Exploration of Hydrogeochemical Characterization and Assessment of Organic Pollution Characteristics of Shallow Groundwater near a Chemical Plant That Discharged Sewage Illegally
Abstract
1. Introduction
2. Study Area
3. Methods and Materials
3.1. Sampling and Measurements
3.2. Correlation Analysis
3.3. Principal Component Analysis
3.4. Data Analysis
3.5. Quality Standards and Control Methods
4. Results and Discussion
4.1. General Characteristics of GW Hydrochemistry
4.2. Hydrochemical Types of GW
4.3. Correlation Analysis
4.4. Principal Component Analysis
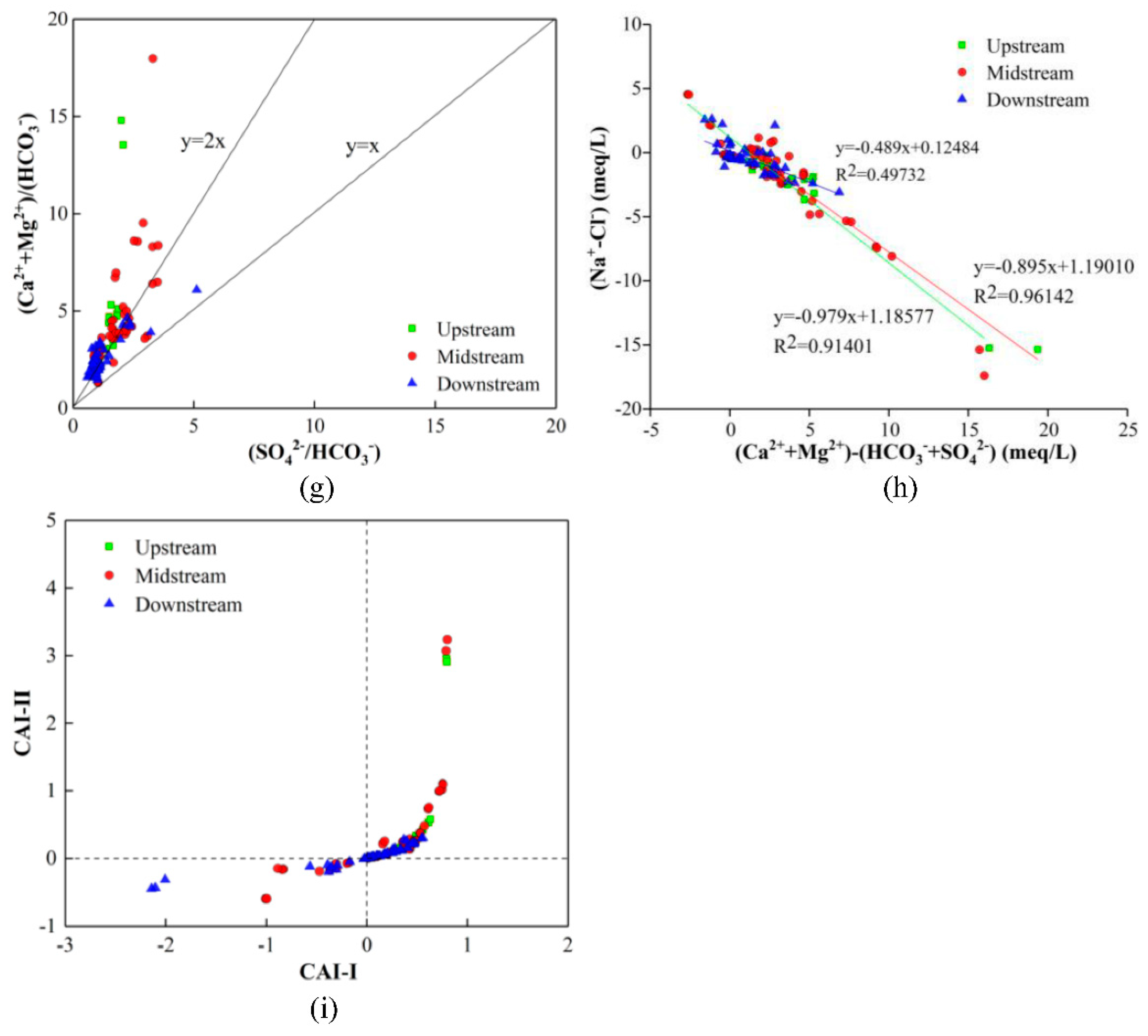
4.5. Natural Processes Controlling the Formation of GW Chemistry
4.6. Mechanisms Responsible for the Formation of GW Hydrochemistry
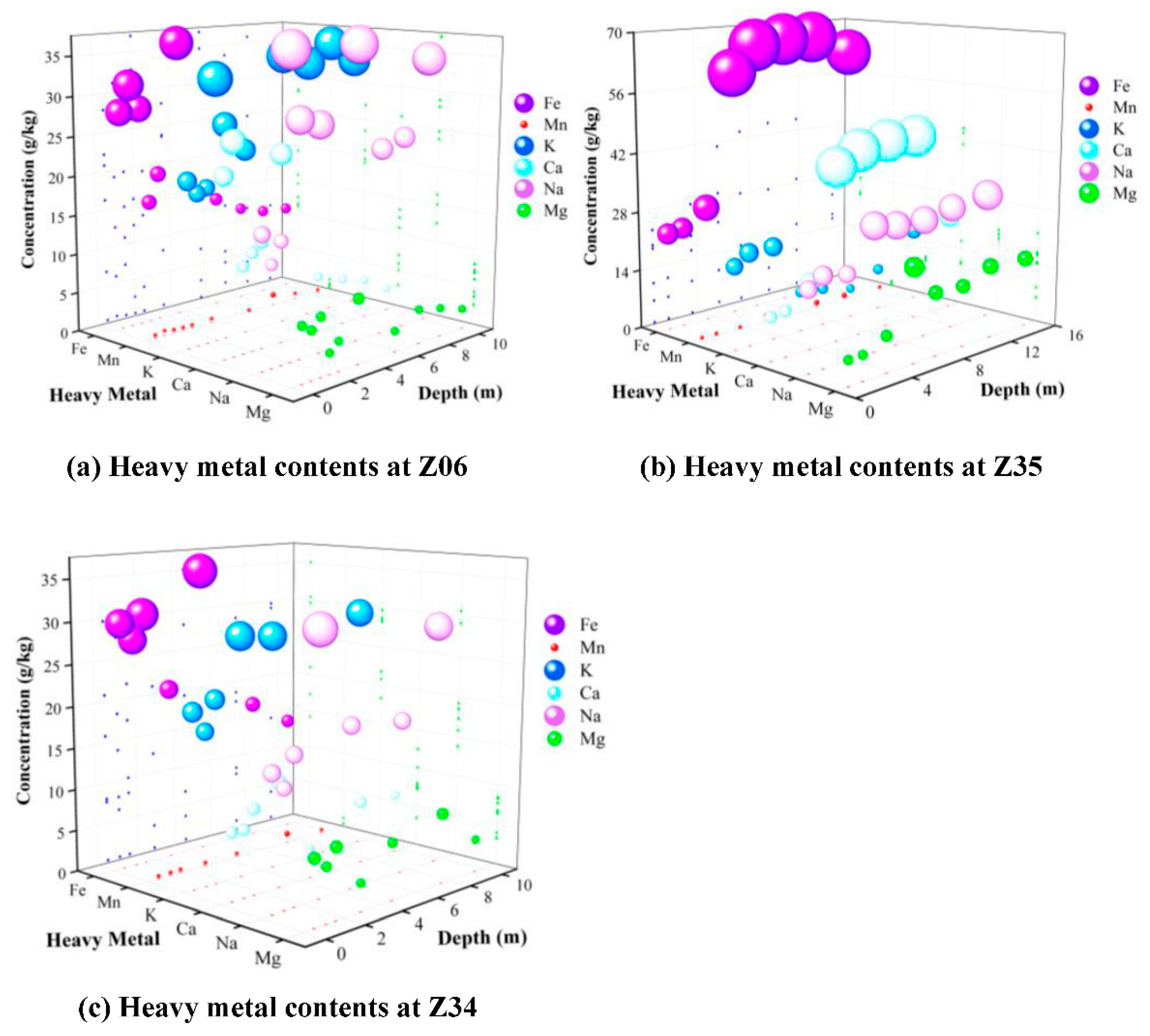
4.7. Detection of Components in Soil
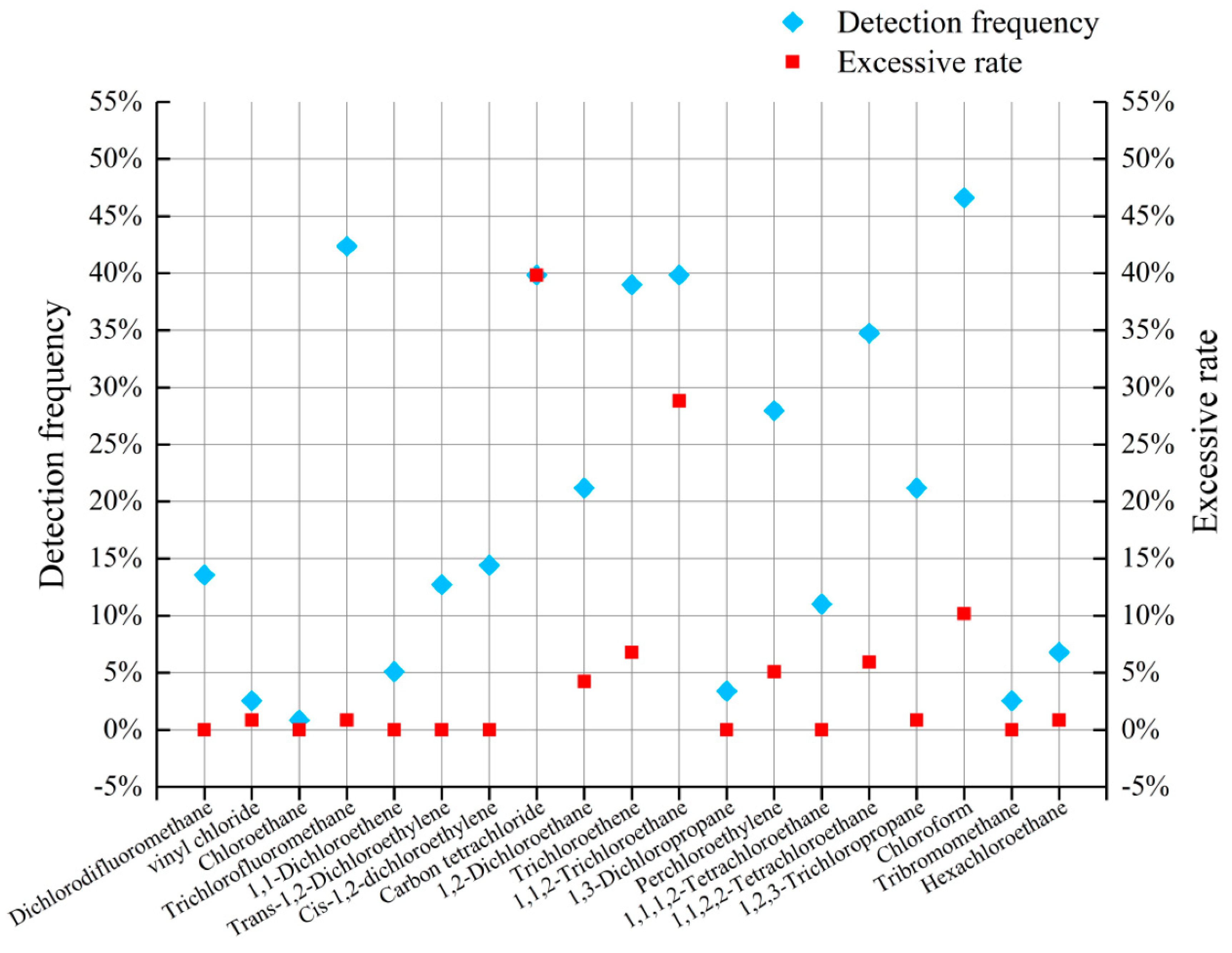
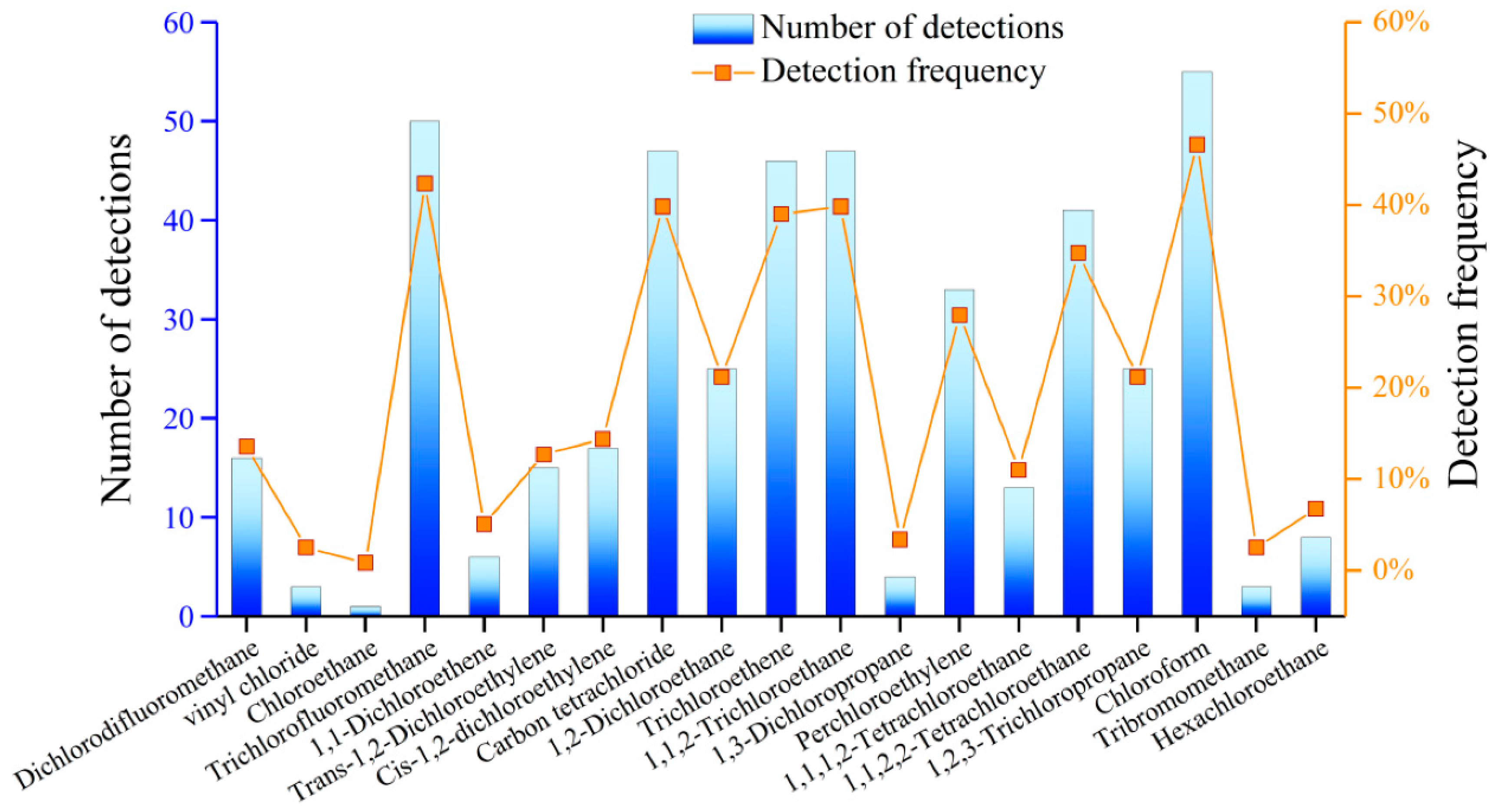
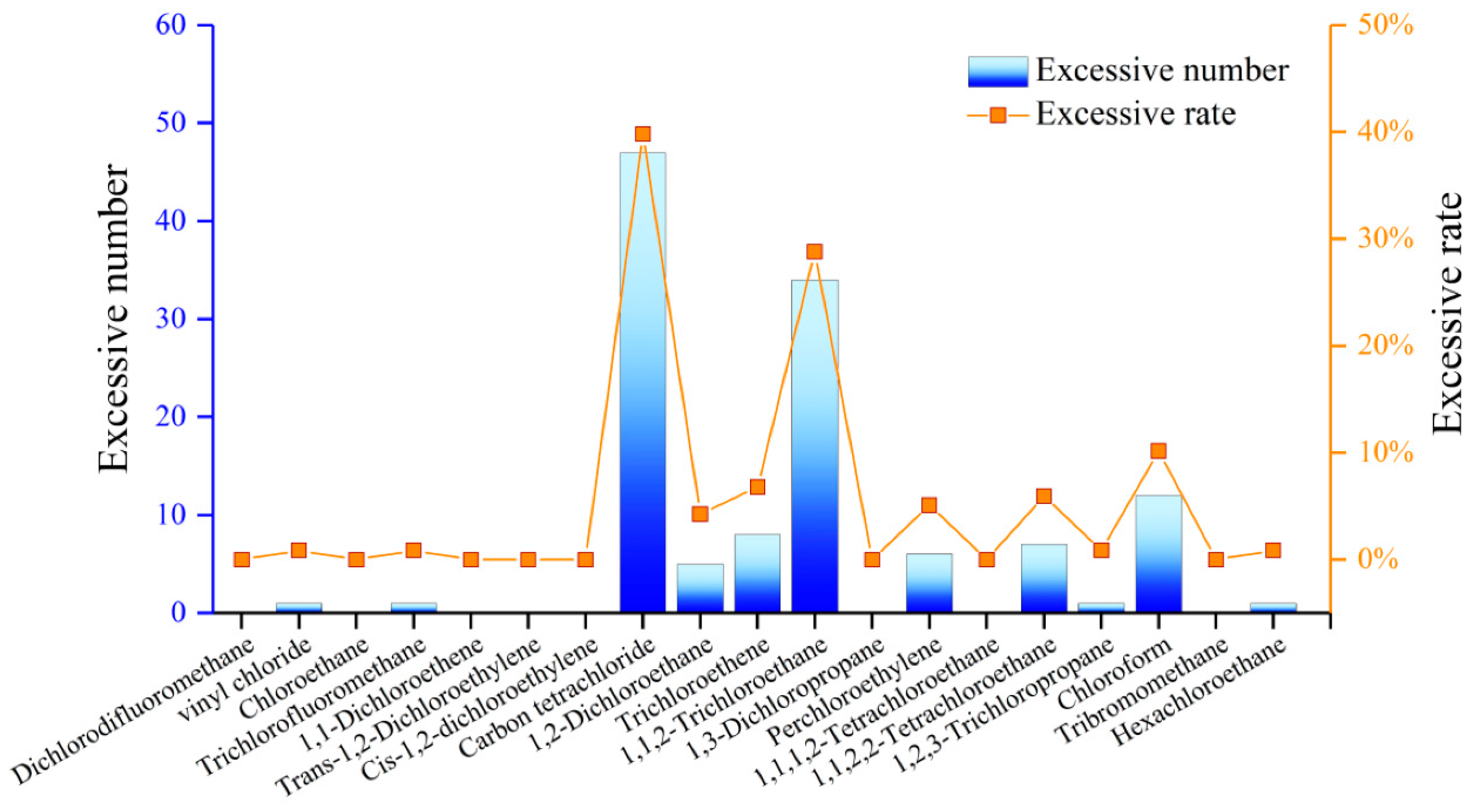
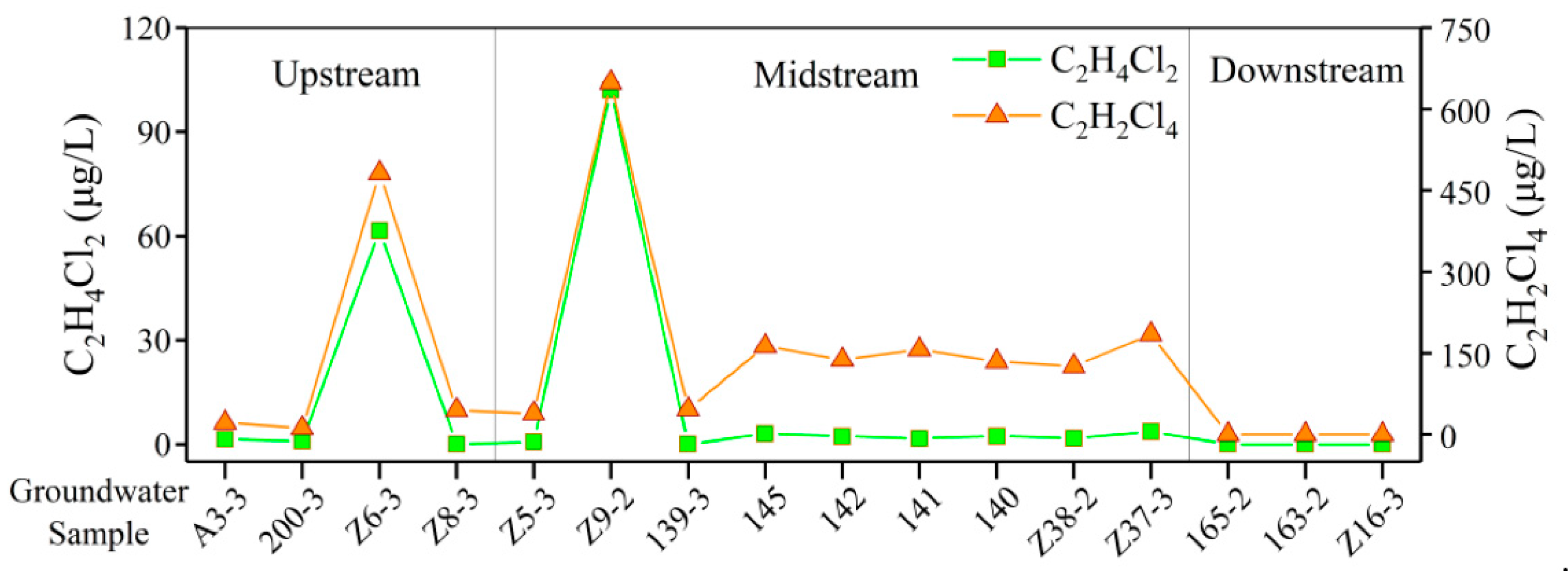
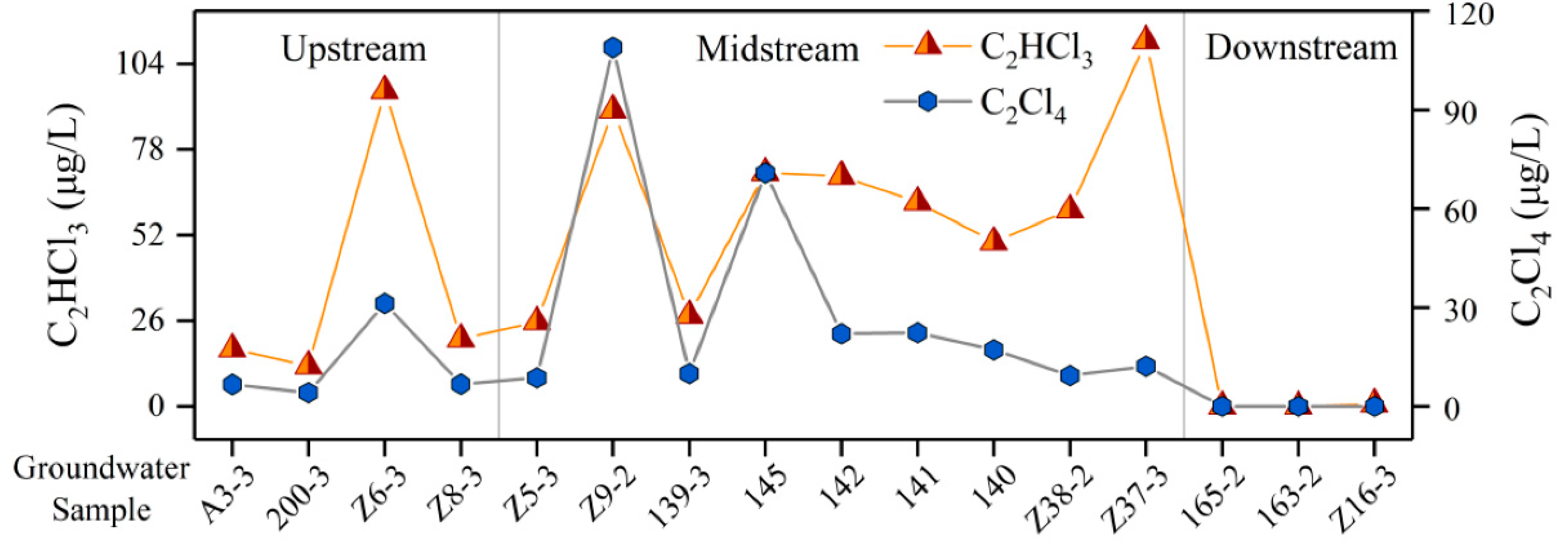
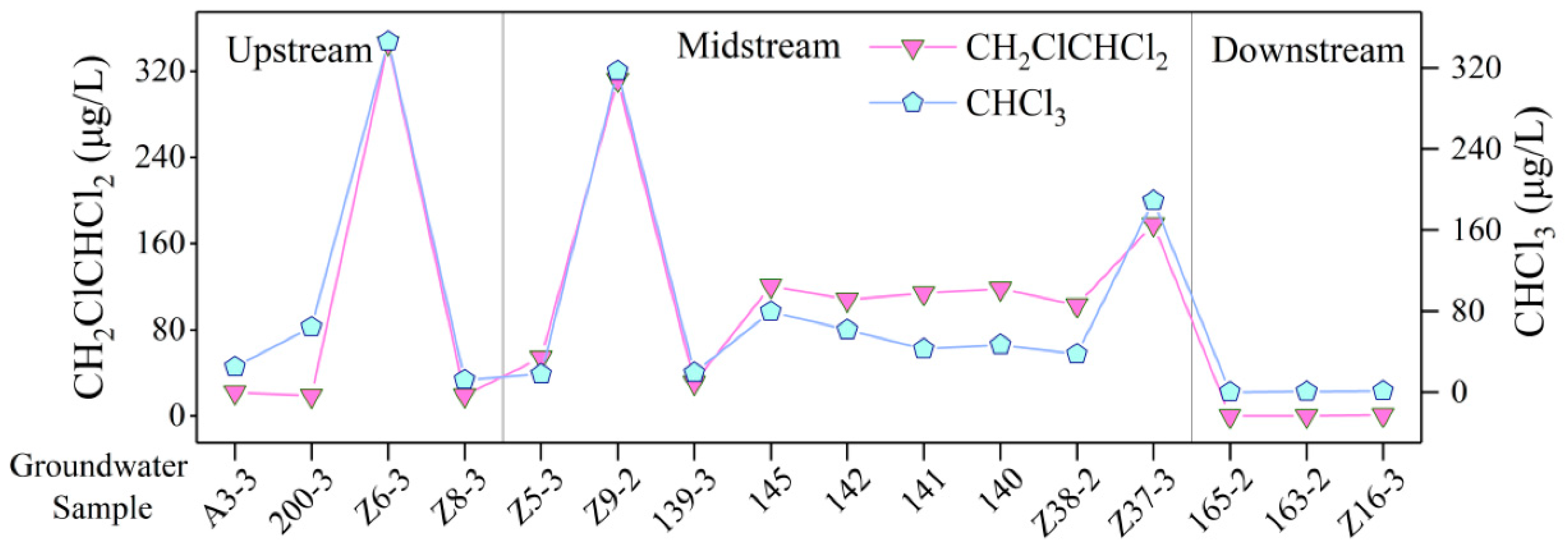
4.8. Detection of Organic Components in GW
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aeschbach-Hertig, W.; Gleeson, T. Regional strategies for the accelerating global problem of groundwater depletion. Nat. Geosci. 2012, 5, 853–861. [Google Scholar] [CrossRef]
- An, D.; Xi, B.D.; Wang, Y.; Xu, D.; Tang, J.; Dong, L.C.; Ren, J.Z.; Pang, C.F. A sustainability assessment methodology for prioritizing the technologies of groundwater contamination remediation. J. Clean. Prod. 2016, 112, 4647–4656. [Google Scholar] [CrossRef]
- Jasechko, S.; Perrone, D.; Befus, K.M.; Cardenas, M.B.; Ferguson, G.; Gleeson, T.; Luijendijk, E.; McDonnell, J.J.; Taylor, R.G.; Wada, Y.; et al. Global aquifers dominated by fossil groundwaters but wells vulnerable to modern contamination. Nat. Geosci. 2017, 10, 425–429. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Qian, H.; Ren, W.H.; Wang, H.K.; Liu, F.X.; Yang, F.X. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Cai, B.M.; Liu, B.B.; Zhang, B. Evolution of Chinese urban household’s water footprint. J. Clean. Prod. 2019, 208, 1–10. [Google Scholar] [CrossRef]
- Paca, J.M.; Santos, F.M.; Pires, J.C.M.; Leitao, A.A.; Boaventura, R.A.R. Quality assessment of water intended for human consumption from Kwanza, Dande and Bengo rivers (Angola). Environ. Pollut. 2019, 254, 113037. [Google Scholar] [CrossRef]
