The Assessment of the Risk Ranking and Mobility Potential Associated with Environmental Resistomes in Wastewater Using Metagenomic Assembly
Abstract
1. Introduction
2. Materials and Methods
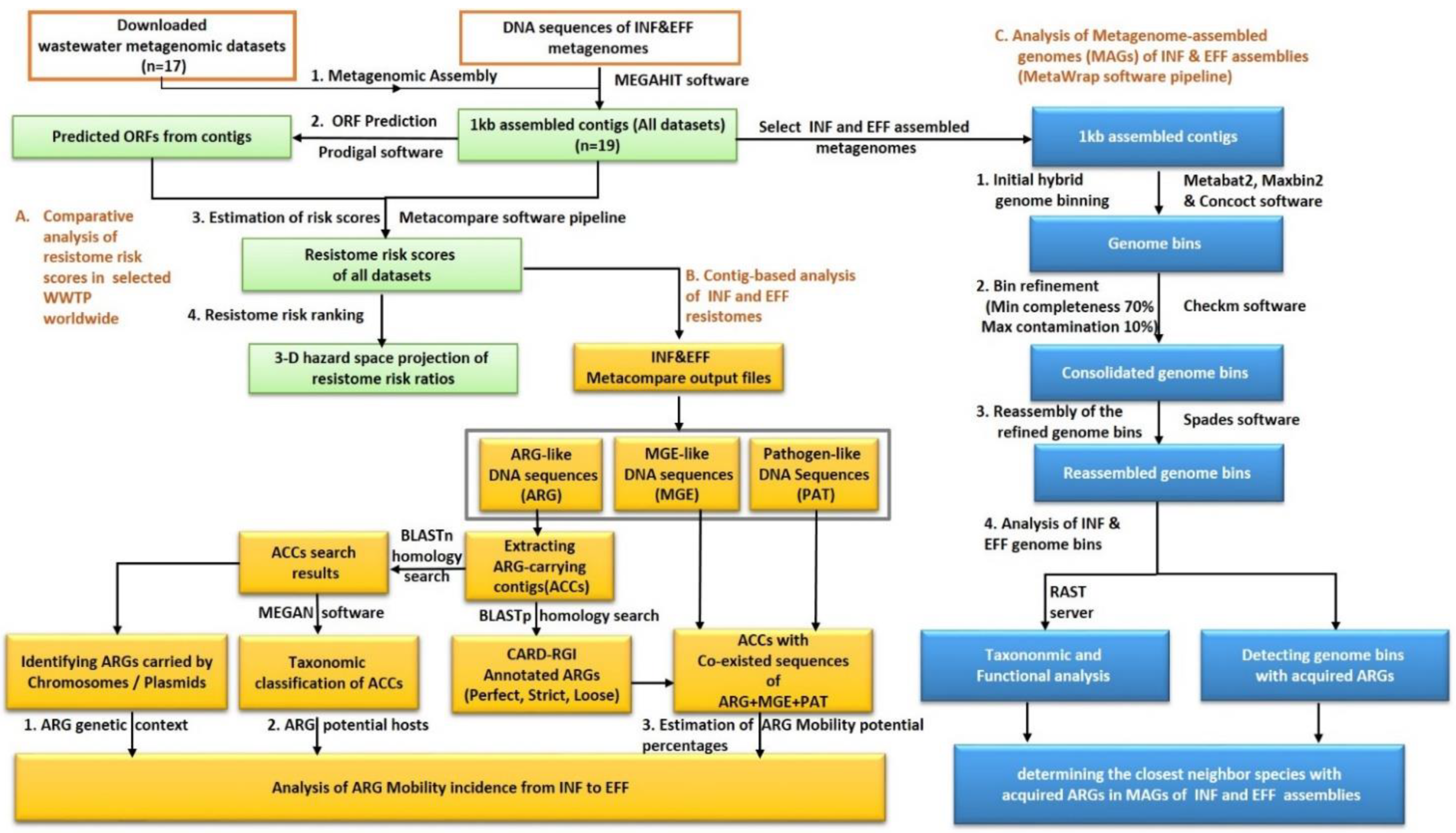
2.1. Metagenomic Assembly and ORF Prediction
2.2. The Comparative Analysis of the Resistome Risk Scores in the Wastewater Samples
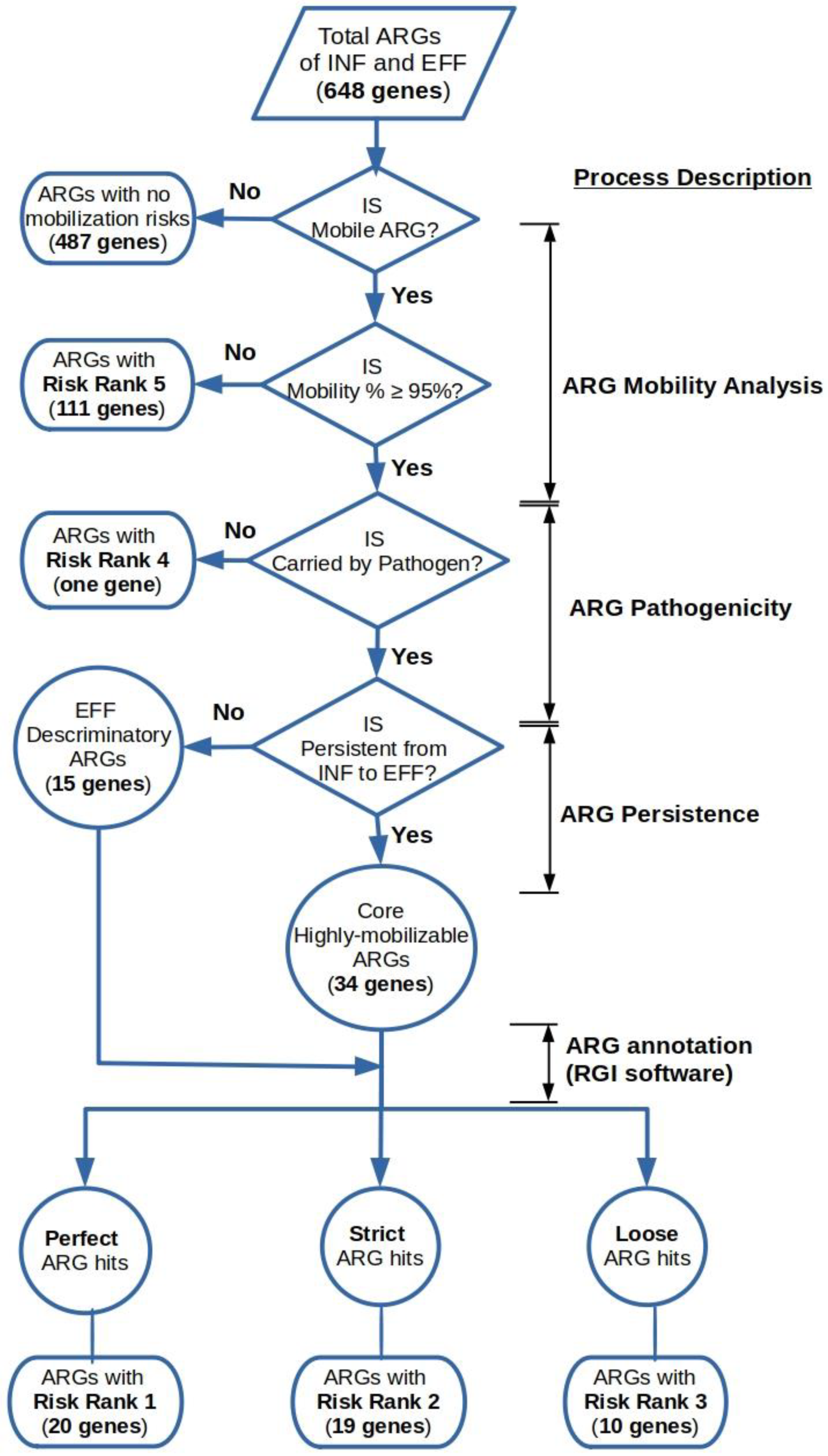
2.3. Determination of the Risk Priority of Mobile ARGs in the Wastewater Influent and Effluent
2.4. Genome Binning of the Influent and Effluent Assembled Metagenomes
3. Results
3.1. Metagenomic Assembly of the Wastewater Metagenomic Datasets
3.2. Estimation of the Comprehensive Risk Scores for the Wastewater Resistomes
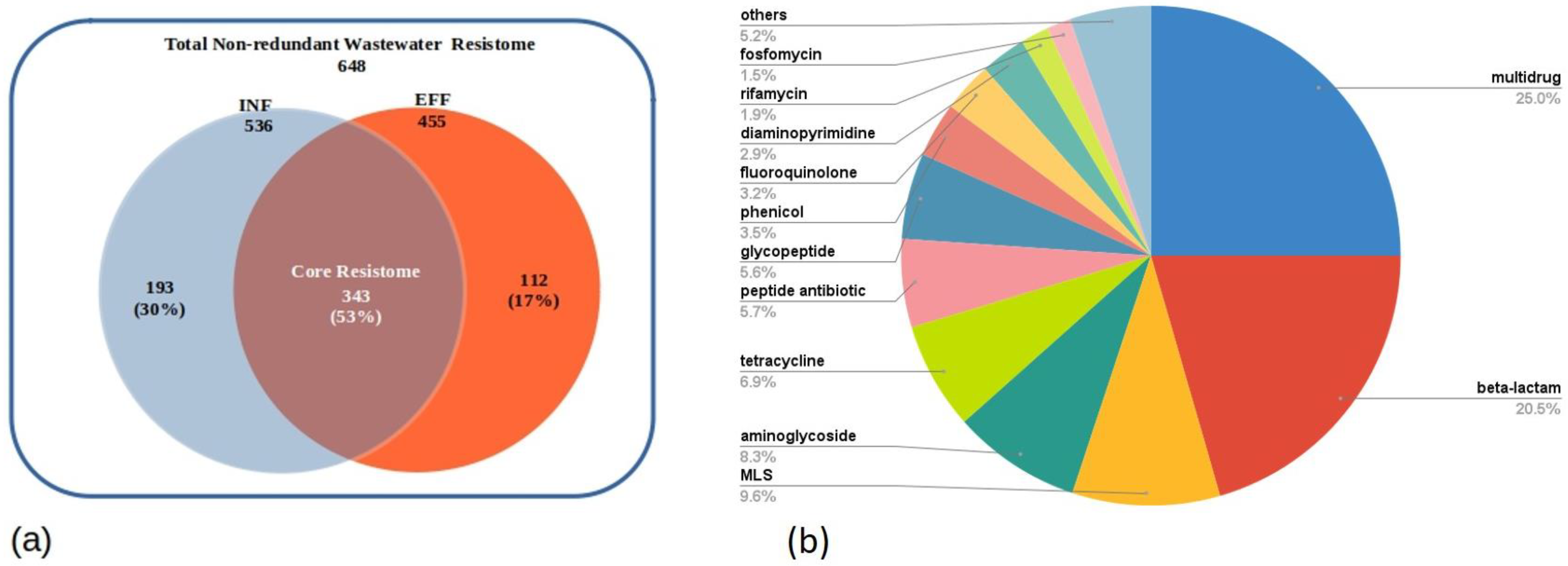
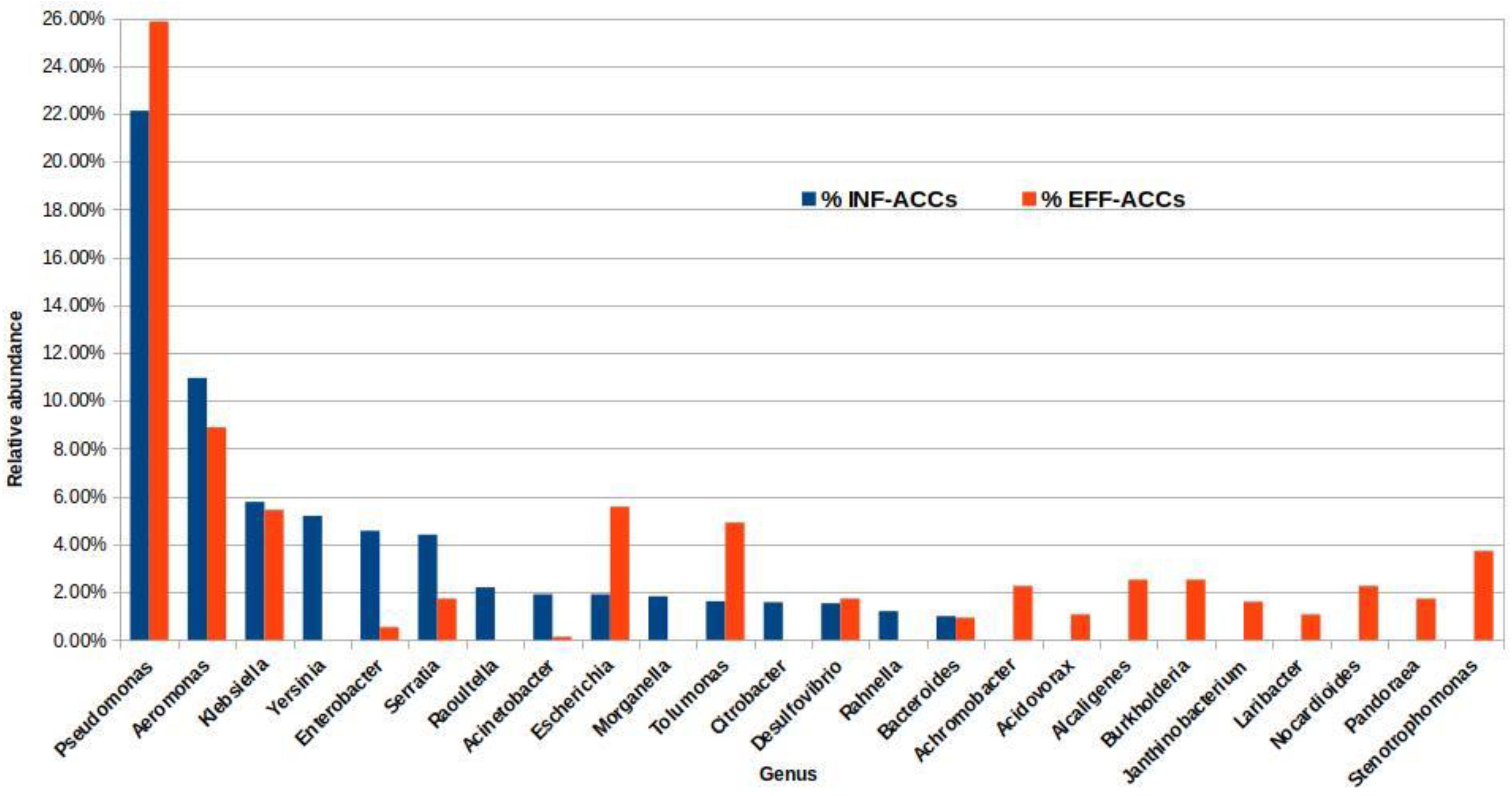
3.3. Resistome Composition of the Wastewater Influent and Effluent of the Assembled Metagenomes
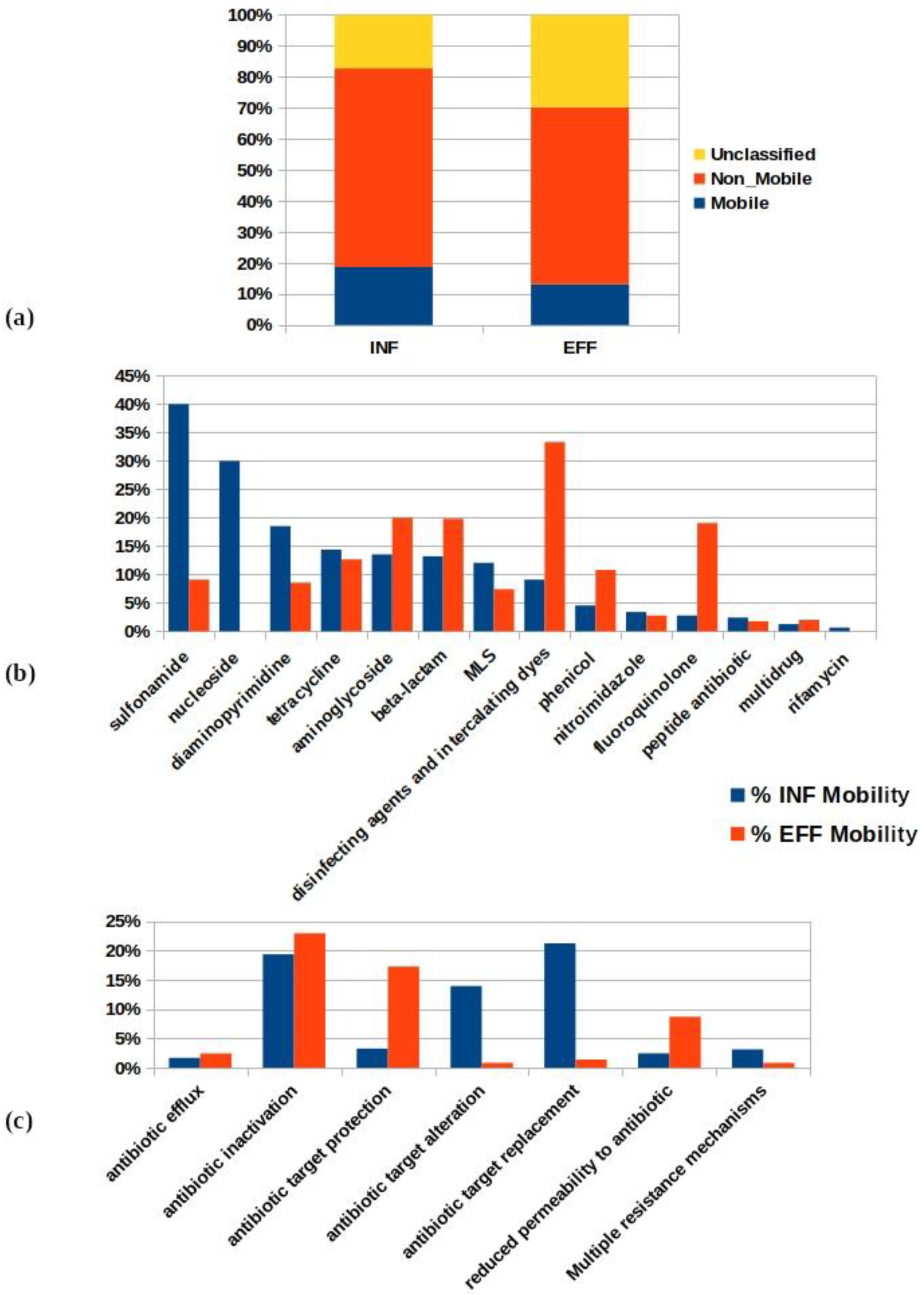
3.4. Evaluation of the Mobility Incidence Percentage of the Antibiotic Resistomes in the WWTP
3.5. Risk Priority Classification of the Wastewater Mobile Resistome
3.6. Genome-Based Analysis of the INF and EFF Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; Volume 10. [Google Scholar]
- Hofer, U. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Rev. Microbiol. 2019, 17, 1124. [Google Scholar]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; El Ghany, M.A. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef]
- Metcalf, W.W.; Griffin, B.M.; Cicchillo, R.M.; Gao, J.; Janga, S.C.; Cooke, H.A.; Circello, B.T.; Evans, B.S.; Martens-Habbena, W.; Stahl, D.A.; et al. Synthesis of Methylphosphonic Acid by Marine Microbes: A Source for Methane in the Aerobic Ocean. Science 2012, 337, 1104–1107. [Google Scholar] [CrossRef]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 2017, 8, 15784. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Genet. 2021, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.; Chowdhury, P.R.; Djordjevic, S.P. Genomic analysis of multidrug-resistant Escherichia coli ST58 causing urosepsis. Int. J. Antimicrob. Agents 2018, 52, 430–435. [Google Scholar] [CrossRef]
