The Importance of Measuring Arsenic in Honey, Water, and PM10 for Food Safety as an Environmental Study: Experience from the Mining and Metallurgical Districts of Bor, Serbia
Abstract
:1. Introduction
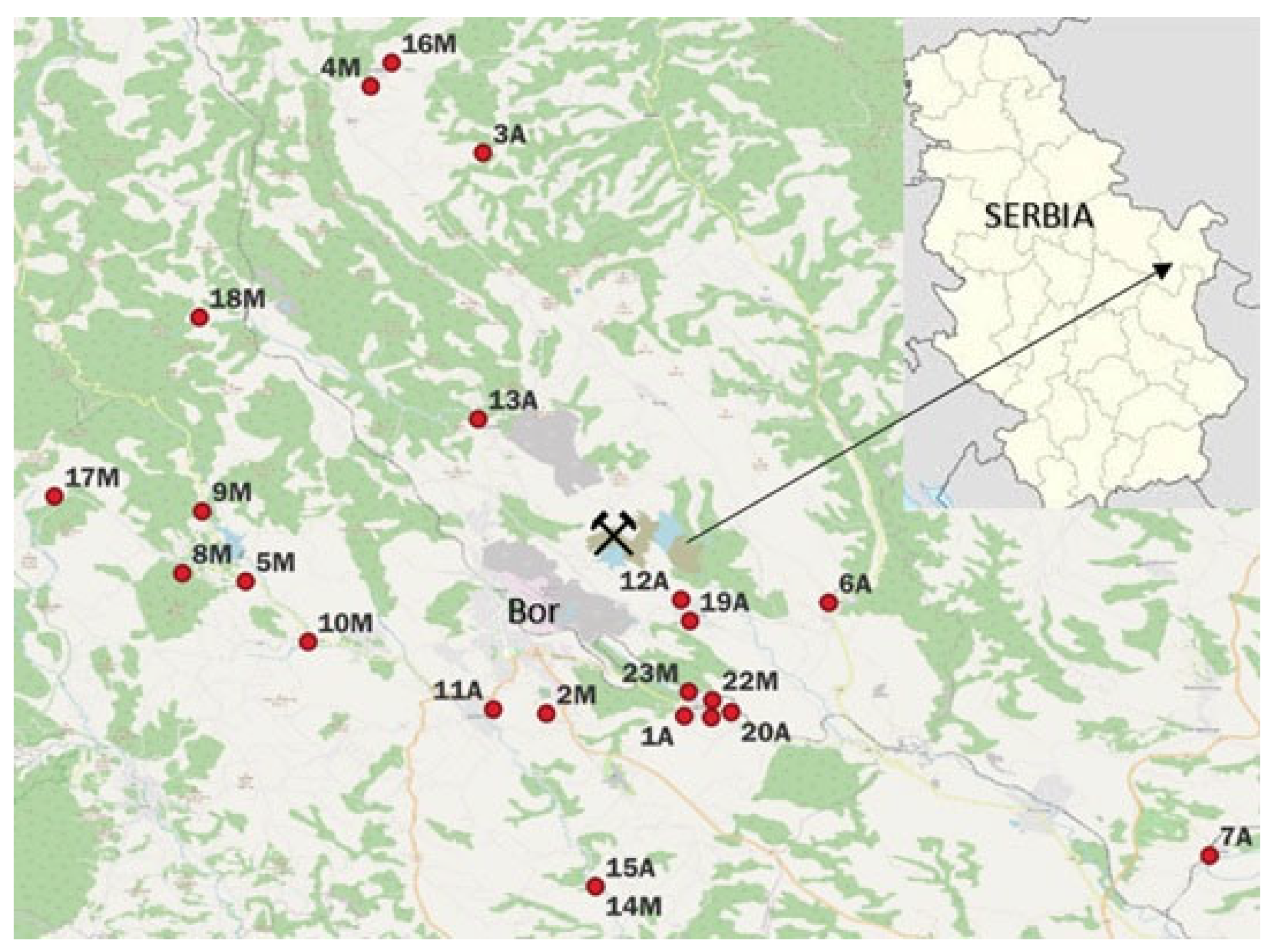
2. Experimental Part and Methodology
2.1. Experimental Part
2.2. Methodology and Main Characteristics of Honey
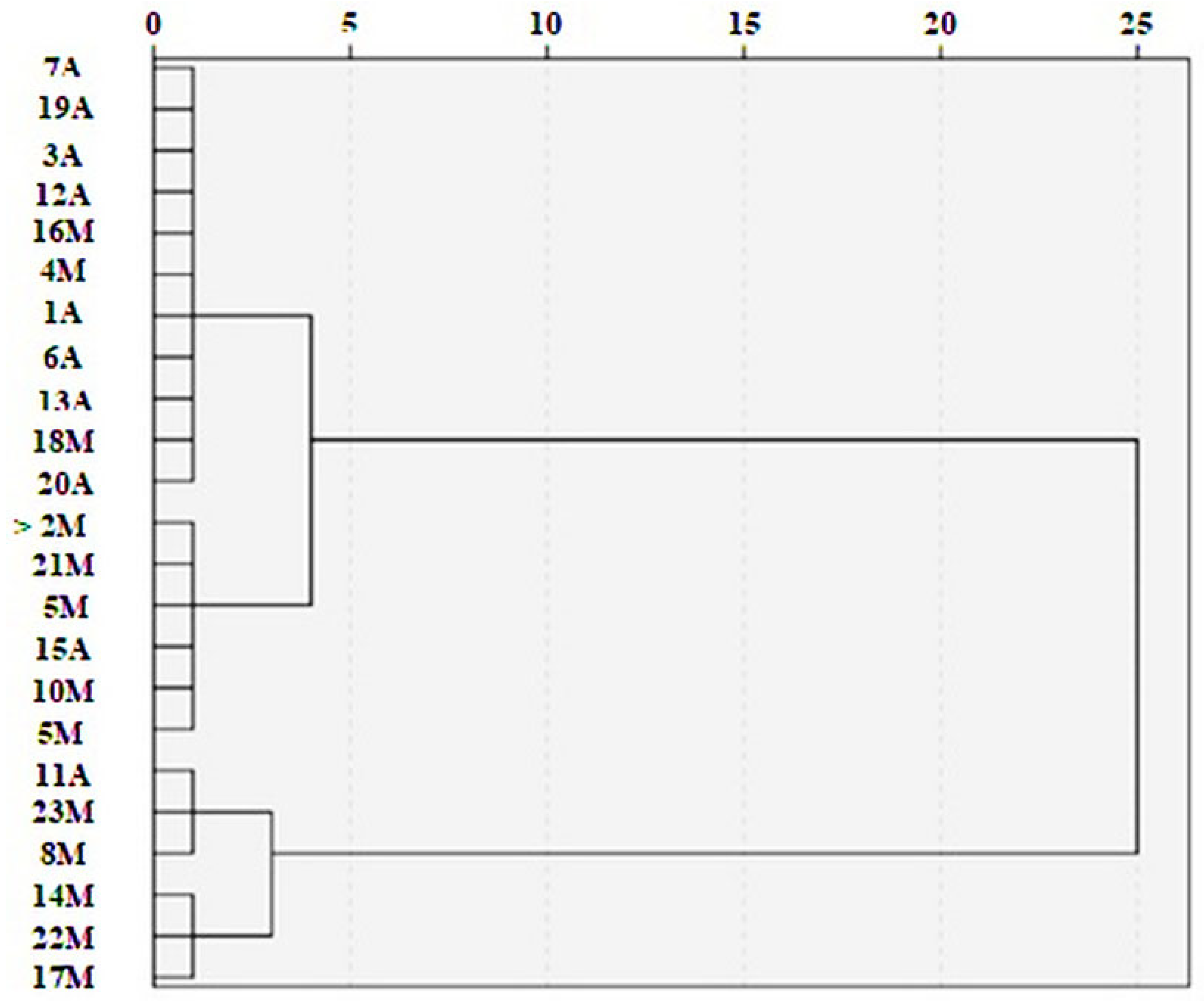
3. Results
3.1. As in Honey and Water
| Location | Distance from the Source of Pollution (km) | As in Honey mg/kg | pH of Honey | Elec. Conductivity of Honey µS/cm | |
|---|---|---|---|---|---|
| Symbol | Name | ||||
| 1A | Slatina | 5.44 | 0.0110 | 3.52 | 163.7 |
