr. 1984, 29, 236–249. [Google Scholar] [CrossRef]
- Agboola, O.; Mokrani, T.; Sadiku, E.R.; Kolesnikov, A.; Olukunle, O.I.; Maree, J.P. Characterization of Two Nanofiltration Membranes for the Separation of Ions from Acid Mine Water. Mine Water Environ. 2017, 36, 401–408. [Google Scholar] [CrossRef]
- Alegbe, M.J.; Ayanda, O.S.; Ndungu, P.; Nechaev, A.; Fatoba, O.O.; Petrik, L.F. Physicochemical characteristics of acid mine drainage, simultaneous remediation and use as feedstock for value added products. J. Environ. Chem. Eng. 2019, 7, 103097. [Google Scholar] [CrossRef]
- Sitorus, B.; Panjaitan, S.D. Biogas recovery from anaerobic digestion process of mixed fruit-vegetable wastes. Energy Procedia 2013, 32, 176–182. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, D.; Gaur, J.P. Removal of Cu(II) and Pb(II) by Pithophora oedogonia:Sorption, desorption and repeated use of the biomass. J. Harzadous Mater. 2008, 152, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ozacar, M. Equilibrium and kinetic modelling of adsorption of Phosphorous on calcined alunite. Adsorption 2003, 9, 125–132. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Lin, C.; Chen, C. Effect of heavy metals on the methanogenic UASB granule. Water Res. 1999, 33, 409–416. [Google Scholar] [CrossRef]
- Yenigün, O.; Kizilgün, F.; Yilmazer, G. Inhibition Effects of Zinc and Copper on Volatile Fatty Acid Production during Anaerobic Digestion. Environ. Technol. 1996, 17, 1269–1274. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Inhibition on Acidogenesis of Dairy Wastewater by Zinc and Copper. Environ. Technol. 2001, 22, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Greben, H.A.; Tjatji, M.; Maree, J. Biological sulphate reduction COD/SO4 ratios using propionate and acetate as the energy source for the biological sulphate removal in Acid Mine Drainage. In Proceedings of the IMWA Conference, Newcastle Upon Tyne, UK, 20–25 September 2004; pp. 1–12. [Google Scholar]
- Greenhalf, C.E.; Nowakowski, D.J.; Bridgwater, A.V.; Titiloye, J.; Yates, N.; Riche, A.; Shield, I. Thermochemical characterisation of straws and high yielding perennial grasses. Ind. Crops Prod. 2012, 36, 449–459. [Google Scholar] [CrossRef]
- Zagury, G.J.; Neculita, C.; Bussiere, B. Passive treatment of acid mine drainage in bioreactors: Short review, applications, and research needs. In Proceedings of the 60th Canadian Geotechnical Conference and 8th Joint CGS/IAH-CNC Specialty Groundwater Conference, Ottawa, ON, Canada, 21–24 October 2007; pp. 1439–1446. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).