- de Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2006, 19, 153–160. [Google Scholar] [CrossRef]
- Łuczkiewicz, A.; Jankowska, K.; Fudala-Książek, S.; Olańczuk-Neyman, K. Antimicrobial resistance of fecal indicators in municipal wastewater treatment plant. Water Res. 2010, 44, 5089–5097. [Google Scholar] [CrossRef]
- da Costa, P.M.; Vaz-Pires, P.; Bernardo, F. Antimicrobial resistance in Enterococcus spp. isolated in inflow, effluent and sludge from municipal sewage water treatment plants. Water Res. 2006, 40, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.-Y.Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Tay, M.; Tan, B.; Le, T.-H.; Haller, L.; Chen, H.; Koh, T.H.; Barkham, T.M.S.; Thompson, J.R.; Gin, K.Y.-H. Characterization of Metagenomes in Urban Aquatic Compartments Reveals High Prevalence of Clinically Relevant Antibiotic Resistance Genes in Wastewaters. Front. Microbiol. 2017, 8, 2200. [Google Scholar] [CrossRef] [PubMed]
- Cacace, D.; Fatta-Kassinos, D.; Manaia, C.M.; Cytryn, E.; Kreuzinger, N.; Rizzo, L.; Karaolia, P.; Schwartz, T.; Alexander, J.; Merlin, C.; et al. Antibiotic resistance genes in treated wastewater and in the receiving water bodies: A pan-European survey of urban settings. Water Res. 2019, 162, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.N.; Scriver, L.; Neudorf, K.D.; Hansen, L.T.; Jamieson, R.C.; Yost, C. Antimicrobial resistance gene surveillance in the receiving waters of an upgraded wastewater treatment plant. FACETS 2018, 3, 128–138. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Liu, S.-S.; Qu, H.-M.; Yang, D.; Hu, H.; Liu, W.-L.; Qiu, Z.-G.; Hou, A.-M.; Guo, J.; Li, J.-W.; Shen, Z.-Q.; et al. Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full-scale wastewater treatment plant. Water Res. 2018, 136, 131–136. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Heinken, A.; Kutt, L.; Ravcheev, D.A.; Bauer, E.; Noronha, A.; Greenhalgh, K.; Jäger, C.; Baginska, J.; Wilmes, P.; et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 2016, 35, 81–89. [Google Scholar] [CrossRef]