| 2M | Novi Gradski | 2.28 | 0.0540 | 3.89 | 467.0 |
| 3A | Tanda | 15.9 | 0.0037 | 3.28 | 378.0 |
| 4M | Gornjane | 18.71 | 0.0157 | 3.82 | 658.0 |
| 5M | Savača | 7.25 | 0.0662 | 3.76 | 892.0 |
| 6A | D.B. Reka | 7.67 | 0.0132 | 3.38 | 246.0 |
| 7A | Trnavac | 19.53 | 0.0033 | 3.57 | 145.3 |
| 8M | TilvaNjagra | 8.95 | 0.1219 | 3.98 | 1017.0 |
| 9M | Borsko Jezero | 8.83 | 0.0622 | 4.02 | 954.0 |
| 10M | Brestovačka Banja | 5.79 | 0.0702 | 3.76 | 676.0 |
| 11A | Brestovac | 4.05 | 0.1137 | 3.68 | 358.0 |
| 12A | Oštrelj | 3.85 | 0.0148 | 3.55 | 129.0 |
| 13A | Krivelj | 6.21 | 0.0359 | 3.62 | 191.5 |
| 14M | Džanovo Polje | 10.08 | 0.1919 | 3.93 | 599.0 |
| 15A | Džanovo Polje | 10.08 | 0.0672 | 3.45 | 166.9 |
| 16M | Gornjane | 19.07 | 0.0151 | 3.98 | 492.0 |
| 17M | Crni Vrh | 12.62 | 0.1505 | 4.11 | 776.0 |
| 18M | Mali Krivelj | 13.28 | 0.0316 | 4.07 | 684.0 |
| 19A | Oštrelj | 4.24 | 0.0034 | 3.63 | 143.0 |
| 20A | Slatina | 6.67 | 0.0265 | 3.52 | 141.6 |
| 21M | Slatina | 6.28 | 0.0491 | 3.79 | 428.0 |
| 22M | Slatina | 6.13 | 0.1763 | 4.09 | 612.0 |
| 23M | Slatina | 5.29 | 0.1167 | 3.99 | 584.0 |
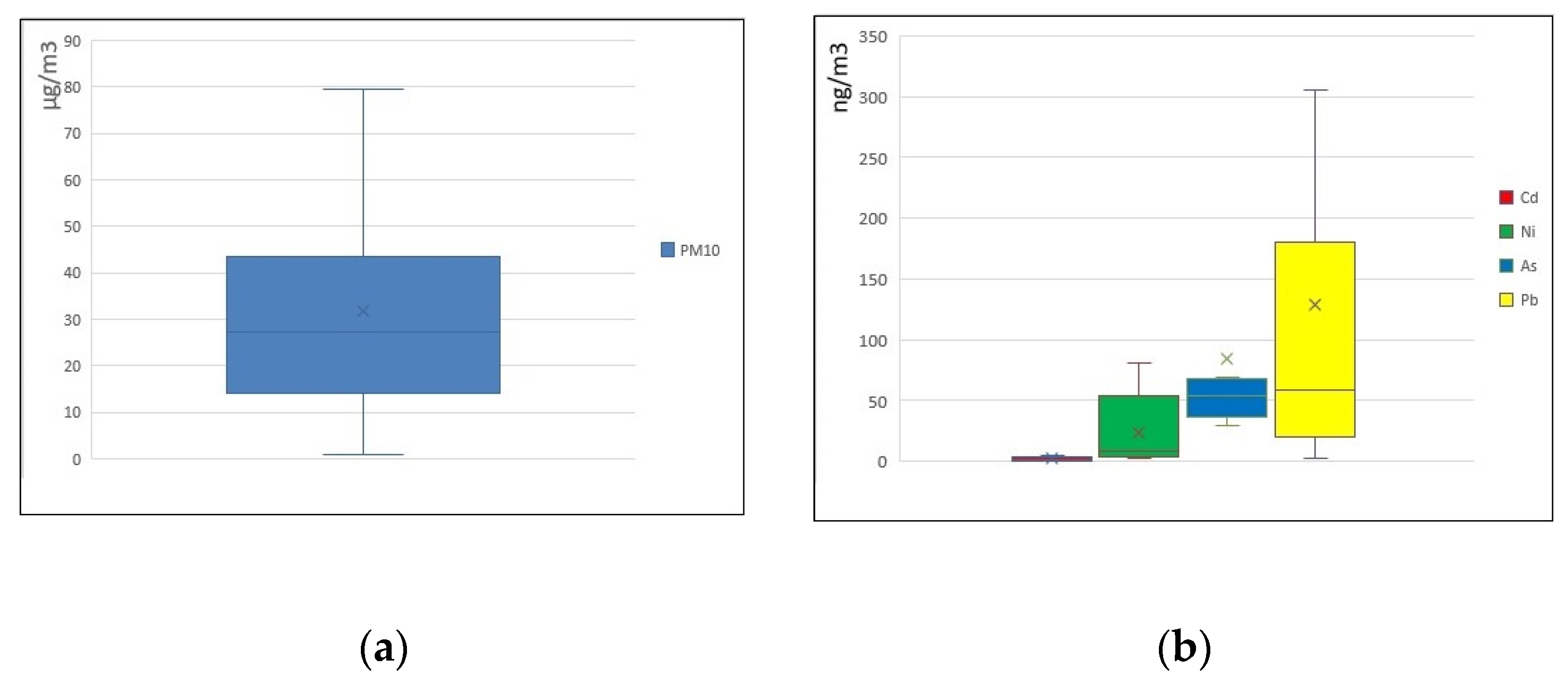
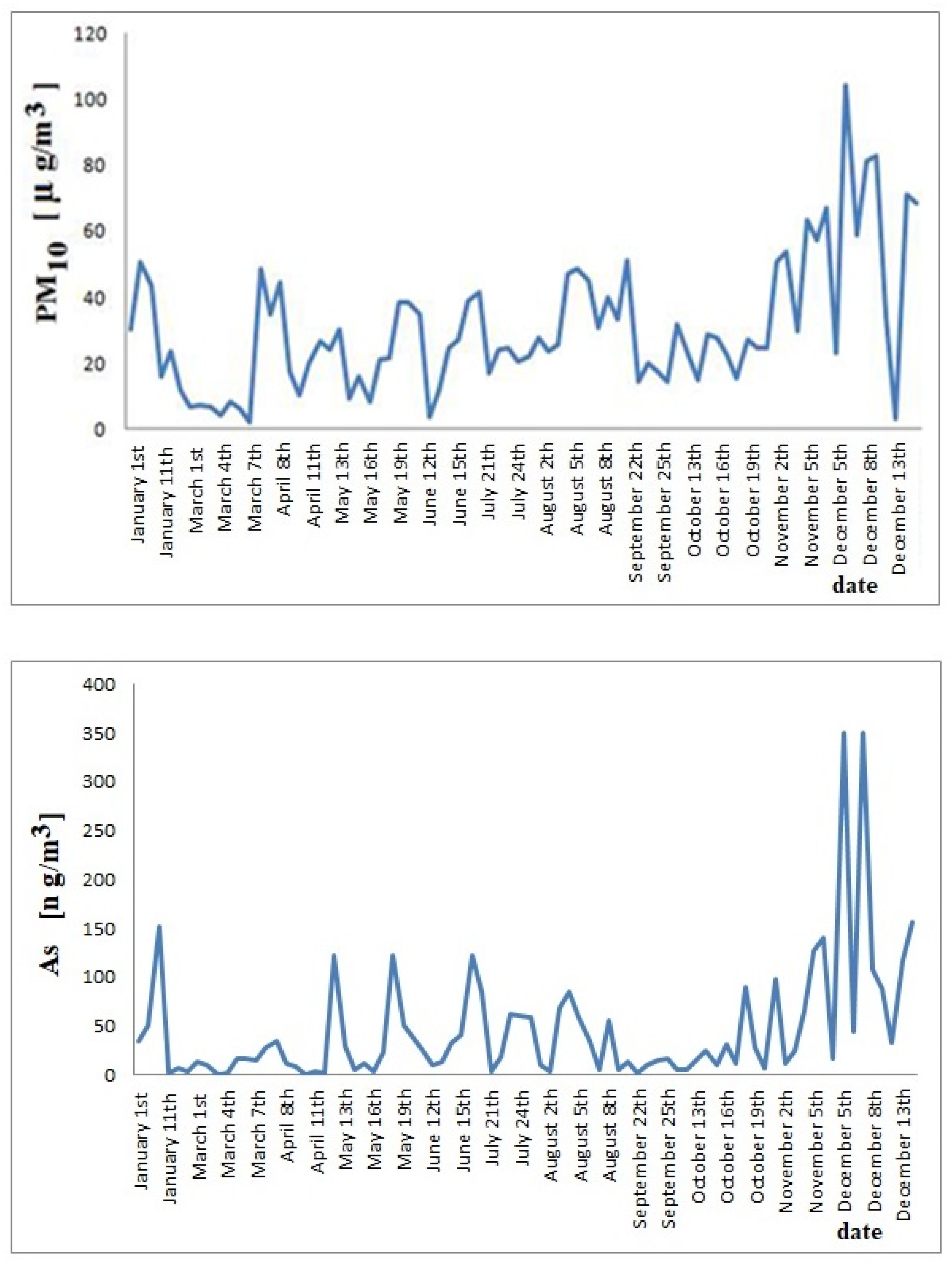
3.2. As and Accompanying Metals in PM10 Particles
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krstić, V. Some Effective Methods for Treatment of Wastewater from Cu Production. In Water Pollution and Remediation: Heavy Metals; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Chapter 12; pp. 313–440. [Google Scholar]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Harris, J.M. Sustainability and sustainable development. Int. Soc. Ecol. Econ. 2003, 1, 1–12. [Google Scholar]
- WCED. World Commission on Environment and Development: Our Common Future; WCED: New York, NY, USA, 1987.
- Do You Know All 17 SDGs? Available online: https://sdgs.un.org/goals (accessed on 24 August 2022).
