Abstract
This study investigates the degradation characteristics, bacterial community structure, and degradation mechanism of rice straw under different levels of nitrogen (N) application and depths of return of 1-mature-winter tillage in paddy fields in a cold zone from the perspective of biodegradation by using the nylon mesh bag in situ culture method. Paludibacteraceae and Ruminococcaceae were the dominant bacteria in the degradation process, and their abundance decreased with the increasing depth of return. The activities of extracellular enzymes associated with the C-cycle (α-glucosidase, β-glucosidase, β-cellobiosidase, and β-xylosidase) were generally higher than those of other functional extracellular enzymes, and straw degradation extracellular enzyme activities generally increased in the middle and late stages (104 d). At an N application rate of 110–150 kg hm−2, the potential functionality of soil showed a quadratic trend with the increasing N application. When the full amount of straw was returned to the field, there was significant spatial heterogeneity in soil potential functionality. Our results showed that the most optimum N application rate was 140 kg hm−2, while the best soil return level (0–15 cm) was observed in the tillage layer under full rice straw return in the cold zone.
1. Introduction
As an important renewable resource, straw contains mineral nutrients and organic matter essential for plant growth and is an important source of organic fertilizer for soil enrichment [1]. China is the largest straw producer, accounting for approximately 1/3 of the global production [2,3,4]. Straw burning or leaving it in the field not only cause energy and material loss from agroecosystems, but also increase greenhouse gas emissions. Therefore, straw return to the field has become an important measure to fertilize soil, promote sustainable agricultural development, and mitigate global climate change. The degradation of returned straw is a mineralization and humification process involving both microorganisms and enzymes [5]. During straw degradation, the composition of the material in straw changes with the degradation time, and the composition and activity of the associated degradation microbial community change [6]. It has been found that bacteria are active during straw degradation, with actinomycetes being the dominant bacterial genus in the late stages of degradation [7,8]. Microorganisms sustain population reproduction and regulate the straw degradation process through various enzymes, including hydrolases that control the primary metabolism of carbon (C) and nitrogen (N), and oxidases that utilize difficult-to-degrade compounds for nutrient acquisition. The dynamics of these indicators reflect the catabolic capacity of straw during its degradation [9]. In addition, extracellular enzyme activity and microbial communities during straw degradation are highly influenced by external factors such as climate, cropping practices, soil type, temperature, and water content in the growing area [10].
Under straw return conditions, microorganisms use the returned straw as a carbon source and must simultaneously absorb inorganic nitrogen sources from the soil to meet their growth requirements. As a material high in carbon and nitrogen, a large amount of inorganic nitrogen in the soil is sequestered by microorganisms under field return conditions, leading to a competition between microorganisms and crops for nitrogen and affecting the nitrogen supply of the soil [11]. Therefore, a nitrogenous fertilizer is often applied under straw return conditions to ensure the rapid decomposition of straw, and crop growth and development [12]. The application of nitrogenous fertilizer accelerates the enzymatic reactions of chemical decomposition of plant residues and the growth kinetics of microorganisms that secrete these digestive enzymes, thereby affecting the material composition of straw residues [13].
An increase in the depth of return will likewise alter the soil environment and affect the rate of straw degradation. Previous studies have investigated the decay characteristics of paddy straw under flooded conditions from the perspective of biodegradation [14,15], but primarily focused on the warm region under double-season rice cropping patterns. The results from this region cannot explain the decay characteristics of returned rice straw under 1-mature tillage system in cool regions. Additionally, straw degradation is inhibited since clay and heavy soil particles under flooded conditions would directly provide better physical protection for plant residues [16,17]. The prolonged anaerobic soil environment in rice fields will affect the growth of microorganisms inhibiting straw degradation and predisposing to incomplete decomposition [18,19]. Furthermore, the degradation pattern of returned straw in the dry field in the cool zone cannot be used to systematically explain the decomposition characteristics of paddy straw. Therefore, an in-depth investigation on enzyme activities, bacterial community structure, and metabolic capacity related to straw degradation in paddy fields in cold and cool zones remains warranted.
This study investigates the effects of nitrogen application and depth on (1) the decomposition rate and material composition of returned straw, (2) the structural characteristics and representative enzyme activities of the bacterial community in returned straw, (3) exploring the multifunctionality to the soil from the perspective of biodegradation. The nylon mesh bag in situ culture method was used in a cool northern rice crop area. The results of the study provide novel insights into the optimization of the straw return strategy and fertilization in single-season rice cropping systems in cool regions.
2. Material and Methods
2.1. Description of the Study Site
The study site was located at the experimental base of Yanbian University in Longjing City, Jilin Province (42°F′18″ N, 129°23′46″ E), where a single-season rice-winter recreational cropping system was used. The test area was located in the mid-temperate monsoon climate zone, with an average annual precipitation of 549.3 mm, an average annual temperature of 5.6 °C, a frost-free period of approximately 126 d, and an annual active cumulative temperature 2650 °C, which belongs to the cool northeast region. The test rice soil was black soil, which was classified as soft soil (Mollisols) according to the USDA soil classification. The basic physical and chemical properties of the soil at the beginning of the experiment are listed in Table 1.

Table 1.
Basic physicochemical properties of the test soil.
2.2. Experimental Design
The straw degradation experiment was conducted using a nylon mesh bag layered in situ incubation method. Mature straw was cut into a 5 cm length to simulate the state of straw after fragmentation, dried at 60 °C for 24 h, and then 13.75 g of rice straw (dry weight) was weighed into a nylon mesh bag (size: 10 cm width, 30 cm length, 0.1 mm aperture) to produce straw bales. The experiment was based on the full amount of straw returned to the field (8800 kg ha−1), with 2 depths of 0–15 cm (TU) and 15–30 cm (TD), combined with five levels of nitrogen application, 110 kg hm−2 (TU1, TD1), 120 kg hm−2 (TU2, TD2), 130 kg hm−2 (TU3, TD3), 140 kg hm−2 (TU4, TD4), and 150 kg hm−2 (TU5, TD5) for a total of 10 treatments. The straw bales were buried diagonally at <45° in 0–15 cm and 15–30 cm soil layers before spring tillage (21 May 2021) to ensure the uniform distribution of straw in the bales and maximum contact with the soil. A nitrogenous fertilizer was applied as 4:4:2 as the base fertilizer: tiller fertilizer: spike fertilizer, a phosphorus fertilizer (P2O5, 70 kg hm−2) was applied as the base fertilizer, and potassium (K2O, 80 kg hm−2) was applied as 5:4:1 as the base fertilizer: tiller fertilizer: spike fertilizer. The test fertilizers were urea (46% N), diammonium phosphate (18% N, 46% P2O5), and potassium sulfate (50% K2O), respectively. Four straw bales were removed from each treatment replicate at 20, 40, 71, 104, and 137 days of the straw return, and the bales were transported to the laboratory on ice. Roots, soil, soil animals, and weeds were removed from the outside of the straw bales. Samples were stored at 4 °C for relevant enzyme activity assays (within 24 h) and −80 °C for the bacterial bioassay analysis, respectively. Partially dried samples were used for the degradation rate and composition fraction determination.
2.3. Determination of Straw Degradation Rate and Composition
Dried straw sample fractions were determined by acid and alkaline liquid washing methods [20]. The straw degradation rate was determined via the weighing method.
