Abstract
Soil acidification is a severe environmental problem around the world. Soil pH buffering capacity (pHBC) is the intrinsic factor affecting the soil acidification rate and is intensively impacted by anthropogenic and natural conditions. However, composite assessments of the effects of land use and soil type on soil pHBC are still limited. Therefore, we collected samples of five soil types (red soil, lateritic red soil, latosol, paddy soil and acid sulphate soil) from two land use patterns of agricultural and adjacent forest fields at different depths (0–10 cm, 10–20 cm and 20–30 cm) in South China, aiming to investigate the effects of land use and soil type on soil pHBC in this region. The results show that land use, soil type and their interactions greatly influence soil pHBC and physico-chemical properties. Forest soils have a significantly higher pHBC (11.40–49.50 mmol·kg−1 soil·unit−1 pH), cation exchange capacity (CEC), exchangeable Al3+ (EAl3+) and clay content than agricultural soils. Acid sulphate soil has the highest pHBC (49.27–117.83 mmol·kg−1 soil·unit−1 pH) values and exchangeable acid (EA) content among all investigated soil types, whereas lateritic red soil has the lowest pHBC (10.56–31.71 mmol·kg−1 soil·unit−1 pH). In agricultural fields, soil pHBC is positively related to CEC, soil organic carbon (SOC) and EA, indicating that agricultural soils may be in a cation exchange buffering stage. The soil pHBC of forest fields is positively correlated with SOC and EAl3+, implying that forest soils may be in the Al buffering stage. In conclusion, soil pHBC would vary with different land use forms and soil types, in which a series of key complex physico-chemical processes and interactions would occur to regulate soil pH buffering capacity.
1. Introduction
With the development of modern industry and agriculture, soil acidification, resulting from acid/nitrogen deposition [1,2], land-use change [3,4] and the abuse of chemical fertilizer [5,6], has become a representative environmental problem around the world. Soil acidification causes intensive soil nutrient leaching and an increase in heavy metal activity, as well as affecting biodiversity, biogeochemical cycling of soil elements and pedogenesis and, ultimately, altering the structure and function of the terrestrial ecosystem [7,8,9]. Therefore, investigating the effects of environmental factors on soil acidification and controlling their mechanisms are imperative issues.
The extent of soil acidification is controlled by the coordinated regulation of environmental acid input and the intrinsic pH buffering capacity (pHBC) of soils [10,11]. However, while numerous studies have focused on the effects of acid input, few have examined the contribution of soil pHBC to soil acidification under different environmental conditions [12]. The pHBC is a natural characteristic in soils, varying with soil physicochemical properties and mineralogy [13,14]. Previous studies have indicated that soil pHBC is greatly affected by several indicators, such as initial pH, cation exchange capacity (CEC), carbonate content and the composition or distribution of aluminum (Al)-bearing minerals [5,15,16]. Therein, soil pH seems to function as an important indicator determining soil pH buffering by controlling soil chemical composition in different pH ranges. Carbonate is largely present in soils beyond pH 7.0 and has become a pivotal component buffering the pH of alkali and near-neutral soils [17]. In soils with a pH between 4.5 and 6.0, CEC and soil organic matter (SOM) play important roles in soil pH buffering [18], whereas in acidic soils with a pH below 4.5, different forms of Al and the interactions between Al and SOM have positive contributions to the soil pH buffering process [19,20]. Ulrich [21] and Bowman et al. [22] qualitatively described the complex buffering system in soils, dividing the buffering system into five subsystems based on the pH range. At pH > 7.5, the dissolution/precipitation of carbonate controls soil pH buffering; at pH 4.5–7.0, cation exchange reactions dominate the soil pH buffering process; at pH < 4.5, different forms of Al regulate soil pH buffering; and at pH < 3.8, iron (Fe) controls the soil pH buffering system [5,21,22]. Moreover, silicate buffering also contributes to soil acid buffering at pH > 5 [5,21].
Land use and soil type are important anthropogenic and natural factors affecting soil properties related to soil pHBC [23,24]. Previous studies reported that intensive agricultural land use could have caused severe soil acidification in Chengdu Plain in China over the last thirty years [25]. Ozlu et al. [26] observed that, compared with chemical fertilizer, manure had a stronger ability to increase soil organic carbon (SOC) storage and preserve soil pH. Jiang et al. [27] found that mature forest possessed lower soil pH and higher contents of SOC, base cations and exchangeable acidic cations than a pioneer forest. Gruba and Mulder [28] suggested that tree species could significantly affect the pH dependency of Al solubility control mechanisms and the intrinsic properties of SOM in a temperate forest. These observations demonstrate the effects of land use on soil properties. However, comparative research examining the effects of different land-use types on soil pHBC has not been conducted. With regard to soil type, different types of soils established under specific geoclimatic conditions, are influenced by parent materials and the extent of soil development [29,30]. Previous studies have observed various effects of parent materials and soil type on soil pHBC and acid-neutralizing capacity. For example, Fujii et al. [31] suggested that the low acid-neutralizing capacity of granite-derived soils and the high buffering capacity of volcanic soils can be ascribed to the difference in these soils’ weatherable mineral content. Wei et al. [32] investigated the effects of simulated acid rain on three types of soil in South China and found that the pH-change responses in red soil (RS) and lateritic red soil (LRS) to acid rain were weaker than those in latosol (LTS). However, in natural conditions, soil type and human interference always exert interactive impacts on soil pHBC and physicochemical properties, and their mechanisms are still poorly understood. Investigating the interactive impacts of land use and soil type on soil pHBC and buffering mechanisms is an important topic for land development and management.