- Kachenko, A.G.; Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Stachnik, Ł.; Korabiewski, B.; Raczyk, J.; Łopuch, M.; Wieczorek, I. Arsenic pollution in Quaternary sediments and water near a former gold mine. Sci. Rep. 2020, 10, 18458. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Ciminelli, V.; Gasparon, M.; Caldeira, C. Health risk apportionment of arsenic from multiple exposure pathways in Paracatu, a gold mining town in Brazil. Sci. Total Environ. 2019, 673, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.K.; Warke, M.; Datta, R.; Sarkar, D. Is Arsenic in Rice a Major Human Health Concern. Curr. Pollut. Rep. 2020, 6, 37–42. [Google Scholar] [CrossRef]
- Formenton, G.; Gregio, M.; Gallo, G.; Liguori, F.; Peruzzo, M.; Innocente, E.; Lava, R.; Masiol, M. PM10-bound arsenic emissions from the artistic glass industry in Murano (Venice, Italy) before and after the enforcement of REACH authorization. J. Hazard. Mater. 2021, 406, 124294. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Lanjwani, M.F.; Tuzen, M. Assessment of arsenic in water, rice and honey samples using new and green vortex-assisted liquid phase microextraction procedure based on deep eutectic solvent: Multivariate study. Microchem. J. 2022, 179, 107541. [Google Scholar] [CrossRef]
- Bastías, J.M.; Jambon, P.; Muñoz, O.; Manquián, N.; Bahamonde, P.; Neira, M. Honey as a bioindicator of arsenic contamination due to volcanic and mining activities in Chile. Chilean J. Agricult. Resear. 2013, 73, 147–153. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Davies, P.J.; Prasad, S. Identifying sources of environmental contamination in European honey bees (Apismellifera) using trace elements and lead isotopic compositions. Environ. Sci. Tech. 2018, 52, 991–1001. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Polo, R.O.; Newman, T.; Lansing, L.; Griffiths, J.; Bilodeau, G.; Rott, M.; Guarna, M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecolog. Ind. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Tong, S.S.; Morse, R.A.; Bache, C.A.; Lisk, D.J. Elemental analysis of honey as an indicator of pollution: Forty-seven elements in honeys produced near highway, industrial, and mining areas. Arch. Environ. Health Int. J. 1975, 30, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Massidda, A.; Tradori, M.; Fiori, G.; Chessa, G. Honey bees as bioindicators of environmental contamination in the industrial and mining area of Sulcis-Iglesiente-Sardinia. REDIA 2007, XC, 115–119. [Google Scholar]
- Zarić, N.M.; Braeuer, S.; Goessler, W. Arsenic speciation analysis in honey bees for environmental monitoring. J. Hazard. Mater. 2022, 432, 128614. [Google Scholar] [CrossRef] [PubMed]
- Maggid, A.D.; Kimanya, M.E.; Ndakidemi, P.A. The contamination and exposure of mercury in honey from Singida, Central Tanzania. Am. J. Res. Commun. 2014, 2, 127–139. [Google Scholar]
- Baloš, M.Ž.; Mihaljev, Ž.; Jakšić, S. Toxic elements as a risk factor for the survival of the honey bees (Apismellifera L.). Arch. Vet. Med. 2021, 14, 5–18. [Google Scholar] [CrossRef]
- Rollin, O.; Bretagnolle, V.; Decourtye, A.; Aptel, J.; Michel, N.; Vaissière, B.E.; Henry, M. Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agricult. Ecosy. Environ. 2013, 179, 78–86. [Google Scholar] [CrossRef]
- Christen, V.; Vogel, M.S.; Hettich, T.; Fent, K. A Vitellogenin Antibody in Honey Bees (Apismellifera): Characterization and Application as Potential Biomarker for Insecticide Exposure. Environ. Toxicol. Chem. 2019, 35, 1074–1083. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohyd. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef]
- Patel, V.; Pauli, N.; Biggs, E.; Barbour, L.; Boruff, B. Why bees are critical for achieving sustainable development. Ambio 2021, 50, 49–59. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Hladun, K.R.; Di, N.; Liu, T.X.; Trumble, J.T. Metal contaminant accumulation in the hive: Consequences for whole-colony health and brood production in the honey bee (Apismellifera L.). Environ. Toxicol. Chem. 2016, 35, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Đurđevac Ignjatović, L.; Krstić, V.; Radonjanin, V.; Jovanović, V.; Malešev, M.; Ignjatović, D.; Đurđevac, V. Application of cement paste in mining works, environmental protection and sustainable development goals in the mining industry. Sustainability 2022, 14, 7902. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Dimitrijević, M.D.; Stevanović, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef]
- Matić, B.I.; Rakić, U.D.; Dejanović, S.M.; Jovanović, V.S.; Jevtić, M.R.; Đonović, N.Ž. Industrially contaminated areas in Serbia as a potential public health threat to the exposed population. Tehnika 2017, 72, 441–447. [Google Scholar] [CrossRef]
- Nikolić, D.; Milošević, N.; Mihajlović, I.; Živković, Ž.; Kovačević, R.; Petrović, N. Multicriteria analysis of soil pollution with heavy metals in vicinity of copper smelting plant in Bor (Serbia). J. Serb. Chem. Soc. 2011, 76, 625–641. [Google Scholar] [CrossRef]
- Urošević, S.; Vuković, M.; Pejčić, B.; Štrbac, N. Mining-metallurgical sources of pollution in eastern Serbia and environmental consciousness. Rev. Contam. 2018, 34, 103–115. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; EPA SW-846, Revision 3; Environmental Protection Agency: Washington, DC, USA, 2004.
