Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings
Abstract
1. Introduction
2. Materials and Methods
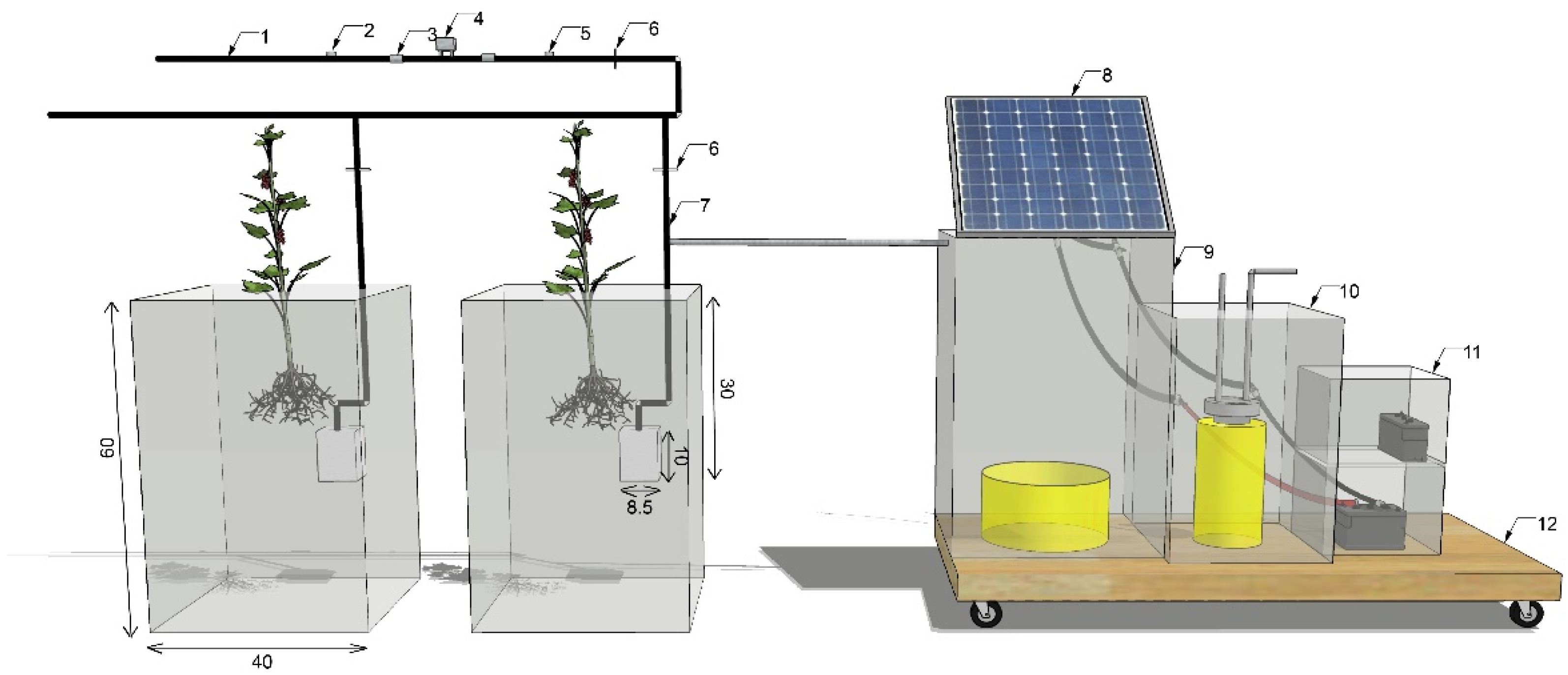
2.1. Experimental Materials and Design
2.2. Soil Sampling
2.3. Determination of Growth Index and Plant Biomass
2.4. Determination of Root Morphological Index
2.5. Soil DNA Extraction and PCR Amplification
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Impact of Aerated Irrigation on the Biomass Accumulation of Grape Seedlings
3.2. Impact of Aerated Irrigation on the Root Morphology of Grape Seedlings in Different Soil Layers
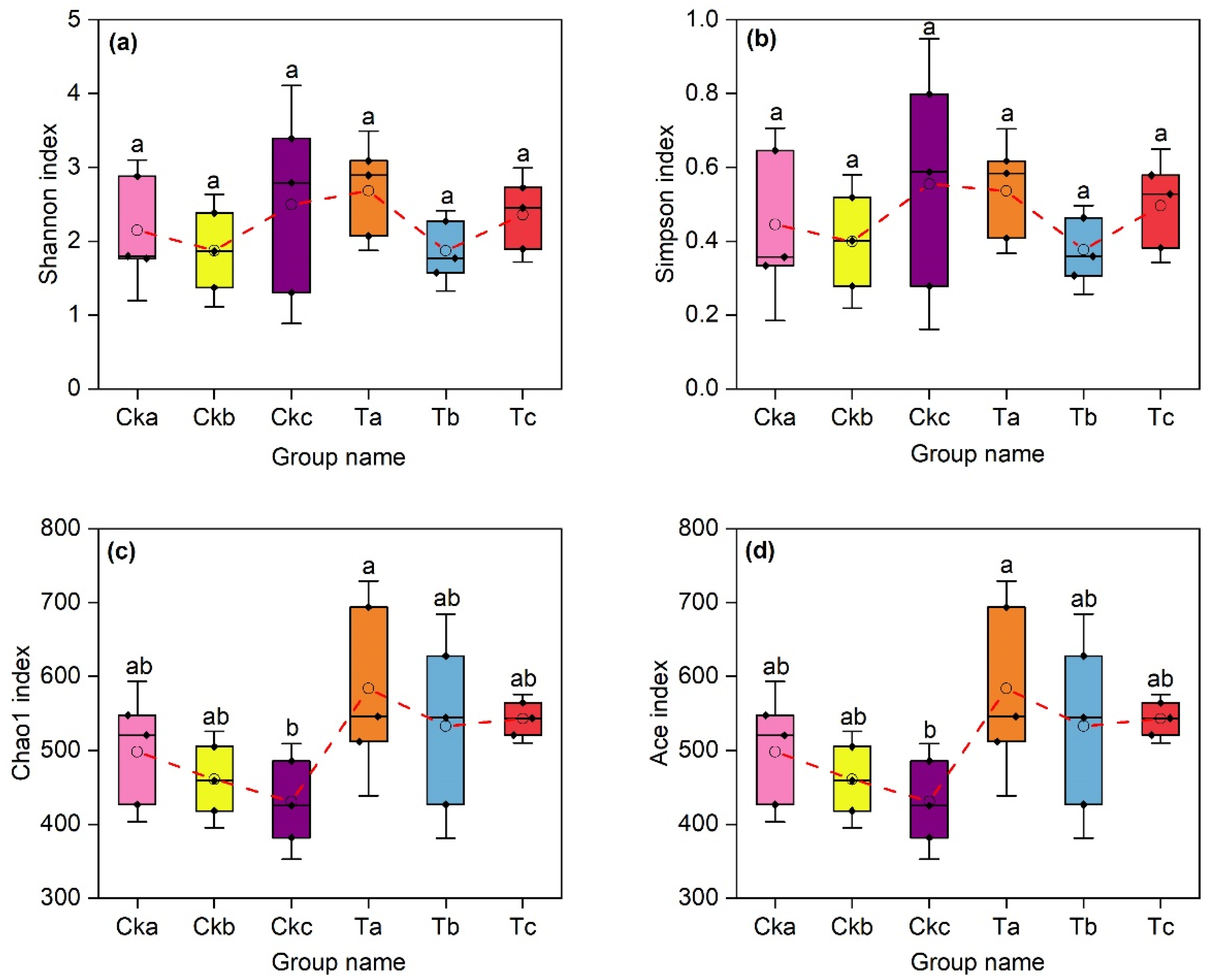
3.3. Impact of Aerated Irrigation on Soil Fungal Community Diversity
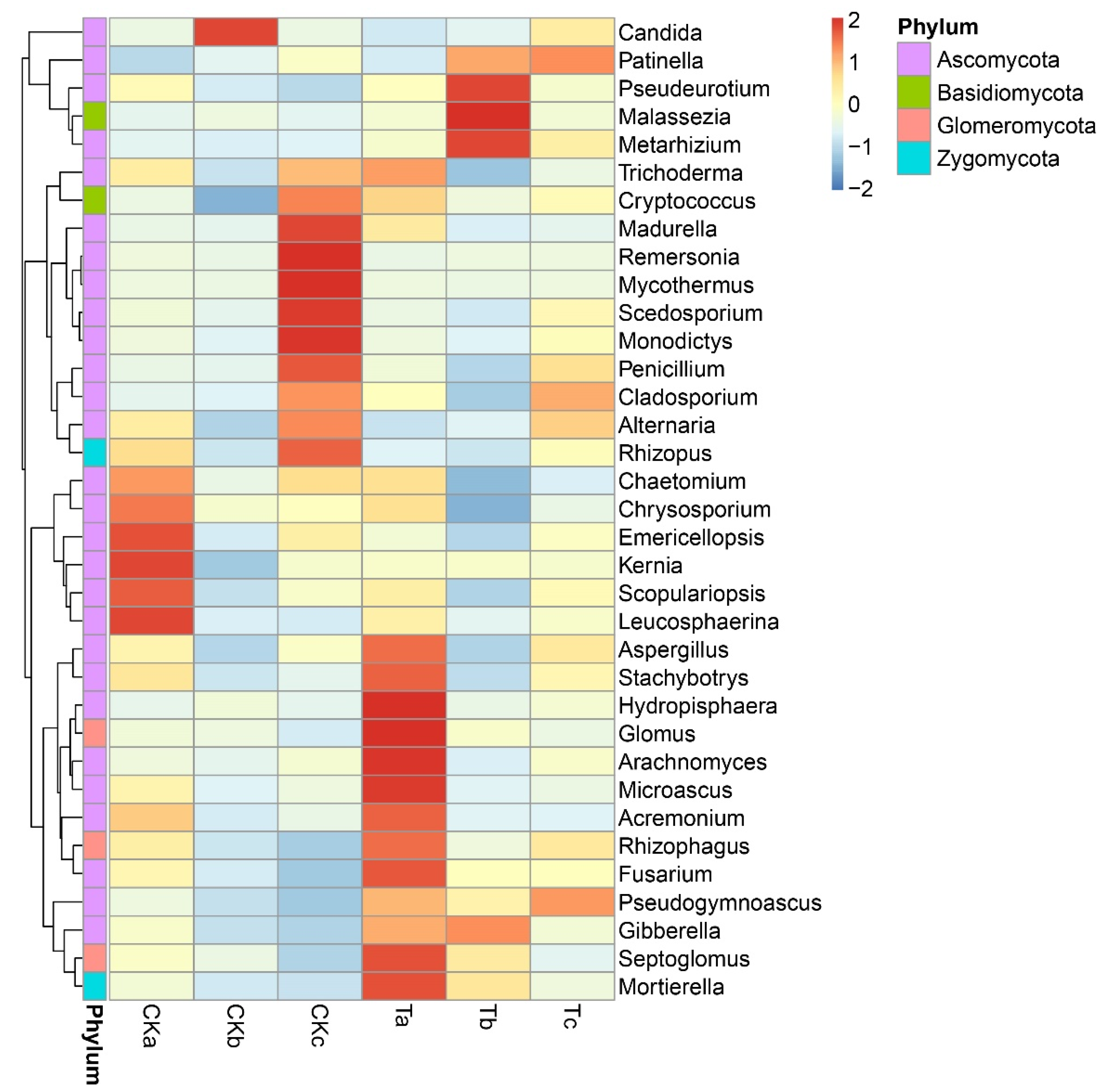
3.4. Impact of Aerated Irrigation on Fungi at Phylum, Class, Order, Family, and Genus Levels
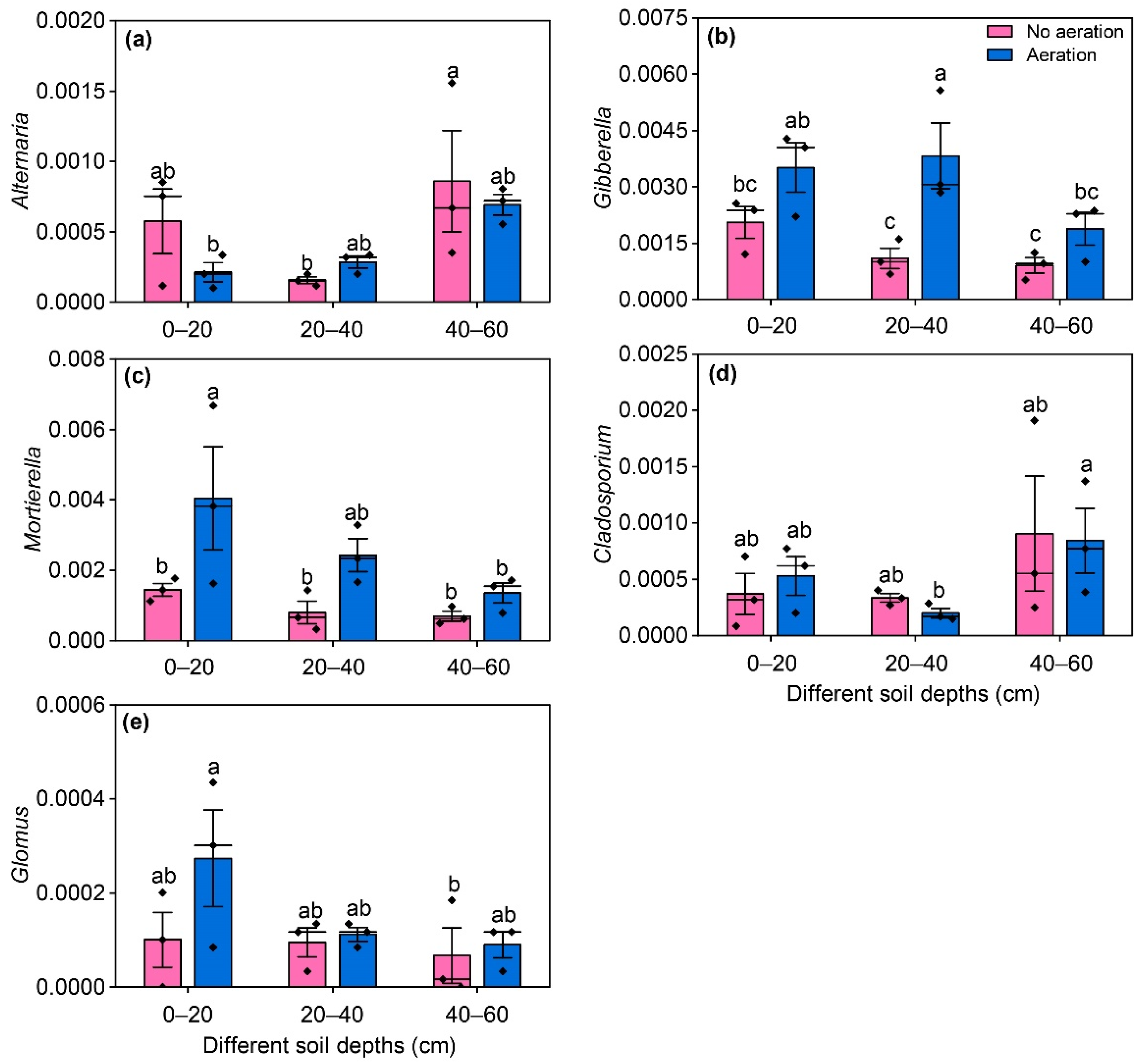
3.5. Impact of Aerated Irrigation on Harmful and Beneficial Fungal Genera
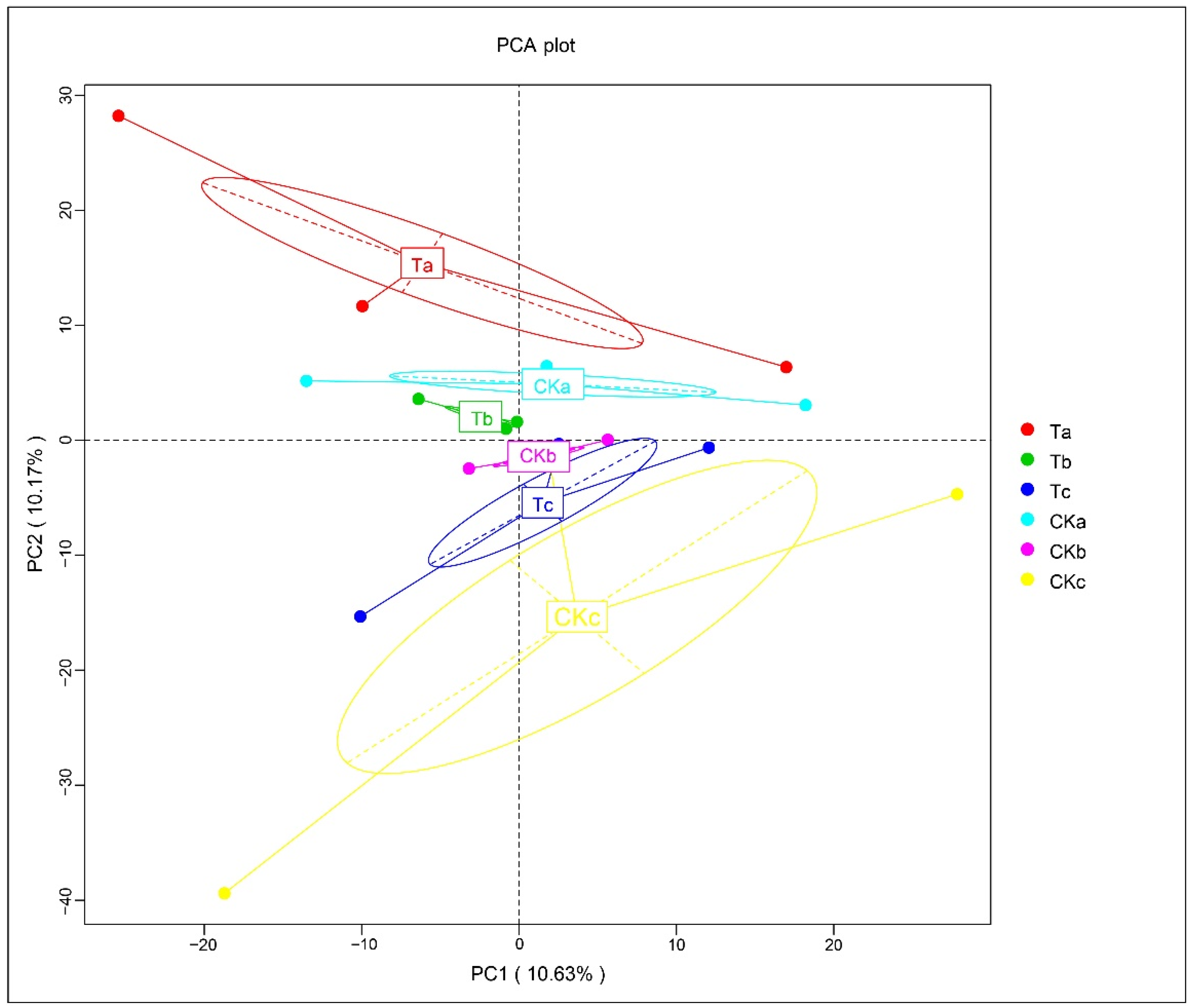
3.6. Impact of Aerated Irrigation on the Fungal Community Structure at Different Soil Depths
4. Discussion
4.1. Impact of Aerated Irrigation on the Biomass and Root Distribution of Grape Seedlings
4.2. Impact of Aerated Irrigation on the Fungal Community Structure in Grape Rhizosphere Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedman, S.P.; Naftaliev, B. A Survey of the aeration status of drip-irrigated orchards. Agric. Water Manag. 2012, 115, 132–147. [Google Scholar] [CrossRef]
- Fiebig, A.; Dodd, I.C. Inhibition of tomato shoot growth by over-irrigation is linked to nitrogen deficiency and ethylene. Physiol. Plant. 2015, 156, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Pandey, S.; Burritt, D.J.; Lam-Son, P.T. Plant responses to low-oxygen stress: Interplay between ROS and NO signaling pathways. Environ. Exp. Bot. 2019, 161, 134–142. [Google Scholar] [CrossRef]