In this study, we collected soil samples from agricultural fields and adjacent forest fields under five typical soil types (RS, LRS, LTS, paddy soil and acid sulphate soil) at different soil depths (0–10 cm, 10–20 cm and 20–30 cm) in South China, aiming to investigate the effects of land use and soil type on soil chemical and physical properties and pH buffering mechanisms in South China.
2. Materials and Methods
2.1. Site Description
Soil samples were collected from Guangdong Province in Southern China (Figure 1 and Table 1), which is predominantly characterized by a typical tropical or subtropical climate. The annual mean temperature of Guangdong Province is 19.0–24.0 °C, and the annual mean precipitation is 1300–2500 mm.
Figure 1.
Spatial distribution of sampling sites in Southern China. (a) The location of the study region in China. (b) Spatial distribution of specific sampling sites. Each icon represents a sampling plot, and each soil type is indicated by one color.

Table 1.
Location of the collected sampling sites in South China.
Five soil types from agricultural lands and the adjacent forest lands, including RS, LRS, LTS, paddy soil (PS, developed from yellow lateritic red soil (YLRS)) and acid sulphate soil (ASS) were collected. For these soil types, RS, LRS, PS/YLRS and LTS belong to Oxisol [32], and ASS belongs to Histosol in US soil taxonomy [33]. The parent materials of RS, LRS, LTS, PS/YLRS and ASS are sandy shale, granite, basalt, sand mudstone and coastal mud deposit, respectively. The cultivated crop in the agricultural fields of RS, LRS and LTS was maize, while in the farmlands of PS and ASS was rice. Unfortunately, due to the extent of high land-development in Haiyan town, Jiangmen City of China, we did not find the appropriate forest without human interference. Thus, for ASS, there was only one land-use type of agricultural fields to be investigated in the present study. At each sampling site, five main plots (50 m × 50 m area) were randomly selected, and five sub-plots (1 m × 1 m area) were established in each main plot (one in the center and four in the corners). Soil samples were collected from each sub-plot at depths of 0–10 cm, 10–20 cm and 20–30 cm, respectively. The soil samples from the same main plot were thoroughly mixed to produce one composite sample.
2.2. Soil Analyses
After air-drying, the soil samples were ground to pass through sieves of 2, 0.25 and 0.15 mm to analyze different soil properties. The soil pHBC was determined by the method of acid/alkali titration as proposed by Nelson and Su [14]. Simply, 5 g of soil sample (passed through a 2 mm sieve) was weighed into 50 mL polyethylene centrifuge tubes and 25 mL of carbon dioxide-free water was added. Then, 0, 1, 2.5, 5 and 10 mL of either HNO3 (0.1 mol·L−1) or NaOH (0.1 mol·L−1) were added into each tube to acidify or alkalify the sample. Calcium chloride solution (1 mL, 0.05 mol·L−1) and chloroform (0.2 mL) were added into each tube to inhibit ionic strength and microbial activities in the solution, respectively. The soil suspensions were intensively mixed, equilibrated on a shaker (300 r·min−1) for 24 h at 25 °C and then incubated for 6 days. The suspension was shaken daily for 2 min during incubation. Soil pH values were measured on the last day with a pH meter (S2 Seven2Go, Mettler Toledo, Germany).
The soil pH was measured with a soil–water ratio of 1:2.5. Soil total nitrogen (TN) and SOC were determined using an elemental analyzer (Elementar Vario III, Germany). The CEC of acidic soil was eluted with 1 mol·L−1 ammonium acetate, then the CEC of substrates was determined by a nitrogen-fixing instrument (KN580, ALVA) [34]. Soil exchangeable acid (EA) is the sum of exchangeable H+ (EH+) and exchangeable Al3+ (EAl3+), which were determined following the KCl-extraction method [34]. Soil texture (clay, silt and sand contents) was measured with the pipette method [34].
2.3. Statistical Analysis
The acid/alkali titration curves were fitted by the sigmoid function (Equation (1)) as proposed by Nelson and Su [14], and then pHBC was calculated by Equation (2) [14]:
where pHmin represents the minimum pH value of a given soil; A represents the amount of acid (−) or alkali (+) solution added to the sample; Amid represents the A value at the inflection point of the curve; a and b are the fitted constants.
