The Source, Transport, and Removal of Chemical Elements in Rainwater in China
Abstract
1. Introduction
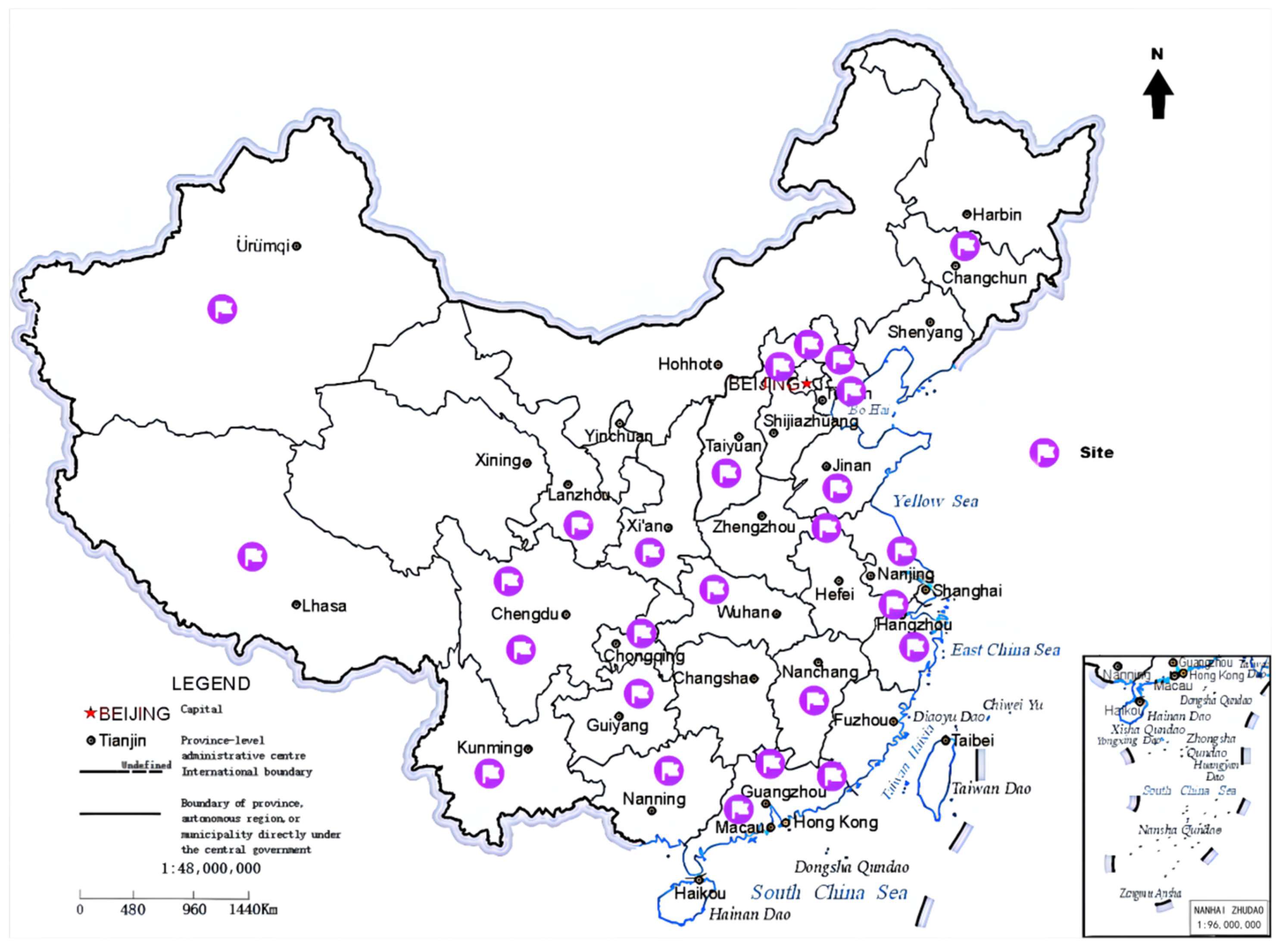
2. Method
3. Results
3.1. The Content of Ions in Rainwater
3.1.1. Regional Change of pH Value in Rainwater
3.1.2. Regional Change of Major Ions Contents in Rainwater
| Site | pH | Ca2+ | Mg2+ | K+ | Na+ | NH+ | F− | Cl− | SO42− | NO3− | SO42−/NO3− | Time | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guizhou, China a | 5.2 | 7.0 | 110.4 | 4.4 | 3.0 | 11.7 | 0.8 | 5.2 | 51.8 | 24.7 | 2.1 | 2019–2020 | Zeng et al. 2020 [43] |
| Jiangxi, China a | 5.8 | 70.7 | 15.1 | 33.4 | 19.9 | 3.5 | 38.4 | 69.4 | 37.9 | 1.8 | 2018–2020 | Li et al. 2022 [44] | |
| Beijing, China a | 6.7 | 59.2 | 76.3 | 56.0 | 33.0 | 141.3 | 100.1 | 66.3 | 59.6 | 1.1 | 2017–2018 | Xu et al. 2020 [55] | |
| Shanghai, China a | 5.5 | 50.3 | 130.1 | 21.1 | 145.0 | 342.0 | 240.0 | 354.0 | 138.0 | 2.6 | 2016–2018 | Luan et al. 2019 [41] | |
| Huzhou, China a | 6.2 | 37.1 | 57.9 | 17.3 | 21.1 | 194.6 | 40.0 | 78.8 | 41.8 | 1.9 | 2016–2018 | Yan et al. 2019 [56] | |
| Hangzhou, China a | 6.2 | 29.2 | 56.8 | 16.8 | 16.5 | 191.1 | 28.0 | 58.5 | 42.3 | 1.4 | 2016–2018 | Yan et al. 2019 [56] | |
| Zhanjiang, China a | 34.4 | 5.1 | 14.6 | 94.2 | 8.9 | 99.3 | 25.3 | 2015–2019 | Zeng et al. 2022 [38] | ||||
| Jiaozhou, China a | 4.8 | 54.7 | 107.0 | 17.2 | 21.9 | 64.1 | 2.6 | 66.0 | 93.7 | 62.9 | 1.5 | 2015–2016 | Xing et al. 2017 [57] |
| Ya’an, China a | 4.4 | 16.5 | 169.6 | 11.4 | 8.5 | 37.9 | 2.7 | 20.9 | 138.4 | 71.4 | 1.9 | 2013–2014 | Li et al. 2016 [2] |
| Nanjing, China a | 5.1 | 15.3 | 127.5 | 8.0 | 20.4 | 247.9 | 61.7 | 135.7 | 58.1 | 2.3 | 2013–2014 | Xue et al. 2014 [58] | |
| Beijing, China a | 4.9 | 21.5 | 346.0 | 9.2 | 53.3 | 273.0 | 12.0 | 50.9 | 357.0 | 42.6 | 8.4 | 2011–2012 | Xu et al. 2015 [9] |
| Xinjiang (Urumqi), China a | 6.3 | 193.0 | 118.9 | 45.1 | 54.4 | 422.3 | 10.0 | 121.5 | 307.6 | 39.1 | 7.9 | 2010–2019 | Zhong et al. 2022 [17] |
| Ya’an, China a | 4.0 | 24.2 | 203.7 | 30.1 | 13.2 | 98.4 | 13.3 | 37.5 | 212.3 | 84.4 | 2.5 | 2010–2011 | Zhao et al. 2013 [16] |
| Jiuzhaigou, China a | 6.0 | 38.0 | 13.4 | 21.2 | 41.1 | 149.8 | 21.0 | 37.2 | 70.5 | 12.7 | 5.6 | 2010–2011 | Qiao et al. 2015 [33] |
| Shenzhen, China a | 4.3 | 36.4 | 14.7 | 2.0 | 11.8 | 18.1 | 45.9 | 59.3 | 23.7 | 2.5 | 2008-2009 | Zhou et al. 2019 [11] | |
| Shenzhen, China a | 4.9 | 10.5 | 24.0 | 1.1 | 2.3 | 21.4 | 0.5 | 19.8 | 38.4 | 12.3 | 3.1 | 2005–2009 | Huang et al. 2010 [59] |
| Guangzhou, China a | 4.5 | 18.0 | 66.0 | 9.0 | 9.0 | 131.0 | 12.0 | 21.0 | 202.0 | 52.0 | 3.9 | 2005–2006 | Huang et al. 2009 [48] |
| Beijing, China a | 6.0 | 234.0 | 191.2 | 248.9 | 84.1 | 3.0 | 2001–2003 | Yang et al. 2004 [60] | |||||
| Chongqing, China b | 5.8 | 20.7 | 223.8 | 60.4 | 40.2 | 595.6 | 30.5 | 96.9 | 717.8 | 90.0 | 8.0 | 2000–2009 | Lu et al. 2013 [18] |
| Tibet, China a | 30.5 | 15.0 | 2.5 | 14.7 | 70.1 | 25.6 | 29.9 | 6.4 | 4.7 | 2021 | Wang et al. 2022 [28] | ||
| Xian, China a | 6.3 | 28.7 | 147.4 | 4.6 | 22.4 | 136.6 | 8.9 | 145.1 | 25.3 | 5.7 | 2017 | Huo et al. 2020 [45] | |
| shenzhen, China a | 5.0 | 23.1 | 17.4 | 5.4 | 6.5 | 28.2 | 1.3 | 27.1 | 22.4 | 18.6 | 1.2 | 2017 | Jian et al. 2019 [61] |
| Wuxi, China a | 5.9 | 21.3 | 48.2 | 8.3 | 14.9 | 70.9 | 1.8 | 4.6 | 10.5 | 4.1 | 2.6 | 2016 | Wang et al. 2021 [62] |
| Lijiang, China c | 6.1 | 1.0 | 20.8 | 2.0 | 10.9 | 50.1 | 0.6 | 2.0 | 23.7 | 7.0 | 3.4 | 2012 | Niu et al. 2014 [3] |
| Hangzhou, China a | 4.7 | 18.9 | 39.8 | 7.1 | 13.4 | 158.0 | 8.3 | 32.2 | 125.0 | 35.9 | 3.5 | 2011 | Wu et al. 2021 [63] |
| Xi’an, China a | 6.6 | 31.1 | 229.8 | 13.8 | 36.6 | 425.6 | 28.7 | 38.7 | 489.7 | 128.8 | 3.8 | 2010 | Lu et al. 2011 [13] |
| Chengdu, China a | 5.1 | 1.4 | 150.5 | 6.6 | 16.2 | 196.6 | 6.2 | 8.9 | 212.8 | 156.2 | 1.4 | 2008 | Wang & Han 2011 [29] |
| Beijing, China a | 5.3 | 8.5 | 174.0 | 6.7 | 38.5 | 291.0 | 10.5 | 67.8 | 270.0 | 139.0 | 1.9 | 2008 | Xu et al. 2012 [19] |
| Puding, China a | 5.4 | 10.8 | 33.1 | 9.1 | 3.9 | 155.8 | 2.8 | 54.5 | 152.4 | 17.0 | 9.0 | 2008 | Wu et al. 2012 [64] |
| Beijing, China a | 5.1 | 25.0 | 185.6 | 17.7 | 40.4 | 607.2 | 15.7 | 104.0 | 315.8 | 109.0 | 2.9 | 2006 | Xu & Han 2009 [40] |
| Shanghai, China a | 4.5 | 50.1 | 80.7 | 14.9 | 29.6 | 204.0 | 58.3 | 200.0 | 49.8 | 4.0 | 2004 | Zhang et al. [31] | |