- LaPara, T.M.; Burch, T.R.; McNamara, P.J.; Tan, D.T.; Yan, M.; Eichmiller, J.J. Tertiary-Treated Municipal Wastewater is a Significant Point Source of Antibiotic Resistance Genes into Duluth-Superior Harbor. Environ. Sci. Technol. 2011, 45, 9543–9549. [Google Scholar] [CrossRef]
- Pruden, A.; Arabi, M.; Storteboom, H.N. Correlation Between Upstream Human Activities and Riverine Antibiotic Resistance Genes. Environ. Sci. Technol. 2012, 46, 11541–11549. [Google Scholar] [CrossRef]
- Jäger, T.; Hembach, N.; Elpers, C.; Wieland, A.; Alexander, J.; Hiller, C.; Krauter, G.; Schwartz, T. Reduction of Antibiotic Resistant Bacteria During Conventional and Advanced Wastewater Treatment, and the Disseminated Loads Released to the Environment. Front. Microbiol. 2018, 9, 2599. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater treatment: Current strategies and future challenges. Sci. Total Environ. 2021, 783, 146964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Matamoros, S.; van Hattem, J.M.; Arcilla, M.S.; Willemse, N.; Melles, D.C.; Penders, J.; Vinh, T.N.; Hoa, N.T.; Bootsma, M.C.J.; van Genderen, P.J.; et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep. 2017, 7, 15364. [Google Scholar] [CrossRef]
- Aminov, R.I. Horizontal Gene Exchange in Environmental Microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Watkinson, A.; Murby, E.; Costanzo, S. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef]
- Martínez, J.L.; Coque, T.M.; Baquero, F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Genet. 2014, 13, 116–123. [Google Scholar] [CrossRef]
- Li, A.-D.; Li, L.-G.; Zhang, T. Exploring antibiotic resistance genes and metal resistance genes in plasmid metagenomes from wastewater treatment plants. Front. Microbiol. 2015, 6, 1025. [Google Scholar] [CrossRef]