- Niu, W.Q.; Guo, Q.; Zhou, X.B.; Helmers, M.J. Effect of aeration and soil water redistribution on the air permeability under subsurface drip irrigation. Soil Sci. Soc. Am. J. 2012, 76, 815–820. [Google Scholar] [CrossRef]
- Licausi, F.; Giuntoli, B.; Perata, P. Similar and yet different: Oxygen sensing in animals and plants. Trends Plant Sci. 2020, 25, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Kläring, H.P.; Zude, M. Sensing of tomato plant response to hypoxia in the root environment. Sci. Hortic. 2009, 122, 17–25. [Google Scholar] [CrossRef]
- Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; El-Sawy, M.B.I. The effects of root aeration and different soil conditioners on the nutritional values, yield, and water productivity of potato in clay loam soil. Agronomy 2019, 9, 418. [Google Scholar] [CrossRef]
- Yang, X.; Fan, J.; Ge, J.; Luo, Z. Effect of irrigation with activated water on root morphology of hydroponic rice and wheat seedlings. Agronomy 2022, 12, 1068. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Wang, C.; Zou, H.F.; Wang, H.X.; Li, H.L.; Sun, H.T.; Yu, D.H. The effects of aerated irrigation on soil respiration and the yield of the maize root zone. Sustainability 2022, 14, 4378. [Google Scholar] [CrossRef]
- Cao, X.S.; Li, H.P.; Zheng, H.X.; Feng, Y.Y.; Cheng, Z.Z.; Zhao, Q.H. Effects of aerated irrigation on soil fertility and crop growth in root zone. Agric. Res. Arid. Areas 2020, 38, 183–189. [Google Scholar] [CrossRef]
- Heuberger, H.; Livet, J.; Schnitzler, W. Effect of soil aeration on nitrogen availability and growth of selected vegetables—Preliminary results. Acta. Hortic. 2001, 56, 147–154. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Su, N.; Midmore, D.J. Oxygation unlocks yield potentials of crops in oxygen limited soil environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar] [CrossRef]
