Synthesis of Novel MOF-5 Based BiCoO3 Photocatalyst for the Treatment of Textile Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of MOF-5
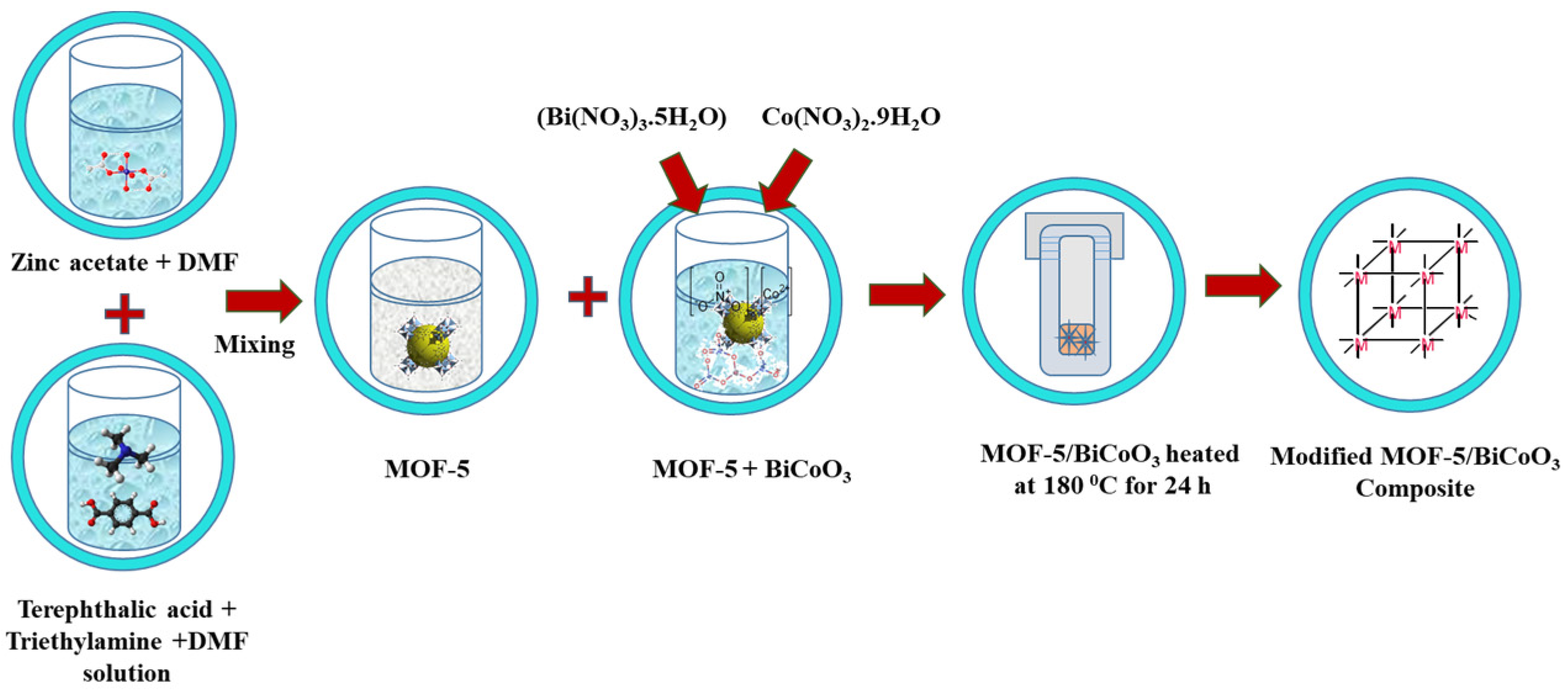
2.3. Preparation of MOF-5/BiCoO3 Composite
2.4. Characterization of Samples
2.5. Photocatalytic Degradation Experiment
2.6. Role of Radical Trapping Scavengers
2.7. Recycling of Photocatalyst
3. Results and Discussion
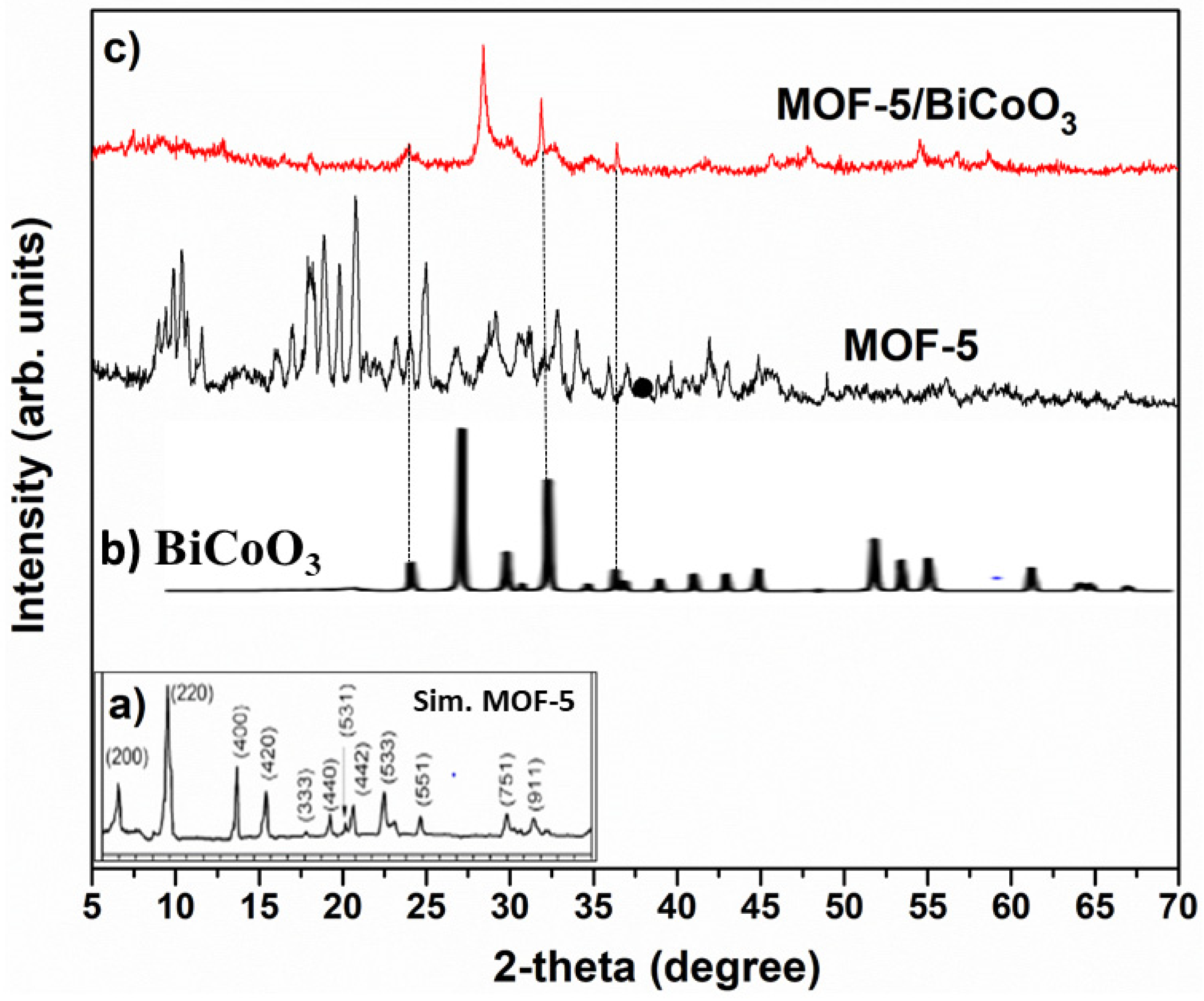
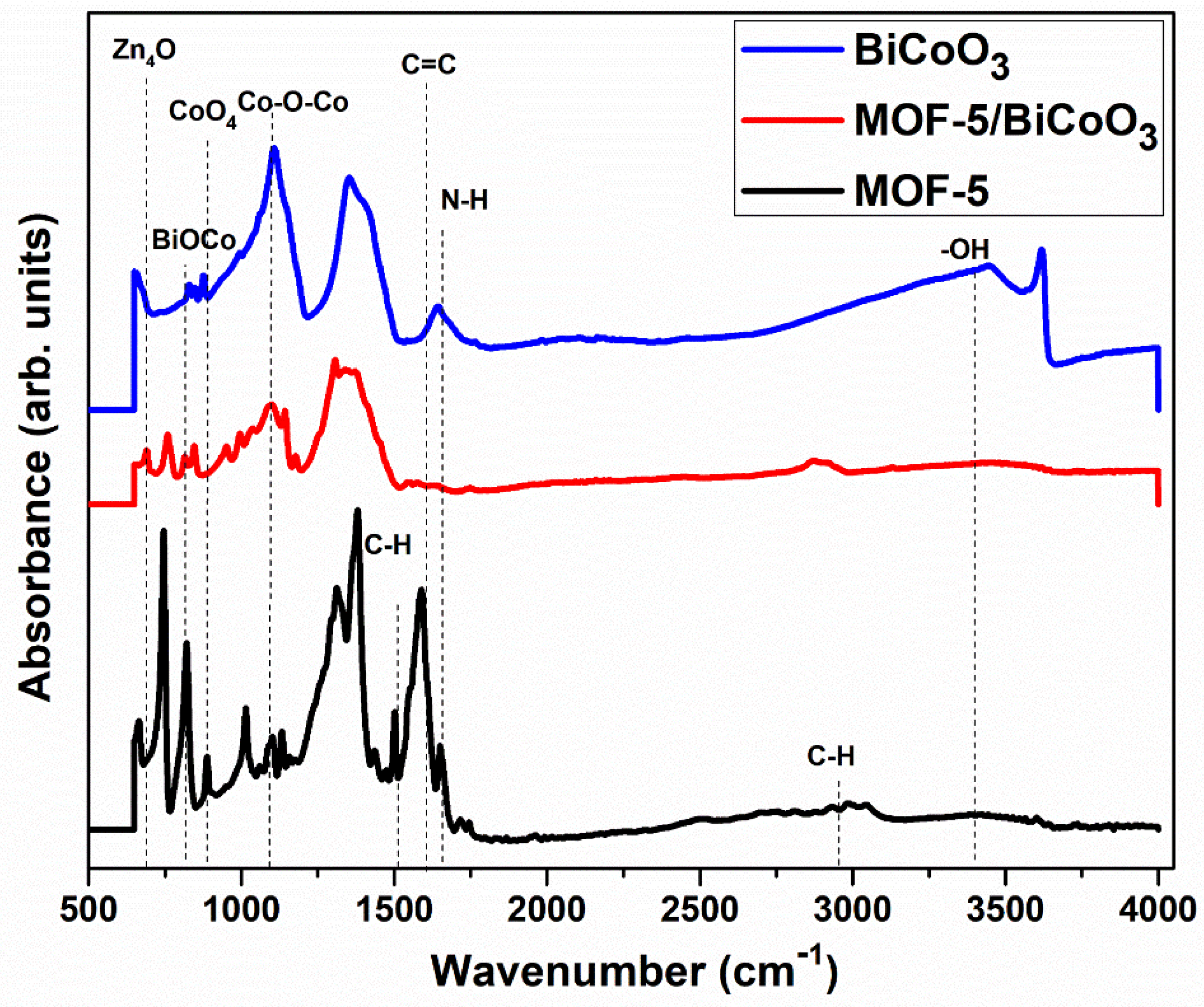
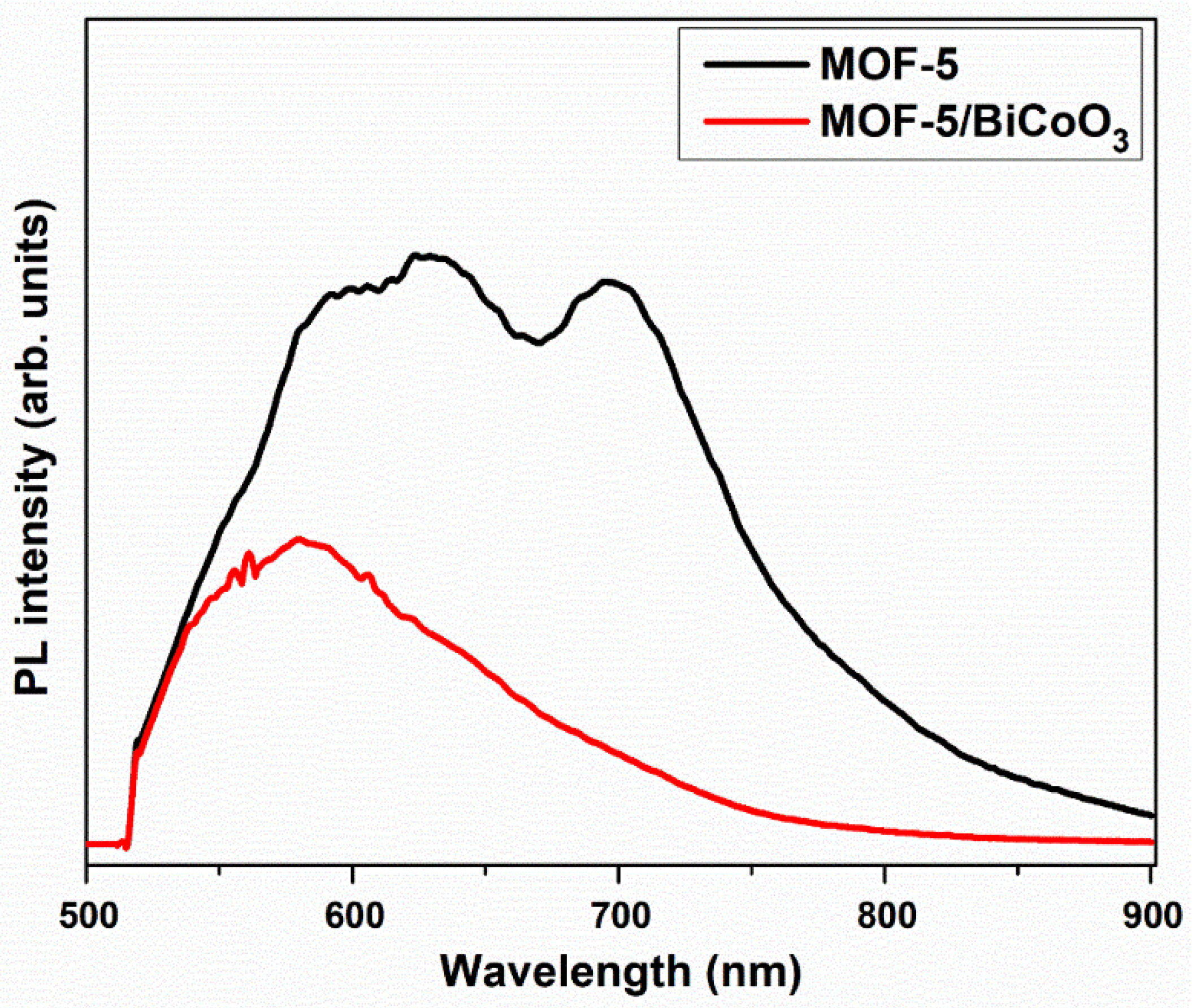
3.1. Evaluation of Crystallinity, Morphological, Functional Groups, Specific Surface-Area, and Optical Properties of Prepared Samples
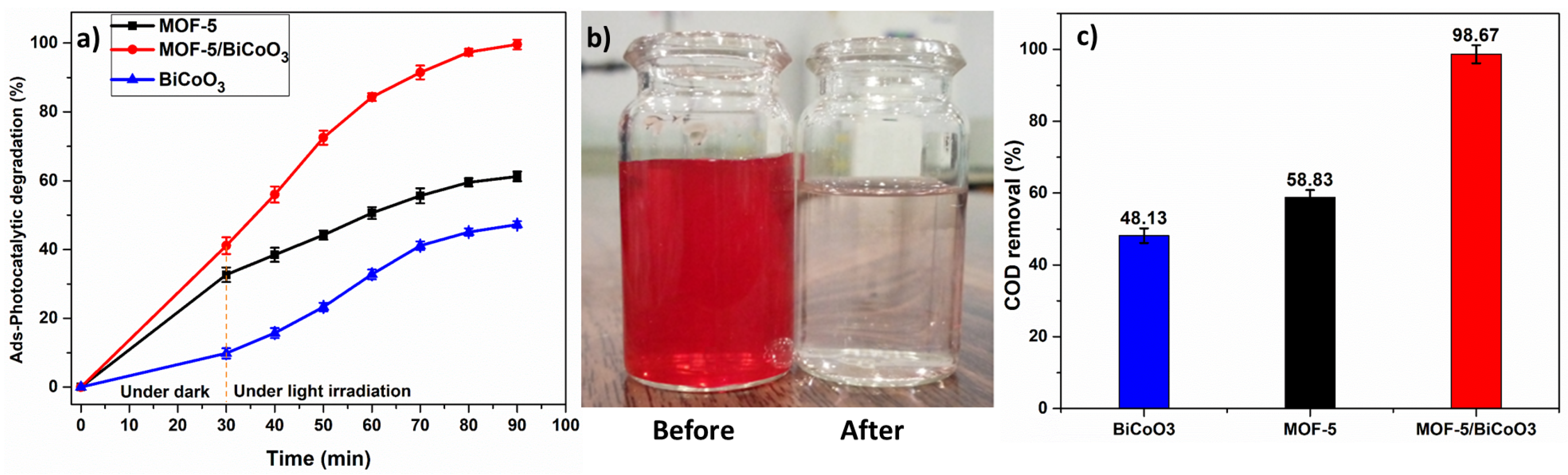
3.2. Photocatalytic Degradation Performance and COD Removal
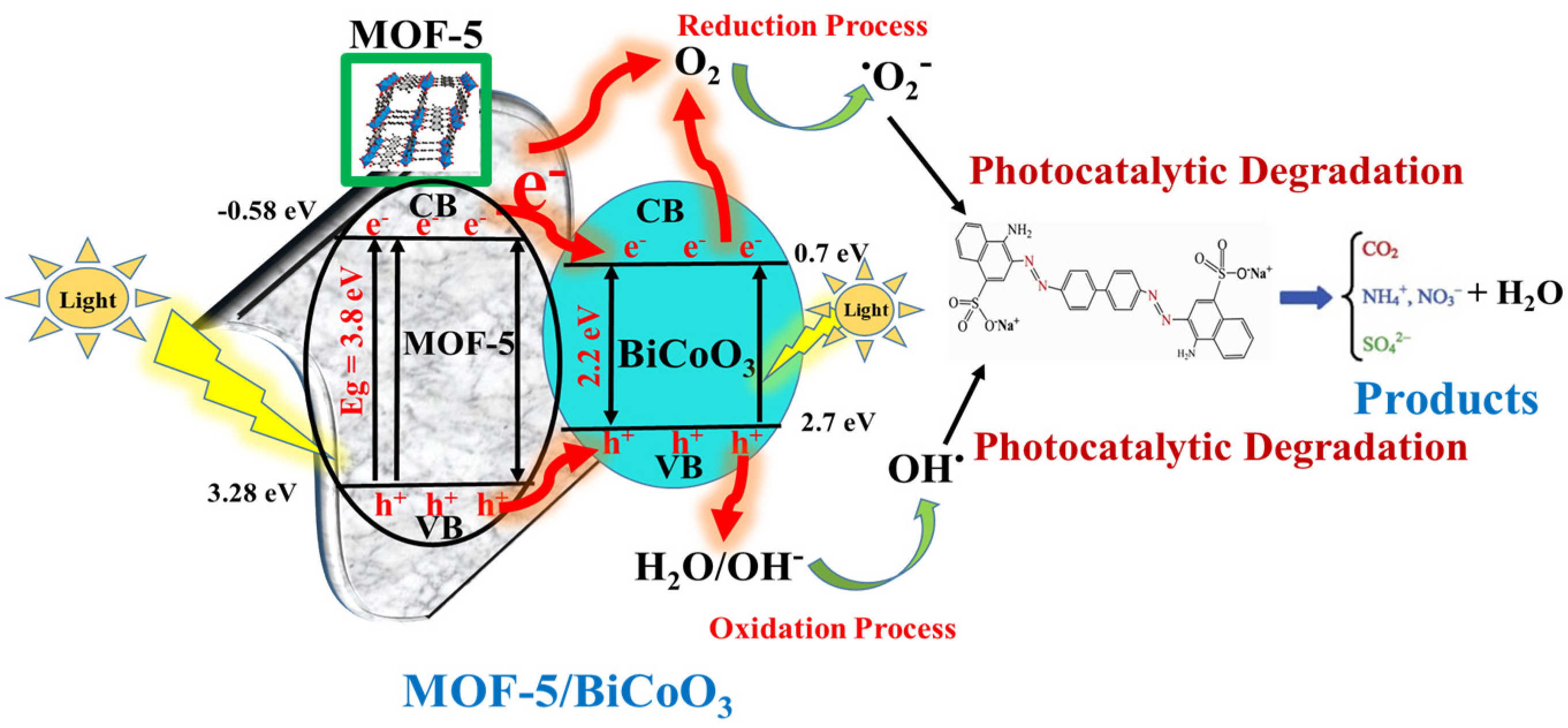
3.3. Photocatalytic Reaction Mechanism
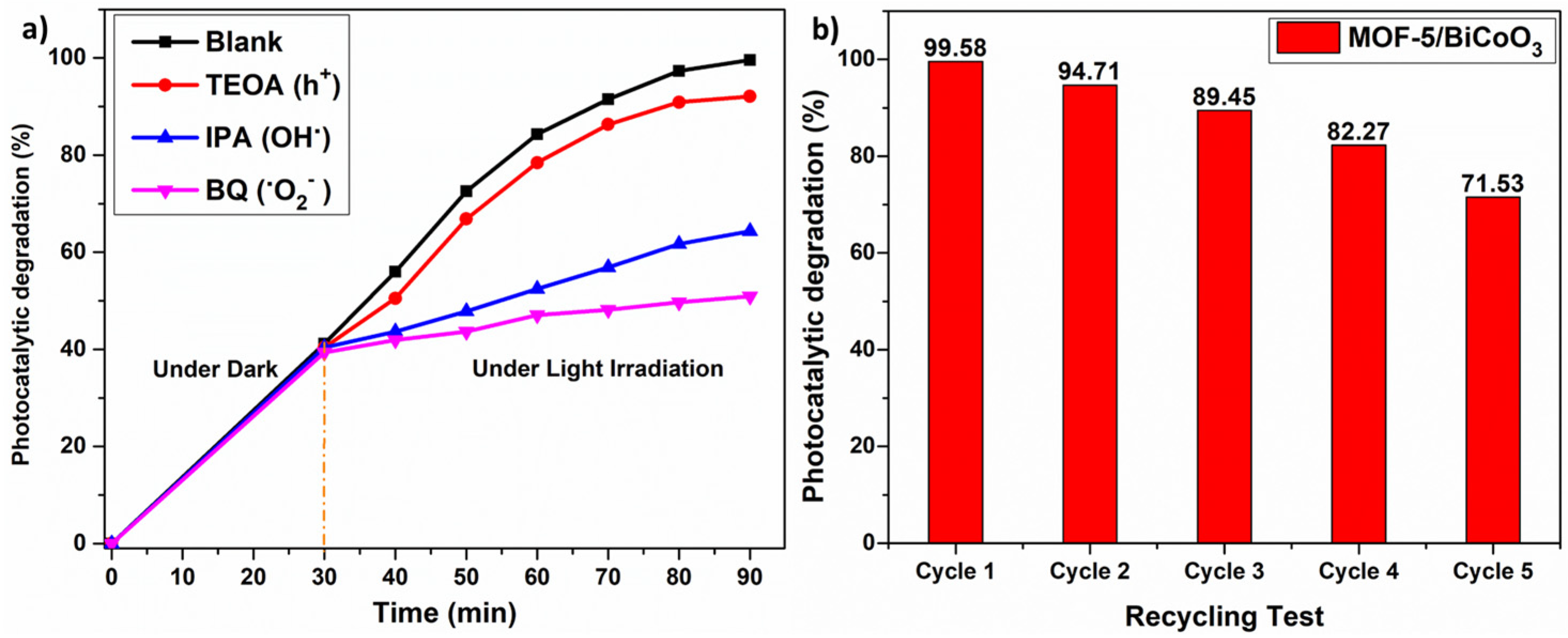
3.4. Radical Trapping and Reusability Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| MOF | Metal Organic Framework |
| CR | Congo red |
| BQ | Benzene Quinone |
| TEOA | Triethanolamine (TEOA), |
| IPA | Isopropanol (IPA) |
| COD | Chemical Oxygen Demand |
References
- Zhou, F.Y.; Mao, J.N.; Peng, X.L.; Hong, B.; Xu, J.C.; Zeng, Y.X.; Han, Y.B.; Ge, H.L.; Wang, X.Q. Magnetically Separable Ni/g-C3N4 Nanocomposites for Enhanced Visible-Light Photocatalytic Degradation of Methylene Blue and Ciprofloxacin. Diam. Relat. Mater. 2022, 126, 109070. