Prediction of Potential Habitats of Zanthoxylum armatum DC. and Their Changes under Climate Change
Abstract
1. Introduction
2. Materials and Methods
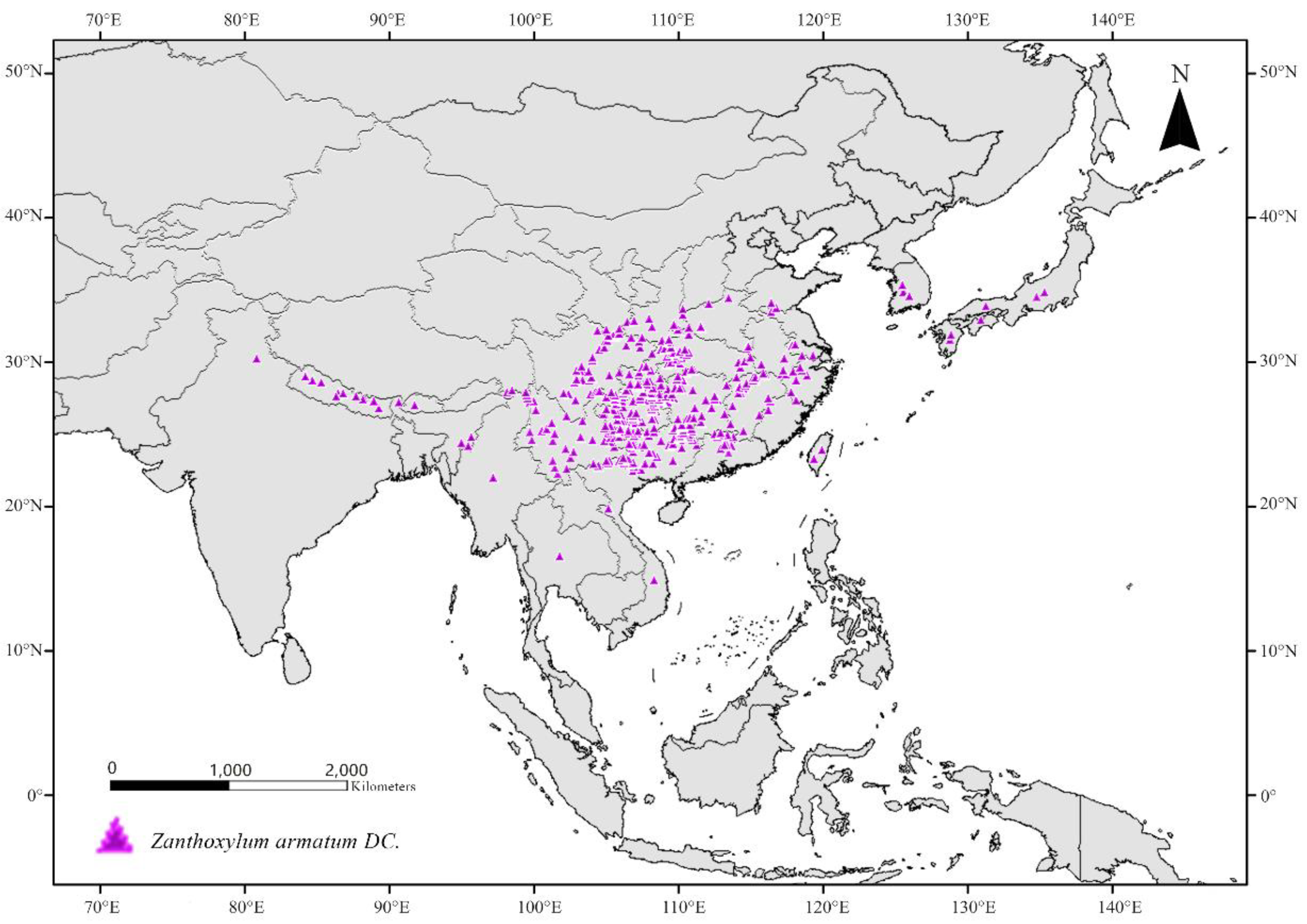
2.1. Species Distribution Point Data
2.2. Environmental Variables