2.4. DNA Extraction, Sequencing Library Preparation, and Bioinformatics
The straw decay-associated microorganisms were sequenced via 16S rDNA high-throughput sequencing: samples to be tested were melted on ice, centrifuged, and mixed thoroughly. DNA was extracted from the straw microbiome using the PowerSoil DNA Isolation Kit (MoBio Laboratories, Inc., San Diego, CA, USA), while DNA quality and concentration were determined using a Nanodrop 2000 (ThermoFisher Scientific, Inc., Waltham, MA, USA). The quality-checked samples were stored at −20 °C for subsequent experiments.
The straw sample DNA was used as the template, and the region of the assay was the bacterial 16S rDNA V3-V4 region. Primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used for amplification. The PCR amplification system (25 μL) was 12.5 μL 2xTaq Plus Master Mix, 1 μL of each upper and lower primer, 7.5-X μL of double distilled water, 3 μL of BSA (2 ng μL−1), and the X (30 ng) DNA template. The amplification procedure was: 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 60 s, 72 °C extensions for 7 min, and end at 4 °C. The PCR products were amplified on an ABI 9700 PCR instrument (ThermoFisher Scientific, Inc., Waltham, MA, USA), and the amplified target bands were detected via 1% agarose gel electrophoresis and purified using the Agencourt AMPure XP (Beckman Coulter, Inc., Pasadena, CA, USA) nucleic acid purification kit.
The PCR products were used to construct microbial diversity sequencing libraries using the NEB Next Ultra II DNA Library Prep Kit (New England Biolabs, Inc., Ipswich, MA, USA) for library construction and the Illumina Miseq PE300 (Illumina, Inc., San Diego, CA, USA) for library construction at Beijing Ovison Gene Technology Co. (Illumina, Inc., San Diego, CA, USA) high-throughput sequencing platform for paired-end sequencing. The raw sequences were uploaded to the NCBI SRA database.
Miseq sequencing was performed to obtain pair-end (PE) double-end sequence data, and the measured Fastq data were quality-controlled using Trimmomatic (v0.36), Pear (v0.9.6), Flash (v1.20), and Search (v2.7.1). The final quality of the Fasta data was obtained via processing. The specific parameters were as follows: (1) quality control of Fastq data using Trimmomatic and Pear. For Trimmomatic, a sliding window strategy was used with a window size of 50 bp, an average quality value of 20, and a minimum retained sequence length of 120, while Pear was used to remove sequences with N. (2) Flash and Pear were used to splice (merge) the two end sequences according to the overlapping relationship of PE, with a minimum overlap of 10 bp and a mismatch rate of 0.1; the Fasta sequence was obtained. (3) The chimeras of the Fasta sequence were removed using the Uchime method according to the known database, while the unknown database was removed using the Denovo method, along with short sequences that do not meet the requirements. The representative sequences of OTUs were classified and identified using the SILVA database (SILVA 138).
2.5. Assessment of Soil Multifunctionality via Averaging and Multiple-Threshold Approaches
In this study, the activities of eight extracellular enzymes associated with C and N cycling and redox reactions of straw were determined (Table 2). Among them, hydrolytic enzymes associated with straw decay (β-glucosidase, α-glucosidase, β-xylosidase, β-cellulodiglucosidase, acetylaminoglucosidase, and leucine aminopeptidase) were determined using a fluorescent microplate enzyme assay technique [21,22,23]. Phenol oxidase and peroxidase assays were performed via the spectrophotometric method [24].

Table 2.
Types of extracellular enzymes related to straw decomposition and their related substrates, enzyme function, and enzymes’ international system classification number (EC).
2.6. Statistical Analyses
Data were organized and graphically plotted using Excel 2020, and Origin2021. An alpha diversity analysis of the data was performed using Qiime version v.1.8.0 [25]. Bacterial alpha diversity was assessed using Chao1, observed_species, PD_whole_tree, Shannon (H). Bar plots of species composition were drawn using a statistical analysis to observe community structure and its variation at the sample family level [26]. A beta diversity analysis between groups was performed using PLS-DA. A lefse analysis used the linear discriminant analysis (LDA) effect size algorithm of Segata et al. [27]. All biomarkers used in this study met the following criteria: (i) LDA score threshold (log10 value) ≥ 3.0 for discriminant features; and (ii) alpha value ≤ 0.05 for the intergroup factorial Kruskal–Wallis test. A multifactor ANOVA was performed using IBM SPSS Statistics 23, using Duncan’s comparison method, and the t-test. Comparing soil multifunctionality levels, the effect of the N application and depth of return on soil multifunctionality was explored via two-way ANOVA (mean method). A correlation analysis of eight extracellular enzymes was performed using the “eggcorn” package of R 4.0.5. Soil functionality was calculated using the averaging method (Z-score conversion) [28,29,30], which provides interpretable results only at high levels of the multifunctionality index [31]; therefore, only periods of high functionality were analyzed using the averaging method (data from 104 d of straw return were used) using the R language “Plspm” software package to construct a partial least squares path model (PLS-PM) to determine the relationship between microbial diversity and soil multifunctionality when multiple functions are considered simultaneously (based on the averaging method). The model assessed direct and indirect associations between bacterial abundance, bacterial composition, C-cycle-related enzyme activity, N-cycle-related enzyme activity, oxidation-related enzyme activity, and soil multifunctionality.
3. Results
3.1. Changes in Ambient Temperature and Soil Water Content during the Growing Season
The air and the soil temperatures of each layer varied greatly throughout the experiment period, with higher temperatures in July, while the ambient temperature continued to drop after July, and the air temperature and soil temperature decreased significantly into October (Figure 1). The maximum temperature difference between the soil layers reached more than 10 °C during the whole growing season, and the temperature of each soil layer decreased with increasing depth before mid-August, while the temperature differences between soil layers were large; the temperature of each soil layer increased with increasing depth after mid-August, while the soil temperature was similar between the tillage layer (0–15 cm) and sub-tillage layer (15–30 cm). At the same time, with the increase in soil depth, the variation of soil temperature gradually decreased during the whole reproductive period; the variation of soil temperature in the sub-tillage layer (15–30 cm) was the smallest. The soil moisture content also differed significantly among the different soil layers, and the moisture content of the 15–30 cm soil layer was lower compared to that of the 0–15 cm layer. The soil water content was low in the early stage of the experiment and increased after July 14; the difference in water content among layers became increasingly obvious, with the maximum being nearly two-fold. From September 7 to October 10, the soil water content in each layer gradually decreased, while the water content in the 15–30 cm soil layer gradually stabilized to approximately 30%.
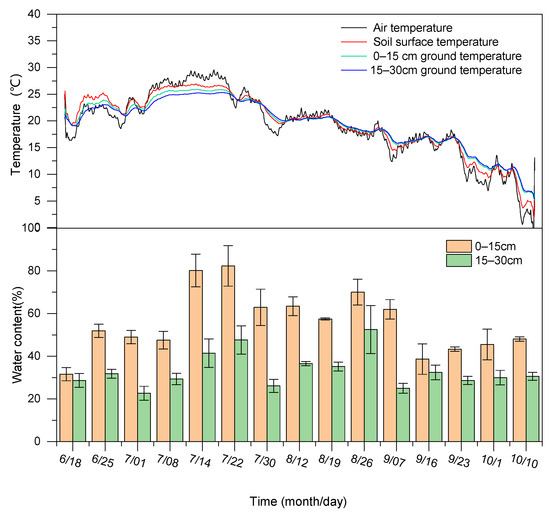
Figure 1.
Changes in water content and temperature in each soil layer and air.