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Khan, N.; Imran, M.; Ali, J.; Ul, Z.; Khan, H.; Gamal, A.; Hussien, S. Nano-Zerovalent Manganese/Biochar Composite for the Adsorptive and Oxidative Removal of Congo-Red Dye from Aqueous Solutions. J. Hazard. Mater. 2021, 403, 123854. [Google Scholar] [CrossRef]
- Tabasum, B.; Dhagale, P.R.; Nitnaware, K.M.; Nikule, H.A.; Nikam, T.D. New Chemical Products Formation from Textile Dye Degradation, Chitinolytic and Antioxidant Activity in New Strain Nbpc5-18 of Cellulosimicrobium Sp. TH-20. J. Environ. Chem. Eng. 2019, 7, 103114. [Google Scholar] [CrossRef]
- Suleman, M.; Zafar, M.; Ahmed, A.; Rashid, M.U.; Hussain, S.; Razzaq, A.; Mohidem, N.A.; Fazal, T.; Haider, B.; Park, Y.K. Castor Leaves-Based Biochar for Adsorption of Safranin from Textile Wastewater. Sustainability 2021, 13, 6926. [Google Scholar] [CrossRef]
- Singh, S.; Perween, S.; Ranjan, A. Dramatic Enhancement in Adsorption of Congo Red Dye in Polymer-Nanoparticle Composite of Polyaniline-Zinc Titanate. J. Environ. Chem. Eng. 2021, 9, 105149. [Google Scholar] [CrossRef]
- Koohi, P.; Rahbar-kelishami, A.; Shayesteh, H. Efficient Removal of Congo Red Dye Using Fe3O4/NiO Nanocomposite: Synthesis and Characterization. Environ. Technol. Innov. 2021, 23, 101559. [Google Scholar] [CrossRef]