| Tie Shan Ping, China a | 4.1 | 3.0 | 76.0 | 8.0 | 9.0 | 58.0 | 11.0 | 184.0 | 35.0 | 5.3 | 2003 | Aas et al. 2007 [65] | |
| Cai Jia Tang, China a | 4.3 | 7.0 | 112.0 | 10.0 | 10.0 | 60.0 | 11.0 | 155.0 | 60.0 | 2.6 | 2003 | Aas et al. 2007 [65] | |
| Lei Gong Shan, China a | 5.2 | 7.0 | 110.4 | 4.4 | 3.0 | 11.7 | 0.8 | 5.2 | 51.8 | 24.7 | 2.1 | 2003 | Aas et al. 2007 [65] |
| Liu Chong Guan, China a | 5.8 | 70.7 | 15.1 | 33.4 | 19.9 | 3.5 | 38.4 | 69.4 | 37.9 | 1.8 | 2003 | Aas et al. 2007 [65] | |
| Li Xi He, China a | 6.7 | 59.2 | 76.3 | 56.0 | 33.0 | 141.3 | 100.1 | 66.3 | 59.6 | 1.1 | 2003 | Aas et al. 2007 [65] | |
| Chongqing, China a | 5.5 | 50.3 | 130.1 | 21.1 | 145.0 | 342.0 | 240.0 | 354.0 | 138.0 | 2.6 | 2002 | Zhou et al. 2003 [49] | |
| Ahvaz, Iran a | 6.2 | 37.1 | 57.9 | 17.3 | 21.1 | 194.6 | 40.0 | 78.8 | 41.8 | 1.9 | 2014–2015 | Naimabadi et al. 2018 [66] | |
| India a | 6.2 | 29.2 | 56.8 | 16.8 | 16.5 | 191.1 | 28.0 | 58.5 | 42.3 | 1.4 | 2012–2018 | Majumdar et al. 2022 [22] | |
| Oleiros, Spain b | 34.4 | 5.1 | 14.6 | 94.2 | 8.9 | 99.3 | 25.3 | 2011–2012 | Moreda-Piñeiro et al. 2014 [66] | ||||
| Pune, India a | 4.8 | 54.7 | 107.0 | 17.2 | 21.9 | 64.1 | 2.6 | 66.0 | 93.7 | 62.9 | 1.5 | 2006–2009 | Budhavant et al. 2011 [37] |
| Newark, USA a | 4.4 | 16.5 | 169.6 | 11.4 | 8.5 | 37.9 | 2.7 | 20.9 | 138.4 | 71.4 | 1.9 | 2006–2007 | Song and Gao 2009 [67] |
| Ghor Es-Safi, Jordan b | 5.1 | 15.3 | 127.5 | 8.0 | 20.4 | 247.9 | 61.7 | 135.7 | 58.1 | 2.3 | 2006–2007 | Al-Khashman 2009 [42] | |
| Mexico a | 4.9 | 21.5 | 346.0 | 9.2 | 53.3 | 273.0 | 12.0 | 50.9 | 357.0 | 42.6 | 8.4 | 2001–2002 | Baez et al. 2007 [68] |
| Hamedan, Iran a | 6.3 | 193.0 | 118.9 | 45.1 | 54.4 | 422.3 | 10.0 | 121.5 | 307.6 | 39.1 | 7.9 | 2014 | Peikam et al. 2021 [10] |
| Kushkabad, Iran a | 4.0 | 24.2 | 203.7 | 30.1 | 13.2 | 98.4 | 13.3 | 37.5 | 212.3 | 84.4 | 2.5 | 2014 | Peikam et al. 2021 [10] |
| Nemat Abad, Iran a | 6.0 | 38.0 | 13.4 | 21.2 | 41.1 | 149.8 | 21.0 | 37.2 | 70.5 | 12.7 | 5.6 | 2014 | Peikam et al. 2021 [10] |
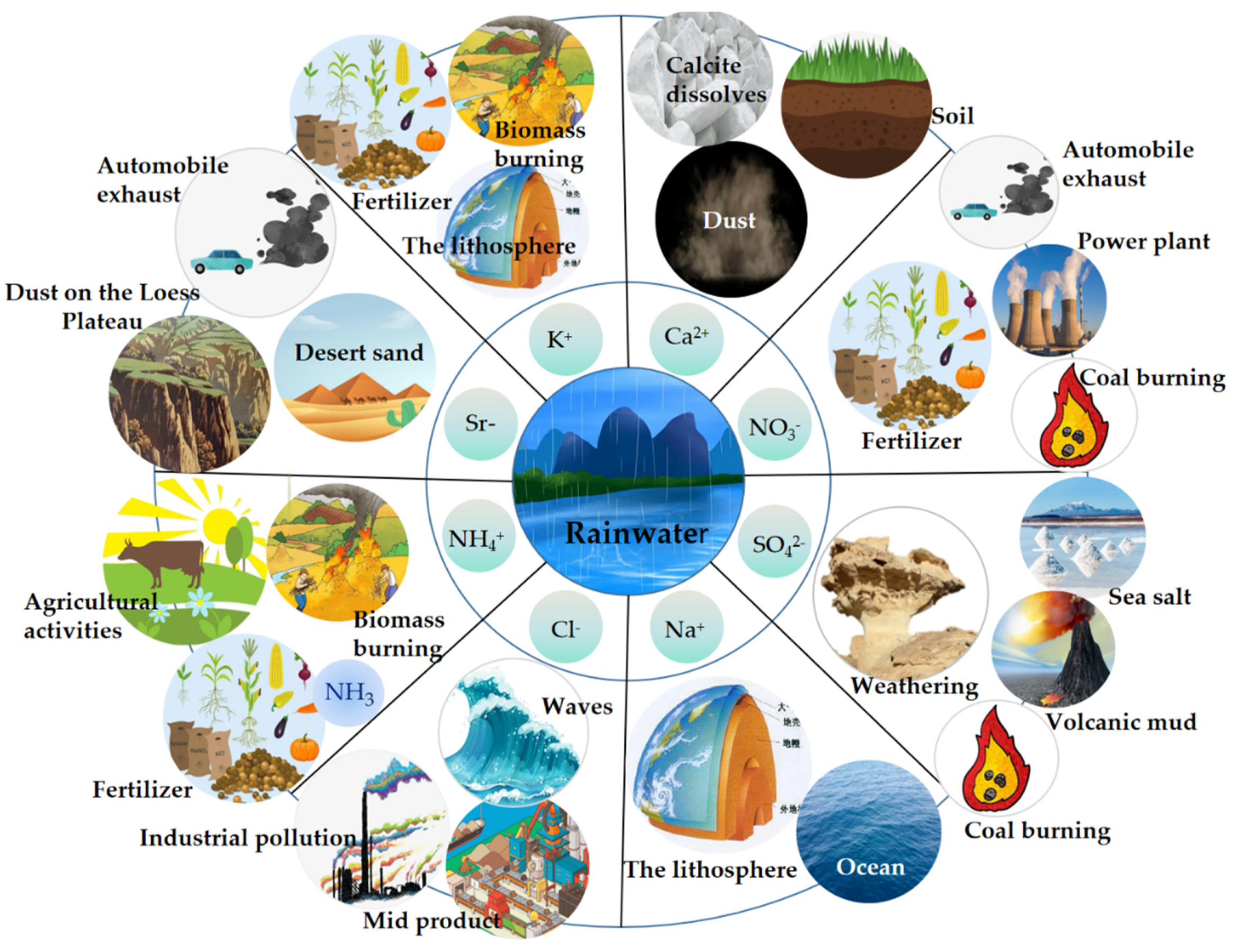
3.2. Sources of Nutrient Elements in Rainwater
3.2.1. Sources of Nutrient Elements in Rainwater
3.2.2. Distribution of Pollutants in Rainwater in Different Regions
3.2.3. Seasonal Variation of Elements Content in Rainwater
3.2.4. Effects of Dust Storms on the Composition of Rainwater
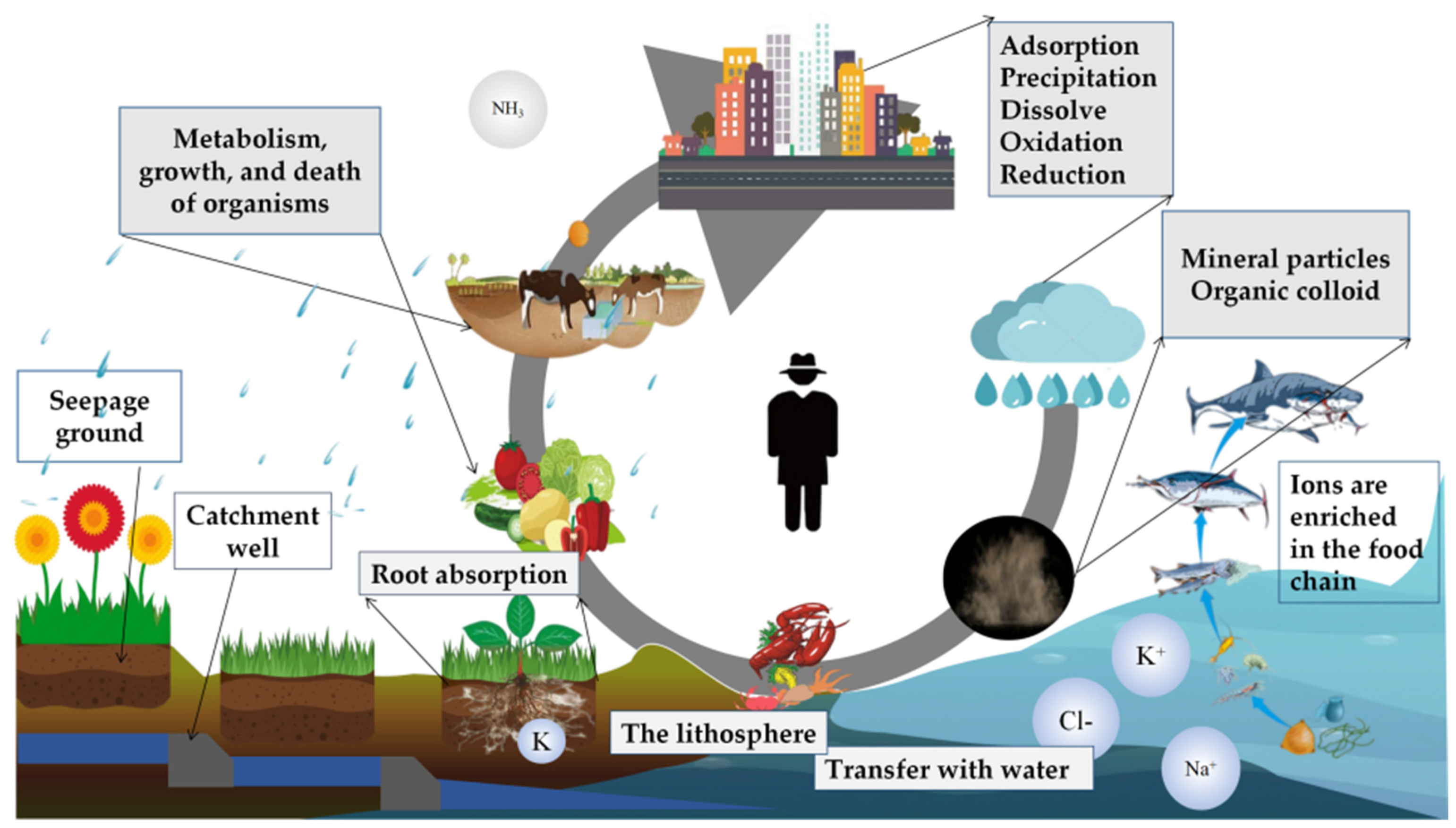
3.3. Rainwater Nutrient Transport
3.4. The Removal Method of Pollutants in Rainwater
3.4.1. Research Progress and Methods of Nutrient Removal in Rain Water
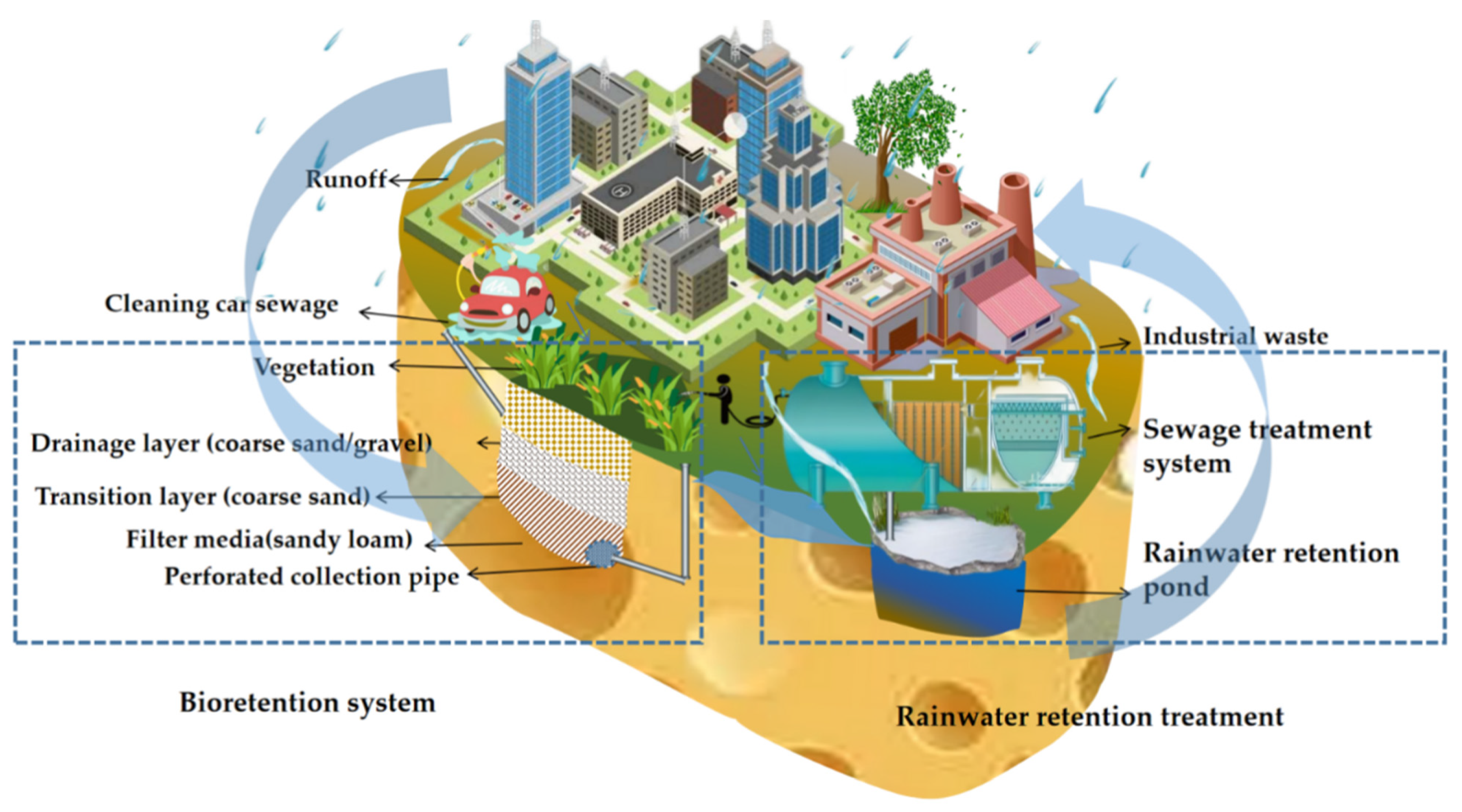
3.4.2. Bioretention Systems Treatment
3.4.3. Rainwater Storage Tank Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bisht, D.S.; Srivastava, A.K.; Joshi, H.; Ram, K.; Singh, N.; Naja, M.; Srivastava, M.K.; Tiwari, S. Chemical characterization of rainwater at a high-altitude site “Nainital” in the central Himalayas, India. Environ. Sci. Pollut. R 2017, 24, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, M.; Shu, M.; Ho, S.S.H.; Liu, Z.; Wang, X.; Zhao, X. Chemical characteristics of rainwater in Sichuan basin, a case study of Ya’an. Environ. Sci. Pollut. R 2016, 23, 13088–13099. [Google Scholar] [CrossRef]
- Niu, H.; He, Y.; Lu, X.X.; Shen, J.; Du, J.; Zhang, T.; Pu, T.; Xin, H.; Chang, L. Chemical composition of rainwater in the Yulong Snow Mountain region, Southwestern China. Atmos. Res. 2014, 144, 195–206. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, R.; Tunved, P.; Singh, S.; Panicker, A.S. Significant cooling effect on the surface due to soot particles over Brahmaputra River Valley region, India: An impact on regional climate. Sci. Total Environ. 2016, 562, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Chate, D.M.; Bisht, D.S.; Srivastava, M.K.; Padmanabhamurty, B. Rainwater chemistry in the North Western Himalayan Region, India. Atmos. Res. 2012, 104–105, 128–138. [Google Scholar] [CrossRef]
- Jawad Al Obaidy, A.H.M.; Joshi, H. Chemical composition of rainwater in a tropical urban area of northern India. Atmos. Environ. 2006, 40, 6886–6891. [Google Scholar] [CrossRef]
- Nadzir, M.S.M.; Lin, C.Y.; Khan, M.F.; Latif, M.T.; Dominick, D.; Hamid, H.H.A.; Mohamad, N.; Maulud, K.N.A.; Wahab, M.I.A.; Kamaludin, N.F.; et al. Characterization of rainwater chemical composition after a Southeast Asia haze event: Insight of transboundary pollutant transport during the northeast monsoon. Environ. Sci. Pollut. R 2017, 24, 15278–15290. [Google Scholar] [CrossRef]