3.2. Degradation Rate and Composition of Returned Straw in Different Periods under Different Fertilizer Applications and Depth of Return to the Field
The degradation rate of returned straw was approximately 55% in the 0–15 cm tillage layer and approximately 50% in the 15–30 cm layer during the test period (Figure 2). The straw degradation rate was faster at the early (0–20 d) and mid-late (71–137 d) stages of field return. The average degradation of straw was approximately 20% at the early stage of return (0–20 d) and 10% at the middle and late stages (71–137 d). The straw degradation was slow in the middle and early stages (20–71 d) and late stage (104–137 d). The straw degradation rate showed a decreasing trend with increasing depth of returning straw at the same period and at the same N application rate. The degradation rate of straw returned to the field was higher at high N application rates (TU4, TU5, TD4, and TD5).
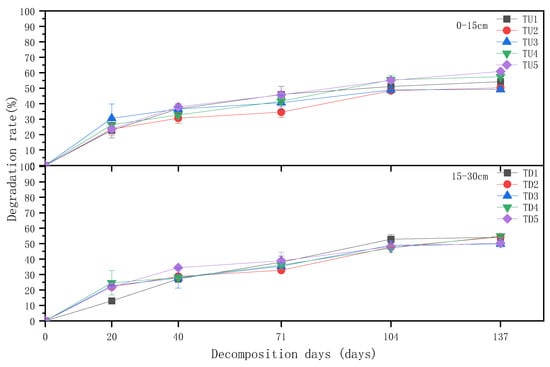
Figure 2.
Changes in degradation rate of straw returned to the field by different treatments.
The percentage of soluble matter in the straw residue at the early stage of returning (0–20 d) decreased significantly by approximately 20%, while the percentage of cellulose increased significantly by nearly 15% (Figure 3). As the return time increased (20–137 d), the proportion of soluble substances and lignin in the returned straw residue increased, while the proportion of cellulose and hemicellulose decreased. The percentage of hemicellulose and lignin fluctuated less during the test period. In the middle and late stages of return (71–104 d), the proportion of soluble matter increased by approximately 5% and the proportion of lignin increased by nearly 10%; the proportion of cellulose and hemicellulose decreased by nearly 5% on average. The percentage of soluble matter generally decreased, and the percentage of lignin generally increased with the increasing depth of return to the field for the same period and application rate. The percentage of soluble matter was higher, while the percentage of lignin was lower in all treatments (TU4, TU5, TD4, and TD5) under high N application.
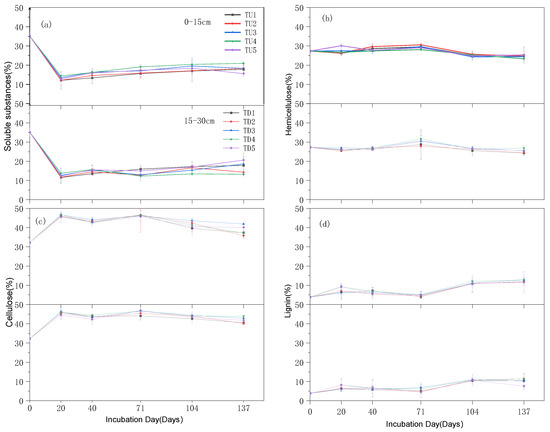
Figure 3.
Changes in the content of main components of returned straw in different periods: (a) percentage of soluble material in straw residue; (b) percentage of hemicellulosic material in straw residue; (c) percentage of cellulose in straw residue; (d) percentage of lignin in straw residue.
3.3. Effect of Fertilizer Application and Depth of Return on Straw-Degrading Bacteria
Alpha diversity of microorganisms in straw tended to increase with the increasing time of returning to the field (Figure 4a). The mean values of chao1 and PD whole tree were relatively stable and slightly increased in the early stage of returning to the field (20–40 d), while the observed species slightly decreased, and the Shannon increased significantly. Alpha diversity of each treatment increased significantly from 40 to 104 d and reached the maximum value of each index (chao1, PD whole tree, observed species, and Shannon) at 104 d. The changes in bacterial alpha diversity of each treatment were relatively stable and slightly decreased in the later stage of field return (104–137 d). Bacterial alpha diversity in the returned straw tended to decrease with the increasing N application in the low N application range (110–120 kg hm−2) and increase with the increasing N application in the high N application range (140–150 kg hm−2) (Figure 4b). The indicators (chao1, PD whole tree, observed species, and Shannon) were higher at 110, 130, and 150 kg hm−2 of the N application, and chao1 reached its maximum at 130 kg hm−2 of the N application. The values of each index (chao1, PD whole tree, observed species, Shannon) of alpha diversity of returned straw bacteria were similar at different depths of return (Figure 4c).
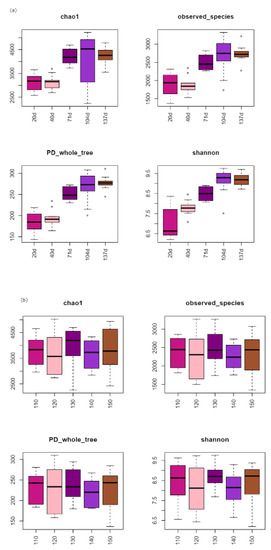
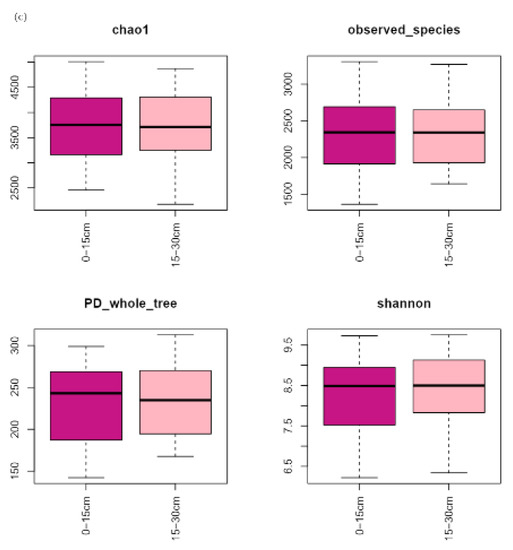
Figure 4.
Soil bacterial α-diversity of different field return treatments: (a) time of straw return, (b) N application management grouping, (c) different return depths.
Conversely, the beta diversity of the straw flora appeared more clearly zoned at different return times (Figure 5a), with 20 and 40 d of the return being closer together, 71 and 104 d being closer together, and 137 d of the return being further apart from the other return times. The beta diversity of the three treatments with 110–130 kg hm−2 of the N application showed more obvious partitioning, and the treatments with a high N application (140 kg hm−2, 150 kg hm−2) had more similar decomposing microflora (Figure 5b). There was clear zoning of beta diversity of straw-degrading bacteria at different return depths (Figure 5c).
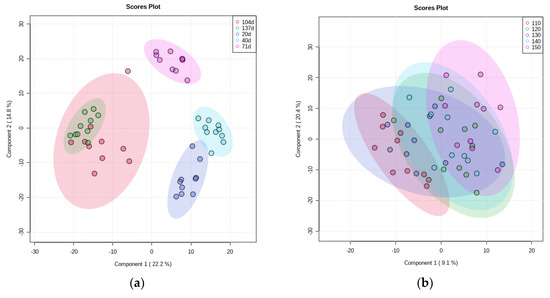
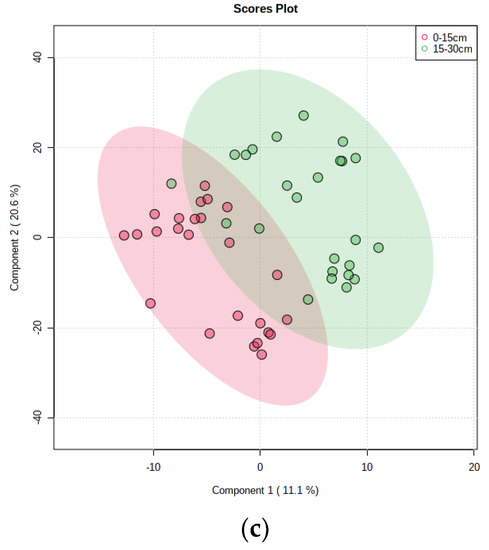
Figure 5.