- Fazal, T.; Faisal, A.; Mushtaq, A.; Hafeez, A.; Javed, F.; Alaud Din, A.; Rashid, N.; Aslam, M.; Rehman, M.S.; Rehman, F. Macroalgae and Coal-Based Biochar as a Sustainable Bioresource Reuse for Treatment of Textile Wastewater. Biomass Convers. Biorefinery 2019, 11, 1491–1506. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Ullah, A.; Riaz, N.; Aslam, M.; Kim, J. Photocatalytic Systems as an Advanced Environmental Remediation: Recent Developments, Limitations and New Avenues for Applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Ur Rehman, M.S.; Faisal, A.; Rehman, F. Integrating Adsorption and Photocatalysis: A Cost Effective Strategy for Textile Wastewater Treatment Using Hybrid Biochar-TiO2 Composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Yadav, A.; Mandal, M.K.; Dubey, K.K. TiO2 Based Photocatalysis: A Valuable Approach for the Removal of Pharmaceuticals from Aquatic Environment. Int. J. Environ. Sci. Technol. 2022, 2010. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S.; Hameed, B.H. Epigrammatic Progress and Perspective on the Photocatalytic Properties of BiVO4-Based Photocatalyst in Photocatalytic Water Treatment Technology: A Review. J. Mol. Liq. 2018, 268, 438–459. [Google Scholar] [CrossRef]
- Leichtweis, J.; Silvestri, S.; Carissimi, E. New Composite of Pecan Nutshells Biochar-ZnO for Sequential Removal of Acid Red 97 by Adsorption and Photocatalysis. Biomass Bioenergy 2020, 140, 105648. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Guo, Q.; Wang, Y.; Li, C. Preparation of a Heterogeneous Catalyst CuO-Fe2O3/CTS-ATP and Degradation of Methylene Blue and Ciprofloxacin. Coatings 2022, 12, 559. [Google Scholar] [CrossRef]