- Lü, P.; Han, G.; Wu, Q. Chemical characteristics of rainwater in karst rural areas, Guizhou Province, Southwest China. Acta Geochim. 2017, 36, 572–576. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Liu, W.; Liang, C.; Ji, J.; Zhao, T.; Zhang, X. Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city, China. Atmos. Res. 2015, 164–165, 278–285. [Google Scholar] [CrossRef]
- Peikam, E.N.; Jalali, M. Chemical composition of rainwater at an urban and two rural stations in the west of Iran, Hamedan. Environ. Earth Sci. 2021, 80, 605. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Z.; Liu, W.; Wu, Y.; Zhao, T.; Jiang, H.; Zhang, X.; Zhang, J.; Zhou, L.; Wang, Y. Chemical composition of precipitation in, a coastal mega-city in South China: Influence of urbanization and anthropogenic activities on acidity and ionic composition. Sci. Total Environ. 2019, 662, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Hopke, P.K.; Thimmaiah, D.; Dumka, U.C.; Srivastava, A.K.; Bisht, D.S.; Rao, P.S.P.; Chate, D.M.; Srivastava, M.K.; Tripathi, S.N. Nature and Sources of Ionic Species in Precipitation across the Indo-Gangetic Plains, India. Aerosol. Air Qual. Res. 2016, 16, 943–957. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.Y.; Li, N.; Yang, G.; Luo, D.; Chen, J. Chemical characteristics of spring rainwater of Xi’an city, NW China. Atmos. Environ. 2011, 45, 5058–5063. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Alonso-Rodríguez, E.; Moscoso-Pérez, C.; Blanco-Heras, G.; Turnes-Carou, I.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmos. Environ. 2014, 88, 30–38. [Google Scholar] [CrossRef]
- Xiao, H.; Xiao, H.; Long, A.; Wang, Y.; Liu, C. Chemical composition and source apportionment of rainwater at Guiyang, SW China. J. Atmos. Chem. 2013, 70, 269–281. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Liu, Z.; Chen, B.; Huang, J.; Cai, L.; Deng, S. Chemicalcomposition and sources of rainwater collected at a semi-rural site inYa’an, Southwestern China. Atmos. Clim. Sci. 2013, 3, 486–496. [Google Scholar]
- Zhong, Y.; Li, X.; Fan, Z.; Ayitken, M.; Li, S.; Liu, X. Chemical Composition Characteristics and Source Contributions of Precipitation in Typical Cities on the North Slope of Tianshan Mountain in Xinjiang during 2010–2019. Atmosphere 2022, 13, 646. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, L.; Li, L.; Yang, F.; Yang, Q.; Wei, S.; Ouyang, W.; He, K.; Chen, G. Chemical composition of precipitation and its spatiotemporal variations in the Three Gorges Reservoir Region. Acta Sci. Circum. 2013, 6, 1682–1689. [Google Scholar]
- Xu, Z.; Tang, Y.; Ji, J. Chemical and strontium isotope characterization of rainwater in Beijing during the 2008 Olympic year. Atmos. Res. 2012, 107, 115–125. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, H.; Zhang, Q.; Zhang, X. Chemical characteristics of rainwater in northeast China, a case study of Dalian. Atmos. Res. 2012, 116, 151–160. [Google Scholar] [CrossRef]
- Qu, R.; Han, G. A critical review of the variation in rainwater acidity in 24 Chinese cities during 1982–2018. Elem. Sci. Anthr. 2021, 9, 142. [Google Scholar] [CrossRef]
- Majumdar, A.; Samanta, D.; Das, R. Chemical Characteristics and Trends of Indian Summer Monsoon Rainfall: A Review. Aerosol. Air Qual. Res. 2022, 22, 220019. [Google Scholar] [CrossRef]
- Meera, V.; Ahammed, M.M. Water quality of rooftop rainwater harvesting systems: A review. J. Water Supply: Res. Technol. 2006, 55, 257–268. [Google Scholar] [CrossRef]
- Minixhofer, P.; Stangl, R. Green Infrastructures and the Consideration of Their Soil-Related Ecosystem Services in Urban Areas—A Systematic Literature Review. Sustainability 2021, 13, 3322. [Google Scholar] [CrossRef]
- Lu, G.; Wang, L. An Integrated Framework of Green Stormwater Infrastructure Planning—A Review. Sustainability 2021, 13, 13942. [Google Scholar] [CrossRef]
- Chebana, F.; Ouarda, T.B.M.J. Multivariate non-stationary hydrological frequency analysis. J. Hydrol. 2021, 593, 125907. [Google Scholar] [CrossRef]
- Burgan, H.I.; Vaheddoost, B.; Aksoy, A.H. Frequency Analysis of Monthly Runoff in Intermittent Rivers. World Environ. Water Resour. Congr. (EWRI 2017) 2017, 2017, 327–334. [Google Scholar]