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme. In Proceedings of the Twenty-Fourth Session, Geneva, Switzerland, 2–7 July 2001. [Google Scholar]
- Tasić, V.; Maluckov, B.; Apostolovski-Trujić, T.; Kovačević, R.; Živković, M.; Lazović, I. Particulate matter concentration (PM10 and PM2.5) in naturally ventilated offices in Bor, (Serbia), FactaUniversitatis Series: Working Living. Environ. Protect. 2015, 12, 279–287. [Google Scholar]
- CEN/TC 264 N 799; Ambient Air Quality—Standard Method for the Measurement of Pb, Cd, As and Ni in PM10 Fraction of Suspended Particulate Matter; iTeh Inc: Newark, NJ, USA, 2006.
- Adamovic, D.; Ishiyama, D.; Djordievski, S.; Ogawa, Y.; Stevanovic, Z.; Kawaraya, H.; Sato, H.; Obradovic, L.J.; Marinkovic, V.; Petrovic, J.; et al. Estimation and comparison of the environmental impacts of acid mine drainage-bearing river water in the Bor and Majdanpek porphyry copper mining areas in Eastern Serbia. Resour. Geol. 2021, 71, 123–143. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apicult. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Jakšić, S.; Popov, N.; Polače, V. Characterization of Serbian sunflower honeys by their physicochemical characteristics. Food Feed Res. 2021, 48, 1–8. [Google Scholar] [CrossRef]
- Directive 2008/50/EC on ambient air quality and cleaner air for Europe (CAFÉ) EU, 11/06/2008. 2008/50/EC Council directive on ambient air quality and cleaner air for Europe. Off. J. L 2008, 152, 1–44.
- WHO. Review of Evidence on Health Aspects of Air Pollution-REVIHAAP Proyect. Technical Report. 2013. Available online: http://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report.pdf (accessed on 24 August 2022).
- Jovašević-Stojanović, M.; Bartonova, A.; Topalović, D.; Lazović, I.; Pokrić, B.; Ristovski, Z. On the use of small and cheaper sensors and devices for indicative citizen-based monitoring of respirable particulate matter. Environ. Polut. 2015, 206, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Šerbula, S.; Milosavljević, J.; Kalinović, J.; Kalinović, T.; Radojević, A.; Apostolovski-Trujić, T.; Tasić, V. Arsenic and SO2 hotspot in South-Eastern Europe: An overview of the air quality after the implementation of the flash smelting technology for copper production. Sci. Total Environ. 2021, 777, 145981. [Google Scholar] [CrossRef] [PubMed]
- Tasić, V.; Kovačević, R.; Maluckov, B.; Apostolovski-Trujić, T.; Matić, B.; Cocić, M.; Šteharnik, M. The content of As and heavy metals in TSP and PM10 near copper smelter in Bor, Serbia, Water. Air Soil Pollut. 2017, 228, 230. [Google Scholar] [CrossRef]
- Yadav, R.; Sahu, L.K.; Nisar, S.; Jaaffrey, A.; Beig, G. Temporal Variation of Particulate matter (PM) and potential sources at an urban site of Udaipur in Western India. Aerosol Air Quality Res. 2014, 14, 1613–1629. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Satsang, P.G.; Masih, J.; Taneja, A. Metal concentration of PM2.5 and PM10 particles and seasonal variations in urban and rural environment of Agra, India. Sci. Total Environ. 2009, 407, 6196–6204. [Google Scholar] [CrossRef]
- Yli-Tuomi, T.; Hopke, P.K.; Paatero, P.; Basunia, M.S.; Landsberger, S.; Viisanen, Y.; Paatero, J. Atmospheric aerosol over Finnish Arctic: Source analysis by the multilinear engine and the potential source contribution function. Atmos. Environ. 2003, 37, 4381–4392. [Google Scholar] [CrossRef]
- Gidhagen, L.; Kahelin, H.; Schmidt-Thomé, P.; Johansson, C. Anthropogenic and natural levels of arsenic in PM10 in central and Northern Chile. Atmos. Environ. 2002, 36, 3803–3817. [Google Scholar] [CrossRef]
- Hedberg, E.; Gidhagen, L.; Johansson, C. Source contributions to PM10 and arsenic concentrations in Central Chile using positive matrix factorization. Atmos. Environ. 2005, 39, 549–561. [Google Scholar] [CrossRef]