- Yang, J.; Luo, X. Ag-Doped TiO2 Immobilized Cellulose-Derived Carbon Beads: One-Pot Preparation, Photocatalytic Degradation Performance and Mechanism of Ceftriaxone Sodium. Appl. Surf. Sci. 2021, 542, 148724. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Kong, X.; Yao, K.; Liu, L.; Zhang, J.; Guo, Z.; Xu, W.; Fang, Z.; Liu, Y. Photocatalytic Performance of the MOF-Coating Layer on SPR- Excited Ag Nanowires. ACS Omega 2021, 6, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.K. Reduced Graphene Oxide (RGO) on TiO2 for an Improved Photoelectrochemical (PEC) and Photocatalytic Activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Younis, S.A.; Serp, P.; Nassar, H.N. Photocatalytic and Biocidal Activities of ZnTiO2 Oxynitride Heterojunction with MOF-5 and g-C3N4: A Case Study for Textile Wastewater Treatment under Direct Sunlight. J. Hazard. Mater. 2020, 410, 124562. [Google Scholar] [CrossRef]
- Silvestri, S.; Gonçalves, M.G.; Da Silva Veiga, P.A.; Matos, T.T.D.S.; Peralta-Zamora, P.; Mangrich, A.S. TiO2 Supported on Salvinia Molesta Biochar for Heterogeneous Photocatalytic Degradation of Acid Orange 7 Dye. J. Environ. Chem. Eng. 2019, 7, 102879. [Google Scholar] [CrossRef]
- Wang, C.C.; Yi, X.H.; Wang, P. Powerful Combination of MOFs and C3N4 for Enhanced Photocatalytic Performance. Appl. Catal. B Environ. 2019, 247, 24–48. [Google Scholar] [CrossRef]