- Wang, W.; Guan, L.; Zhao, J.; Sha, Z.; Fang, J. Chemical Compositions of Rainfall Water in Nyingchi City, Tibet. Atmosphere 2022, 13, 1021. [Google Scholar] [CrossRef]
- Wang, H.; Han, G. Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos. Res. 2011, 99, 190–196. [Google Scholar] [CrossRef]
- Haihua, W. Chemical composition of fine particulate matter of sandstorm and its toxicological study. J. Environ. Health 2011, 1, 42–44. [Google Scholar]
- Zhang, M.; Wang, S.; Wu, F.; Yuan, X.; Zhang, Y. Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos. Res. 2007, 84, 311–322. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Liu, W.; Zhao, T.; Zhang, X.; Jiang, H.; Yu, C.; Zhou, L.; Zhou, X. Chemical compositions of precipitation at three non-urban sites of Hebei Province, North China: Influence of terrestrial sources on ionic composition. Atmos. Res. 2016, 181, 115–123. [Google Scholar] [CrossRef]
- Qiao, X.; Xiao, W.; Jaffe, D.; Kota, S.H.; Ying, Q.; Tang, Y. Atmospheric wet deposition of sulfur and nitrogen in Jiuzhaigou National Nature Reserve, Sichuan Province, China. Sci. Total Environ. 2015, 511, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.P.; Balasubramanian, R.; Wu, C.D. Chemical characterization of rainwater at Singapore. Chemosphere 2003, 51, 747–755. [Google Scholar] [CrossRef]
- Kaskaoutis, D.G.; Kumar, S.; Sharma, D.; Singh, R.P.; Kharol, S.K.; Sharma, M.; Singh, A.K.; Singh, S.; Singh, A.; Singh, D. Effects of crop residue burning on aerosol properties, plume characteristics, and long-range transport over northern India. J. Geophys. Res. Atmos. 2014, 119, 5424–5444. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Kumari, K.M.; Srivastava, S.S. Measurement of NO2, HNO3, NH3 and SO2 and related particulate matter at a rural site in Rampur, India. Atmos. Environ. 2003, 37, 4837–4846. [Google Scholar] [CrossRef]
- Budhavant, K.B.; Rao, P.S.P.; Safai, P.D.; Ali, K. Influence of local sources on rainwater chemistry over Pune region, India. Atmos. Res. 2011, 100, 121–131. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, X.; Li, Z.; Chen, F.; Luo, H.; He, G.; Deng, Z.; Chen, C.; Lao, Q. Influence of Typhoons on Chemical Makeup of Rainwater in Zhanjiang, China. Aerosol. Air Qual. Res. 2022, 22, 210210. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.; Li, S.; Wang, Z.; Wu, Q.; Qin, C.; Yan, Z. Determining rainwater chemistry to reveal alkaline rain trend in Southwest China: Evidence from a frequent-rainy karst area with extensive agricultural production. Environ. Pollut. 2020, 266, 115166. [Google Scholar] [CrossRef]
- Xu, Z.; Han, G. Chemical and strontium isotope characterization of rainwater in Beijing, China. Atmos. Environ. 2009, 43, 1954–1961. [Google Scholar] [CrossRef]
- Luan, Z. The Chemical Characteristics and Environmental Significance of Atmospheric Precipitation in Shanghai Area. Master’s Thesis, East China Normal University, Shanghai, China, 2019. [Google Scholar]
- Al-Khashman, O.A. Chemical characteristics of rainwater collected at a western site of Jordan. Atmos. Res. 2009, 91, 53–61. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Wu, Q.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290, 106782. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Jiang, P.; Fu, C. Rainwater chemistry in a subtropical high-altitude mountain site, South China: Seasonality, source apportionment and potential factors. Atmos. Environ. 2022, 268, 118786. [Google Scholar] [CrossRef]
- Hou, S.; Chenchen, Q.; Cheng, D. Analysis on chemical composition of precipitation and its source apportionment in Xi’an City. Environ. Chem. 2020, 41, 2384–2394. [Google Scholar]
- Xu, Z.; Li, Y.; Tang, Y.; Han, G. Chemical and strontium isotope characterization of rainwater at an urban site in Loess Plateau, Northwest China. Atmos. Res. 2009, 94, 481–490. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, S.; Zhang, G.; Luo, J.; Lu, S. Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos. Res. 2009, 94, 462–469. [Google Scholar] [CrossRef]
- Huang, D.; Xu, Y.; Peng, P.; Zhang, H.; Lan, J. Chemical composition and seasonal variation of acid deposition in Guangzhou, South China: Comparison with precipitation in other major Chinese cities. Environ. Pollut. 2009, 157, 35–41. [Google Scholar] [CrossRef]
- The chemical composition of precipitation analysis in Chongqing city. Chongqing Environ. Sci. 2003, 25, 112–114.