- Du, Y.D.; Niu, W.Q.; Gu, X.B.; Zhang, Q.; Cui, B.J.; Zhao, Y. Crop yield and water use efficiency under aerated irrigation: A meta-analysis. Agric. Water Manag. 2018, 210, 158–164. [Google Scholar] [CrossRef]
- Niu, W.Q.; Zang, X.; Jia, Z.X.; Shao, H.B. Effects of rhizosphere ventilation on soil enzyme activities of potted tomato under different soil water stress. CLEN–Soil Air Water 2021, 40, 225–232. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, U.D.; Cui, B.J.; Sun, J.; Wang, J.; Wu, M.L.; Niu, W.Q. Aerated irrigation offsets the negative effects of nitrogen reduction on crop growth and water-nitrogen utilization. J. Clean. Prod. 2021, 313, 127917. [Google Scholar] [CrossRef]
- Cui, B.J.; Niu, W.Q.; Du, Y.D.; Zhang, Q. Response of yield and nitrogen use efficiency to aerated irrigation and N application rate in greenhouse cucumber. Sci. Hortic. 2020, 265, 109220. [Google Scholar] [CrossRef]
- Zhu, Y.; Dyck, M.; Cai, H.J.; Song, L.B.; Chen, H. The effects of aerated irrigation on soil respiration, oxygen, and porosity. J. Integr. Agric. 2019, 18, 2854–2868. [Google Scholar] [CrossRef]
- Pang, J.; Han, Q.S.; Zhou, S.; Li, H.H.; Song, J.W.; Liu, H. Effects of water-air interaction on the growth, yield and water use efficiency of greenhouse tomato. J. Irrig. Drain. 2022, 41, 87–94. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Sun, J.L.; Jiang, Y.; Hu, D.G.; Yang, X.; Dong, M.M.; Yu, K.; Yu, S.L. Effect of rhizosphere aeration by subsurface drip irrigation with tanks on the growth of ‘Red Globe’ grape seedling and its absorption, distribution and utilization of urea-15N. Sci. Hortic. 2018, 236, 207–213. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.X.; Niu, W.Q.; Wang, J.W. Impact of post-infiltration soil aeration at different growth stages of sub-surface trickle-irrigated tomato plants. Int. Agrophys. 2016, 30, 331–337. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Png, G.K.; Bardgett, R.D.; Baquerizo, M.D. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.W.; van Moorsel, S.J.; Hahl, T.; De Luca, E.; De Devn, G.B.; Wagg, C.; Niklaus, P.A.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
- Dubey, M.; Hadadi, N.; Pelet, S.; Carraro, N.; Johnson, D.R.; Meer, J.R. Environmental connectivity controls diversity in soil microbial communities. Commun. Biol. 2021, 4, 492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, H.J.; Song, L.B.; Chen, H. Aerated irrigation promotes soil respiration and microorganism abundance around tomato rhizosphere. Soil Sci. Soc. Am. J. 2019, 83, 1343–1355. [Google Scholar] [CrossRef]
- Jia, T.; Yao, Y.H.; Guo, T.Y.; Wang, R.H.; Chai, B.F. Effects of plant and soil characteristics on phyllosphere and rhizosphere fungal communities during plant development in a copper tailings dam. Front. Microbiol. 2020, 11, 556002. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, J.; Ding, Y.; Yu, S.; Lin, H.X.; Yuan, Z.Q.; Li, K.M.; Ou, W.J.; Chen, S.B. Different fertilizers applied alter fungal community structure in rhizospheric soil of cassava (Manihot esculenta Crantz) and increase crop yield. Front. Microbiol. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Yin, C.T.; Vargas, D.M.C.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.B.; Guan, Y.M.; Wu, L.J.; Zhang, L.L.; Pan, X.X.; Zhang, Z.H.; Zhang, Y.Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Hung, P.M.; Wattanachai, P.; Kasem, S.; Poeaim, S. Efficacy of chaetomium species as biological control agents against phytophthora nicotianae root rot in citrus. Mycobiology 2015, 43, 288–296. [Google Scholar] [CrossRef]