- Mo, Z.; Tai, D.; Zhang, H.; Shahab, A. A Comprehensive Review on the Adsorption of Heavy Metals by Zeolite Imidazole Framework (ZIF-8) Based Nanocomposite in Water. Chem. Eng. J. 2022, 443, 136320. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Santaclara, J.G.; Oar-Arteta, L.; van Koppen, L.; Osadchii, D.Y.; Gascon, J.; Kapteijn, F. Photocatalytic Properties of TiO 2 and Fe-Doped TiO 2 Prepared by Metal Organic Framework-Mediated Synthesis. Chem. Eng. J. 2019, 360, 75–88. [Google Scholar] [CrossRef]
- Yao, T.; Tan, Y.; Zhou, Y.; Chen, Y.; Xiang, M. Preparation of Core-Shell MOF-5/Bi2WO6 Composite for the Enhanced Photocatalytic Degradation of Pollutants. J. Solid State Chem. 2022, 308, 122882. [Google Scholar] [CrossRef]
- Lu, G.; Chu, F.; Huang, X.; Li, Y.; Liang, K.; Wang, G. Recent Advances in Metal-Organic Frameworks-Based Materials for Photocatalytic Selective Oxidation. Coord. Chem. Rev. 2022, 450, 214240. [Google Scholar] [CrossRef]
- Sun, W.; Wang, W.; Chen, D.; Zhang, G.; Cheng, Z.; Wang, Y. First-Principles Investigation on Tunable Electronic Properties and Magnetism by Polarization in PbTiO3/BiFeO3 2D Ferroelectric Heterostructures. J. Mater. Chem. C 2019, 7, 463–473. [Google Scholar] [CrossRef]
- Lopes, J.A.; Sabino, E.B.; Raimundo, R.A.; Ribeiro, D. (Bi13Co11)Co2O40-Co3O4 Composites: Synthesis, Structural and Magnetic Properties. J. Alloys Compd. 2021, 852, 156991. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Peng, C.; Zhu, L.; Zhang, Y.; Cheng, H.; Cui, J.; Wu, Q.; Lu, Y. Activating the Lattice Oxygen in (Bi0.5Co0.5)2O3 by Vacancy Modulation for Efficient Electrochemical Water Oxidation. J. Mater. Chem. A 2020, 8, 13150–13159. [Google Scholar] [CrossRef]