- Devic, G.; Djordjevic, D.; Sakan, S. Natural and anthropogenic factors affecting the groundwater quality in Serbia. Sci. Total Environ. 2014, 468–469, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Wang, M.; Gao, Z.J.; Chen, Q.; Wu, G.W.; Li, F.Q. Hydrochemical characteristics and water quality assessment of groundwater in the Yishu River basin. Acta Geophys. 2020, 68, 877–889. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhang, Z.J.; Fei, Y.H.; Chen, H.H.; Qian, Y.; Dun, Y. Investigation of quality and pollution characteristics of groundwater in the Hutuo River Alluvial Plain, North China Plain. Environ. Earth Sci. 2016, 75, 581. [Google Scholar] [CrossRef]
- Pignotti, E.; Dinelli, E.; Birke, M. Geochemical characterization and rare earth elements anomalies in surface- and groundwaters of the Romagna area (Italy). Rendiconti Lincei 2017, 28, 265–279. [Google Scholar] [CrossRef]
- Babiker, I.S.; Mohamed, M.A.A.; Hiyama, T. Assessing groundwater quality using GIS. Water Resour. Manag. 2007, 21, 699–715. [Google Scholar] [CrossRef]
- Bethencourt, M.; Fernandez-Montblanc, T.; Izquierdo, A.; Gonzalez-Duarte, M.M.; Munoz-Mas, C. Study of the influence of physical, chemical and biological conditions that influence the deterioration and protection of Underwater Cultural Heritage. Sci. Total Environ. 2018, 613–614, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Zhan, H.; Lu, X.C.; Liu, Y.R.; Huang, L.X.; Wei, Z.R. Biodegradation of carbon tetrachloride from groundwater in an upflow solid-phase biofilm system. RSC Adv. 2020, 10, 7500–7508. [Google Scholar] [CrossRef]
- Li, P.Y.; Qian, H.; Wu, J.H.; Zhang, Y.Q.; Zhang, H.B. Major Ion Chemistry of Shallow Groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- de Andrade, E.M.; Palacio, H.A.; Souza, I.H.; de Oliveira Leao, R.A.; Guerreiro, M.J. Land use effects in groundwater composition of an alluvial aquifer (Trussu River, Brazil) by multivariate techniques. Environ. Res. 2008, 106, 170–177. [Google Scholar] [CrossRef]
- Abelson, P.H. Groundwater contamination. Science 1984, 224, 673. [Google Scholar] [CrossRef][Green Version]
- Bulut, O.F.; Duru, B.; Cakmak, O.; Gunhan, O.; Dilek, F.B.; Yetis, U. Determination of groundwater threshold values: A methodological approach. J. Clean. Prod. 2020, 253, 120001. [Google Scholar] [CrossRef]