β diversity analysis based on PLS-DA. (a) Time of straw return, (b) N application management grouping, (c) different return depths.
Paludibacteraceae and Ruminococcaceae of Firmicutes were the dominant bacterial genera in the straw degradation process. The abundance of Paludibacteraceae decreased gradually with time in the early stage (20–71 d), and the abundance of Caulobacteraceae increased gradually in the late stage of straw degradation (104–137 d) (Figure 6a). Under a low N application (110–130 kg hm−2), Paludibacteraceae showed a decreasing trend with the increasing N application, while the abundance of unidentified gradually increased, and under a high N application (140–150 kg hm−2), the abundance of Paludibacteraceae gradually decreased with the increasing N application. The abundance of Prevotellaceae, Spirochaetaceae, and Clostridiaceae-1 gradually increased (Figure 6b). The abundance of Paludibacteraceae and Clostridiacea-1 showed a decreasing trend with an increasing depth of field return, and the relative abundance of Spirochaetaceae gradually increased (Figure 6c).
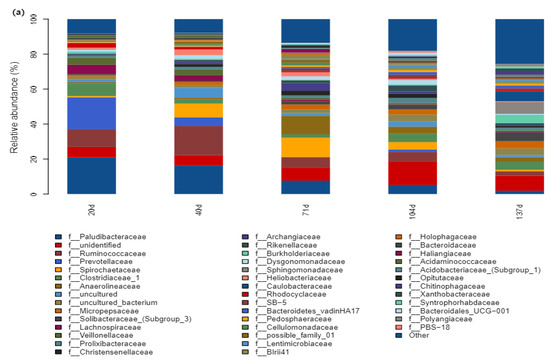
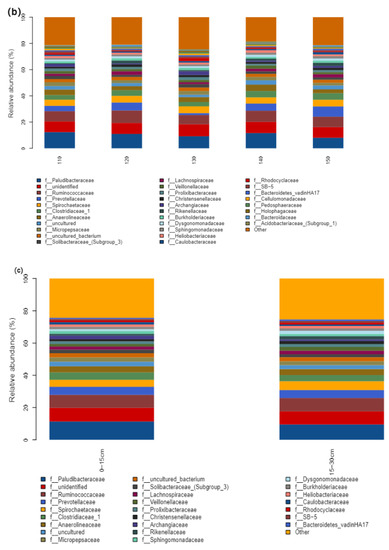
Figure 6.
Bar plot analysis of species composition. (a) Time of straw return, (b) grouping of nitrogen application management, (c) different depths of return; the figure shows the information of species with relative abundance above 1%.
The LEF Se analysis for different lengths of return to the field yielded a total of 328 differential flora for each treatment (Figure 7). In the middle and late stages of straw decomposition, the longer the return time, the more microbial groups were significantly different, with 37 different groups at 20 d (1 at phylum level, 4 at phylum level, 4 at order level, 10 at family level, 14 at genus level, 4 at species level); 18 at 40 d (2 at phylum level, 2 at order level, 2 at order level, 2 at family level, 8 at genus level, 2 at species level); 41 at 71 d (3 at the phylum level, 3 at the order level, 9 at (2 at phylum level); 41 at 71 d (3 at phylum level, 3 at phylum level, 9 at order level, 11 at family level, 11 at genus level, 4 at species level); 52 at 104 d (5 at phylum level, 7 at order level, 11 at order level, 18 at family level, 9 at genus level, 2 at species level); 180 at 137 d (8 at the phylum level), 14 at (35 at the phylum level, 35 at the order level, 48 at the family level, 65 at the genus level, and 10 at the species level). The bacterial community structure was influenced by the amount of nitrogen applied, with significantly different communities at both 130 kg hm−2 and 150 kg hm−2, with Parabacteroides being the dominant group at the genus level for the 150 kg hm−2 treatment, while Geobacteraceae (family level), Geobacter (genus level) and Desulfur Geobacteraceae (family level), Geobacter (genus level) and Desulfuromonadales (order level) were the significantly different microbial groups for the 130 kg hm−2 N treatment. Straw-decomposing bacteria were significantly affected by different depths of return, where Cyanobacteria, Melainabacteria, and Gastranaerophilales were significant groups at 0–15 cm depth of return, while Rikenellaceae, Dysgonomonadaceae, and Gastranaerophilales were significant groups in the 15–30 cm soil layer. Dysgonomonadaceae, and Acetobacteroides were the dominant groups, and the LDA value of Rikenellaceae was the largest.
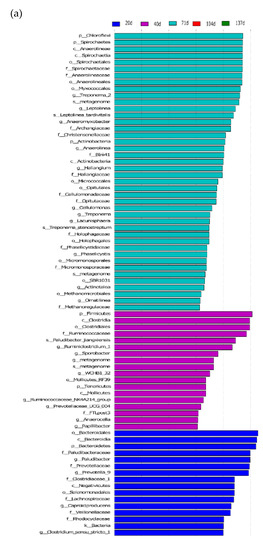
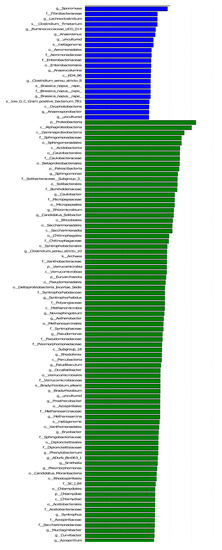
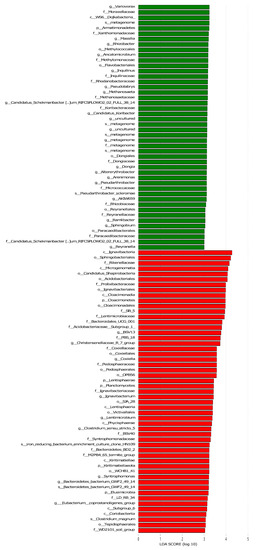
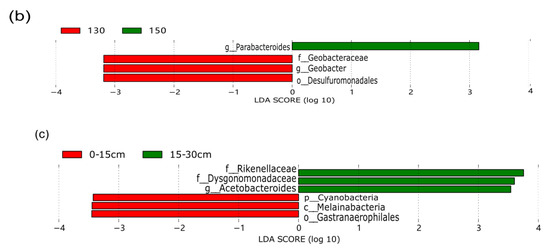
Figure 7.
Linear discriminant analysis (LDA) effect size (LEf Se) was used to determine the main phylotypes of bacterial communities under straw return time, fertilizer application management, and return depth. (a) Straw return time, (b) N application management groups, (c) different return depths; the graphs show species with different LDA scores ≥2, i.e., statistically different biomarkers show species with significantly different abundance in different groups; the length of the bar graph represents the effect size of the significantly different species.