- Parsley, L.C.; Consuegra, E.J.; Kakirde, K.S.; Land, A.M.; Harper, W.F.; Liles, M.R. Identification of Diverse Antimicrobial Resistance Determinants Carried on Bacterial, Plasmid, or Viral Metagenomes from an Activated Sludge Microbial Assemblage. Appl. Environ. Microbiol. 2010, 76, 3753–3757. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.-X.; Ye, L. Plasmid Metagenome Reveals High Levels of Antibiotic Resistance Genes and Mobile Genetic Elements in Activated Sludge. PLoS ONE 2011, 6, e26041. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Pruden, A.; Chen, C.; Heath, L.S.; Xia, K.; Zhang, L. MetaCompare: A computational pipeline for prioritizing environmental resistome risk. FEMS Microbiol. Ecol. 2018, 94, fiy079. [Google Scholar] [CrossRef] [PubMed]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hultman, J.; Waldrop, M.; Mackelprang, R.; David, M.M.; McFarland, J.W.; Blazewicz, S.J.; Harden, J.W.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Deng, Y.; Ma, L.; Wang, Y.; Chan, L.Y.; Zhang, T. Exploration of the antibiotic resistome in a wastewater treatment plant by a nine-year longitudinal metagenomic study. Environ. Int. 2019, 133, 105270. [Google Scholar] [CrossRef]
- Ali, O.S.; Hozayen, W.G.; Almutairi, A.S.; Edris, S.A.; Abulfaraj, A.A.; Ouf, A.A.; Mahmoud, H.M. Metagenomic Analysis Reveals the Fate of Antibiotic Resistance Genes in a Full-Scale Wastewater Treatment Plant in Egypt. Sustainability 2021, 13, 11131. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.-M.; Leung, C.-M.; Ting, H.-F.; Sadakane, K.; Yamashita, H.; Lam, T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Fresia, P.; Antelo, V.; Salazar, C.; Giménez, M.; D’Alessandro, B.; Afshinnekoo, E.; Mason, C.; Gonnet, G.H.; Iraola, G. Urban metagenomics uncover antibiotic resistance reservoirs in coastal beach and sewage waters. Microbiome 2019, 7, 35. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC. A Quality Control Tool for High Throughput Sequence Data 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 16 September 2022).