- Liu, D.; Qi, X.; Li, M.; Zhu, W.F.; Zhang, L.L.; Faiz, M.A.; Khan, M.I.; Li, T.X.; Cui, S. A resilience evaluation method for a combined regional agricultural water and soil resource system based on Weighted Mahalanobis distance and a Gray-TOPSIS model. J. Clean. Prod. 2019, 229, 667–679. [Google Scholar] [CrossRef]
- Grimmeisen, F.; Lehmann, M.F.; Liesch, T.; Goeppert, N.; Klinger, J.; Zopfi, J.; Goldscheider, N. Isotopic constraints on water source mixing, network leakage and contamination in an urban groundwater system. Sci. Total Environ. 2017, 583, 202–213. [Google Scholar] [CrossRef]
- Lin, S.S.; Shen, S.L.; Zhou, A.; Lyu, H.M. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total Environ. 2021, 751, 141618. [Google Scholar] [CrossRef]
- Liu, J.T.; Gao, Z.J.; Wang, Z.Y.; Xu, X.Y.; Su, Q.; Wang, S.; Qu, W.L.; Xing, T.J. Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula, China. Environ. Monit. Assess. 2020, 192, 384. [Google Scholar] [CrossRef] [PubMed]
- Jandu, A.; Malik, A.; Dhull, S.B. Fluoride and nitrate in groundwater of rural habitations of semiarid region of northern Rajasthan, India: A hydrogeochemical, multivariate statistical, and human health risk assessment perspective. Environ. Geochem. Health 2021, 43, 3997–4026. [Google Scholar] [CrossRef]
- Mandal, R.; Das, A.; Sudheer, A.K.; Kumar, S.; Verma, S.; Gaddam, M.; Deshpande, R.D. Sources, controls, and probabilistic health risk assessment of fluoride contamination in groundwater from a semi-arid region in Gujarat, Western India: An isotope-hydrogeochemical perspective. Environ. Geochem. Health 2021, 43, 4043–4059. [Google Scholar] [CrossRef]
- He, Z.; Han, D.; Song, X.; Yang, S. Impact of human activities on coastal groundwater pollution in the Yang-Dai River plain, northern China. Environ. Sci. Pollut. Res. 2020, 27, 37592–37613. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.; Sener, E.; Varol, S. Hydro-chemical and microbiological pollution assessment of irrigation water in Kizilirmak Delta (Turkey). Environ. Pollut. 2020, 266, 115214. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Luo, Q.; Wang, D.H.; Gao, J.J.; Wei, Z.; Wang, Z.J.; Zhou, H.D.; Mazumder, A. Simultaneous assessments of occurrence, ecological, human health, and organoleptic hazards for 77 VOCs in typical drinking water sources from 5 major river basins, China. Environ. Pollut. 2015, 206, 64–72. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, S.R.; Zhao, X.R.; Li, X.; Qiao, X.C.; Dionysiou, D.D.; Zheng, B.H. Distribution characteristics and health risk assessment of volatile organic compounds in the groundwater of Lanzhou City, China. Environ. Geochem. Health 2020, 42, 3609–3622. [Google Scholar] [CrossRef]