3.4. Effect of Fertilizer Application and Field Return Depth on the Degradability of Returned Straw
The dynamics of C and N-related extracellular enzyme activities during the degradation of straw under different N applications and fielding depth treatments are shown in Figure 8. αG, βG, CBH, NAG, and BX had higher activities in the early stage of fielding (20–40 d). The activities of the 5 extracellular enzymes, except for αG, showed a decreasing trend in the middle of the return period (40–104 d); meanwhile, 8 extracellular enzyme activities significantly increased in the middle and late return period (104 d), when the activities of 6 enzymes, except for αG and βG, reached their maximum values, and the activities of the 8 enzymes decreased after 137 d of return. Extracellular enzyme activities were generally higher for straw degradation with a nitrogen application of 140 kg hm−2 (TU4, TD4). The activities of the eight extracellular enzymes tended to decrease with the increasing depth of return. βG, βX, NAG, and CBH slowly decreased with increasing soil water content in the early stages of straw decay (20–104 d) (Figure 1 and Figure 8b–d). The activities of extracellular enzymes associated with the C-cycle (αG, βG, βX, and CBH) were generally higher than those of other functional extracellular enzymes. Phox and Perox were involved in straw lignin degradation and had high activities at the late stage of degradation (104 d) (Figure 8g,h). Collectively, Phox had the lowest activity during the test period (Figure 2g–h).
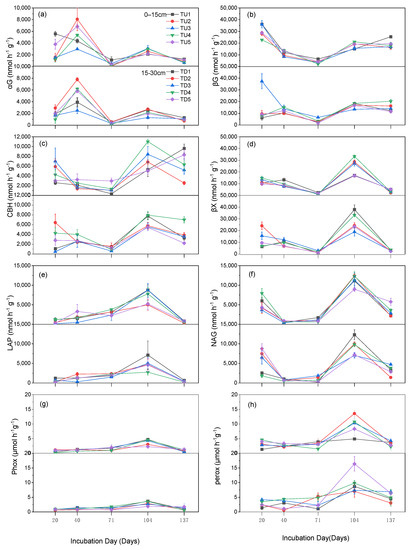
Figure 8.
Changes in the activities of eight extracellular enzymes in different soil layers during the experimental period. Vertical bars indicate the standard error of the mean (n = 3). (a) Change in αG activity; (b) change in βG activity; (c) change in CBH activity; (d) change in βX activity; (e) change in LAP activity; (f) change in NAG activity; (g) change in Phox activity; (h) change in Perox activity.
As shown in Table 3, nitrogen application significantly affected the activities of C-cycle-related extracellular enzymes (αG, βX, βG, and CBH). The depth of return to the field highly significantly affected the activities of C-cycle-related βG, CBH, and N-cycle-related LAP. The time of return was an important factor affecting straw degradation and significantly influenced the activities of the eight extracellular enzymes. The coupling effect of the N application and depth of return, and the coupling effect of the N application and time of return did not affect LAP. Except for LAP, all extracellular enzyme activities were highly significantly influenced by environmental factors of straw decay (nitrogen application, depth of return, and degradation time).

Table 3.
Significance analysis of the effects of fertilizer application, straw return depth, decomposition duration, and their interactions on the activities of eight enzymes.
A two-way ANOVA showed that the fertilizer application regime had a greater effect on soil multifunctionality compared to the depth of return, explaining up to 71% of the variation in soil multifunctionality (Figure 9b). N application significantly affected soil potential functionality, which increased with the increasing N application (110–140 kg hm−2) and was significantly higher in each soil layer at 140 kg hm−2 than in the other treatments, before decreasing at 150 kg hm−2; however, it was still significantly higher than that in the 110–130 kg hm−2 treatments (Figure 9a). The depth of return explained 19% of the variation in soil multifunctionality (Figure 9b), while the same functional variation was observed in different soil layers in response to the N application, and the functional index was significantly higher in the surface soil (0–15 cm) than in the lower soil (15–30 cm) at the same N application (Figure 9a).
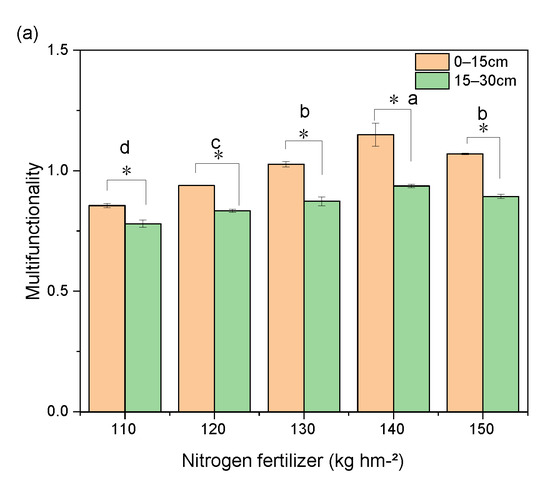
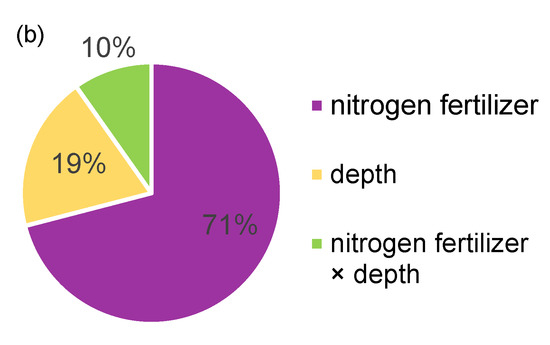
Figure 9.
Factors affecting soil multifunctionality. (a) potential functionality of different soil layers with different nitrogen application rates. The average method was used to calculate soil multifunction. The error bar represents the standard deviation, The small letters indicate the significant difference between different nitrogen application rates. * It indicates that there is a significant difference between different returning depths. (b) effects of different returning measures on potential functions of soil.
3.5. PLS-PM Analysis
To better integrate the complex interrelationships among straw return measures, bacterial community, soil function, and soil multifunctionality, we constructed a partial least squares path model (PLS-PM) (Figure 10). The indirect effects of coupled N application and straw return measures on soil multifunctionality were attributed to changes in soil bacterial species richness and bacterial composition. The PLS-PM explained 71% of the potential functional changes in soil due to straw decay after a return; the N application had a significantly strong positive effect on bacterial species richness and was the most important factor affecting soil multifunctionality, with a total effect of 0.63 on soil multifunctionality (Table 4). Conversely, the depth of return had a negative effect on the bacterial community structure and bacterial richness, with a total effect of −0.24 on soil multifunctionality, indicating that the depth of return of 0–15 cm was more favorable to improve potential soil functionality compared to 15–30 cm. Bacterial abundance had a strong positive effect on soil multifunctionality, with a total effect of 0.62, while N application was the most important factor affecting soil multifunctionality, with a total effect of 0.66.
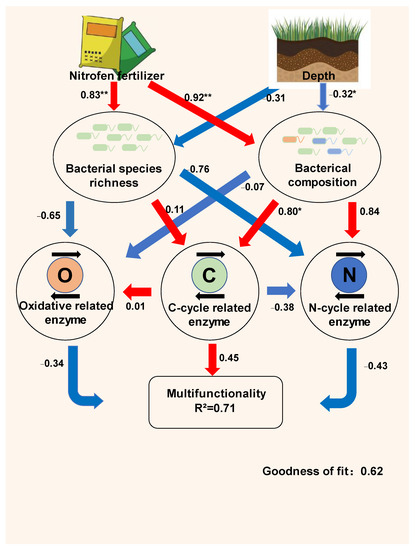
Figure 10.
Relationship between different factors on soil multifunctionality. The numbers adjacent to the arrows indicate coefficients. The red and blue lines indicate positive and negative relationships, respectively. R2 indicates the proportion of explained variance. The mean method was used to calculate the multifunctionality of the soil. * p < 0.05, ** p < 0.01.

Table 4.
Direct and indirect relationship between variables. After 1000 times of guidance, the path coefficients are calculated using PLS-PM.