- Matin, G.; Kargar, N.; Buyukisika, H.B. Bio-monitoring of cadmium, lead, arsenic and mercury in industrialdistricts of Izmir, Turkey by using honey bees, propolisand pine treeleaves. Ecol. Eng. 2016, 90, 331–335. [Google Scholar] [CrossRef]
- Bromenshenk, J.J.; Carlson, S.T.; Simpson, J.C.; Thomas, J.M. Pollution monitoring of Puget Sound with honey bees. Science 1985, 227, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Kalnins, A.A.; Detroy, B.F. Effect of wood preservative treatment of beehives on honey bees and hive products. J. Agric. Food Chem. 1984, 31, 1176–1180. [Google Scholar] [CrossRef]
- Steen, J.; Kraker, J.; Grotenhuis, T. Spatial and temporal variation of metal concentrations in adult honeybees (Apismellifera L.). Environ. Monit. Assess. 2012, 184, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Doker, S.; Aydemir, O.; Uslu, M. Evaluation of digestion procedures for trace element analysis of cankiri, Turkey honey by inductively coupled plasma mass spectrometry. Analyt. Lett. 2014, 47, 2080–2094. [Google Scholar] [CrossRef]
- Ataide de Oliveira, F.; Trópia de Abreu, A.; de Oliveira Nascimento, N.; Santos Froes-Silva, R.E.; Antonini, Y.; Nalini, H.A.; Carvalho de Lena, J. Evaluation of matrix effect on the determination of rare earth elements and As, Bi, Cd, Pb, Se and In in honey and pollen of native Brazilian bees (Tetragoniscaangustula—Jataí) by Q-ICP-MS. Talanta 2017, 162, 488–494. [Google Scholar] [CrossRef]
- Ilić, I.; Bogdanović, D.; Živković, D.; Milošević, N.; Todorović, B. Optimization of heavy metals total emission, case study: Bor. Atmos. Res. 2011, 101, 450–459. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Arsenic; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1998. [Google Scholar]
- SEPA. State of Environment in the Republic of Serbia during 2016. Available online: http://www.sepa.gov.rs/# (accessed on 20 March 2023).
- Antonijević, M.M.; Dimitrijević, M.D.; Milić, S.M.; Nujkić, M.M. Metal concentrations in the soils and native plants surrounding the old flotation tailings pond of the Copper Mining and Smelting Complex Bor (Serbia). J. Environ. Monitor. 2012, 14, 866–877. [Google Scholar] [CrossRef]
- Nikolić, T.V.; Kojić, D.; Orčić, S.; Batinić, D.; Vukašinović, E.; Blagojević, P.D.; Pura, J. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105. [Google Scholar] [CrossRef]
- Deborah, S.R.; Villafuerte, L.; Otisc, G.; Palmer, M.R. Biogeography of Apiscerana F. and A. nigrocincta Smith: Insights from mtDNA studies. Apidologie 2000, 31, 265–279. [Google Scholar]
- Bratu, I.; Georgescu, C. Chemical contamination of bee honey–identifying sensor of the environment pollution. J. Cent. Eur. Agricul. 2005, 6, 95–98. [Google Scholar]
- Boryło, A.; Romańczyk, G.; Wieczorek, J.; Strumińska-Parulska, D.; Kaczor, M. Radioactivity of honey from northern Poland. J. Radioanal. Nucl. Chem. 2019, 319, 289–296. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Asmaryan, S.; Hovsepyan, A.; Merendino, N. Carcinogenic and non-carcinogenic risk assessment of trace elements and POPs in honey from Shirak and Syunik regions of Armenia. Chemosphere 2020, 239, 124809. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ayuso, E.; Abad-Valle, P. Trace element levels in an area impacted by old mining operations and their relationship with beehive products. Sci. Total Environ. 2017, 599, 671–678. [Google Scholar] [CrossRef]
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Ghini, S.; Medrzycki, P.; Grilenyoni, F.; Bortolotti, L.; Gattavecchia, E.; Celli, G. Honey bees and bee products as monitors of the environmental contamination. Apiacta 2003, 38, 63–70. [Google Scholar]
- Lončar, D.; Paunković, J.; Jovanović, V.; Krstić, V. Environmental and social responsibility of companies across European Union countries—Panel data analysis. Sci. Total Environ. 2019, 657, 287–296. [Google Scholar] [CrossRef]