- Moparthi, S.; Burrows, M.; Mgbechi-Ezeri, J.; Agindotan, B. Fusarium spp. associated with root rot of pulse crops and their cross-pathogenicity to cereal crops in montana. Plant Dis. 2021, 105, 548–557. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, S.; Liu, Z.; Li, K.M.; Ou, W.J.; Chen, S.B. Drought analysis during the growth stages of grape in the main grape-growing regions in China. Theor. Appl. Climatol. 2022, 8, 4070. [Google Scholar] [CrossRef]
- Smith, P.; Haber, H.; Popp, A.; Erb, K.H.; Lauk, C.; Harper, R.; Tubiello, F.N.; Pinto, A.D.S.; Jafari, M.; Sohi, S.; et al. How much land-based greenhouse gas mitigation can be achieved without compromising food security and environmental goals? Global Change Biol. 2013, 19, 2285–2302. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.U.; Yang, W.J.; Cao, B. Effect of aerated irrigation on growth of two potted fruit trees. No. Hortic. 2014, 20, 72–75. [Google Scholar]
- Yu, K.; Yu, S.L.; Liu, H.F.; Wang, W.J.; Xu, W.B.; Bai, Z. Design of underground storage drip irrigation system and its influence on the growth and water use efficiency of Cabernet Sauvignon grapes. J. Fruit Sci. 2014, 31, 386–393. [Google Scholar] [CrossRef]
- Yu, K.; Yu, S.L.; Liu, H.F.; Wang, W.J.; Bai, Z.C.; Sun, J.L. Effects of alternate drip irrigation in different root zones on root cap growth of Cabernet Sauvignon grape seedlings. Chin. Soc. Agron. Eng. 2015, 31, 113–120. [Google Scholar] [CrossRef]
- Xie, H.X.; Cai, H.J.; Zhang, Z.H. Evaluation of comprehensive benefit in greenhouse vmuskmelon under aeration irrigation. T. Chin. Soc. Agric. Manuf. 2010, 41, 79–83. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Gan, Y.T.; Wang, R.Y.; Niu, J.Y.; Zhao, H.; Yan, Q.G.; Li, G.C. Phenological trends in winter wheat and spring cotton in response to climate changes in northwest China. Agric. For. Meteorol 2008, 148, 1242–1251. [Google Scholar] [CrossRef]