- Pan, C.; Wang, Z.; Lou, Y.; Zhang, Y.; Dong, Y. The Construction of a Wide-Spectrum-Responsive and High-Activity Photocatalyst, Bi25CoO40, via the Creation of Large External Dipolesation of Large External Dipoles. J. Mater. Chem. A 2021, 3, 3616–3627. [Google Scholar] [CrossRef]
- Qin, X.; Qiang, T.; Chen, L.; Wang, S. Construction of 3D N-CQD/MOF-5 Photocatalyst to Improve the Photocatalytic Performance of MOF-5 by Changing the Electron Transfer Path. Microporous Mesoporous Mater. 2021, 315, 110889. [Google Scholar] [CrossRef]
- Rodríguez, N.A.; Parra, R.; Grela, M.A. Structural Characterization, Optical Properties and Photocatalytic Activity of MOF-5 and Its Hydrolysis Products: Implications on Their Excitation Mechanism. RSC Adv. 2015, 5, 73112–73118. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Zou, J.; Xiong, X.; Zeng, X.; Zhou, J. Photocatalytic Performance of a Novel MOF/BiFeO3 Composite. Materials 2017, 10, 1161. [Google Scholar] [CrossRef]
- Zhao, H.; Song, H.; Chou, L. Nickel Nanoparticles Supported on MOF-5: Synthesis and Catalytic Hydrogenation Properties. Inorg. Chem. Commun. 2012, 15, 261–265. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, J.; Zhou, Y.; Tang, H. Preparation of a Zinc-Based Metal–Organic Framework (MOF-5)/BiOBr Heterojunction for Photodegradation of Rhodamine B. React. Kinet. Mech. Catal. 2021, 134, 1003–1015. [Google Scholar] [CrossRef]