The mixed-effects model was used to test the significances of the main and interaction effects among land use, soil type, soil depth. A one-way analysis of variance (ANOVA) was used to test the significance levels of soil properties within the soil type and depth, while a paired-samples t-test was used to detect the significance levels in different land-use types. The Duncan method was used for post hoc multiple comparisons when the data met the assumption of homogeneity of variances; otherwise, the Tamhane’s T2 method was used. The relationship between soil pHBC and soil properties was analyzed by the mixed-effects model. Due to the deficiency of data from ASS in the forest fields, only the data from four types of soils (including RS, LRS, LTS and PS/YLRS) were tested in the mixed-effects models.
The statistical analyses of the mixed-effects model were implemented using IBM SPSS Statistics 17.0 (IBM Corp., Armonk, NY, USA) and R statistical software (R 4.0.2). The fittings of the sigmoid function were conducted in SigmaPlot 14.0 (Systat Software Inc., San Jose, CA, USA), and the significance level of all data was set at p < 0.05. The figures were drawn by Origin 9.0, Sigmoaplot 14.0, Adobe Photoshop CC 2019 and Adobe Illustrator CC 2019.
3. Results
3.1. Variations in Soil pHBC and Physicochemical Properties
The mixed-effects model showed that soil pHBC was significantly affected by land use (p < 0.001), soil type (p < 0.001), soil depth (p < 0.001) and their interactions (p < 0.05 for all, Table 2). The significant variations between land-use types mainly originated from LRS at the 10–20 cm depth and PS/YLRS at all depths (Figure 2). For agricultural soils, ASS had the highest pHBC values (49.27–117.83 mmol H+·kg−1 soil·unit−1 pH), while the pHBC values of RS, LRS, LTS and PS ranged between 10.56–33.55 mmol H+·kg−1 soil·unit−1 pH (Figure 2). For forest soils, LRS had the lowest pHBC values (11.40–30.55 mmol H+·kg−1 soil·unit−1 pH), while YLRS had relatively high pHBC values (27.50–39.52 mmol H+·kg−1 soil·unit−1 pH, Figure 2). Under different soil types, the pHBC values in forest soils of LRS (at 10–20 cm depth) and PS/YLRS (at all depths) were significantly higher than those of agricultural soils (Figure 2). The pHBC values in forest soils of RS (at all depths) and LRS (at 0–10 cm and 20–30 cm depths) were higher than those of agricultural soils, but the variations were not significant (Figure 2). At different soil depths, the significant decline in soil pHBC with an increasing depth only occurred in the RS and LRS of agricultural soils, while the pHBC in forest soils did not differ in the soil depths (Figure 2).

Table 2.
Mixed-effect models for the effects of land use, soil type, soil depth and their interactions in soil pHBC and physicochemical properties. *: p < 0.05, **: p < 0.01, ***: p < 0.001, NS: nonsignificant. The digits represent F values.
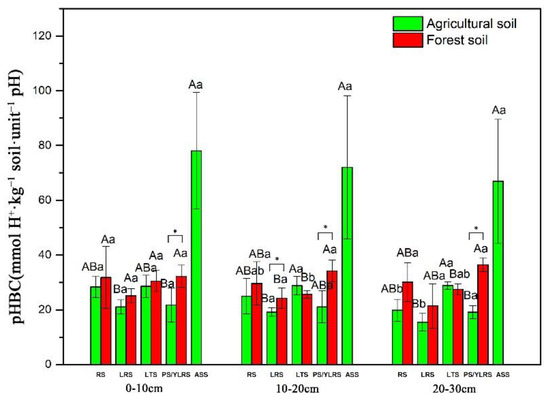
Figure 2.
pHBC values of different land-use and soil types, including red soil (RS), lateritic red soil (LRS), latosol (LTS), paddy soil (PS)/yellow lateritic red soil (YLRS) and acid sulphate soil (ASS). All data presented are the means of five replicates ± standard deviations. The uppercase letters indicate significant differences between soil types in the same land-use type and depth (p < 0.05). The lowercase letters indicate significant differences between depths in the same land-use and soil types (p < 0.05). The symbol “*” indicates the significant differences between agricultural fields and forest fields for the same soil type and depth (p < 0.05).
Land use, soil type, soil depth and their interactions significantly affected pH, CEC, C:N ratio, EA, EAl3+, clay and silt, while the dominant factors affecting SOC were land use and soil depth (Table 2). On the other hand, the soil TN, EH+ and sand were substantially influenced by soil type and depth (Table 2). In agricultural soils, ASS had the lowest pH values (pH 3.05–3.88), while the pH values of RS, LRS, LTS and PS ranged between 3.55–5.95 (Figure 3a). The soil pH of RS was lower than that of LRS, LTS and PS in agricultural fields (Figure 3a). In forest soils, LRS had the lowest pH values (pH 3.66–4.70), while LTS had the highest pH values (pH 4.88–5.86, Figure 3a). For most of the soil types, the pH in agricultural soils was significantly higher than that in forest soils (Figure 3a).