- Wen, G.J.; Cai, H.J.; Chen, X.M.; Liu, H.Y. Influence of aeration irrigation on growth and fruit quality of greenhouse tomato. J. New A. F. Univ. Nat. Sci. 2013, 41, 113–118, 124. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.J.; Song, L.B.; Shang, Z.H.; Chen, H. Comprehensive evaluation of different oxygation treatments based on fruit yield and quality of greenhouse tomato. Sci. Agric. Sinica 2020, 53, 2241–2252. [Google Scholar] [CrossRef]
- Mustroph, A.; Albrecht, G. Tolerance of crop plants to oxygen deficiency stress: Fermenting activity and photosynthetic capacity of entire seedlings under hypoxia and anomia. Physiol. Plant. 2003, 117, 508–520. [Google Scholar] [CrossRef]
- Zhu, J.J.; Xu, N.; Siddique, K.H.M.; Zhang, Z.H.; Niu, W.Q. Aerated drip irrigation improves water and nitrogen uptake efficiencies of tomato roots with associated changes in the antioxidant system. Sci. Hortic. 2022, 306, 111471. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.Q.; Xu, J.; Zhang, R.C.; Wang, J.W.; Zhang, M.Z. Aerated irrigation enhancing quality and irrigation water use efficiency of muskmelon in plastic greenhouse. T. Chin. Soc. Agric. Eng. 2016, 32, 147–154. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Djalovic, I.; Kumar, S.; Siddique, K.H.M. Root-omics for drought tolerance in cool-season grain legumes. Physiol. Plant. 2021, 172, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Essah, S.Y.; Holm, D.G. Air injection of subsurface drip irrigation water improves tuber yield and quality of russet potato. Am. J. Potato Res. 2020, 97, 432–438. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.Q.; Xu, J.; Wang, J.W.; Zhang, M.Z.; Lv, W. Root morphology of greenhouse produced muskmelon under sub-surface drip irrigation with supplemental soil aeration. Sci. Hortic. 2016, 201, 287–294. [Google Scholar] [CrossRef]
- Feng, Z.P.; Yang, G.G.; Guo, E.H.; Cui, Q.F.; Pei, B.; Yang, X.T. Effect of air root pruning on biomass allocation and root growth in Platycladus orientalis seedlings. Acta Ecol. Sin. 2017, 37, 7854–7861. [Google Scholar]