4. Discussion
4.1. Effect of Straw Return to the Field in Cooler Areas on Decomposing Bacteria
Returned straw can provide soil microorganisms with sufficient amounts of easily decomposable substrate, and the decay process of returned straw is a microbial-driven biochemical process [32]. In this study, we found that microorganisms showed different community structures at different decomposition lengths (Figure 4, Figure 5, Figure 6 and Figure 7), with Bacteroidales, Firmicutes, and other bacteria that decompose plant fibers and other polysaccharides as the main dominant groups in the early stage of straw return (20–40 d); in the middle stage of return (71 d), Chloroflexi, Anaerolineae, and other bacteria that decompose carbohydrates were the dominant flora; in the late stage of straw return (104–137 d), Proteobecteria and Sphingobacteriales, which decompose aromatic compounds that are difficult to decompose, were the dominant microorganisms (Figure 6a). This is due to the fact that microorganisms are guided by the substances released from the returned straw at different periods to favor the decomposition of specific compounds, changing their community structure with the chemical composition of the residues at different periods of straw decomposition (Figure 3) [33,34,35,36,37,38].
Fertilizer application affects the microbial community of soil nutrient turnover, and Wu et al. [39] found that the soil bacterial community structure was more sensitive to straw return with fertilizer treatment than fertilizer application alone, and γ-amastigotes showed different degrees of response characteristics to different fertilizer treatments. In this experiment, microbial diversity and community structure fluctuated to different degrees under different nitrogen applications (Figure 4, Figure 5, Figure 6 and Figure 7), which were similar to the results of previous studies [40], indicating that the addition of exogenous nitrogenous fertilizer can regulate the carbon to nitrogen ratio of the environment in which microorganisms live [41]. Geobacteraceae and Parabacteroides were the dominant genera at high N application rates (130 kg hm−2, 150 kg hm−2) (Figure 9), and Parabacteroides facilitated further cellulose decomposition, while Geobacteraceae facilitated the decomposition of organic matter by promoting the heterotrophic process in rice soils. The iron reduction process facilitates the metabolism of organic matter [42]. It was further shown that high nitrogen application can reduce the carbon to nitrogen ratio, thus promoting the decomposition of straw and the release of nutrients.
Bacterial diversity increased with deeper straw decomposition (Figure 4), and the depth of return affected the microbial community structure and activity through changes in soil temperature and water content (Figure 1, Figure 5 and Figure 7), and the tillage layer (0–15 cm) provided a suitable soil environment and aeration for microorganisms compared to the deeper soil layer of 15–30 cm; this ensured that Cyanobacteria, Melainabacteria, and Gastranaerophilales, which grow in an aerobic environment, were the prominent flora, while aerobic or partly anaerobic bacteria such as Rikenellaceae, Dysgonomonadaceae, and Acetobacteroides were the dominant genera in 15–30 cm (30 cm of the dominant genera (Figure 7)). The decomposition rate of crop straw under anaerobic conditions is usually slow and incomplete compared to aerobic conditions [19].
4.2. Effect of Straw Return to the Field in Cooler Areas on Potential Soil Functions
Straw closely combines its own properties with the ability to absorb nutrients due to microbial metabolism through extracellular enzyme activities associated with the decomposition process [43]. The decomposition of straw after returning to the field is a process of the variability of C and N elements, so the determination of the dynamics of C and N-related enzyme activities can reveal to some extent the process of C and N effectiveness during decomposition [44,45,46]. It is generally believed that straw will be preferentially decomposed by water-soluble compounds such as sugar and starch and easily decomposed substances under the action of acetylaminoglucosidase and leucoaminopeptidase in the pre-decomposition stage, and the activities of the two enzymes will decrease with the decrease of degraded substrates [47,48]. However, the results of the present experimental study did not fully conform to the above-mentioned pattern, and the percentage of soluble material decreased rapidly in the early stage of returning to the field (Figure 3). Nevertheless, the characteristics of changes in the activities of the two enzymes were significantly different. For example, although the changes in the activity of acetylaminoglucosidase in the early (about 0–40 d) and middle (40–71 d) stages of decomposition were consistent with the results of previous studies, it still showed strong enzymatic activity in the middle and late stages; meanwhile, the activity of leucoaminopeptidase kept increasing in the early and middle stages of straw decomposition, and the highest period of enzymatic activity appeared in the middle and late stages of decomposition (Figure 8). This is due to the fact that straw-decomposing microorganisms are sensitive to soil’s water content and temperature [49,50], and the higher water content and soil temperature in the middle stage of decomposition (20–71 days) reduce the related enzyme activity to some extent and inhibit the degradation rate of the corresponding substances (Figure 1). In the middle and late stages of decomposition (about 104 d), the returned straw provided a better soil environment for decomposition-related extracellular enzymes and microorganisms, and under the coupling effect of both, the straw entered a rapid decomposition period, and the water-soluble compounds and easily decomposed substances that had not been completely degraded in the early stage provided substrates for acetylaminoglucosidase and leucovorin aminopeptidase, making the extracellular enzyme activity increase. Compared with the degradation of returned straw under warm-temperate flooding conditions, the rapid decomposition period of paddy straw in cooler regions of northeast China was delayed by nearly 30 d. Meanwhile, the C-cycle-related enzyme activities of returned straw under double-season rice cultivation practices in southern China continued to trend upward in the front course of the rapid decomposition period [51]. In the present study, the addition of exogenous nitrogenous fertilizer increased acetylaminoglucosidase and leucoaminopeptidase to some extent, but excessive nitrogen application (TU5 and TD5) had an inhibitory effect on enzyme activity. The reason for this is that excessive exogenous nitrogen fertilization increases the effectiveness of carbon and nitrogen required for microbial growth, and excessive inorganic nitrogen effectiveness can have an inhibitory effect on the N-related enzyme activity (Figure 10) [52].
With the depletion of easily decomposable substances, difficult to degrade macromolecules such as cellulose, hemicellulose, and lignin enter a period of rapid degradation (Figure 3), and the degradation of these substances requires the synergistic cooperation of multiple enzymes [53], which also leads to a significant increase in the activity of four extracellular enzymes associated with C conversion (α-glucosidase, β-glucosidase, β-xylosidase, and β-cellulodiglucosidase) at a later stage (Figure 8). During cellulose catabolism, β-cellulodiglucosidase initially breaks down cellulose into oligosaccharides, which are further broken down into small molecules of glucose by α-glucosidase and β-glucosidase [54,55]. β-xylosidase plays an important role in xylose degradation, degrading xylan and oligosaccharides into small molecules of xylose [56]. Hydrolases are mainly bound to particulate organic matter, and hydrolases are released for decomposition along with the breakdown of particulate organic matter. In this experiment, phenoloxidase and peroxidase did not show a regular increase under the application of exogenous nitrogenous fertilizer and even showed inhibition, which is similar to the results of previous studies [57]. Lower phenoloxidase facilitates carbon fixation [58] and protects organic carbon material containing phenols in an anaerobic environment [59].
The environment of straw decomposition has a huge impact on it, and generally, a soil temperature of 20–30 °C and soil moisture content of 60–80% WHC are most suitable for the decomposition of organic material [46,56]. Temperature and moisture content affect the straw decomposition process by influencing microbial activity and thus the straw decomposition process, and temperature indirectly affects the decomposition of organic material by affecting the production of extracellular enzymes by microorganisms [60]. The lower soil temperature and water content in the later stages of the return period inhibited the degradation rate of straw (Figure 2), and the percentage of lignin and other substances in the residual straw increased in this period with the preferential degradation of soluble substances and other components (Figure 3). It was found that the enzymatic activity of paddy straw returned to the field was higher in the cooler regions of northeast China compared to southern China when entering the rapid decomposition period, presumably due to the soil environment in northeast China providing more abundant microorganisms for straw fractionation [51]. Within a certain range, microbial activity was positively correlated with temperature, and the microbial production of extracellular enzymes was inhibited when substrate depletion was insufficient. It is therefore hypothesized that the lower water content (Figure 1) and soil nutrients (Table 1) at 15–30 cm affected the microbial activity, resulting in a generally lower activity of the relevant enzymes corresponding to the deep straw.