- Okuda, T.; Iwase, T.; Ueda, H.; Suda, Y.; Tanaka, S.; Dokiya, Y.; Fushimi, K.; Hosoe, M. Long-term trend of chemical constituents in precipitation in Tokyo metropolitan area, Japan, from 1990 to 2002. Sci. Total Environ. 2005, 339, 127–141. [Google Scholar] [CrossRef]
- Bao, H.; Jenkins, K.A.; Khachaturyan, M.; Díaz, G.C. Different sulfate sources and their post-depositional migration in Atacama soils. Earth Planet Sci. Lett. 2004, 224, 577–587. [Google Scholar] [CrossRef]
- Yalcin, K.; Wake, C.P.; Kang, S.; Kreutz, K.J.; Whitlow, S.I. Seasonal and spatial variability in snow chemistry at Eclipse Icefield, Yukon, Canada. Ann. Glaciol. 2006, 43, 230–238. [Google Scholar] [CrossRef]
- Legrand, M.R.K.S. Origins and variations of nitrate in south polar precipitation. J. Geophys. Res. 1990, 95, 3493–3507. [Google Scholar] [CrossRef]
- Zhang, N.; He, Y.; Cao, J.; Ho, K.; Shen, Z. Long-term trends in chemical composition of precipitation at Lijiang, southeast Tibetan Plateau, southwestern China. Atmos. Res. 2012, 106, 50–60. [Google Scholar] [CrossRef]
- Xu, W.; Wen, Z.; Shang, B.; Dore, A.J.; Tang, A.; Xia, X.; Zheng, A.; Han, M.; Zhang, L.; Zhao, Y.; et al. Precipitation chemistry and atmospheric nitrogen deposition at a rural site in Beijing, China. Atmos. Environ. 2020, 223, 117253. [Google Scholar] [CrossRef]
- Yan, W. Precipitation chemical characteristics and nitrate source analysis in typical cities in southeastern China. Master Thesis, Zhejiang University of Technology, Hangzhou, China, 2019. [Google Scholar]
- Xing, J.; Song, J.; Yuan, H.; Li, X.; Li, N.; Duan, L.; Qu, B.; Wang, Q.; Kang, X. Chemical characteristics, deposition fluxes and source apportionment of precipitation components in the Jiaozhou Bay, North China. Atmos. Res. 2017, 190, 10–20. [Google Scholar] [CrossRef]
- Xue, Y. Relationship between the Characteristics of Precipitation and Air Quality in Nanjing. Master Thesis, Nanjing Agricultural College, Nanjing, China, 2014. [Google Scholar]
- Huang, X.; Li, X.; He, L.; Feng, N.; Hu, M.; Niu, Y.; Zeng, L. 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos. Res. 2010, 97, 185–193. [Google Scholar] [CrossRef]
- Fumo, Y.; Kebin, H.; Yu, L.; Yongliang, M.; Xuechun, Y. Chemical characters of atmospheric precipitation in Beijing in years of 2001–2003. China Environ. Sci. 2004, 24, 538–541. [Google Scholar]
- Bingyan, J.; Yao, W.; Shaoai, L.; Tianjia, L.; Long, H. Chemical compositions and sources of precipitation in Shenzhen from 2010 to 2017. Environ. Chem. 2019, 38, 1872–1881. [Google Scholar]
- Wang, B.; Luo, X.; Liu, D.; Su, Y.; Wu, Z. The effect of construction dust and agricultural fertilization on the precipitation chemical composition during summer in the Yangtze River Delta area, China. Atmos. Pollut. Res. 2021, 12, 101121. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Xu, Y.; Xu, Z.; Zhou, X.; Zhou, L. Multiple isotopic tracing for sulfate and base cation sources of precipitation in Hangzhou city, Southeast China: Insights for rainwater acidification mechanism. Environ. Pollut. 2021, 288, 117770. [Google Scholar] [CrossRef]
- Wu, Q.; Han, G.; Tao, F.; Tang, Y. Chemical composition of rainwater in a karstic agricultural area, Southwest China: The impact of urbanization. Atmos. Res. 2012, 111, 71–78. [Google Scholar] [CrossRef]
- Aas, W.; Shao, M.; Jin, L.; Larssen, T.; Zhao, D.; Xiang, R.; Zhang, J.; Xiao, J.; Duan, L. Air concentrations and wet deposition of major inorganic ions at five non-urban sites in China, 2001–2003. Atmos. Environ. 2007, 41, 1706–1716. [Google Scholar] [CrossRef]
- Naimabadi, A.; Shirmardi, M.; Maleki, H.; Teymouri, P.; Goudarzi, G.; Shahsavani, A.; Sorooshian, A.; Babaei, A.A.; Mehrabi, N.; Baneshi, M.M.; et al. On the chemical nature of precipitation in a populated Middle Eastern Region (Ahvaz, Iran) with diverse sources. Ecotox Env. Safe 2018, 163, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Gao, Y. Chemical characteristics of precipitation at metropolitan Newark in the US East Coast. Atmos. Environ. 2009, 43, 4903–4913. [Google Scholar] [CrossRef]
- Báez, A.; Belmont, R.; García, R.; Padilla, H.; Torres, M.C. Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos. Res. 2007, 86, 61–75. [Google Scholar] [CrossRef]
- Behera, S.N.; Betha, R.; Huang, X.; Balasubramanian, R. Characterization and estimation of human airway deposition of size-resolved particulate-bound trace elements during a recent haze episode in Southeast Asia. Environ. Sci. Pollut. R 2015, 22, 4265–4280. [Google Scholar] [CrossRef]
- Hou, H.; Takamatsu, T.; Koshikawa, M.K.; Hosomi, M. Trace metals in bulk precipitation and throughfall in a suburban area of Japan. Atmos. Environ. 2005, 39, 3583–3595. [Google Scholar] [CrossRef]
- Zunckel, M.; Saizar, C.; Zarauz, J. Rainwater composition in northeast Uruguay. Atmos. Environ. 2003, 37, 1601–1611. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Victor, T.; Begum, R. Impact of biomass burning on rainwater acidity and composition in Singapore. J. Geophys. Res. Atmos. 1999, 104, 26881–26890. [Google Scholar] [CrossRef]
- Ghude, S.D.; Fadnavis, S.; Beig, G.; Polade, S.D.; van der A, R.J. Detection of surface emission hot spots, trends, and seasonal cycle from satellite-retrieved NO2 over India. J. Geophys. Res. 2008, 113, D20305. [Google Scholar] [CrossRef]
- Han, F.; Yang, Z.; Liu, X.; Di, F. Impact assessment and protection of outstanding landscape integrity in a natural heritage site: Fairy valley, Kanas Nature Reserve, Xinjiang, China. J. Mt. Sci.-Engl. 2011, 8, 46–52. [Google Scholar] [CrossRef]
- Santos, P.S.M.; Otero, M.; Santos, E.B.H.; Duarte, A.C. Chemical composition of rainwater at a coastal town on the southwest of Europe: What changes in 20years? Sci. Total Environ. 2011, 409, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.P.; Tiwari, S.; Matwale, J.L.; Pervez, S.; Tunved, P.; Safai, P.D.; Srivastava, A.K.; Bisht, D.S.; Singh, S.; Hopke, P.K. Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos. Environ. 2016, 146, 90–99. [Google Scholar] [CrossRef]
- Chen, X.; Mulder, J. Atmospheric deposition of nitrogen at five subtropical forested sites in South China. Sci. Total Environ. 2007, 378, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Laouali, D.; Galy-Lacaux, C.; Diop, B.; Delon, C.; Orange, D.; Lacaux, J.P.; Akpo, A.; Lavenu, F.; Gardrat, E.; Castera, P. Long term monitoring of the chemical composition of precipitation and wet deposition fluxes over three Sahelian savannas. Atmos. Environ. 2012, 50, 314–327. [Google Scholar] [CrossRef]