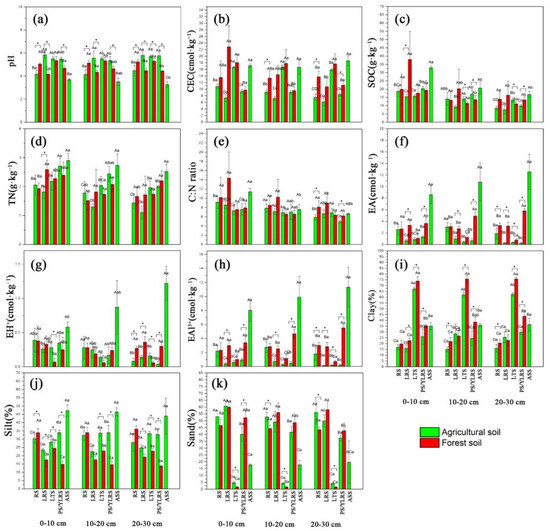
Figure 3.
Soil physicochemical properties under two land-use types and five soil types (n = 5). All data presented are the means of five replicates ± standard deviations. The abbreviations of RS, LRS, LTS, PS/YLRS and ASS represent red soil, lateritic red soil, latosol, paddy soil/yellow lateritic red soil and acid sulphate soil, respectively. The abbreviations of CEC, SOC, TN, EA, EH+ and EAl3+ represent cation exchangeable capacity, soil organic carbon, total nitrogen, exchangeable acid, exchangeable H+ ion and exchangeable Al3+ ion, respectively. The uppercase letters indicate significant differences between soil types in the same land-use type and depth (p < 0.05). The lowercase letters indicate significant differences between depths in the same land-use type and soil type (p < 0.05). The symbol “*” indicates the significant differences between agricultural fields and forest fields in the same soil type and depth (p < 0.05).
Soil texture was significantly influenced by land use, soil type, soil depth and their interactions (Table 2 and Figure 3). The LTS had the highest clay content, while RS and LRS had relatively low clay contents under each land-use type (Figure 3i). In the agricultural soils, ASS had the highest silt content, while LRS had the lowest silt content at all soil depths (Figure 3j). In forest soils, RS had the highest silt content at all soil depths (Figure 3). There were significant variations in silt content between land-use types in RS, LRS, LTS and PS/YLRS, especially at 0–10 cm soil depth (Figure 3j). Under land-use types, LTS had the lowest sand content (1.18–5.69%), ASS had a relatively low sand content (9.19–44.43%), while the sand content in RS, LRS and PS/YLRS was relatively high (34.35–69.11%) (Figure 3k). The significant variations in sand content between land-use types were present in RS (at 10-20 cm and 20-30 cm depths), LTS (at all depths) and PS/YLRS (at 0-10 cm depth), and the variations also occurred in the soil depths of LRS (in agricultural fields) and YLRS (in forest fields) (Figure 3k).
3.2. Correlation between Soil pHBC and Physicochemical Properties
The mixed-effects model showed that the pHBC of agricultural soil was positively correlated with CEC (R2 = 0.581, p < 0.001), SOC (R2 = 0.161, p < 0.001), TN (R2 = 0.122, p = 0.001), C:N ratio (R2 = 0.172, p < 0.001), EA (R2 = 0.267, p < 0.001), EH+ (R2 = 0.202, p < 0.001) and EAl3+ (R2 = 0.211, p = 0.001), while it was negatively related with soil pH (R2 = 0.190, p = 0.008, Figure 4). In forest soils, soil pHBC was positively correlated with SOC (R2 = 0.044, p = 0.020), TN (R2 = 0.059, p = 0.004), C:N ratio (R2 = 0.049, p = 0.028), EA (R2 = 0.119, p = 0.017) and EAl3+ (R2 = 0.113, p = 0.018), while it was not related to pH and CEC (Figure 4). The effects of the soil texture on soil pHBC were not recorded in either agricultural or forest fields (Figure 4).
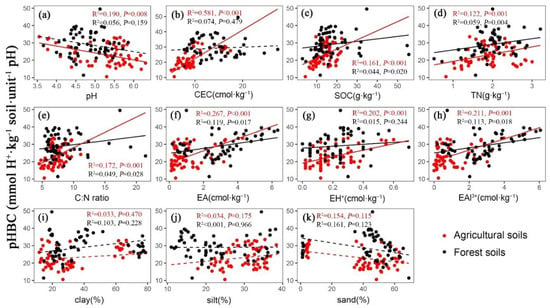
Figure 4.
The relationship between soil pHBC and physicochemical properties in agricultural (n = 60) and forest (n = 60) fields. The abbreviations of CEC, SOC, TN, EA, EH+ and EAl3+ represent cation exchangeable capacity, soil organic carbon, total nitrogen, exchangeable acid, exchangeable H+ ion and exchangeable Al3+ ion, respectively.