- Jones, D.L.; Hinsinger, P. The rhizosphere: Complex by design. Plant Soil 2008, 312, 1–6. [Google Scholar] [CrossRef]
- Mo, A.S.; Qiu, Z.Q.; He, Q.; Wu, H.Y.; Zhou, X.B. Effect of continuous monocropping of tomato on soil microorganism and microbial biomass carbon communications in soil. Commun. Soil Sci. Plan. 2016, 47, 1069–1077. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Li, W.; Lu, P.; Xu, T.Y.; Pan, K. Three preceding crops increased the yield of and inhibited clubroot disease in continuously monocropped chinese cabbage by regulating the soil properties and rhizosphere microbial community. Microorganisms 2022, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.J.; Niu, Z.R.; Song, S.Z.; Zhao, Y. Water stress affects the frequency of Firmicutes, Clostridiales and Lysobacter in rhizosphere soils of greenhouse grape. Agric. Water Manag. 2019, 226, 105776. [Google Scholar] [CrossRef]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.; Egbeagu, U.U.; Yu, S.; Zhao, L.Y.; Li, D.T.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef]
- Pinzari, F.; Ceci, A.; Samra, N.A.; Canfora, L.; Maggi, O.; Persiani, A. Phenotype MicroArray system in the study of fungal functional diversity and catabolic versatility. Res. Microbiol. 2016, 167, 710–722. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [PubMed]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, A.J.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, pathogenicity, and fungicide sensitivity of Alternaria isolates associated with preharvest fruit drop in California citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Chen, L.; Li, F.; Zhang, C.Z.; Ma, D.H.; Cai, Z.J.; Zhang, J.B. Effects of Mortierella on nutrient availability and straw decomposition in soil. Acta Pedol. Sin. 2022, 59, 206–217. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun. Soil Sci. Plan. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Tamayo-vélez, Á.; Osorio, N.W. Soil Fertility Improvement by litter decomposition and inoculation with the fungus Mortierella sp. in avocado plantations of colombia. Commun. Soil Sci. Plan. 2018, 49, 1417420. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wu, X.H.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fert. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Latef, A.A.H.A. Influence of arbuscular mycorrhizal fungi and copper on growth, accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.). Mycorrhiza 2011, 21, 495–503. [Google Scholar] [CrossRef]



| Treatment | Root Weight (g) | Branch Weight (g) | Leaf Weight (g) | Total Biomass (g) | Root–Shoot Ratio |
|---|---|---|---|---|---|
| Aeration | 59.48 ± 4.23 a | 51.64 ± 6.14 a | 30.05 ± 2.32 a | 141.17 ± 8.76 a | 0728 a |
| No aeration | 53.47 ± 5.85 b | 45.46 ± 3.05 b | 24.44 ± 1.21 b | 123.37 ± 9.91 b | 0.765 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xi, J.; Lv, Q.; Wang, J.; Yu, K.; Zhao, F. Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings. Sustainability 2022, 14, 12719. https://doi.org/10.3390/su141912719
Zhang H, Xi J, Lv Q, Wang J, Yu K, Zhao F. Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings. Sustainability. 2022; 14(19):12719. https://doi.org/10.3390/su141912719
Chicago/Turabian StyleZhang, Huanhuan, Jinshan Xi, Qi Lv, Junwu Wang, Kun Yu, and Fengyun Zhao. 2022. "Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings" Sustainability 14, no. 19: 12719. https://doi.org/10.3390/su141912719
APA StyleZhang, H., Xi, J., Lv, Q., Wang, J., Yu, K., & Zhao, F. (2022). Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings. Sustainability, 14(19), 12719. https://doi.org/10.3390/su141912719