- Tsal, J.; Lin, K.; Chen, C.; Ding, J.; Chon, C.; Chiang, H. Chemical constituents in particulate emissions from an integrated iron and steel facility. J. Hazard. Mater. 2007, 147, 111–119. [Google Scholar]
- Başak, B.; Alagha, O. Trace metals solubility in rainwater: Evaluation of rainwater quality at a watershed area, Istanbul. Environ. Monit. Assess 2010, 167, 493–503. [Google Scholar] [CrossRef]
- Başak, B.; Alagha, O. The chemical composition of rainwater over Büyükçekmece Lake, Istanbul. Atmos Res 2004, 71, 275–288. [Google Scholar] [CrossRef]
- Qiu, Y.; Felix, J.D. Hurricane/tropical storm rainwater chemistry in the US (from 2008 to 2019). Sci. Total Environ. 2021, 798, 149009. [Google Scholar] [CrossRef]
- Hua, W.; Maning; Xiaojing, Y.; Kaiming, Y.; Guilin, H. Chemical composition of rainwater and anthropogenic influences in Chengdu. Earth Environ. 2010, 38, 49–53. [Google Scholar]
- Schlesinger, W.H.; Hartley, A.E. A global budget for atmospheric NH3. Biogeochemistry 1992, 15, 191–211. [Google Scholar] [CrossRef]
- Galy-Lacaux, C.; Carmichaël, G.R.; Song, C.H.; Lacaux, P.; Modi, A.I. that heterogeneous processes involving terrigenous compounds are important and play a. J. Geophys. Res. 2001, 106, 12559–12578. [Google Scholar] [CrossRef]
- Awasthi, A.; Agarwal, R.; Mittal, S.K.; Singh, N.; Singh, K.; Gupta, P.K. Study of size and mass distribution of particulate matter due to crop residue burning with seasonal variation in rural area of Punjab, India. J. Environ. Monit. 2011, 13, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, S.; Tiwari, S.; Kanawade, V.P.; Bisht, D.S. Variation between near-surface and columnar aerosol characteristics during the winter and summer at Delhi in the Indo-Gangetic Basin. J. Atmos. Sol-Terr. Phy. 2012, 77, 57–66. [Google Scholar] [CrossRef]
- Bove, M.C.; Brotto, P.; Cassola, F.; Cuccia, E.; Massabò, D.; Mazzino, A.; Piazzalunga, A.; Prati, P. An integrated PM2.5 source apportionment study: Positive Matrix Factorisation vs. the chemical transport model CAMx. Atmos. Environ. 2014, 94, 274–286. [Google Scholar] [CrossRef]
- Tsamos, P.; Kolias, P.; Lambropoulou, D.; Noli, F. Distribution and temporal variability of uranium and toxic metal(loid)s in snow and rainwater from an oil industry and urban area in Thessaloniki-Greece. Sci. Total Environ. 2022, 838, 155604. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Cui, L.; Meng, Y.; Fu, H. Spatial and temporal variation of inorganic ions in rainwater in Sichuan province from 2011 to 2016. Environ. Pollut. 2019, 254, 112941. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, C. Chemical characteristics of water-soluble components in TSP over Guiyang, SW China, 2003. Atmos. Environ. 2004, 38, 6297–6306. [Google Scholar] [CrossRef]
- Kang, S.; Mayewski, P.A.; Qin, D.; Sneed, S.A.; Ren, J.; Zhang, D. Seasonal differences in snow chemistry from the vicinity of Mt. Everest, central Himalayas. Atmos. Environ. 2004, 38, 2819–2829. [Google Scholar] [CrossRef]
- Coelho, C.H.; Allen, A.G.; Fornaro, A.; Orlando, E.A.; Grigoletto, T.L.B.; Campos, M.L.A.M. Wet deposition of major ions in a rural area impacted by biomass burning emissions. Atmos. Environ. 2011, 45, 5260–5265. [Google Scholar] [CrossRef]
- Okumura, M.; Yamada, S.; Oshima, Y.; Ishikawa, N. Characteristics of paralytic shellfish poisoning toxins derived from short-necked clams (Tapes japonica) in Mikawa Bay. Nat. Toxins 1994, 2, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Wang, G.; Yang, G.; Luo, L. Identification of sand and dust storm source areas in Iran. J. Arid. Land. 2015, 7, 567–578. [Google Scholar] [CrossRef]
- Wijesiri, B.; Bandala, E.; Liu, A.; Goonetilleke, A. A Framework for Stormwater Quality Modelling under the Effects of Climate Change to Enhance Reuse. Sustainability 2020, 12, 10463. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Engling, G.; Zhang, R.; Yang, Y.; Cao, J.; Zhu, C.; Wang, Q.; Luo, L. Chemical composition of PM2.5 in an urban environment in Chengdu, China: Importance of springtime dust storms and biomass burning. Atmos. Res. 2013, 122, 270–283. [Google Scholar] [CrossRef]
- Gong, S.L.; Zhang, X.Y.; Zhao, T.L.; McKendry, I.G.; Jaffe, D.A.; Lu, N.M. Characterization of soil dust aerosol in China and its transport and distribution during 2001 ACE-Asia: 2. Model simulation and validation. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Cyranoski, D. China plans clean sweep on dust storms. Nature 2003, 421, 101. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Liu, C.; Ma, J.; He, H. A case study of Asian dust storm particles: Chemical composition, reactivity to SO2 and hygroscopic properties. J. Environ. Sci. 2012, 24, 62–71. [Google Scholar] [CrossRef]
- Broomandi, P.; Dabir, B.; Bonakdarpour, B.; Rashidi, Y. Identification of dust storm origin in South –West of Iran. J. Environ. Health Sci. 2017, 15, 16. [Google Scholar] [CrossRef]
- Naimabadi, A.; Ghadiri, A.; Idani, E.; Babaei, A.A.; Alavi, N.; Shirmardi, M.; Khodadadi, A.; Marzouni, M.B.; Ankali, K.A.; Rouhizadeh, A.; et al. Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the Middle Eastern Dust (MED) storms in Ahvaz, Iran. Environ. Pollut. 2016, 211, 316–324. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Wang, Y.; Zhao, X.; Li, J.; Wang, Z.; An, Z. Chemical composition of dust storms in Beijing and implications for the mixing of mineral aerosol with pollution aerosol on the pathway. J. Geophys. Res. 2005, 110, D24209. [Google Scholar] [CrossRef]
- Ganor, E.; Foner, H.A.; Brenner, S.; Neeman, E.; Lavi, N. The chemical composition of aerosols settling in Israel following dust storms. Atmos. Environment. Part A. Gen. Top. 1991, 25, 2665–2670. [Google Scholar] [CrossRef]
- Cereceda-Balic, F.; Gala-Morales, M.D.L.; Palomo-Marín, R.; Fadic, X.; Vidal, V.; Funes, M.; Rueda-Holgado, F.; Pinilla-Gil, E. Spatial distribution, sources, and risk assessment of major ions ad trace elements in rainwater at Puchuncaví Valley, Chile: The impact of industrial activities. Atmos. Pollut. Res. 2020, 11, 99–109. [Google Scholar] [CrossRef]
- Li Ran, L.J.Z.W. Overview of heavy metal pollution in water environment. Sichuan Environ. 1997, 1, 19–23. [Google Scholar]