The results showed that the CEC was positively correlated with the SOC in both agricultural (R2 = 0.043, p < 0.001) and forest fields (R2 = 0.235, p < 0.001), while clay was not significantly related to CEC in either of the two land-use types (Figure 5). The soil pH was negatively correlated with SOC in forest fields (R2 = 0.019, p = 0.036), while a clear relationship between pH and SOC was not observed in agricultural fields (Figure 5c). Soil pH had no relation with clay content in either agricultural or forest fields (Figure 5d).
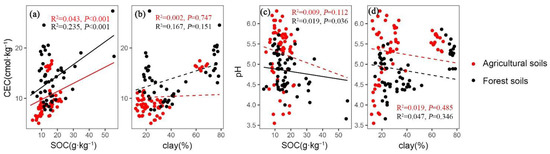
Figure 5.
The relationships between four pairs of soil physicochemical properties (i.e., (a) CEC and SOC, (b) CEC and clay, (c) pH and SOC, and (d) pH and clay) under different land-use types (n = 60). The abbreviations of CEC and SOC represent cation exchangeable capacity and soil organic carbon.
4. Discussion
4.1. Responses of Soil pH Buffering Capacity and Physicochemical Properties to Land Use and Soil Type
In this study, we observed that forest soils had significantly lower pH values, higher pHBC, CEC, EA and EAl3+ than agricultural soils (Figure 2 and Figure 3). This phenomenon is partly in accordance with previous studies. Jiang et al. [35] observed that natural forest soils in subtropics had low soil pH and high content of Al compounds. Alvarez et al. [3] also suggested that tree could decrease soil pH, increase EA and soil total acidity in the Argentine Pampas. This was ascribed to the decomposition of plant litter, the dissociation of H+ ions from the functional groups of SOM and the release of the secretions in rhizosphere [28,35,36]. Meanwhile, soil acidification may facilitate the retention of SOC via inhibiting microbial activities [37]. The negative relationship between the pH and SOC of forest soils has demonstrated their interactions to some extent (Figure 5c). However, the retention and properties of SOM was highly dependent on vegetation type and the extent of human interference. In present study, we observed that although the SOC contents in the two land-use types were at a similar level, the CEC in forest soils was significantly higher than that in agricultural soils (Figure 3b). This indicated that the SOM in forest soils could provide more CEC than that in agricultural soils, which was rarely proposed by previous studies. Luo et al. [17] suggested that the contribution of SOC to soil pHBC might be affected by the charge properties of SOC. Wang et al. [38] observed that land use could significantly alter the chemical composition of SOM, via the complex interactions among SOC, soil texture and pH. Thus, the different cation exchange properties of SOM in the two land-use types may be ascribed to the complex interactive factors by land-use type, and the SOM in forest soils may have more carboxyl and hydroxyl than agricultural soils.
The decline in soil pH could cause the dissolution of Al from the soil solid phase, which promoted free Al3+ to occupy cation-binding sites and form EAl3+ [28,36]. The increase in EAl3+ content implied the rise in Al-compound content in the soil solution. Therefore, forest soils might possess more Al compounds than agricultural soils. Forest soils in the present study may have already been in the Al buffering stage. In addition, in terms of the pH values of each soil type, the ASS in the present study had an extremely low pH, high EAl3+ content and high soil pHBC, which may imply that the buffering capacity of the Al buffering system was stronger than that of the cation exchange or silicate buffering systems.
Soil type was another important factor affecting soil properties. In this study, ASS had the lowest soil pH, the highest TN, EA (including EH+ and EAl3+) and silt contents and relatively high CEC and SOC contents (Figure 3). This might be because ASS originated from long-term waterlogged environments, which caused sulfate to reduce into sulfide, while Fe was reduced from Fe3+ to Fe2+, and finally facilitated the formation of iron sulfide or metastable iron sulfide [39,40]. Iron sulfide’s exposure to the air facilitates the production of sulfuric acid, which causes severe soil acidification (pH < 4) [41]. The extremely low soil pH enhances heavy metal availability, inhibits microbial activities and further influences SOC mineralization. In addition, the chemical properties of ASS may cause the sand to transform into silt.
In our study, the LTS was derived from basalt with a relatively high pH, low EA and EAl3+ content, and had the highest clay content and lowest sand content among the investigated soil types (Figure 3), which was partly in accordance with the previous studies [42,43,44]. This might be because LTS was the most highly weathered soil among the investigated soil types, which was located in tropical regions and formed Fe/Al oxides in soil minerals. Negatively charged clay minerals overlap with positively charged Fe/Al oxides in LTS, which causes the decline in the net charge, further decreases EA on the surface of clay minerals and improves soil pH [45,46]. In addition, previous studies have suggested that high SOC storage was an important feature in basalt-derived soils, because of the close binding between SOC and minerals [47]. However, we did not observe a distinct positive correlation between SOC and clay content in LTS. This might be because the basalt-derived soils mentioned in the previous studies were located in temperate regions and contained a 2:1 mineral type with plenty of permanent charges, leading to the strong binding with SOC and inhibiting SOC mineralization [18]. On the contrary, the clay minerals of highly weathered soils in the (sub)tropics are dominated by a 1:1 mineral type, presenting poor binding with SOC [18]. Moreover, a (sub)tropical monsoon climate might facilitate SOC mineralization, which resulted in the relatively low SOC content of LTS in the current study.