- Harun, H.H.; Kasim, M.R.M.; Nurhidayu, S.; Ash’aari, Z.H.; Kusin, F.M.; Karim, M.K.A. Association of Physicochemical Characteristics, Aggregate Indices, Major Ions, and Trace Elements in Developing Groundwater Quality Index (GWQI) in Agricultural Area. Int. J. Environ. Res. Public Health 2021, 18, 4562. [Google Scholar] [CrossRef]
- Ramanathan, A.L. Seasonal variation in the major ion chemistry of Pandoh Lake, Mandi District, Himachal Pradesh, India. Appl. Geochem. 2007, 22, 1736–1747. [Google Scholar] [CrossRef]
- Singh, C.K.; Kumar, A.; Shashtri, S.; Kumar, A.; Kumar, P.; Mallick, J. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 2017, 175, 59–71. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—A case study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Boonkaewwan, S.; Sonthiphand, P.; Chotpantarat, S. Mechanisms of arsenic contamination associated with hydrochemical characteristics in coastal alluvial aquifers using multivariate statistical technique and hydrogeochemical modeling: A case study in Rayong province, eastern Thailand. Environ. Geochem. Health 2021, 43, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, C.; Xu, Y.; Zhao, Z.; Luo, Z.; Liu, J. Assessment of the water quality of groundwater in Bohai Rim and the controlling factors-a case study of northern Shandong Peninsula, north China. Environ. Pollut. 2021, 285, 117482. [Google Scholar] [CrossRef]
- Abdul-Wahab, D.; Adomako, D.; Abass, G.; Adotey, D.K.; Anornu, G.; Ganyaglo, S. Hydrogeochemical and isotopic assessment for characterizing groundwater quality and recharge processes in the Lower Anayari catchment of the Upper East Region, Ghana. Environ. Dev. Sustain. 2021, 23, 5297–5315. [Google Scholar] [CrossRef]
- Gao, Z.J.; Liu, J.T.; Li, F.Q.; Wang, M.; Feng, J.G.; Wu, G.W. Hydrochemical Characteristics and Temporal Variations of Geothermal Water Quality in Tangtou, Shandong, China. Water 2019, 11, 1643. [Google Scholar] [CrossRef]
- Liu, J.T.; Gao, Z.J.; Wang, M.; Li, Y.Z.; Ma, Y.Y.; Shi, M.J.; Zhang, H.Y. Study on the dynamic characteristics of groundwater in the valley plain of Lhasa City. Environ. Earth Sci. 2018, 77, 646. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Jang, Y.C.; Townsend, T. Sulfate leaching from recovered construction and demolition debris fines. Adv. Environ. Res. 2001, 5, 203–217. [Google Scholar] [CrossRef]