- Ćirić, J.; Spirić, D.; Baltić, T.; Lazić, I.B.; Trbović, D.; Parunović, N.; Petronijević, R.; Đorđević, V. Honey bees and their products as indicators of environmental element deposition. Biol. Trace Elem. Res. 2021, 199, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.; Attard, E. Honeybees and their products as bioindicators for heavy metal pollution in Malta. Acta Bras. 2020, 4, 60–69. [Google Scholar] [CrossRef]
- Murashova, E.A.; Tunikov, G.M.; Nefedova, S.A.; Karelina, O.A.; Byshova, N.G.; Serebryakova, O.V. Major factors determining accumulation of toxic elements by bees and honey products. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2020, 11, 3. [Google Scholar]
- Linderhof, V.; Meeske, M.; Diogo, V.; Sonneveld, A. The Impact of Water-Related Pollution on Food Systems in Transition: The Case of Northern Vietnam. Sustainability 2021, 13, 1945. [Google Scholar] [CrossRef]
| Matrix | As (μg g−1) | Comments | Ref. |
|---|---|---|---|
| honeybee matrix | 0.00456 | Injection volume: 20 L (5 L of palladium chloride and magnesium nitrate were added as a regulatory mixture) | [48] |
| 0.00151 | |||
| 0.00446 | |||
| 0.00284 | |||
| adult honeybees | <0.50–12.50 | 72 sites (rural–urban) | [49] |
| <0.10 | Hives without CCA 1 | [50] | |
| 0.67–0.83 | to large industrial areas | [51] | |
| propolis matrix | 146.24 | Argon, 250; 30 s; 110 °C; 1 °C s−1 | [48] |
| 43.55 | Argon, 250; 30 s; 130 °C; 15 °C s−1 | ||
| 578.46 | Argon, 250; 20 s; 1250 °C; 10 °C s−1 | ||
| 28.81 | Argon, 250; 3 s; 2250 °C; 1 °C s−1 | ||
| 18.99 | Argon, 250; 3 s; 2450 °C; 1 °C s−1 | ||
| adult honeybees | 0.77–1.11 | Hives with CCA 3 urban site close | [50] |
| honey samples from Cankırı City, Turkey | 14.7 ± 4.7 | Mean ± standard deviation (n = 7, k = 4) | [52] |
| honey samples (μg kg−1) | 13.83 ± 1.90 | 2 LOD = 0.54 μg L−1 3 LOQ = 1.8 μg L−1 4 BEC = 0.35 Spiked sample s= 3.232 μg g−1 | [53] |
| 4.23 ± 0.06 | |||
| 1.70 ± 0.01 | |||
| 1.70 ± 0.01 | |||
| 1.70 ± 0.01 | |||
| Pollen samples (μg kg−1) | 361.30 ± 18.88 | 2 LOD = 0.0036 μg L−1 3 LOQ = 0.012 μg L−1 4 BEC = 0.13 Spiked samples = 3.163 μg g−1 | [53] |
| 330.16 ± 4.49 | |||
| 93.21 ± 25.16 | |||
| 51.10 ± 22.19 | |||
| 7.00 ± 0.45 | |||
| meadow honey from Bor, Serbia, with the highest As conc. (μg kg−1) | 191.9 | pH = 3.93; el. cond. = 599.0 µS cm−1 | This work |
| 150.1 | pH = 4.11; el. cond. = 776.0 µS cm−1 | ||
| 176.3 | pH = 4.09; el. cond. = 612.0 µS cm−1 | ||
| 116.7 | pH = 3.99; el. cond. = 584.0 µS cm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sovrlić, Z.; Tošić, S.; Kovačević, R.; Jovanović, V.; Krstić, V. The Importance of Measuring Arsenic in Honey, Water, and PM10 for Food Safety as an Environmental Study: Experience from the Mining and Metallurgical Districts of Bor, Serbia. Sustainability 2022, 14, 12446. https://doi.org/10.3390/su141912446
Sovrlić Z, Tošić S, Kovačević R, Jovanović V, Krstić V. The Importance of Measuring Arsenic in Honey, Water, and PM10 for Food Safety as an Environmental Study: Experience from the Mining and Metallurgical Districts of Bor, Serbia. Sustainability. 2022; 14(19):12446. https://doi.org/10.3390/su141912446
Chicago/Turabian StyleSovrlić, Zorica, Snežana Tošić, Renata Kovačević, Violeta Jovanović, and Vesna Krstić. 2022. "The Importance of Measuring Arsenic in Honey, Water, and PM10 for Food Safety as an Environmental Study: Experience from the Mining and Metallurgical Districts of Bor, Serbia" Sustainability 14, no. 19: 12446. https://doi.org/10.3390/su141912446
APA StyleSovrlić, Z., Tošić, S., Kovačević, R., Jovanović, V., & Krstić, V. (2022). The Importance of Measuring Arsenic in Honey, Water, and PM10 for Food Safety as an Environmental Study: Experience from the Mining and Metallurgical Districts of Bor, Serbia. Sustainability, 14(19), 12446. https://doi.org/10.3390/su141912446