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.-L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Leplae, R.; Lima-Mendez, G.; Toussaint, A. ACLAME: A CLAssification of Mobile genetic Elements, update 2010. Nucleic Acids Res. 2010, 38, D57–D61. [Google Scholar] [CrossRef]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2016, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Ligges, U.; Maechler, M. scatterplot3d-AnRPackage for Visualizing Multivariate Data. J. Stat. Softw. 2003, 8, 1–20. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2014. [Google Scholar]
- Zhao, R.; Yu, K.; Zhang, J.; Zhang, G.; Huang, J.; Ma, L.; Deng, C.; Li, X.; Li, B. Deciphering the mobility and bacterial hosts of antibiotic resistance genes under antibiotic selection pressure by metagenomic assembly and binning approaches. Water Res. 2020, 186, 116318. [Google Scholar] [CrossRef]
- Ju, F.; Beck, K.; Yin, X.; Maccagnan, A.; McArdell, C.S.; Singer, H.P.; Johnson, D.R.; Zhang, T.; Bürgmann, H. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 2019, 13, 346–360. [Google Scholar] [CrossRef]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN Community Edition—Interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef]
- Uritskiy, G.V.; DiRuggiero, J.; Taylor, J. MetaWRAP—A flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-W.; Tang, Y.-H.; Tringe, S.G.; Simmons, A.B.; Singer, S.W. MaxBin: An automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.D.; Froula, J.; Egan, R.; Wang, Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 2015, 3, e1165. [Google Scholar] [CrossRef] [PubMed]
- Alneberg, J.; Bjarnason, B.S.; de Bruijn, I.; Schirmer, M.; Quick, J.; Ijaz, U.Z.; Loman, N.J.; Andersson, A.F.; Quince, C. CONCOCT: Clustering contigs on coverage and composition. arXiv 2013. [Google Scholar] [CrossRef]
- Song, W.-Z.; Thomas, T. Binning_refiner: Improving genome bins through the combination of different binning programs. Bioinformatics 2017, 33, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Ng, C.; Tan, B.; Jiang, X.-T.; Gu, X.; Chen, H.; Schmitz, B.W.; Haller, L.; Charles, F.R.; Zhang, T.; Gin, K. Metagenomic and Resistome Analysis of a Full-Scale Municipal Wastewater Treatment Plant in Singapore Containing Membrane Bioreactors. Front. Microbiol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Majeed, H.J.; Riquelme, M.V.; Davis, B.C.; Gupta, S.; Angeles, L.; Aga, D.S.; Garner, E.; Pruden, A.; Vikesland, P.J. Evaluation of Metagenomic-Enabled Antibiotic Resistance Surveillance at a Conventional Wastewater Treatment Plant. Front. Microbiol. 2021, 12, 657954. [Google Scholar] [CrossRef] [PubMed]
- Sentchilo, V.; Mayer, A.P.; Guy, L.; Miyazaki, R.; Tringe, S.; Barry, K.; Malfatti, S.; Goessmann, A.; Robinson-Rechavi, M.; van der Meer, J.R. Community-wide plasmid gene mobilization and selection. ISME J. 2013, 7, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-N.; Gaston, J.M.; Dai, C.L.; Zhao, S.; Poyet, M.; Groussin, M.; Yin, X.; Li, L.-G.; van Loosdrecht, M.C.M.; Topp, E.; et al. An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat. Commun. 2021, 12, 4765. [Google Scholar] [CrossRef] [PubMed]
- Hultman, J.; Tamminen, M.; Pärnänen, K.; Cairns, J.; Karkman, A.; Virta, M. Host range of antibiotic resistance genes in wastewater treatment plant influent and effluent. FEMS Microbiol. Ecol. 2018, 94, fiy038. [Google Scholar] [CrossRef] [PubMed]
- Jacquiod, S.; Brejnrod, A.; Morberg, S.M.; Abu Al-Soud, W.; Sørensen, S.J.; Riber, L. Deciphering conjugative plasmid permissiveness in wastewater microbiomes. Mol. Ecol. 2017, 26, 3556–3571. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Korobeynikov, A.I. Metagenomic Data Assembly—The Way of Decoding Unknown Microorganisms. Front. Microbiol. 2021, 12, 613791. [Google Scholar] [CrossRef]
- Antipov, D.; Raiko, M.; Lapidus, A.; Pevzner, P.A. Plasmid detection and assembly in genomic and metagenomic data sets. Genome Res. 2019, 29, 961–968. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Blackwell, G.A.; Hall, R.M. Mobilisation of a small Acinetobacter plasmid carrying an oriT transfer origin by conjugative RepAci6 plasmids. Plasmid 2019, 103, 36–44. [Google Scholar] [CrossRef]