- van den Brand, T.P.H.; Roest, K.; Chen, G.-H.; Brdjanovic, D.; van Loosdrecht, M.C.M. Adaptation of Sulfate-Reducing Bacteria to Sulfide Exposure. Environ. Eng. Sci. 2016, 33, 242–249. [Google Scholar] [CrossRef]
- Pant, R.R.; Zhang, F.; Rehman, F.U.; Wang, G.X.; Ye, M.; Zeng, C.; Tang, H.D. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci. Total Environ. 2018, 622, 770–782. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.F.; Yang, L.H.; Han, D.M.; Zhang, Y.H.; Ma, Y.; Bu, H.M. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef]
- Li, P.Y.; Wu, J.H.; Qian, H.; Zhang, Y.T.; Yang, N.A.; Jing, L.J.; Yu, P.Y. Hydrogeochemical Characterization of Groundwater in and Around a Wastewater Irrigated Forest in the Southeastern Edge of the Tengger Desert, Northwest China. Expo. Health 2016, 8, 331–348. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Ion, I.; Ion, A.C.; Calin, M.R.; Radulescu, I.; Bogdan, D. Assessment of Chemical Parameters and Natural Radionuclides Concentrations in Carbonated Natural Mineral Water and Contribution to Radiation Dose. Rom. J. Phys. 2019, 64, 804. [Google Scholar]
- Liu, X.; Wang, X.L.; Zhang, L.; Fan, W.Y.; Yang, C.; Li, E.H.; Wang, Z. Impact of land use on shallow groundwater quality characteristics associated with human health risks in a typical agricultural area in Central China. Environ. Sci. Pollut. Res. 2021, 28, 1712–1724. [Google Scholar] [CrossRef]
- Sohrabi, M.; Mehrjerdi, M.Z.; Karimi, S.; Tavallali, V. Using gypsum and selenium foliar application for mineral biofortification and improving the bioactive compounds of garlic ecotypes. Ind. Crop. Prod. 2020, 154, 112742. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Xu, P.P.; Qian, H.; Yang, F.X. Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: Assessment based on multivariate statistical approach and human health risk. Sci. Total Environ. 2020, 741, 140460. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigation and Development; UNESCO: Paris, France, 1965; pp. 54–83. [Google Scholar]
- Li, P.Y.; Wu, J.H.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 15. [Google Scholar] [CrossRef]
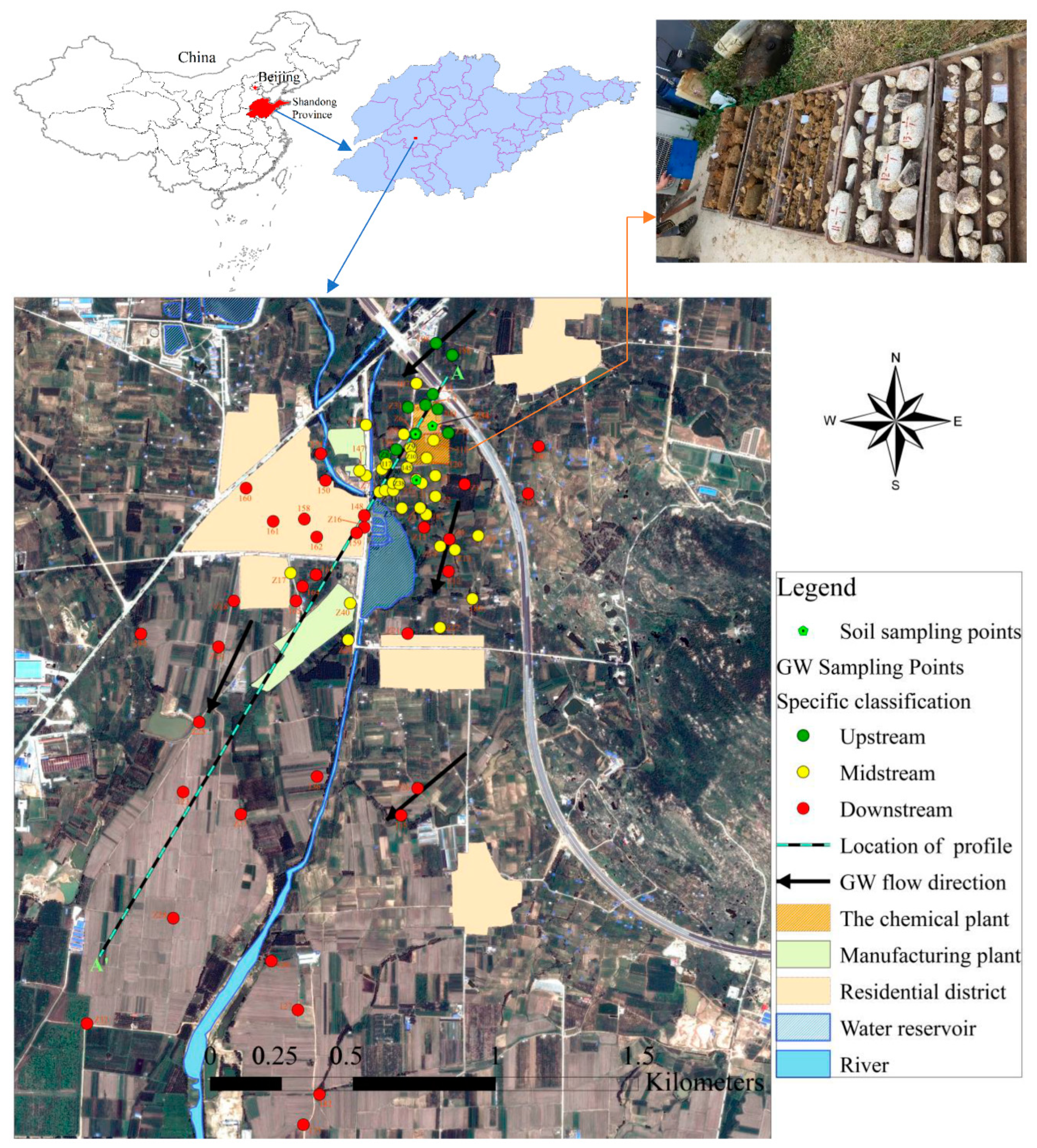
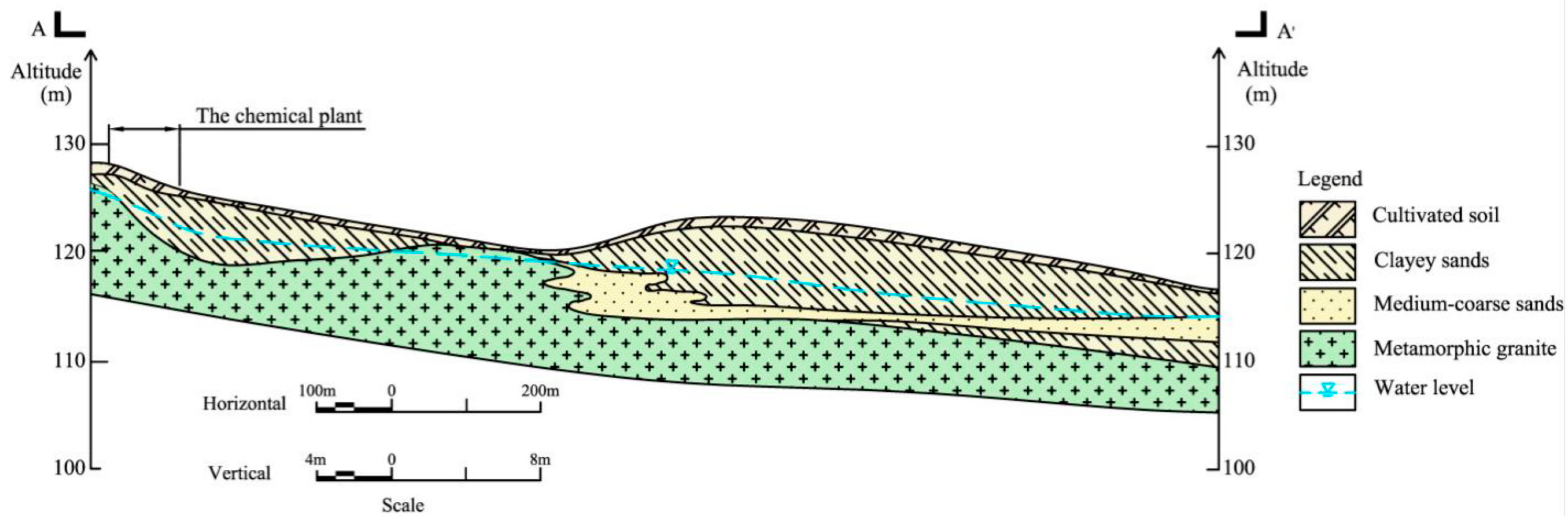
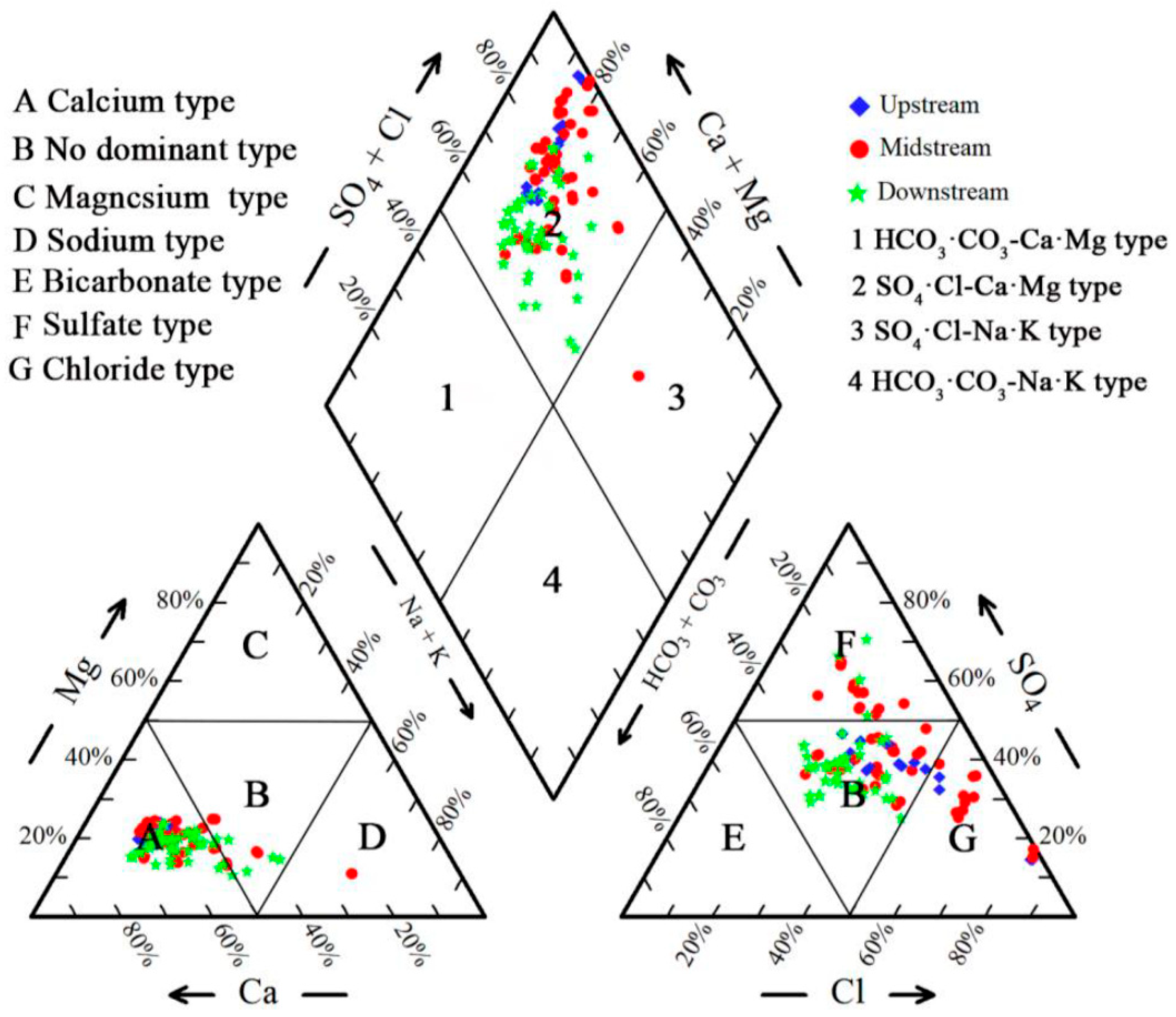
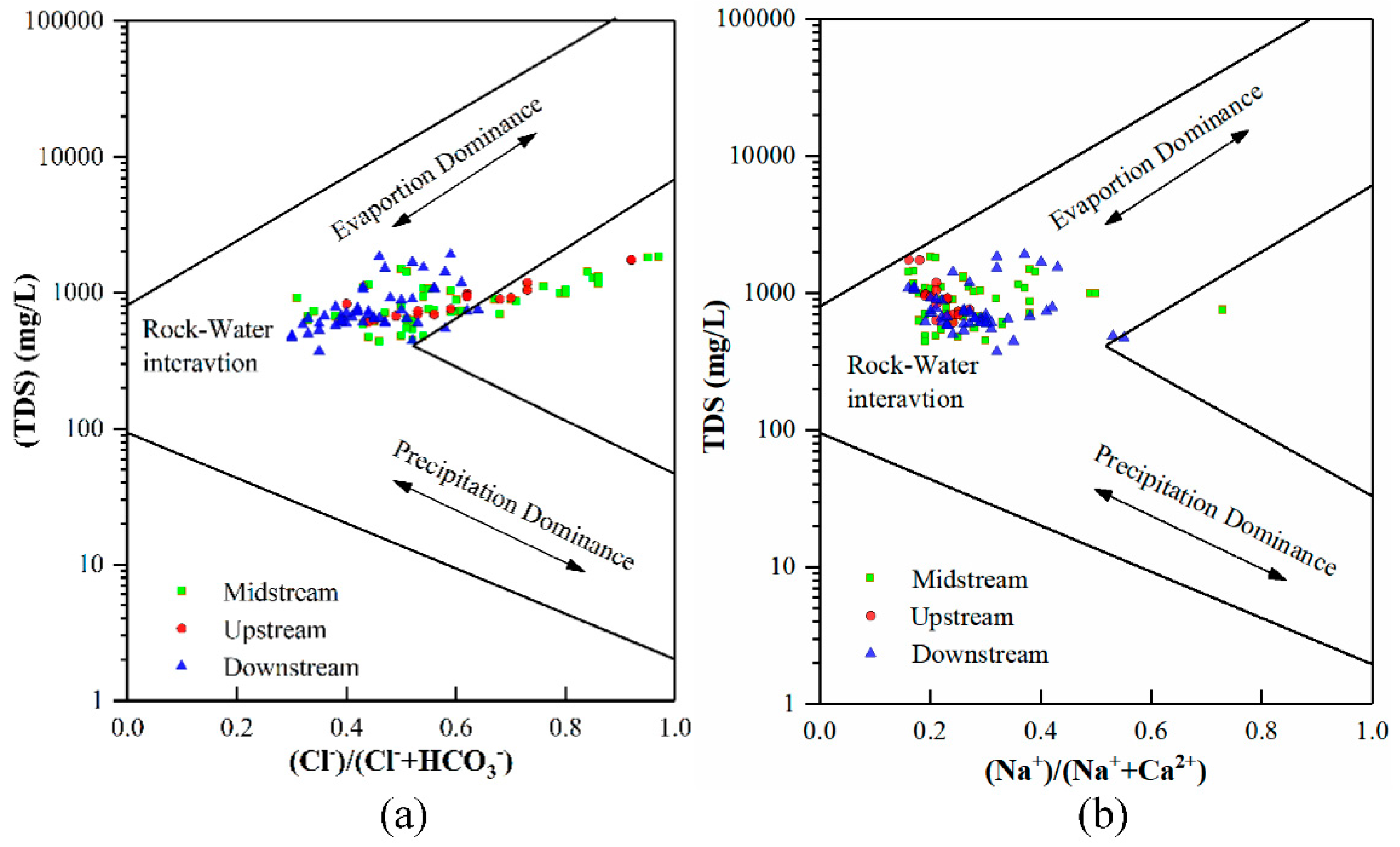
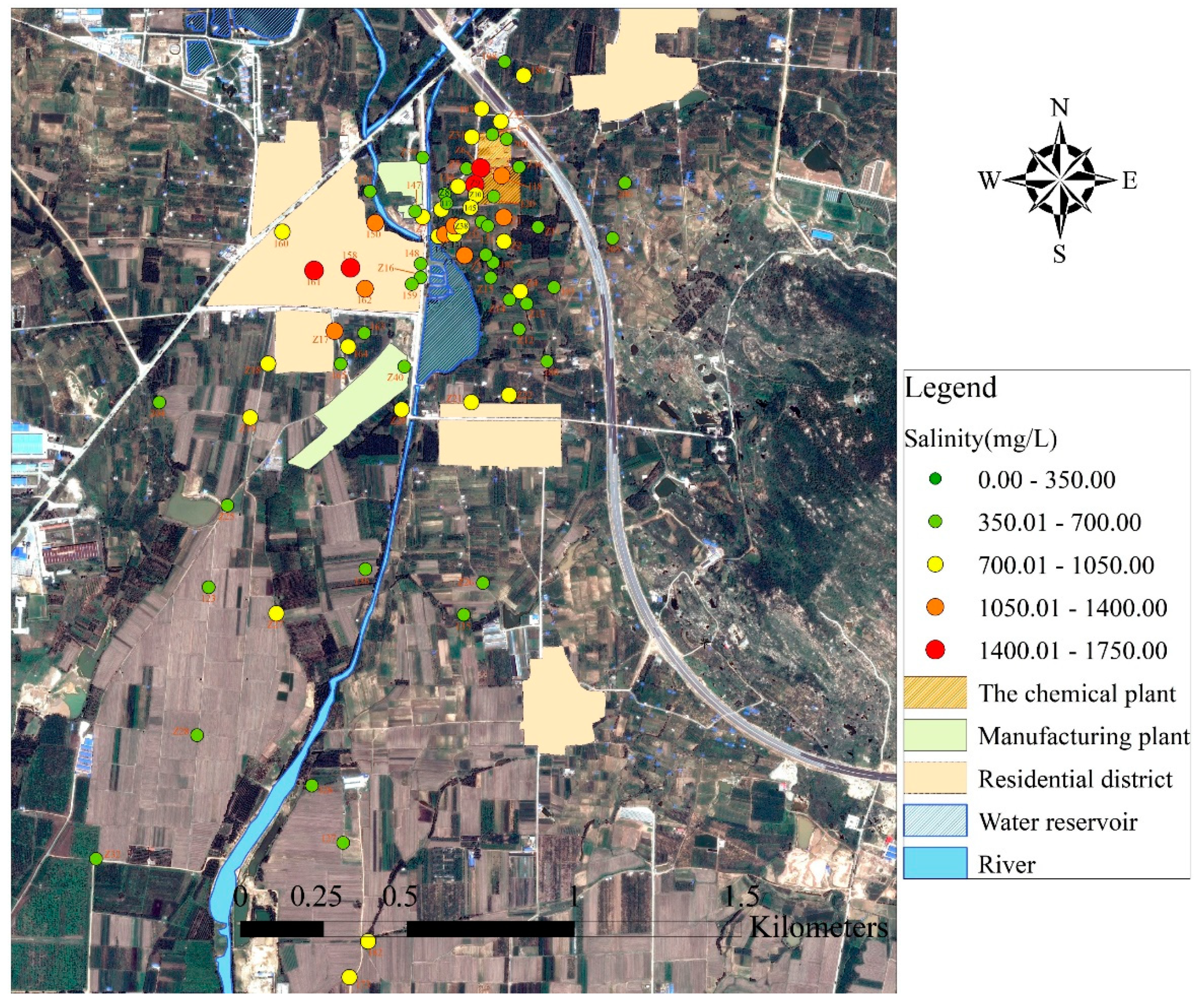
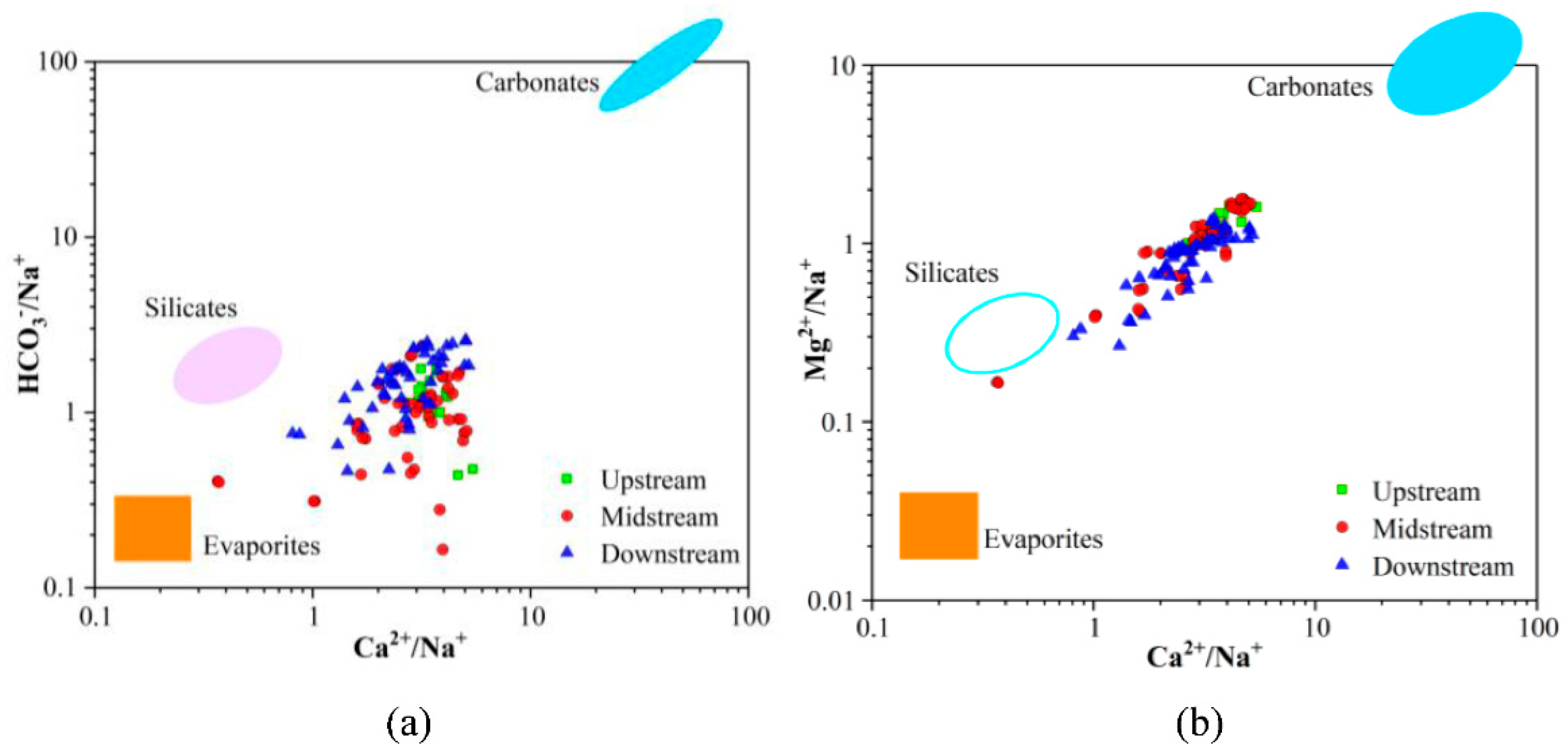
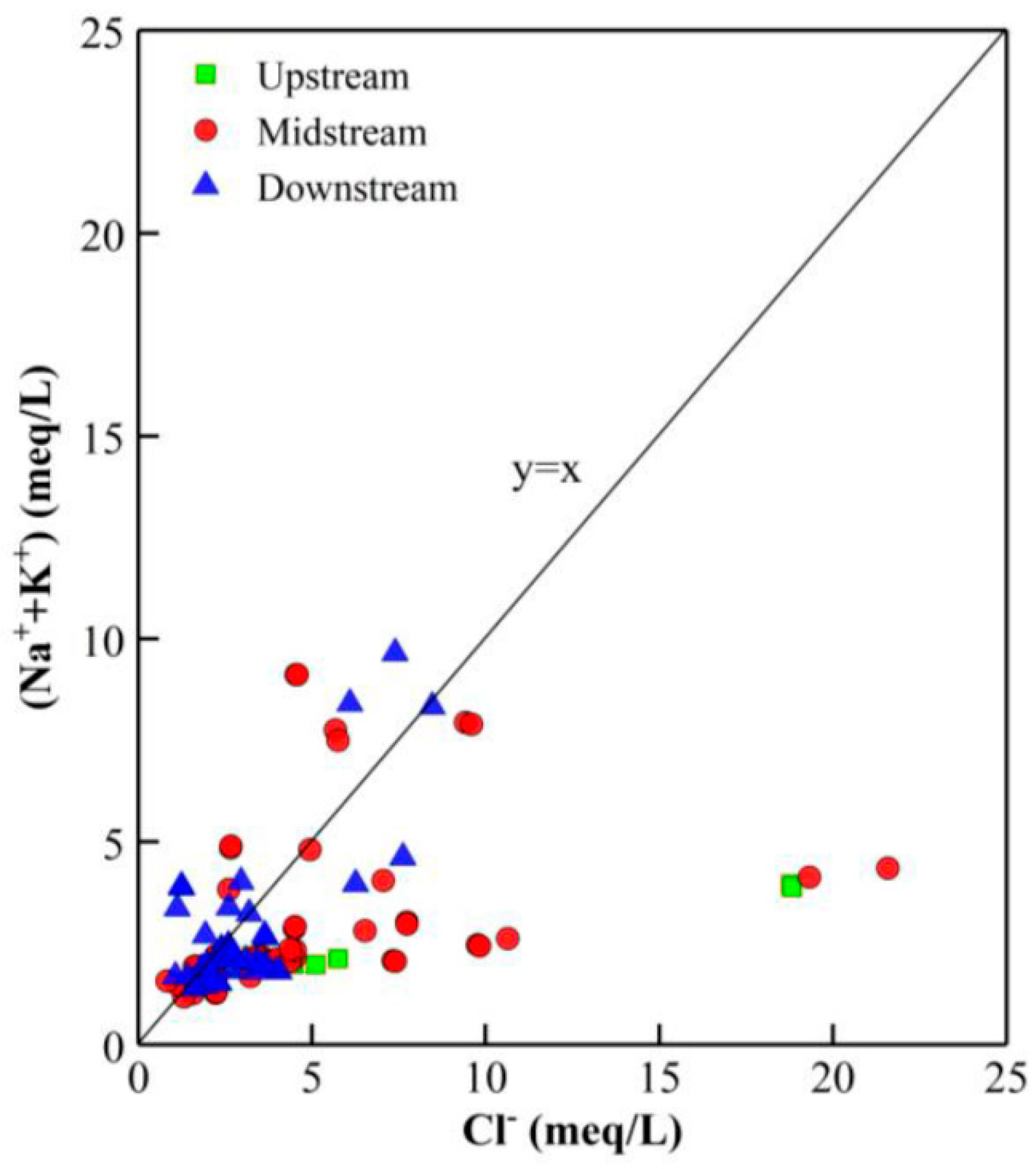