Previous investigations have reported that granite-derived soils always present a low acid-neutralization capacity, high sand content and poor SOC storage [48,49]. In the present study, the lowest soil pHBC and highest sand content were observed in granite-derived LRS (Figure 2 and Figure 3), which is partly in accordance with previous studies. The low pHBC of LRS might be due to the low content of weathered minerals [49]. The low SOC content in agricultural LRS and relatively high SOC content in forest soils implied the effects of land use on SOC storage in granite-derived LRS (Figure 3c).
The RS and PS/YLRS were both derived from sandy shale in the present study; however, they had different responses of pH values to land-use types (Figure 3a). We supposed that, in RS, the lower soil pH of agricultural soils may be caused by the fertilization during cultivated practice, resulting in soil acidification [6], while the higher soil pH in the PS/YLRS of agricultural soils resulted from the changes in soil oxidation-reduction potential under the continual change in flood–dry conditions [50].
4.2. Effects of Land-Use Type on Soil pH Buffering Patterns
Our results show that agricultural and forest soils seem to be in different buffering stages. In agricultural soils, soil pHBC was positively correlated with CEC and SOC (Figure 4), implying that agricultural soils were in a cation exchange buffering stage [17,51]. The positive correlation between pHBC and EA implied the low base saturation of soils, which was the feature of highly weathered acidic soils [52]. The positive relationship between pHBC and EH+ implied that H+ ions still have advantages in the competition with Al3+ for binding sites, and play an important role in regulating soil pH [16]. In forest soils, soil pHBC was positively correlated with SOC and EAl3+, but was not related to CEC (Figure 4), although the contribution of SOC to CEC was demonstrated (Figure 5a). This might be because the buffering of SOM to soil pH was not only dependent on providing CEC, but also on the interactions with Al. Skyllberg [53] suggested that SOM and Al3+ could form Al–SOM complexes under acidic conditions. The complexation/decomplexation of Al–SOM compounds was an important mechanism in the soil pH buffering system during Al buffering stage of acidic soils [32,54]. Therefore, we inferred that forest soils in the present study might be in the Al buffering stage.
Based on the results of this study, we considered that land-use type impacted soil pHBC via affecting SOC turnover, soil pH, Al activities and the interactions of Al–SOM–pH. Soil texture was affected by land-use type to some extent; however, the dominant factor of soil texture is soil type, which is intensively affected by parent materials. Land use, soil type and their interactions greatly impact soil properties, further affecting soil pHBC.
5. Conclusions
This study showed that land use and soil type could significantly affect soil pHBC and physico-chemical properties. Forest soils had higher pHBC (11.40–49.50 mmol·kg−1 soil·unit−1 pH), CEC, EAl3+ and clay contents than agricultural soils, while the pH values of forest soils (on average: 4.84) were significantly lower than those of agricultural soils (on average: 5.28). Acid sulphate soil had the highest pHBC (49.27–117.83 mmol·kg−1 soil·unit−1 pH), EA content and the lowest soil pH, whereas latosol had the highest clay content among all the investigated soil types. Moreover, land-use type considerably changed soil pH buffering patterns. Agricultural soils might be in the cation exchange buffering stage, while forest soils might be in the Al buffering stage. In addition, this study also indicated that the forest soils had a higher pHBC which is helpful to stabilize soil pH and a greater capacity to keep soil CEC and nutrients than the agricultural soils in South China.
Author Contributions
Conceptualization, J.Z., J.Y. and H.W.; methodology, J.Y., Z.S. and H.L.; formal analysis, J.Y. and Z.S.; writing—original draft preparation, J.Y.; writing—review and editing, J.Y., J.Z., H.W. and A.I.A.; visualization, J.Y., Z.S. and Y.Y.; supervision, J.Z.; project administration, J.Z. and H.W.; funding acquisition, J.Z. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers U1701236, 32071641), the Joint Team Project of Guangdong Laboratory for Lingnan Modern Agriculture (grant number NT2021010) and Guangdong Science and Technology Project (grant number 2019B030301007 and 2021A1515012507).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the analysis in this study are available upon request from the corresponding author.