- Che, Y.; Xu, X.; Yang, Y.; Břinda, K.; Hanage, W.; Yang, C.; Zhang, T. High-resolution genomic surveillance elucidates a multilayered hierarchical transfer of resistance between WWTP- and human/animal-associated bacteria. Microbiome 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Moralez, J.; Szenkiel, K.; Hamilton, K.; Pruden, A.; Lopatkin, A.J. Quantitative analysis of horizontal gene transfer in complex systems. Curr. Opin. Microbiol. 2021, 62, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Šilha, D.; Vacková, B.; Šilhová, L. Occurrence of virulence-associated genes in Arcobacter butzleri and Arcobacter cryaerophilus isolates from foodstuff, water, and clinical samples within the Czech Republic. Folia Microbiol. 2018, 64, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-K.; Chen, J.-H.; Yang, L.; Li, A.-R.; Su, D.-H.; Lin, Y.-P.; Chen, D.-Q. Emergence and genomic analysis of MDR Laribacter hongkongensis strain HLGZ1 from Guangzhou, China. J. Antimicrob. Chemother. 2017, 73, 643–647. [Google Scholar] [CrossRef]
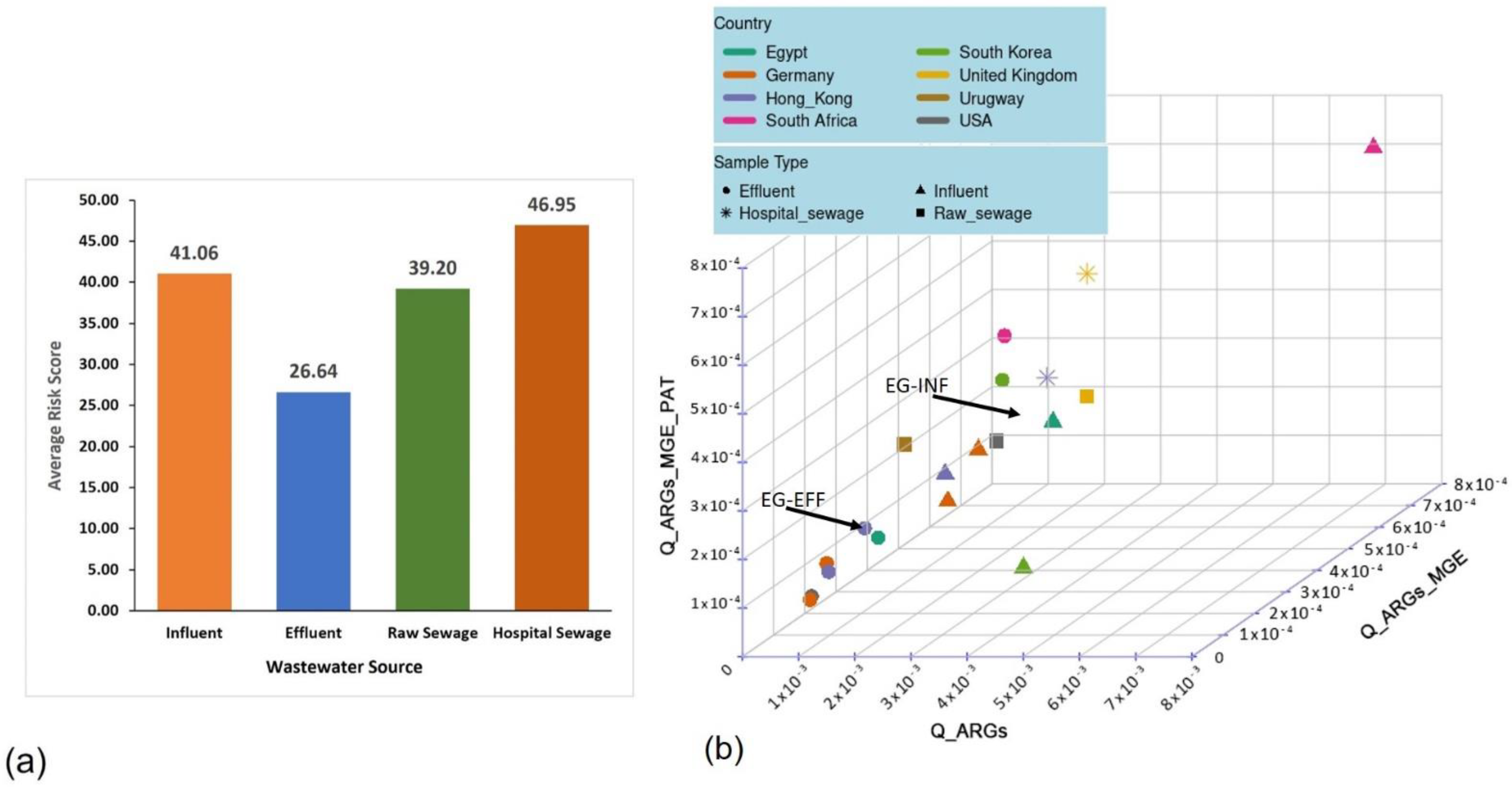

| Sample Name | Number of Input Contigs | % ACCs | % ACCs + MGEs | % ACCs + MGEs + PAT | Risk Score | Sample Source | Country | Continent |
|---|---|---|---|---|---|---|---|---|
| SK_EFF | 145,531 | 0.48% | 0.0004 | 0.0004 | 37.89 | Effluent | South Korea | Asia |
| SA_EFF | 59,712 | 0.41% | 0.05% | 0.05% | 35.51 | Effluent | South Africa | Africa |
| EG_EFF | 410,198 | 0.29% | 0.02% | 0.02% | 26.80 | Effluent | Egypt | Africa |
| HK_EFF | 191,621 | 0.23% | 0.02% | 0.02% | 24.89 | Effluent | Hong Kong | Asia |
| HK_EFF2 | 66,117 | 0.17% | 0.01% | 0.01% | 22.56 | Effluent | Hong Kong | Asia |
| DE_EFF | 82,507 | 0.15% | 0.01% | 0.01% | 22.07 | Effluent | Germany | Europe |
| USA_EFF | 163,101 | 0.15% | 0.01% | 0.01% | 21.69 | Effluent | United States of America | North America |
| DE_EFF2 | 148,405 | 0.15% | 0.01% | 0.01% | 21.68 | Effluent | Germany | Europe |
| UK_HS | 108,598 | 0.61% | 0.06% | 0.05% | 48.56 | Hospital sewage | United Kingdom | Europe |
| DE_HS2 | 143,088 | 0.63% | 0.04% | 0.04% | 45.33 | Hospital sewage | Germany | Europe |
| SA_INF | 45,534 | 1.44% | 0.07% | 0.07% | 50.25 | Influent | South Africa | Africa |
| EG_INF | 334,605 | 0.72% | 0.03% | 0.03% | 47.87 | Influent | Egypt | Africa |
| SK_INF | 159,229 | 0.76% | 0.02% | 0.01% | 44.37 | Influent | South Korea | Asia |
| DE_INF | 93,644 | 0.50% | 0.03% | 0.03% | 36.79 | Influent | Germany | Europe |
| DE_INF2 | 168,139 | 0.47% | 0.02% | 0.02% | 34.32 | Influent | Germany | Europe |
| HK_INF | 236,067 | 0.42% | 0.03% | 0.03% | 32.75 | Influent | Hong Kong | Asia |
| UK_RS | 135,578 | 0.81% | 0.04% | 0.04% | 52.29 | Raw sewage | United Kingdom | Europe |
| USA_RS | 202,631 | 0.55% | 0.03% | 0.03% | 39.46 | Raw sewage | United States of America | North America |
| UR_RS | 67,082 | 0.23% | 0.03% | 0.03% | 25.86 | Raw sewage | Uruguay | South America |
| Parameter | INF | EFF |
|---|---|---|
| Total ACCs by MetaCompare software (% of total assembled contigs) | 2409 (0.7%) | 1196 (0.3%) |
| Total RGI-annotated ACCs (% of total ACCs) | 2386 (99%) | 1189 (99%) |
| ACCs with one ARG (% of total ACCs) | 1429 (60%) | 705 (60%) |
| ACCs with more than one ARG (% of total ACCs) | 980 (40%) | 484 (40%) |
| Total annotated ARG ORFs in ACCs | 3891 | 1996 |
| Total non-redundant ARGs | 536 | 455 |
| Perfect ARG hits (% of total ARGs) | 64 (2%) | 56 (3%) |
| Strict ARG hits (% of total ARGs) | 415 (11%) | 174 (9%) |
| Loose ARG hits (% of total ARGs) | 3412 (87%) | 705 (88%) |
| ARG Risk Priority Level | ARG Name | Drug Class | INF Mobility, % | EFF Mobility, % | ARG Annotation in the Effluent | ||
|---|---|---|---|---|---|---|---|
| Potential Pathogenic Host (PATRIC BLASTn Best Hit) | ARG Genomic Context (NCBI BLASTn Best Hit) | RGI Cutoff Category | |||||
| Level 1 (20 genes) | aadA6 | aminoglycoside antibiotic | 0% | 100% | Pseudomonas aeruginosa | plasmid | Perfect |
| ANT(2″)-Ia | aminoglycoside antibiotic | 0% | 100% | Escherichia coli | plasmid | Perfect | |