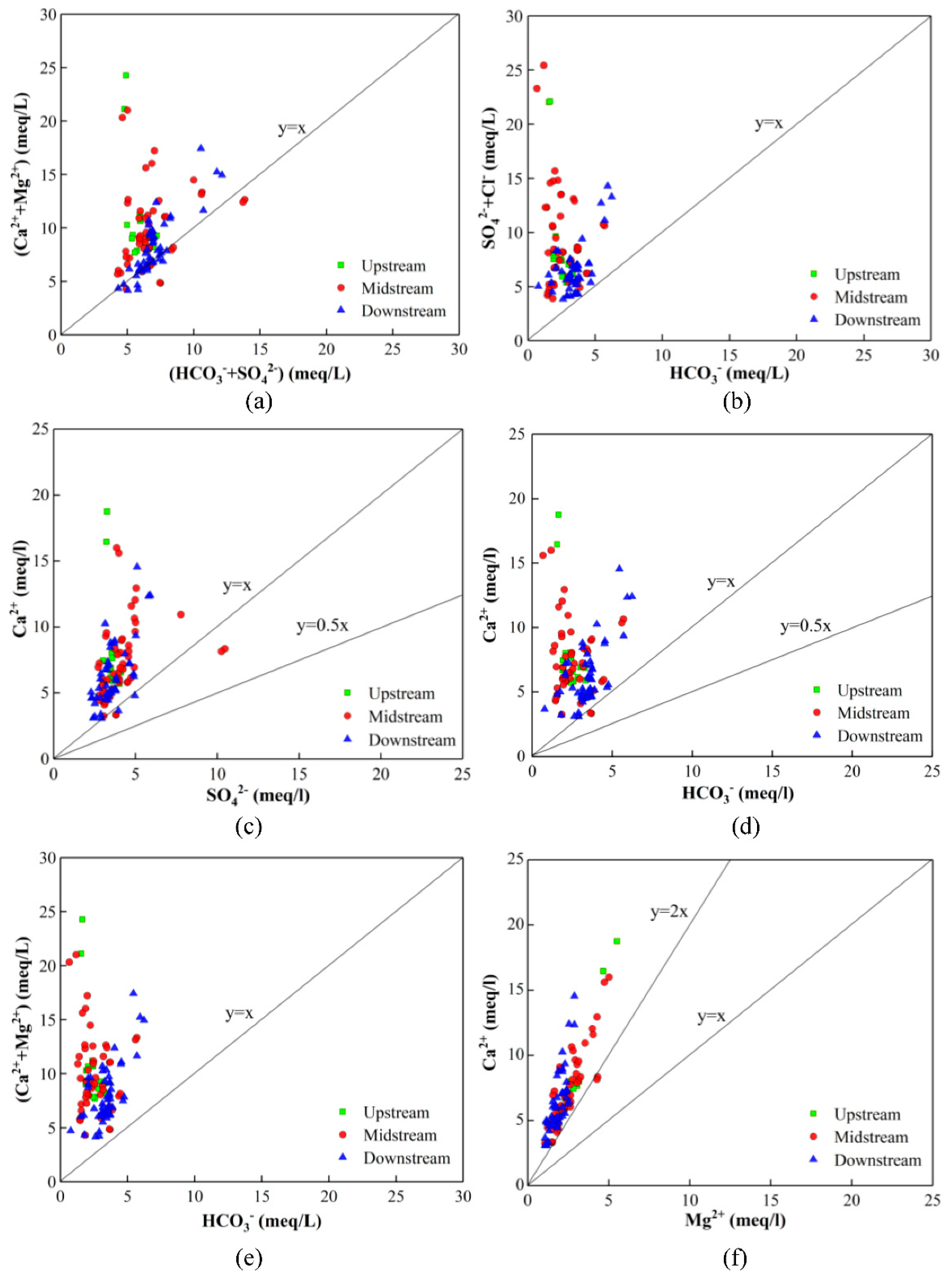

| Groundwater Classification | Sample Number | Sampling Depth (m) | Sample Number | Sampling Depth (m) | Sample Number | Sampling Depth (m) |
|---|---|---|---|---|---|---|
| Upstream | Z31-1 | 4.5 | 200-2 | 11.5 | Z36-2 | 10.4 |
| Z31-2 | 6.6 | 200-3 | 19.8 | Z36-3 | 16.5 | |
| Z31-3 | 9.1 | Z6-2 | 7.3 | 138 | 5.4 | |
| Z33 | 5.3 | Z6-3 | 10.4 | Z8-2 | 12 | |
| C3-2 | 7.7 | 186 | 6.5 | Z8-3 | 20.8 | |
| C3-3 | 11.8 | 108 | 6.4 | |||
| Midstream | Z5-1 | 3.7 | Z2-2 | 9.3 | Z14-3 | 14.8 |
| Z5-2 | 7.8 | Z2-3 | 12.8 | Z13 | 12.8 | |
| Z5-3 | 12.2 | 143 | 3.3 | Z22-2 | 9.6 | |
| 107 | 4.2 | 142 | 4.4 | Z22-3 | 11.3 | |
| Z9-2 | 11.1 | 141 | 4.7 | 103 | 3.2 | |
| Z9-3 | 17.6 | 140 | 5.1 | 180 | 4.4 | |
| Z10-2 | 10.3 | Z38-2 | 7.6 | Z20-2 | 4.8 | |
| Z10-3 | 16 | Z38-3 | 11.2 | Z20-3 | 6.3 | |
| 120-2 | 13.2 | Z35-2 | 12.5 | Z40-2 | 4.6 | |
| 120-3 | 26 | Z35-3 | 19.8 | Z40-3 | 6.6 | |
| 118 | 48 | 121 | 5.6 | Z17-2 | 5.7 | |
| 139-2 | 5.6 | 102 | 6.8 | Z17-3 | 7.4 | |
| 139-3 | 7.2 | Z37-2 | 8.1 | Z39-2 | 9.5 | |
| 117 | 4 | Z37-3 | 12.2 | Z39-3 | 15.8 | |
| 145 | 5.3 | Z3-2 | 8.6 | Z7-2 | 5.9 | |
| Z1-2 | 9 | Z3-3 | 13.1 | Z7-3 | 8.8 | |
| Z1-3 | 12.8 | Z14-2 | 11.5 | 147 | 20.5 | |
| Downstream | Z11-2 | 9.6 | 162 | 4.9 | 135 | 9.2 |
| Z12-3 | 15.1 | 158 | 30.2 | 136 | 4.7 | |
| Z4 | 12.3 | 161 | 19.3 | Z27-2 | 13.5 | |
| Z15-2 | 8.3 | 160 | 35.5 | Z27-3 | 21 | |
| Z15-3 | 11.8 | 151 | 3.8 | 173-2 | 8.3 | |
| Z21-2 | 6 | 150 | 1.4 | 173-3 | 9.3 | |
| Z21-3 | 7.3 | Z18-2 | 17.2 | 182-2 | 6.7 | |
| 165-2 | 4.4 | Z18-3 | 27 | 182-3 | 7.6 | |
| 165-3 | 5.6 | Z19-2 | 12.1 | Z29-2 | 16.7 | |
| 163-2 | 4.3 | Z19-3 | 16.4 | Z29-3 | 25.8 | |
| 163-3 | 5.3 | Z25-2 | 10.9 | Z32-2 | 16.8 | |
| 164 | 3.8 | Z25-3 | 14.4 | Z32-3 | 25.8 | |
| 148-2 | 4.4 | 123 | 6.8 | 127 | 5.7 | |
| 148-3 | 5.4 | Z28-2 | 13 | 126 | 3.1 | |
| Z16-2 | 8.7 | Z28-3 | 17.8 | 205 | 4.1 | |
| Z16-3 | 13.7 | 204 | 4.9 | 206 | 6.2 | |
| 159 | 4.2 | Z26 | 11.6 |
| Parameters | Units | Minimum | Maximum | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|
| pH | 5.77 | 8.08 | 6.95 | 0.34 | 4.92 | |
| TDS | mg/L | 374.00 | 1910.00 | 874.98 | 349.33 | 39.92 |
| Cl− | mg/L | 29.60 | 765.00 | 147.20 | 127.54 | 86.64 |
| SO42− | mg/L | 110.00 | 502.00 | 183.94 | 57.65 | 31.34 |
| NO3− | mg/L | 0.00 | 98.30 | 21.02 | 18.69 | 88.88 |
| HCO32− | mg/L | 40.00 | 380.00 | 177.38 | 67.39 | 37.99 |
| Ca2+ | mg/L | 61.60 | 375.00 | 140.39 | 57.75 | 41.14 |