Acknowledgments
Huimin Xiang, Xiaoran Shan, Jiawen Zhong and Saifei Li are appreciated for their helps on soil collections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, J.; Luo, W.; Liu, H.; Feng, X.; Zhang, Y.; Wang, R.; Xu, Z.; Zhang, Y.; Jiang, Y. Precipitation-mediated responses of soil acid buffering capacity to long-term nitrogen addition in a semi-arid grassland. Atmos. Environ. 2017, 170, 312–318. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Global Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Gimenez, A.; Pagnanini, F.; Recondo, V.; Gangi, D.; Caffaro, M.; de Paepe, J.L.; Berhongaray, G. Soil acidity in the Argentine Pampas: Effects of land use and management. Soil Till. Res. 2020, 196, 104434. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Global Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Tao, L.; Li, F.-B.; Liu, C.-S.; Feng, X.-H.; Gu, L.-L.; Wang, B.-R.; Wen, S.-L.; Xu, M.-G. Mitigation of soil acidification through changes in soil mineralogy due to long-term fertilization in southern China. Catena 2019, 174, 227–234. [Google Scholar] [CrossRef]
- Zamanian, K.; Kuzyakov, Y. Contribution of soil inorganic carbon to atmospheric CO2: More important than previously thought. Global Chang. Biol. 2019, 25, e1–e3. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B. Production (via N-fertilization) and correction (by liming) of acidity in soils contribute a huge efflux of CO2 to atmosphere: Real or arbitrary. Global Chang. Biol. 2018, 24, 3280–3281. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent responses of soil buffering capacity to long-term N deposition in three typical tropical forests with different land-use history. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, T.T.H. The contribution of various components to pH buffering capacity of Acrisols in Southeastern Vietnam. Commun. Soil Sci. Plan. 2019, 50, 1170–1177. [Google Scholar] [CrossRef]
- Lieb, A.M.; Darrouzet-Nardi, A.; Bowman, W.D. Nitrogen deposition decreases acid buffering capacity of alpine soils in the southern Rocky Mountains. Geoderma 2011, 164, 220–224. [Google Scholar] [CrossRef]
- Aitken, R.L.; Moody, P.W. The effect of valence and ionic strength on the measurement of pH buffer capacity. Aust. J. Soil Res. 1994, 32, 975–984. [Google Scholar] [CrossRef]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Aust. J. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- Johnson, C.E. Cation exchange properties of acid forest soils of the northeastern USA. Eur. J. Soil Sci. 2002, 2, 271–282. [Google Scholar] [CrossRef]
- Li, W.; Johnson, C.E. Relationships among pH, aluminum solubility and aluminum complexation with organic matter in acid forest soils of the Northeastern United States. Geoderma 2016, 271, 234–242. [Google Scholar] [CrossRef]
- Luo, W.T.; Nelson, P.N.; Li, M.-H.; Cai, J.P.; Zhang, Y.Y.; Zhang, Y.G.; Yang, S.; Wang, R.Z.; Wang, Z.W.; Wu, Y.N.; et al. Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 2015, 12, 7047–7056. [Google Scholar] [CrossRef]
- Aitken, R.L.; Moddy, P.W.; Mckinley, P.G. Lime requirement of acidic Queensland soils. I. relationships between soil properties and pH buffer capacity. Aust. J. Soil Res. 1990, 28, 695–701. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Cheng, P.; Liu, D.; Ma, S.; Zhang, C.; Liu, T.; Tao, L. Surface charge properties of variable charge soils influenced by environmental factors. Appl. Clay Sci. 2020, 189, 105522. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J.; Brożek, S. Modelling the pH dependency of dissolved calcium and aluminium in O, A and B horizons of acid forest soils. Geoderma 2013, 206, 85–91. [Google Scholar] [CrossRef]
- Ulrich, B. Natural and anthropogenic components of soil acidification. J. Plant Nutr. Soil Sc. 1986, 149, 702–717. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Tang, X.; Hu, J.; Lu, Y.; Qiu, J.; Dong, Y.; Li, B. Soil C, N, P stocks and stoichiometry as related to land use types and erosion conditions in lateritic red soil region, south China. Catena 2022, 210, 105888. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Gao, Y.; Yang, Y.; Gao, Y.; Xu, Z. Removal of aluminum from rare-earth leaching solutions via a complexation-precipitation process. Hydrometallurgy 2020, 191, 105220. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Yu, X.; Dai, T.; Peng, Y.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.; Li, B.; et al. Soil acidification of the soil profile across Chengdu Plain of China from the 1980s to 2010s. Sci. Total Environ. 2020, 698, 134320. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of soil organic carbon, pH, electrical conductivity, and water stable aggregates to long-term annual manure and inorganic fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.-P.; Yu, M.; Li, K.; Shao, Y.; Yan, J. Responses of soil buffering capacity to acid treatment in three typical subtropical forests. Sci. Total Environ. 2016, 563–564, 1068–1077. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, H.Y.H.; Searle, E.B.; Sardans, J.; Ciais, P.; Peñuelas, J.; Huang, Z. Whole soil acidification and base cation reduction across subtropical China. Geoderma 2020, 361, 114107. [Google Scholar] [CrossRef]