- Long, J.; Tan, D.; Zhou, Y.; Zhou, D.; Luo, Y.; Bin, D.; Wang, Z.; Wang, J.; Lei, M. The leaching of antimony and arsenic by simulated acid rain in three soil types from the world’s largest antimony mine area. Environ. Geochem. Health 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Kondo, Y.; Kita, K.; Takegawa, N.; Nishi, N.; Kashihara, T.; Kawakami, S.; Kudoh, S.; Blake, D.; Shirai, T.; et al. Measurements of reactive nitrogen produced by tropical thunderstorms during BIBLE-C. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Ridley, B.A.; Dye, J.E.; Walega, J.G.; Zheng, J.; Grahek, F.E.; Rison, W. On the production of active nitrogen by thunderstorms over New Mexico. J. Geophys. Res. Atmos. 1996, 101, 20985–21005. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water purification by freezing and gas hydrate processes, and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+). J. Mol. Liq. 2016, 223, 1021–1031. [Google Scholar] [CrossRef]
- Schipper, P.N.M.; Bonten, L.T.C.; Plette, A.C.C.; Moolenaar, S.W. Measures to diminish leaching of heavy metals to surface waters from agricultural soils. Desalination 2008, 226, 89–96. [Google Scholar] [CrossRef]
- Characklis, G.W.; Wiesner, M.R. Particles, metals, and water quality in runoff from large urban watershed. J. Environ. Eng. 1997, 123, 753–759. [Google Scholar] [CrossRef]
- Rossi, L.; Chèvre, N.; Fankhauser, R.; Krejci, V. Probabilistic environmental risk assessment of urban wet-weather discharges: An approach developed for Switzerland. Urban Water J. 2009, 6, 355–367. [Google Scholar] [CrossRef]
- Kayhanian, M.; Suverkropp, C.; Ruby, A.; Tsay, K. Characterization and prediction of highway runoff constituent event mean concentration. J. Environ. Manag. 2007, 85, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yan, L.; Junqi, L. Urban Rainwater Quality and Pollution Control at Home and Abroad. Water Supply Sewerage 2003, 29, 38–42. [Google Scholar]
- Walaszek, M.; Bois, P.; Laurent, J.; Lenormand, E.; Wanko, A. Urban stormwater treatment by a constructed wetland: Seasonality impacts on hydraulic efficiency, physico-chemical behavior and heavy metal occurrence. Sci. Total Environ. 2018, 637–638, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Birch, G.F.; Matthai, C.; Fazeli, M.S.; Suh, J.Y. Efficiency of a constructed wetland in removing contaminants from stormwater. Wetlands 2004, 24, 459–466. [Google Scholar] [CrossRef]
- Gregoire, B.G.; Clausen, J.C. Effect of a modular extensive green roof on stormwater runoff and water quality. Ecol. Eng. 2011, 37, 963–969. [Google Scholar] [CrossRef]
- Carleton, J.N.; Grizzard, T.J.; Godrej, A.N.; Post, H.E.; Lampe, L.; Kenel, P.P. Performance of a Constructed Wetlands in Treating Urban Stormwater Runoff. Water Environ. Res. 2000, 72, 295–304. [Google Scholar] [CrossRef]
- Scholes, L.; Revitt, D.M.; Ellis, J.B. The Fate of Stormwater Priority Pollutants in BMPs; Public Report; DayWater Project: London, UK, 2005. [Google Scholar]
- Al-Rubaei, A.M.; Engström, M.; Viklander, M.; Blecken, G. Long-term hydraulic and treatment performance of a 19-year old constructed stormwater wetland—Finally maturated or in need of maintenance? Ecol. Eng. 2016, 95, 73–82. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Chou, C.C.; Pan, C.T. Heavy metal removal within pilot-scale constructed wetlands receiving river water contaminated by confined swine operations. Desalination 2009, 249, 368–373. [Google Scholar] [CrossRef]
- Bressy, A.; Gromaire, M.C.; Lorgeoux, C.; Saad, M.; Leroy, F.; Chebbo, G. Towards the determination of an optimal scale for stormwater quality management: Micropollutants in a small residential catchment. Water Res. 2012, 46, 6799–6810. [Google Scholar] [CrossRef]
- Schmitt, N.; Wanko, A.; Laurent, J.; Bois, P.; Molle, P.; Mosé, R. Constructed wetlands treating stormwater from separate sewer networks in a residential Strasbourg urban catchment area: Micropollutant removal and fate. J. Environ. Chem. Eng. 2015, 3, 2816–2824. [Google Scholar] [CrossRef]
- Teemusk, A.; Mander, Ü. The Use Of Greenroofs For The Mitigation Of Environmental Problems in Urban Areas. WIT Trans. Ecol. Environ. 2006, 93, 15. [Google Scholar]
- Lee, J.H.; Bang, K.W.; Ketchum, L.H.; Choe, J.S.; Yu, M.J. First flush analysis of urban storm runoff. Sci. Total Environ. 2002, 293, 163–175. [Google Scholar] [CrossRef]
- Deletic, A. The first flush load of urban surface runoff. Water Res. 1998, 32, 2462–2470. [Google Scholar] [CrossRef]
- Sansalone, J.J.; Cristina, C.M. First Flush Concepts for Suspended and Dissolved Solids in Small Impervious Watersheds. J. Environ. Eng. 2004, 130, 1301–1314. [Google Scholar] [CrossRef]
- Gill, L.W.; Ring, P.; Casey, B.; Higgins, N.; Johnston, P.M. Long term heavy metal removal by a constructed wetland treating rainfall runoff from a motorway. Sci. Total Environ. 2017, 601–602, 32–44. [Google Scholar] [CrossRef]
- Sajn Slak, A.; Bulc, T.G.; Vrhovsek, D. Comparison of nutrient cycling in a surface-flow constructed wetland and in a facultative pond treating secondary effluent. Water Sci. Technol. 2005, 51, 291–298. [Google Scholar] [CrossRef]
- Sparkman, S.A.; Hogan, D.M.; Hopkins, K.G.; Loperfido, J.V. Modeling Watershed-Scale Impacts of Stormwater Management with Traditional versus Low Impact Development Design. J. Am. Water Resour. Assoc. 2017, 53, 1081–1094. [Google Scholar] [CrossRef]
- Yanping, Q.; Hongcui, L.; Yonghua, H. Study on total phosphorus and chemical oxygen demand in initial rainwater treated by fly ash adsorption. Shandong Sci. 2019, 32, 80–85. [Google Scholar]
- Czemiel Berndtsson, J. Green roof performance towards management of runoff water quantity and quality: A review. Ecol. Eng. 2010, 36, 351–360. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Liu, Y.; Ma, R.; Lu, S.; Lu, X.; Liu, L.; Liang, H. Gravity-driven membrane bioreactor coupled with electrochemical oxidation disinfection (GDMBR-EO) to treat roofing rainwater. Chem. Eng. J. 2022, 427, 131714. [Google Scholar] [CrossRef]
- Anoob, F.; Meera, V. Optimization of operational parameters for the treatment of roof-harvested rainwater with biologically synthesised nanosilver coated on sand. Water Supply 2022, 22, 1120–1130. [Google Scholar] [CrossRef]
- Hathaway, A.M.; Hunt, W.F.; Jennings, G.D. A Field Study of Green Roof Hydrologic and Water Quality Performance. T Asabe 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Mazer, G.; Booth, D.; Ewing, K. Limitations to vegetation establishment and growth in biofiltration swales. Ecol. Eng. 2001, 17, 429–443. [Google Scholar] [CrossRef]
- Osman, M.; Wan Yusof, K.; Takaijudin, H.; Goh, H.W.; Abdul Malek, M.; Azizan, N.A.; Ab Ghani, A.; Sa Id Abdurrasheed, A. A Review of Nitrogen Removal for Urban Stormwater Runoff in Bioretention System. Sustainability 2019, 11, 5415. [Google Scholar] [CrossRef]