| Mg2+ | mg/L | 13.00 | 67.10 | 27.73 | 10.46 | 37.70 |
| K+ | mg/L | 0.95 | 80.50 | 4.97 | 9.80 | 197.23 |
| Na+ | mg/L | 25.90 | 219.00 | 61.64 | 39.17 | 63.55 |
| Mn+ | mg/L | 0.16 | 5090.48 | 233.49 | 727.95 | 311.77 |
| pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | TDS | EC | NO3− | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 0.033 | −0.003 | −0.533 ** | −0.517 ** | −0.587 ** | −0.039 | 0.486 ** | −0.382 ** | −0.378 ** | −0.154 |
| K+ | 1 | 0.410 ** | 0.301 ** | 0.138 | 0.224 * | 0.157 | 0.452 ** | 0.472 ** | 0.466 ** | 0.469 ** | |
| Na+ | 1 | 0.325 ** | 0.273 ** | 0.392 ** | 0.400 ** | 0.383 ** | 0.467 ** | 0.610 ** | 0.397 ** | ||
| Ca2+ | 1 | 0.845 ** | 0.875 ** | 0.366 ** | 0.016 | 0.830 ** | 0.904 ** | 0.483 ** | |||
| Mg2+ | 1 | 0.804 ** | 0.518 ** | −0.179 | 0.675 ** | 0.725 ** | 0.206 * | ||||
| Cl− | 1 | 0.162 | −0.191 * | 0.767 ** | 0.850 ** | 0.228 * | |||||
| SO42− | 1 | 0.086 | 0.366 ** | 0.386 ** | 0.197 * | ||||||
| HCO3− | 1 | 0.141 | 0.239 ** | 0.393 ** | |||||||
| TDS | 1 | 0.875 ** | 0.554 ** | ||||||||
| EC | 1 | 0.599 ** | |||||||||
| NO3− | 1 |
| Parameter | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| EC | 0.969 | 0.104 | −0.054 |
| Ca2+ | 0.933 | −0.188 | −0.058 |
| TDS | 0.911 | 0.052 | −0.091 |
| Cl− | 0.861 | −0.358 | −0.122 |
| Mg2+ | 0.819 | −0.371 | 0.264 |
| NO3− | 0.581 | 0.448 | −0.307 |
| Na+ | 0.575 | 0.462 | 0.239 |
| HCO3− | 0.119 | 0.876 | −0.042 |
| pH | −0.493 | 0.646 | 0.26 |
| K+ | 0.473 | 0.573 | −0.257 |
| SO42− | 0.466 | 0.122 | 0.802 |
| Explained variance (%) | 49.249 | 20.518 | 9.347 |
| Item | Fe | Mn | K | Ca | Na | Mg | |
|---|---|---|---|---|---|---|---|
| Z34 | Mean (mg/kg) | 25,057.14 | 486.14 | 25,114.29 | 8127.14 | 20,614.29 | 6360.00 |
| Maximum (mg/kg) | 35,100 | 1140 | 29,700 | 12,500 | 30,600 | 8370 | |
| Minimum (mg/kg) | 14,200 | 343 | 18,300 | 3490 | 13,800 | 3260 | |
| SD | 7670.48 | 289.04 | 4647.38 | 2758.48 | 6567.45 | 2027.76 | |
| Z35 | Mean (mg/kg) | 49,287.50 | 732.88 | 14,495.00 | 29,262.50 | 25,400.00 | 13,005.00 |
| Maximum (mg/kg) | 66,500 | 1270 | 21,900 | 44,900 | 30,200 | 21,800 | |
| Minimum (mg/kg) | 23,100 | 309 | 6970 | 10,100 | 18,600 | 5810 | |
| SD | 20,435.29 | 349.57 | 6280.97 | 15,850.10 | 4816.04 | 5770.15 | |
| Z06 | Mean (mg/kg) | 20,800 | 540.7 | 28,000 | 12,948 | 25,710 | 4762 |
| Maximum (mg/kg) | 36,000 | 1400 | 35,800 | 26,100 | 37,000 | 8070 | |