| aadA2 | aminoglycoside antibiotic | 75% | 100% | Pseudomonas aeruginosa | plasmid | Perfect | |
| AAC(6′)-Il | aminoglycoside antibiotic | 100% | 100% | Pseudomonas aeruginosa | plasmid | Perfect | |
| AAC(6′)-IIa | aminoglycoside antibiotic | Ud | 100% | Pseudomonas aeruginosa | integron | Perfect | |
| GES-2 | beta-lactam | 50% | 100% | Pseudomonas aeruginosa | integron | Perfect | |
| CMY-4 | beta-lactam | 100% | 100% | Escherichia coli | plasmid | Perfect | |
| OXA-2 | beta-lactam | 100% | 100% | Shigella sonnei | plasmid | Perfect | |
| PER-3 | beta-lactam | 100% | 100% | Acinetobacter baumannii | plasmid | Perfect | |
| CfxA2 | beta-lactam | Ud | 100% | Chlamydia trachomatis | bacteriophage | Perfect | |
| TEM-181 | beta-lactam | Ud | 100% | Shigella sonnei | plasmid | Perfect | |
| VEB-9 | beta-lactam | Ud | 100% | Pseudomonas aeruginosa | plasmid | Perfect | |
| dfrB5 | diaminopyrimidine antibiotic | 0% | 100% | Pseudomonas aeruginosa | integron | Perfect | |
| QnrS2 | fluoroquinolone antibiotic | 0% | 100% | Vibrio parahaemolyticus | plasmid | Perfect | |
| QnrVC4 | fluoroquinolone antibiotic | 100% | 100% | Vibrio parahaemolyticus | plasmid | Perfect | |
| QnrS1 | fluoroquinolone antibiotic | 100% | 100% | Escherichia coli | plasmid | Perfect | |
| mphE | MLS | 0% | 100% | Acinetobacter pittii | plasmid | Perfect | |
| MCR-5.1 | peptide antibiotic | 100% | 100% | Pseudomonas aeruginosa | plasmid | Perfect | |
| cmlA5 | phenicol antibiotic | 0% | 100% | Pseudomonas aeruginosa | integron | Perfect | |
| catI | phenicol antibiotic | 100% | 100% | Acinetobacter baumannii | plasmid | Perfect | |
| Level 2 (19 genes) | aadA17 | aminoglycoside antibiotic | 0% | 100% | Pseudomonas aeruginosa | plasmid | Strict |
| aadA16 | aminoglycoside antibiotic | 50% | 100% | Vibrio cholerae | plasmid | Strict | |
| aadA15 | aminoglycoside antibiotic | 100% | 100% | Pseudomonas aeruginosa | integron | Strict | |
| ANT(3″)-IIa | aminoglycoside antibiotic | 100% | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| aadA | aminoglycoside antibiotic | Ud | 100% | Yersinia enterocolitica | plasmid | Strict | |
| aadA10 | aminoglycoside antibiotic | Ud | 100% | Escherichia coli | plasmid | Strict | |
| AAC(6′)-Ib9 | aminoglycoside antibiotic | Ud | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| OXA-129 | beta-lactam | 100% | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| OXA-1 | beta-lactam | Ud | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| NPS-1 | beta-lactam | Ud | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| OXA-663 | beta-lactam | Ud | 100% | Pseudomonas aeruginosa | integron | Strict | |
| dfrA1 | diaminopyrimidine antibiotic | 0% | 100% | Escherichia coli | plasmid | Strict | |
| dfrA14 | diaminopyrimidine antibiotic | 100% | 100% | Escherichia coli | plasmid | Strict | |
| ErmB | MLS | 0% | 100% | Staphylococcus aureus | plasmid | Strict | |
| lnuF | MLS | 100% | 100% | Salmonella enterica | integron | Strict | |
| lnuB | MLS | Ud | 100% | Staphylococcus aureus | ICE | Strict | |
| floR | phenicol antibiotic | 33% | 100% | Pseudomonas aeruginosa | plasmid | Strict | |
| tetM | tetracycline antibiotic | 0% | 100% | Streptococcus sp. | transposon | Strict | |
| tetX | tetracycline antibiotic | 100% | 100% | Salmonella enterica | plasmid | Strict | |
| Level 3 (10 genes) | ANT(9)-Ia | aminoglycoside antibiotic | 0% | 100% | Staphylococcus aureus | plasmid | Loose |
| aadA6/aadA10 | aminoglycoside antibiotic | 100% | 100% | Escherichia coli | plasmid | Loose | |
| TLA-3 | beta-lactam | 100% | 100% | Escherichia coli | plasmid | Loose | |
| OXA-15 | beta-lactam | Ud | 100% | Pseudomonas aeruginosa | transposon | Loose | |
| OXA-898 | beta-lactam | Ud | 100% | Burkholderia glumae | plasmid | Loose | |
| norB | fluoroquinolone antibiotic | 14% | 100% | Pseudomonas aeruginosa | plasmid | Loose | |
| lsaB | MLS | 0% | 100% | Staphylococcus aureus | ICE | Loose | |
| mef(D) | MLS | Ud | 100% | Burkholderia cenocepacia | plasmid | Loose | |
| efpA | multidrug | Ud | 100% | Pseudomonas monteilii | plasmid | Loose | |
| tetA(P) | tetracycline antibiotic | 50% | 100% | Clostridium butyricum | plasmid | Loose | |