- Shrestha, P.; Hurley, S.E.; Wemple, B.C. Effects of different soil media, vegetation, and hydrologic treatments on nutrient and sediment removal in roadside bioretention systems. Ecol. Eng. 2018, 112, 116–131. [Google Scholar] [CrossRef]
- Duchemin, M.; Hogue, R. Reduction in agricultural non-point source pollution in the first year following establishment of an integrated grass/tree filter strip system in southern Quebec (Canada). Agric. Ecosyst. Environ. 2009, 131, 85–97. [Google Scholar] [CrossRef]
- Baoshan, W.; Tinglin, H.; Xiaobao, N.; Beibei, C. Research on the control of initial rainwater runoff pollution by ecological green space. China Water Supply Drain. 2010, 26, 11–13. [Google Scholar]
- Barrett, M.E.; Limouzin, M.; Lawler, D.F. Effects of Media and Plant Selection on Biofiltration Performance. J. Environ. Eng. 2013, 139, 462–470. [Google Scholar] [CrossRef]
- Wang, S.; Lin, X.; Yu, H.; Wang, Z.; Xia, H.; An, J.; Fan, G. Nitrogen removal from urban stormwater runoff by stepped bioretention systems. Ecol. Eng. 2017, 106, 340–348. [Google Scholar] [CrossRef]
- Xiong, J.; Li, G.; Zhu, J.; Li, J.; Yang, Y.; An, S.; Liu, C. Removal characteristics of heavy metal ions in rainwater runoff by bioretention cell modified with biochar. Environ. Technol. 2021, 1–13. [Google Scholar] [CrossRef]
- Tao, C.; Ben, Z.; Jianfeng, L.; Mengzi, H. Kinetics of nutrient uptake by plants in water. Environ. Eng. 2018, 36, 21–25. [Google Scholar]
- Davis, A.P.; Shokouhian, M.; Ni, S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001, 44, 997–1009. [Google Scholar] [CrossRef]
- Palomo, J.L.; López, M.M.; García-Benavides, P.; Velázquez, E.; Martínez-Molina, E. Evaluation of the API 50CH and API ZYM systems for rapid characterization of Clavibacter michiganensis subsp. sepedonicus, causal agent of potato ring rot. Eur. J. Plant Pathol. 2006, 115, 443–451. [Google Scholar] [CrossRef]
- Fujita, S. Full-Fledged Movement on Improvement of the Combined Sewer System and Flood Control Underway in Japan (Keynote Paper). In Proceedings of the International Conference on Urban Drainage, Portland, OR, USA, 8–13 September 2002. [Google Scholar]
- Guihong, F.; Liu, S.W.; Yonglong, H. Discussion on the implementation of diversion system of drainage system in Shenzhen. Water Supply Drain. China 2002, 10, 24–26. [Google Scholar]
- Marszałek, A.; Kamińska, G.; Abdel Salam, N.F. Simultaneous adsorption of organic and inorganic micropollutants from rainwater by bentonite and bentonite-carbon nanotubes composites. J. Water Process Eng. 2022, 46, 102550. [Google Scholar] [CrossRef]
- Jakubowicz, P.; Fitobór, K.; Gajewska, M.; Drewnowska, M. Detection and Removal of Priority Substances and Emerging Pollutants from Stormwater: Case Study of the Kołobrzeska Collector, Gdańsk, Poland. Sustainability 2022, 14, 1105. [Google Scholar] [CrossRef]
- Hadi Hassan Al-Taai, S. Ground water: A study of its importance, its sources, and the causes of its pollution. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Z.; Cao, J.; Zhang, R.; Zhang, L.; Huang, R.J.; Zheng, C.; Wang, L.; Liu, S.; Xu, H.; et al. Variations in PM2.5, TSP, BC, and trace gases (NO2, SO2, and O3) between haze and non-haze episodes in winter over Xi’an, China. Atmos. Environ. 2015, 112, 64–71. [Google Scholar] [CrossRef]
- Shen, Z.; Arimoto, R.; Cao, J.; Zhang, R.; Li, X.; Du, N.; Okuda, T.; Nakao, S.; Tanaka, S. Seasonal variations and evidence for the effectiveness of pollution controls on water-soluble inorganic species in total suspended particulates and fine particulate matter from Xi’an, China. J. Air Waste Manag. Assoc. 2008, 58, 1560–1570. [Google Scholar] [CrossRef]
- Dhanu Radha, S.V.V.; Sabarathinam, C.; Al-Ayyadhi, N.; Al-Ajeel, F.K.; Al-Qallaf, H.; Akber, A. Spatial and temporal variation of dissolved CO2 in rainwater from an arid region with special focus on its association with DIC and pCO2. Environ. Earth Sci. 2022, 81, 113. [Google Scholar] [CrossRef]
- Latif, S.; Alim, M.A.; Rahman, A. Disinfection methods for domestic rainwater harvesting systems: A scoping review. J. Water Process Eng. 2022, 46, 102542. [Google Scholar] [CrossRef]
- Ebraheim, G.; Karbassi, A.R.; Mehrdadi, N. Employing speciation of metals to assess photo-assisted electrochemical efficiency for improving rainwater quality in Tehran, Iran. Int. J. Environ. Sci. Technol. 2022, 19, 261–280. [Google Scholar] [CrossRef]
- Karbassi, A.R.; Ebraheim, G.; Mehrdadi, N. Chemical reducing conditions through the photo-assisted electrochemical process in the treatment of the urban rainwater. Int. J. Hum. Cap. Urban Manag. 2021, 6, 209–224. [Google Scholar]
- Ranaee, E.; Abbasi, A.A.; Tabatabaee Yazdi, J.; Ziyaee, M. Feasibility of Rainwater Harvesting and Consumption in a Middle Eastern Semiarid Urban Area. Water 2021, 13, 2130. [Google Scholar] [CrossRef]
- Kugedera, A.T.; Nyamadzawo, G.; Mandumbu, R.; Nyamangara, J. Potential of field edge rainwater harvesting, biomass transfer and integrated nutrient management in improving sorghum productivity in semi-arid regions: A review. Agroforest Syst. 2022, 96, 909–924. [Google Scholar] [CrossRef]
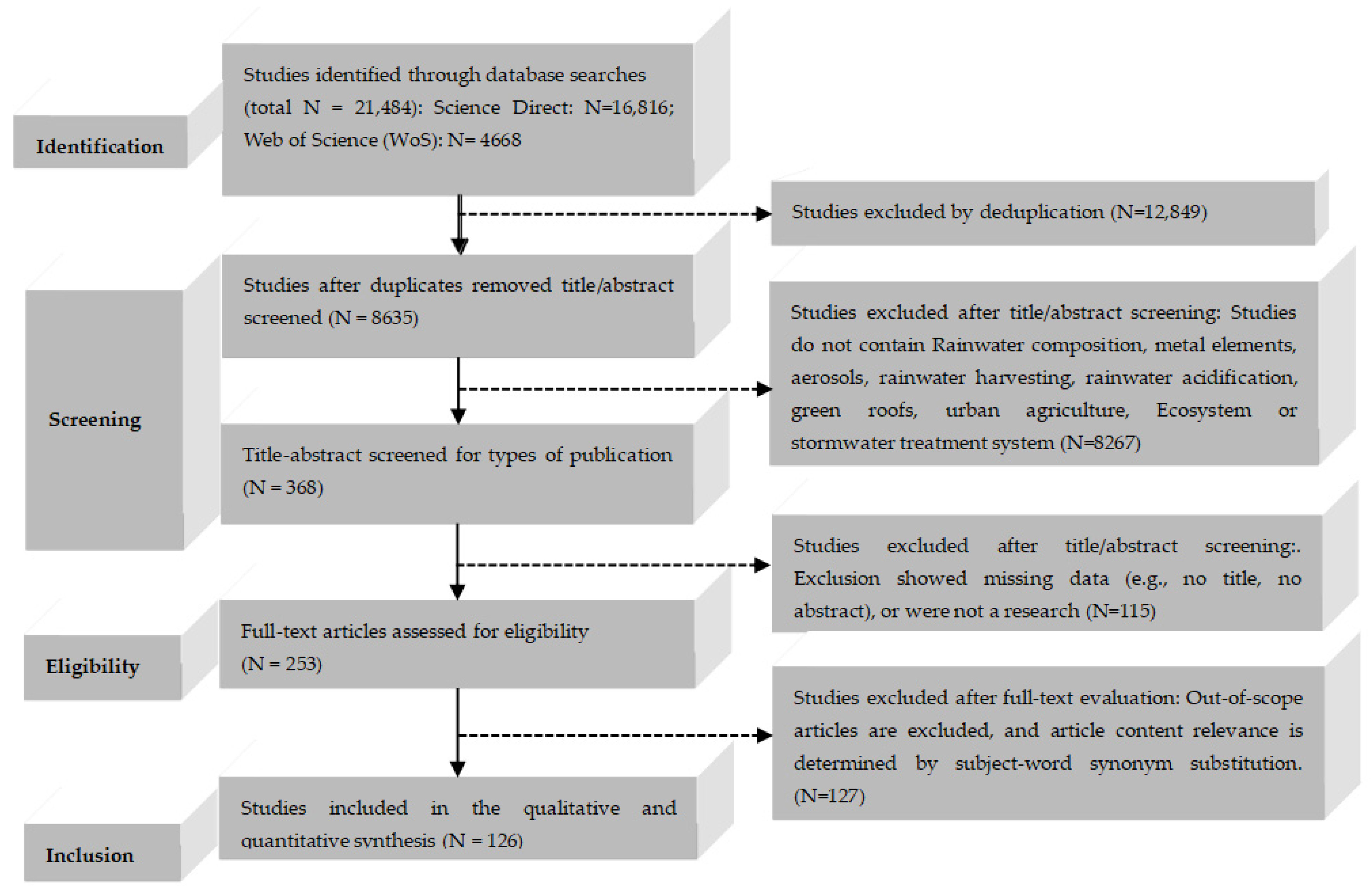
| Phase | Inclusion Criteria | Exclusion Criteria | Total N of Excluded Articles |
|---|---|---|---|
| Identification | Keywords: rainwater, chemical composition, transfer, removal | Duplicates | 12,849 |
| Screening: Title and keyword screening | No title; no abstract; unrelated topic or measures with no relevance to rainwater; missing data; book chapters; conference papers; editorials | 8267 | |
| Screening: Abstract screening | Abstract out of scope; no access, not found; no keywords; language (not English or Chinese) | 115 | |
| Eligibility | Thematic relevance: rainwater, China, chemical composition, organic matter, transfer, collection facility and synonyms | Content out of scope | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Guo, Z. The Source, Transport, and Removal of Chemical Elements in Rainwater in China. Sustainability 2022, 14, 12439. https://doi.org/10.3390/su141912439
Chen D, Guo Z. The Source, Transport, and Removal of Chemical Elements in Rainwater in China. Sustainability. 2022; 14(19):12439. https://doi.org/10.3390/su141912439
Chicago/Turabian StyleChen, Dandan, and Zhongsheng Guo. 2022. "The Source, Transport, and Removal of Chemical Elements in Rainwater in China" Sustainability 14, no. 19: 12439. https://doi.org/10.3390/su141912439
APA StyleChen, D., & Guo, Z. (2022). The Source, Transport, and Removal of Chemical Elements in Rainwater in China. Sustainability, 14(19), 12439. https://doi.org/10.3390/su141912439