| Minimum (mg/kg) | 11,200 | 210 | 19,100 | 2900 | 12,500 | 2550 | |
| SD | 9173.51 | 337.10 | 6741.41 | 8348.62 | 8966.78 | 2106.38 | |
| All | Mean (mg/kg) | 31,714.88 | 586.57 | 22,536.43 | 16,779.21 | 23,908.10 | 8042.33 |
| Compound | Detection Limit (μg/L)/Ratio | Average Detection Concentration (μg/L) | Water Quality Standard (μg/L) | Over-Limit Ratio (%) |
|---|---|---|---|---|
| Trichlorofluoromethane | 0.5/42.37% | 666.12 | 10,000 | 0.85 |
| Carbon tetrachloride | 0.5/39.83% | 2059.82 | 2 | 39.83 |
| Trichloroethene | 0.5/38.98% | 175.74 | 70 | 6.78 |
| 1,1,2-Trichloroethane | 0.5/39.83% | 145.22 | 5 | 28.81 |
| Perchloroethylene | 0.5/27.97% | 157.62 | 40 | 5.08 |
| 1,1,2,2-Tetrachloroethane | 0.5/34.75% | 410.67 | 400 | 5.93 |
| Chloroform | 0.5/46.61% | 69.36 | 60 | 10.17 |
| Compound | Units | Water Quality Standard | Detection Maximum | Detection Minimum |
|---|---|---|---|---|
| Trichlorofluoromethane | μg/L | 10,000 | 20,400 | 0.5 |
| Carbon tetrachloride | μg/L | 2 | 69,500 | 2.2 |
| Trichloroethene | μg/L | 70 | 5900 | 0.6 |
| 1,1,2-Trichloroethane | μg/L | 5 | 3500 | 0.6 |
| Perchloroethylene | μg/L | 40 | 4240 | 0.9 |
| 1,1,2,2-Tetrachloroethane | μg/L | 400 | 11,700 | 1.8 |
| Chloroform | μg/L | 60 | 811 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, H.; Wu, Q.; Liu, B.; Zhou, G. Exploration of Hydrogeochemical Characterization and Assessment of Organic Pollution Characteristics of Shallow Groundwater near a Chemical Plant That Discharged Sewage Illegally. Sustainability 2022, 14, 660. https://doi.org/10.3390/su14020660
Zhan H, Wu Q, Liu B, Zhou G. Exploration of Hydrogeochemical Characterization and Assessment of Organic Pollution Characteristics of Shallow Groundwater near a Chemical Plant That Discharged Sewage Illegally. Sustainability. 2022; 14(2):660. https://doi.org/10.3390/su14020660
Chicago/Turabian StyleZhan, Hao, Qiang Wu, Benhua Liu, and Guangya Zhou. 2022. "Exploration of Hydrogeochemical Characterization and Assessment of Organic Pollution Characteristics of Shallow Groundwater near a Chemical Plant That Discharged Sewage Illegally" Sustainability 14, no. 2: 660. https://doi.org/10.3390/su14020660
APA StyleZhan, H., Wu, Q., Liu, B., & Zhou, G. (2022). Exploration of Hydrogeochemical Characterization and Assessment of Organic Pollution Characteristics of Shallow Groundwater near a Chemical Plant That Discharged Sewage Illegally. Sustainability, 14(2), 660. https://doi.org/10.3390/su14020660