4.3. Effect of Agricultural Management Practices on Soil Functionality
From a functional point of view, soil enzyme activity produced by various microorganisms is the main biological mechanism for organic matter decomposition and nutrient cycling [61]. The direct return of straw to the field will be followed by microbial and enzymatic decomposition, with nitrogen levels being a key factor affecting its biological processes [62]. The application of nitrogenous fertilizer increased the carbon cycle of the soil, which is similar to the results of previous studies, indicating that the application of nitrogenous fertilizer increases the activity of most soil enzymes involved in the mineralization and decomposition of organic carbon and nitrogen [63,64]. An investigation and analysis by Mao Hui [39] based on 65 fertilization experiments worldwide showed that nitrogenous fertilizer application stimulates hydrolytic enzyme activity but also inhibits oxidative enzyme activity, and similar results were also shown in this study (Figure 10). It has been found that soil bacteria will be more sensitive to changes caused by agricultural production practices compared to fungi, and that long-term biochemical and biological responses of soil bacteria to fertilization practices play a more active role in maintaining nutrient turnover and land use sustainability than fungi [24]. In this experiment, the bacterial community structure and species richness occurred to varying degrees in response to both the nitrogenous fertilizer addition and straw return depth under straw return conditions, with the nitrogenous fertilizer application contributing highly significantly to bacterial species richness and community structure (Figure 10) and being the most important factor affecting potential soil functionality under straw return conditions (Table 4). Furthermore, it was found that carbon cycling during straw degradation promotes redox reactions (Figure 10), presumably due to the porous structure created by straw degradation that helps accelerate oxygen transfer [65].
5. Conclusions
Agricultural management practices can affect soil biomes in different ways, leading to changes in farmland function and productivity through soil biological processes. In this study, by developing a PLS-PM model of the straw decomposition process in cold areas, we found that fertilizer application under full straw return to rice fields in cold areas was the most important agricultural measure affecting soil function, and it had a highly significant positive correlation with potential soil functionality. Meanwhile, bacterial species richness and species composition were very sensitive to changes in nitrogen fertilization. The depth of straw return was negatively correlated with soil functionality. In addition, decomposition time can significantly affect soil potential functionality through its effects on extracellular enzyme activity and microorganisms, and the degradation of straw in paddy fields in cooler regions of northeast China is mainly concentrated in the early (0–20 d) and mid-late (71–104 d) stages of field return compared to warm temperate regions. Under the cool environmental conditions in northeast China, a longer return time is required to facilitate the release of nutrients from straw and improve soil functionality.
Author Contributions
Data curation, L.L. and M.F.; Investigation, L.L., M.C. and L.Y.; Methodology, L.L. and J.J.; Supervision, M.F.; Writing—original draft, L.L.; Writing—review & editing, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Jilin Province, China (YDZJ202201ZYTS527); China National Key R&D Program Subproject (2018YFD0300206-2); China Jilin Province Agricultural Science and Technology Innovation Project Postgraduate Fund (CXGC2021RCY031).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, D.X.; Han, Z.Q.; Li, D.P. Effects of straw return under different decay promotion conditions on the dynamics of soil microbial carbon, nitrogen and phosphorus. Chin. J. Appl. Ecol. 2005, 16, 1903–1908. [Google Scholar]
- Lal, R. Residue management, conservation tillage and soil restoration for mitigating greenhouse effect by CO2-enrichment. Soil Till. Res. 1997, 43, 81–107. [Google Scholar] [CrossRef]
- Peng, C.Y.; Luo, H.; Kong, J. Research progress of crop straw resource estimation and utilization status in China. Chin. J. Agric. Resour. Reg. Plan. 2014, 3, 14–20. [Google Scholar] [CrossRef]
- Cui, M.M.; Jiang, L.; Yan, T. Potential evaluation and market assessment on crop straw resource utilization based on resource density. J. China Agric. Univ. 2016, 21, 117–131. [Google Scholar]
- Dou, S. Progress in the formation and transformation of soil humic substances and their microbiological mechanisms. J. Jilin Univ. 2008, 4, 538–547. [Google Scholar]
- Dilly, O.; Bloem, J.; Vos, A.; Munch, J.C. Bacterial diversity during litter decomposition in agricultural soils. Appl. Environ. Microb. 2004, 70, 468–474. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilized by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Marschner, P.; Umar, S.; Baumann, K. The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol. Biochem. 2011, 43, 445–451. [Google Scholar] [CrossRef]
- Zhou, G.X.; Zhang, J.B.; Chen, L.; Zhang, C.Z.; Yu, Z.H. Temperature and straw quality regulate the microbial phospholipid fatty acid composition associated with straw decomposition. Pedosphere 2016, 26, 386–398. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Wang, F.; Jiang, Y.; Zhang, X. Assessing the relative effects of geographic location and soil type on microbial communities associated with straw decomposition. Appl. Environ. Microb. 2013, 79, 3327–3335. [Google Scholar] [CrossRef]
- Rengel, Z. The role of crop residues in improving soil fertility. In Nutrient Cycling in Terrestrial Ecosystems; Springer: Berlin/Heidelberg, Germany, 2007; pp. 183–214. [Google Scholar] [CrossRef]
- Li, W.; Qiao, Y.Q.; Chen, H.; Cao, C.F.; Du, S.Z.; Zhao, Z. Effect of corn straw return with nitrogen fertilizer on the apparent nitrogen gain/loss and yield of winter wheat soil. J. Plant Nutr. Fertil. 2015, 21, 561–570. [Google Scholar]
- Raiesi, F. Carbon and N mineralization as affected by soil cultivation and crop residue in a calcareous wetland ecosystem in Central Iran. Agric. Ecosyst. Environ. 2006, 112, 13–20. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Q.; Ai, C.; Liang, G.; He, P.; Zhou, W. Nitrogen enrichment regulates straw decomposition and its associated microbial community in a double-rice cropping system. Sci. Rep. 2018, 8, 1847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liang, G.Q.; Guo, T.F.; He, P.; Wang, X.B.; Zhou, W. Evident variations of fungal and actinobacterial cellulolytic communities associated with different humified particle-size fractions in a long-term fertilizer experiment. Soil Biol. Biochem. 2017, 113, 1–13. [Google Scholar] [CrossRef]
- Hassink, J.; Bouwman, L.A.; Zwart, K.B.; Brussaard, L. Relationships between habitable pore space, soil biota and mineralization rates in grassland soils. Soil Biol. Biochem. 1993, 25, 47–55. [Google Scholar] [CrossRef]
- Jenkinson, D. Studies on decomposition of plant material in soil. V The effects of plant cover and the soil type on the loss of carbon from 14C-labelled rye grass decomposition under field conditions. J. Soil Sci. 1997, 28, 424–434. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Steiner, J.L.; Unger, P.W. Decomposition and nitrogen dynamics of crop residues: Residue quality and water effects. Soil Sci. Soc. Am. J. 1994, 58, 372–381. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Organic matter accumulation in submerged soils. Adv. Agron. 2004, 81, 169–201. [Google Scholar] [CrossRef]