- Pichler, V.; Gömöryová, E.; Leuschner, C.; Homolák, M.; Abrudan, I.V.; Pichlerová, M.; Střelcová, K.; Filippo, A.D.; Sitko, R. Parent material effect on soil organic carbon concentration under primeval European beech forests at a regional scale. Forests 2021, 12, 405. [Google Scholar] [CrossRef]
- Fujii, K.; Sukartiningsih; Hayakawa, C.; Inagaki, Y.; Kosaki, T. Effects of land use change on turnover and storage of soil organic matter in a tropical forest. Plant Soil 2020, 446, 425–439. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Xiang, H.; Zhang, J.; Li, S.; Yang, J. Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef]
- Zhou, J.M.; Shen, R.F. Dictionary of Soil Science; Science Press: Beijing, China, 2013; p. 114. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Chinese Agriculture Press: Beijing, China, 2000; pp. 163–167. [Google Scholar]
- Jiang, J.; Wang, Y.-P.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Ross, D.S.; Matschonat, G.; Skyllberg, U. Cation exchange in forest soils: The need for a new perspective. Eur. J. Soil Sci. 2008, 59, 1141–1159. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Vogt, R.D.; Mulder, J.; Wang, Y.; Qian, C.; Wang, J.; Zhang, X. Soil acidification as an additional driver to organic carbon accumulation in major Chinese croplands. Geoderma 2020, 366, 114234. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.-K.; Wang, N.; Li, X.-H. Soil acidification of Alfisols as influenced by tea cultivation in eastern China. Pedosphere 2010, 6, 799–806. [Google Scholar] [CrossRef]
- Lindgren, A.; Jonasson, I.K.; Öhrling, C.; Giese, M. Acid sulfate soils and their impact on surface water quality on the Swedish west coast. J. Hydrol-Reg. Stud. 2022, 40, 101019. [Google Scholar] [CrossRef]
- Boman, A.; Åström, M.; Fröjdö, S. Sulfur dynamics in boreal acid sulfate soils rich in metastable iron sulfide—The role of artificial drainage. Chem. Geol. 2008, 255, 68–77. [Google Scholar] [CrossRef]
- Boman, A.; Fröjdö, S.; Backlund, K.; Åström, M.E. Impact of isostatic land uplift and artificial drainage on oxidation of brackish-water sediments rich in metastable iron sulfide. Geochim. Cosmochim. Acta 2010, 74, 1268–1281. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Ouyang, Y.; Hu, R.; Xu, H.; Wang, J. Phosphate-solubilizing bacteria and fungi in relation to phosphorus availability under different land uses for some latosols from Guangdong, China. Catena 2020, 195, 104686. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, X.; Wu, D.; Peng, L.; Fan, C.; Zhang, W.; Li, Q.; Ge, C. Addition of biodegradable microplastics alters the quantity and chemodiversity of dissolved organic matter in latosol. Sci. Total Environ. 2022, 816, 151960. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Oliveira, G.C.; Serafim, M.E.; Silva, É.A.; Ferreira, M.M.; Norton, L.D.; Curi, N. Critical soil moisture range for a coffee crop in an oxidic latosol as affected by soil management. Soil Till. Res. 2015, 154, 103–113. [Google Scholar] [CrossRef]
- Li, J.-Y.; Xu, R.-K.; Zhang, H. Iron oxides serve as natural anti-acidification agents in highly weathered soils. J. Soil Sediment 2012, 12, 876–887. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Li, J. Effect of Fe/Al oxides on desorption of Cd2+ from soils and minerals as related to diffuse layer overlapping. Soil Res. 2011, 49, 231–237. [Google Scholar] [CrossRef]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Mao, X.; van Zwieten, L.; Zhang, M.; Qiu, Z.; Yao, Y.; Wang, H. Soil parent material controls organic matter stocks and retention patterns in subtropical China. J. Soil Sediment 2020, 20, 2426–2438. [Google Scholar] [CrossRef]
- Fujii, K.; Kanetani, S.; Tetsuka, K. Effects of volcanic parent materials on the acid buffering capacity of forest soils on Yakushima Island, Japan. Soil Sci. Plant Nutr. 2020, 66, 680–692. [Google Scholar] [CrossRef]
- Gao, S.; Cao, W.; Zhou, G.; Rees, R.M. Bacterial communities in paddy soils changed by milk vetch as green manure: A study conducted across six provinces in South China. Pedosphere 2021, 31, 521–530. [Google Scholar] [CrossRef]
- Magdoff, F.R.; Bartlett, R.J. Soil pH buffering revisited. Soil Sci. Soc. Am. J. 1985, 49, 145–148. [Google Scholar] [CrossRef]
- Nguyen, T. The pH buffering capacity of Acrisols under cassava production in Southeastern Vietnam, Chau Thanh district. Soil Use Manag. 2018, 34, 554–562. [Google Scholar] [CrossRef]
- Skyllberg, U. pH and solubility of aluminium in acidic forest soils: A consequence of reactions between organic acidity and aluminium alkalinity. Eur. J. Soil Sci. 1999, 50, 95–106. [Google Scholar] [CrossRef]
- Lofts, S.; Simon, B.M.; Tipping, E.; Woof, C. Modelling the solid–solution partitioning of organic matter in European forest soils. Eur. J. Soil Sci. 2001, 52, 215–226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).