| Level 4 (one gene) | OXA-347 | beta-lactam | 100% | 100% | NA | transposon | Perfect |
| Category | Maxbin2 | Metabat2 | Concoct | MetaWRAP Binning (70% Minimum Completeness and 10% Maximum Contamination) | ||||
|---|---|---|---|---|---|---|---|---|
| INF | EFF | INF | EFF | INF | EFF | INF | EFF | |
| Number of binned contigs | 297,403 | 365,355 | 89,792 | 128,060 | 334,602 | 409,577 | 16,449 | 37,923 |
| Total size of binned contigs (Mbp) | 649.59 | 1011.00 | 331.45 | 591.17 | 724.93 | 1108.99 | 73.01 | 260.11 |
| Number of unbinned contigs | 37,202 | 44,824 | 244,812 | 282,137 | 542 | 602 | 318,156 | 372,256 |
| Total size of unbinned contigs (Mbp) | 75.87 | 98.60 | 394.02 | 518.42 | 0.54 | 0.62 | 652.46 | 849.49 |
| % Binned number | 88.88% | 89.07% | 26.84% | 31.22% | 99.84% | 99.85% | 4.92% | 9.25% |
| % Total size binned | 89.54% | 91.11% | 45.69% | 53.28% | 99.93% | 99.95% | 10.06% | 23.44% |
| Number of genome Bins | 292 | 450 | 163 | 100 | 224 | 290 | 35 | 118 |
| Bin_ID | Database | Resistance Gene | Identity | Query/Template Length (bp) | Contig_ID | Position in Contig | Predicted Phenotype | Accession Number |
|---|---|---|---|---|---|---|---|---|
| Bin.101 | macrolide | mdf(A) | 99.35 | 1233/1233 | k141_999869_length_12294_cov_6.7526 | 4976..6208 | Gene is missing from ResFinder database | Y08743 |
| Bin.75 | tetracycline | tet(39) | 100 | 1122/1122 | k141_59336_length_24223_cov_13.4223 | 2428..3549 | Tetracycline resistance | KT346360 |
| Bin.75 | macrolide | mph(E) | 100 | 885/885 | k141_59336_length_24223_cov_13.4223 | 4576..5460 | Macrolide resistance | DQ839391 |
| Bin.75 | macrolide | msr(E) | 100 | 1476/1476 | k141_59336_length_24223_cov_13.4223 | 5516..6991 | Macrolide, lincosamide and streptogramin B resistance | FR751518 |
| Bin.3 | beta-lactam | blaGES-14 | 97.09 | 550/864 | NODE_220_length_549_cov_1.313559 | 1..549 | Beta-lactam resistance | GU207844 |
| Bin.94 | beta-lactam | cfxA6 | 99.7 | 996/996 | k141_539772_length_5362_cov_34.9153 | 333..1328 | Beta-lactam resistance | GQ342996 |
| Bin.94 | tetracycline | tet(Q) | 98.81 | 1681/1926 | k141_1010747_length_2949_cov_30.2689 | 1..1681 | Tetracycline resistance | L33696 |
| Bin.82 | nitroimidazole | nimD | 99.19 | 495/495 | k141_1686426_length_3658_cov_11.0000 | 2911..3405 | Metronidazole (5-nitroimidazole) | X76949 |
| Bin.88 | macrolide | lnu(B) | 99.75 | 804/804 | NODE_176_length_4337_cov_1.714554 | 3114..3917 | Lincosamide resistance | JQ861959 |
| Bin.88 | macrolide | lsa(E) | 99.39 | 1485/1485 | NODE_176_length_4337_cov_1.714554 | 1576..3060 | Gene is missing from ResFinder database | JX560992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, O.S.; Hozayen, W.G.; Almutairi, A.S.; Edris, S.; Karkashan, A.; Abulfaraj, A.A.; Attar, R.; Ouf, A.A.; Abbas, B.; Mahmoud, H.M. The Assessment of the Risk Ranking and Mobility Potential Associated with Environmental Resistomes in Wastewater Using Metagenomic Assembly. Sustainability 2022, 14, 14292. https://doi.org/10.3390/su142114292
Ali OS, Hozayen WG, Almutairi AS, Edris S, Karkashan A, Abulfaraj AA, Attar R, Ouf AA, Abbas B, Mahmoud HM. The Assessment of the Risk Ranking and Mobility Potential Associated with Environmental Resistomes in Wastewater Using Metagenomic Assembly. Sustainability. 2022; 14(21):14292. https://doi.org/10.3390/su142114292
Chicago/Turabian StyleAli, Osama S., Walaa G. Hozayen, Abdulwahab S. Almutairi, Sherif Edris, Alaa Karkashan, Aala A. Abulfaraj, Roba Attar, Amged A. Ouf, Basma Abbas, and Hamada M. Mahmoud. 2022. "The Assessment of the Risk Ranking and Mobility Potential Associated with Environmental Resistomes in Wastewater Using Metagenomic Assembly" Sustainability 14, no. 21: 14292. https://doi.org/10.3390/su142114292
APA StyleAli, O. S., Hozayen, W. G., Almutairi, A. S., Edris, S., Karkashan, A., Abulfaraj, A. A., Attar, R., Ouf, A. A., Abbas, B., & Mahmoud, H. M. (2022). The Assessment of the Risk Ranking and Mobility Potential Associated with Environmental Resistomes in Wastewater Using Metagenomic Assembly. Sustainability, 14(21), 14292. https://doi.org/10.3390/su142114292