- Hajiashra, S.; Motakef, N. Preparation and Evaluation of ZnO Nanoparticles by Thermal Decomposition of MOF-5. Heliyon 2019, 5, e02152. [Google Scholar] [CrossRef]
- Ramachandran, T.; Rajeevan, N.E.; Pradyumnan, P.P. Enhanced Thermoelectric Properties of BiCoO3 by Nickel Substitution. Mater. Sci. Appl. 2013, 4, 816–821. [Google Scholar] [CrossRef][Green Version]
- Reza, M.; Negar, M.; Ashouri, M.F. Nitrate Adsorption from Aqueous Solution by Metal—Organic Framework MOF-5. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 443–449. [Google Scholar] [CrossRef]
- Savic, M.; Marjanovi, B.; Zaso, B.A.; Stojadinovi, S.; Gordana, Ć. Novel Microporous Composites of MOF-5 and Polyaniline with High Specific Surface Area. Synth. Met. 2020, 262, 116348. [Google Scholar] [CrossRef]
- Erdemoǧlu, S.; Aksu, S.K.; Sayilkan, F.; Izgi, B.; Asiltürk, M.; Sayilkan, H.; Frimmel, F.; Güçer, Ş. Photocatalytic Degradation of Congo Red by Hydrothermally Synthesized Nanocrystalline TiO2 and Identification of Degradation Products by LC-MS. J. Hazard. Mater. 2008, 155, 469–476. [Google Scholar] [CrossRef]
- Harun, N.H.; Rahman, M.N.A.; Kamarudin, W.F.W.; Irwan, Z.; Muhammud, A.; Akhir, N.E.F.M.; Yaafar, M.R. Photocatalytic Degradation of Congo Red Dye Based on Titanium Dioxide Using Solar and Uv Lamp. J. Fundam. Appl. Sci. 2018, 10, 832–846. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of Novel Magnetically Separable Nanocomposites Using Graphitic Carbon Nitride, Silver Phosphate and Silver Chloride and Their Applications in Photocatalytic Removal of Different Pollutants Using Visible-Light Irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef]
- Zhai, B.; Chen, Y.; Li, J.; Liang, Y. Two-Dimensional Composite (BiOCl/GO/MOF-5) by Ultrasonic-Assisted Solvothermal Synthesis with Enhanced Photocatalytic Activity. Micro Nano Lett. 2020, 15, 149–154. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, B.; Khan, A.U.; Fazal, T.; Aslam, M.; Qaisrani, N.A.; Ahmed, A. Synthesis of Novel MOF-5 Based BiCoO3 Photocatalyst for the Treatment of Textile Wastewater. Sustainability 2022, 14, 12885. https://doi.org/10.3390/su141912885
Sarwar B, Khan AU, Fazal T, Aslam M, Qaisrani NA, Ahmed A. Synthesis of Novel MOF-5 Based BiCoO3 Photocatalyst for the Treatment of Textile Wastewater. Sustainability. 2022; 14(19):12885. https://doi.org/10.3390/su141912885
Chicago/Turabian StyleSarwar, Bazla, Asad Ullah Khan, Tahir Fazal, Muhammad Aslam, Naeem Akhtar Qaisrani, and Ashfaq Ahmed. 2022. "Synthesis of Novel MOF-5 Based BiCoO3 Photocatalyst for the Treatment of Textile Wastewater" Sustainability 14, no. 19: 12885. https://doi.org/10.3390/su141912885
APA StyleSarwar, B., Khan, A. U., Fazal, T., Aslam, M., Qaisrani, N. A., & Ahmed, A. (2022). Synthesis of Novel MOF-5 Based BiCoO3 Photocatalyst for the Treatment of Textile Wastewater. Sustainability, 14(19), 12885. https://doi.org/10.3390/su141912885