- Ma, X.G.; Li, C.; Yuan, X.F.; Zhu, W.; Wang, S.F.; Procedure, C.Z. Methane production process by fermentation of high solids content straw and cow dung mixture. J. Agric. Eng. 2014, 30, 227–235. [Google Scholar]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soi Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. JoVE 2013, 81, e50961. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Miller, G.E.; Engen, P.A.; Gillevet, P.M.; Shaikh, M.; Sikaroodi, M.; Forsyth, C.B.; Mutlu, E.; Keshavarzian, A. Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PLoS ONE 2016, 11, e0148952. [Google Scholar] [CrossRef] [PubMed]
- Oberauner, L.; Zachow, C.; Lackner, S.; Högenauer, C.; Smolle, K.H.; Berg, G. The ignored diversity: Complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci. Rep. 2013, 3, 1413. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-G´omez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Byrnes, J.E.; Gamfeldt, L.; Isbell, F.; Lefcheck, J.S.; Griffin, J.N.; Hector, A.; Cardinale, B.J.; Hooper, D.U.; Dee, L.E.; Duffy, J.E. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 2014, 5, 111–124. [Google Scholar] [CrossRef]
- Aneja, M.K.; Sharma, S.; Fleischmann, F.; Stich, S.; Heller, W.; Bahnweg, G.; Munch, J.C.; Schloter, M. Microbial Colonization of Beech and Spruce Litter-Influence of decomposition site and plant litter species on the diversity of microbial community. Microb. Ecol. 2006, 52, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tang, X.H.; Lv, J.K.; Luo, Y.; Wei, C. Formation of huminic acid in purple rice soils: A rice straw decomposition experiment. J. Agric. Environ. Sci. 2009, 28, 2596–2602. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Tanahashi, T.; Murase, J.; Matsuya, K.; Asakawa, S.; Kimura, M. Microbial communities responsible for the decomposition of rice straw compost in a Japanese rice paddy field determined by phospholipid fatty acid (PLFA) analysis. Soil Sci. Plant Nutr. 2004, 50, 1229–1236. [Google Scholar] [CrossRef]
- Cox, P.; Wilkinson, S.P.; Anderson, J.M. Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinussylvestris litter. Biol. Fertil. Soils 2001, 33, 246–251. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jacson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, S.H.; Li, S.H.J.; Deng, Y. Advances in molecular ecology of microbial carbon cycle based on functional genes. Microbiol. Bull. 2017, 44, 1676–1689. [Google Scholar] [CrossRef]
- Wu, M.; Qin, H.; Chen, Z.; Wu, J.; Wei, W. Effect of long-term fertilization on bacterial composition in rice paddy soil. Biol. Fertil. Soils 2011, 47, 397–405. [Google Scholar] [CrossRef]
- Whittinghill, K.A.; Currie, W.S.; Zak, D.R.; Burton, A.J.; Pregitzer, K.S. Anthropogenic N deposition increases soil C storage by decreasing the extent of litter decay: Analysis of field observations with an ecosystem model. Ecosystems 2012, 15, 450–461. [Google Scholar] [CrossRef]
- Zhang, S.H.; Shi, Z.L.; Yang, S.J.; Gu, K.; Dai, T.; Wang, F.; Li, X.; Sun, R. Effects of nitrogen application and straw return on nutrient balance and yield of late-sown wheat. J. Appl. Ecol. 2015, 26, 2714–2720. [Google Scholar] [CrossRef]
- Mao, H. Reduction of Dissimilatory Iron in Rice Soils and Its Effect on the Reduction of Chromium (IV); Northwest Agriculture and Forestry University: Xianyang, China, 2005. [Google Scholar]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22, pp. 229–243. [Google Scholar]
- Xia, X.; Zhang, P.; He, L.; Gao, X.; Li, W.; Zhou, Y.; Li, Z.; Li, H.; Yang, L. Effects of tillage managements and maize straw returning on soil microbiome using 16S rDNA sequencing. J. Integr. Plant Biol. 2019, 61, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ge, Y.; Zhu, Q.; Yu, J.; Zhou, Q.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Soil nitrogen balance and nitrogen utilization of winter wheat affected by straw management and nitrogen application in the Yangtze river basin of China. Arch. Agron. Soil Sci. 2019, 65, 1–15. [Google Scholar] [CrossRef]
- Yao, X.H.; Min, H.; Lü, Z.H.; Yuan, H.P. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2005, 42, 120–126. [Google Scholar] [CrossRef]
- McMahon, S.K.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. Soil Sci. Soc. Am. J. 2005, 69, 1238–1247. [Google Scholar] [CrossRef]
- Baumann, K.; Marschner, P.; Smernik, R.J.; Baldock, J.A. Residue chemistry and microbial community structure during decomposition of eucalypt, wheat and vetch residues. Soil Biol. Biochem. 2009, 41, 1966–1975. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.F.; Wang, H.; Zheng, J.B.; Bao, J.X.; Gao, M. Effects of soil moisture and plant residues on organic carbon mineralization in purple rice soils. J. Plant Nutr. Fertil. 2007, 13, 1013–1019. [Google Scholar] [CrossRef]
- Avrahami, S.; Liesack, W.; Conrad, R. Effect of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 2003, 5, 691–705. [Google Scholar] [CrossRef]
- Guo, T. Mechanism of Carbon and Nitrogen Interactions in the Decomposition of Straw in Rice Fields; Chinese Academy of Agricultural Sciences: Beijing, China, 2019. [Google Scholar]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Wang, W.J.; Baldock, J.A.; Dalal, R.C.; Moody, P.W. Decomposition of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol. Biochem. 2004, 36, 2045–2058. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Seidl, V.; Seiboth, B. Plant cell wall and chitin degradation. In Cellulose and Molecular Biology of Filamentous Fungi; Borkovish, K.A., Ebbole, D.J., Eds.; ASM Press: Washington, DC, USA, 2010; Volume 27, pp. 396–413. [Google Scholar]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: The growth rate hypothesis in reverse. Biogeochemistry 2011, 102, 31–43. [Google Scholar] [CrossRef]
- Knob, A.; Terrasan, C.R.F.; Carmona, E.C. p-Xylosidases from filamentous fungi: An overmew. World J. Microbiol. Biotechnol. 2010, 26, 389–407. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.J.; Sun, R.J.; Lin, J.H. Correlation between soil enzyme activity and fertility factors under different long-term fertilization practices. Ecol. Environ. 2008, 2, 688–692. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 2001, 409, 149. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qian, H.; Huang, G.; Fan, Z.; Fang, Y. Straw return to field and its research progress. J. Agron. 2012, 2, 1–4+28. [Google Scholar]
- Muhammad, W.; Vaughan, S.M.; Dalal, R.C.; Menzies, N.W. Crop residues and fertilizer nitrogen influence residue decomposition and nitrous oxide emission from a Vertisol. Biol. Fertil. Soils 2011, 47, 15–23. [Google Scholar] [CrossRef]
- Giacometti, C.; Cavani, L.; Baldoni, G.; Ciavatta, C.; Marzadori, C.; Kandeler, E. Microplate-scalefluorometric soil enzyme assays as tools to assess soil quality in a long-term agriculturalfield experiment. Appl. Soil Ecol. 2014, 75, 80–85. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Dai, X.; Schaeffer, S.; Yang, F.; Radosevich, M.; Xu, L.; Liu, X.; Sun, X. Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci. Total Environ. 2015, 536, 59–67. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe−N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbial fuel cell. Int. J. Agric. Biol. Eng. 2020, 13, 207–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).