2.3. Model Building
3. Results
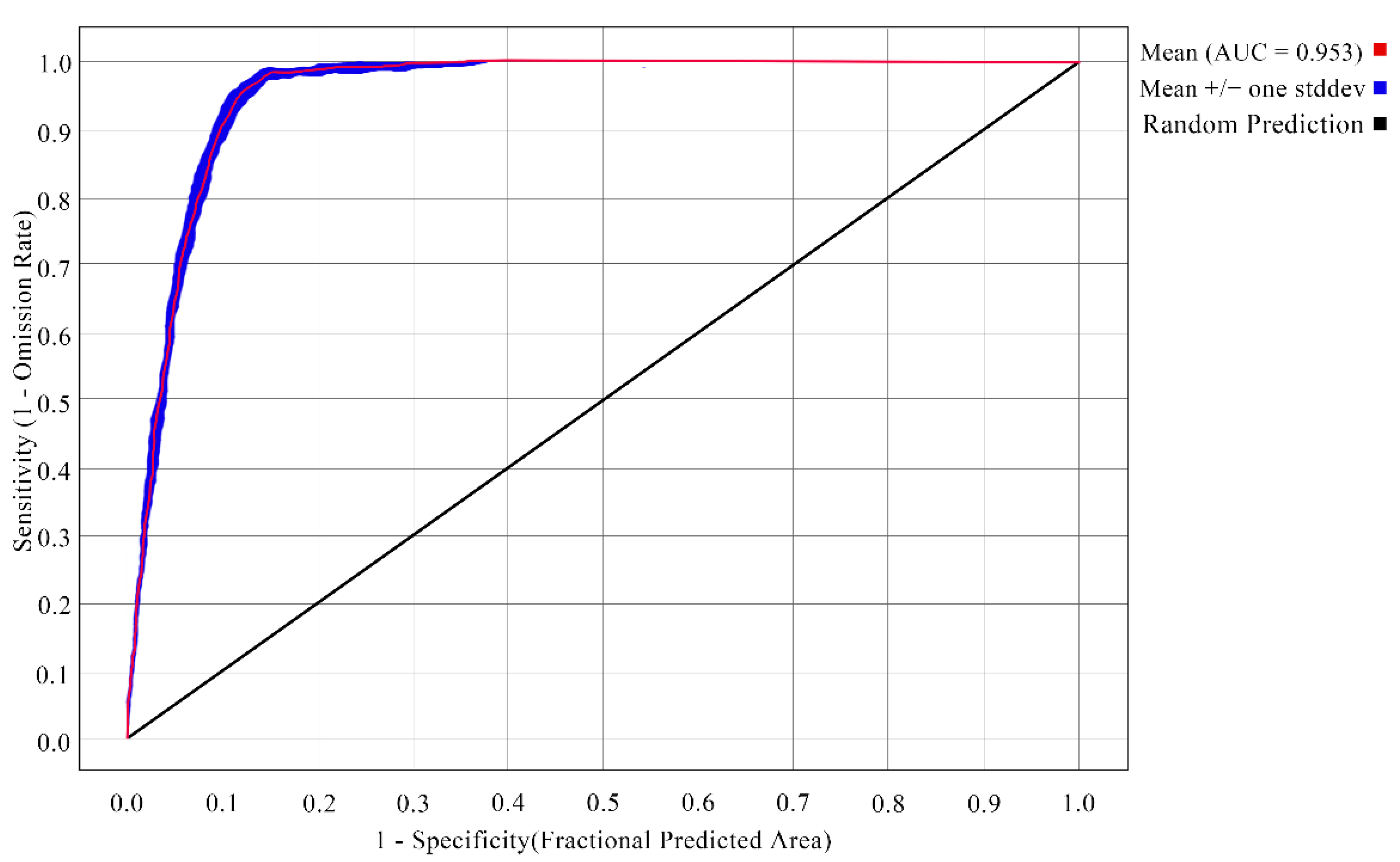
3.1. Evaluation of Model Prediction Accuracy
3.2. Critical Climate Factors Affecting the Geographical Distribution of Z. armatum
3.3. Distribution of Potential Suitable Areas of Z. armatum in 1970–2000
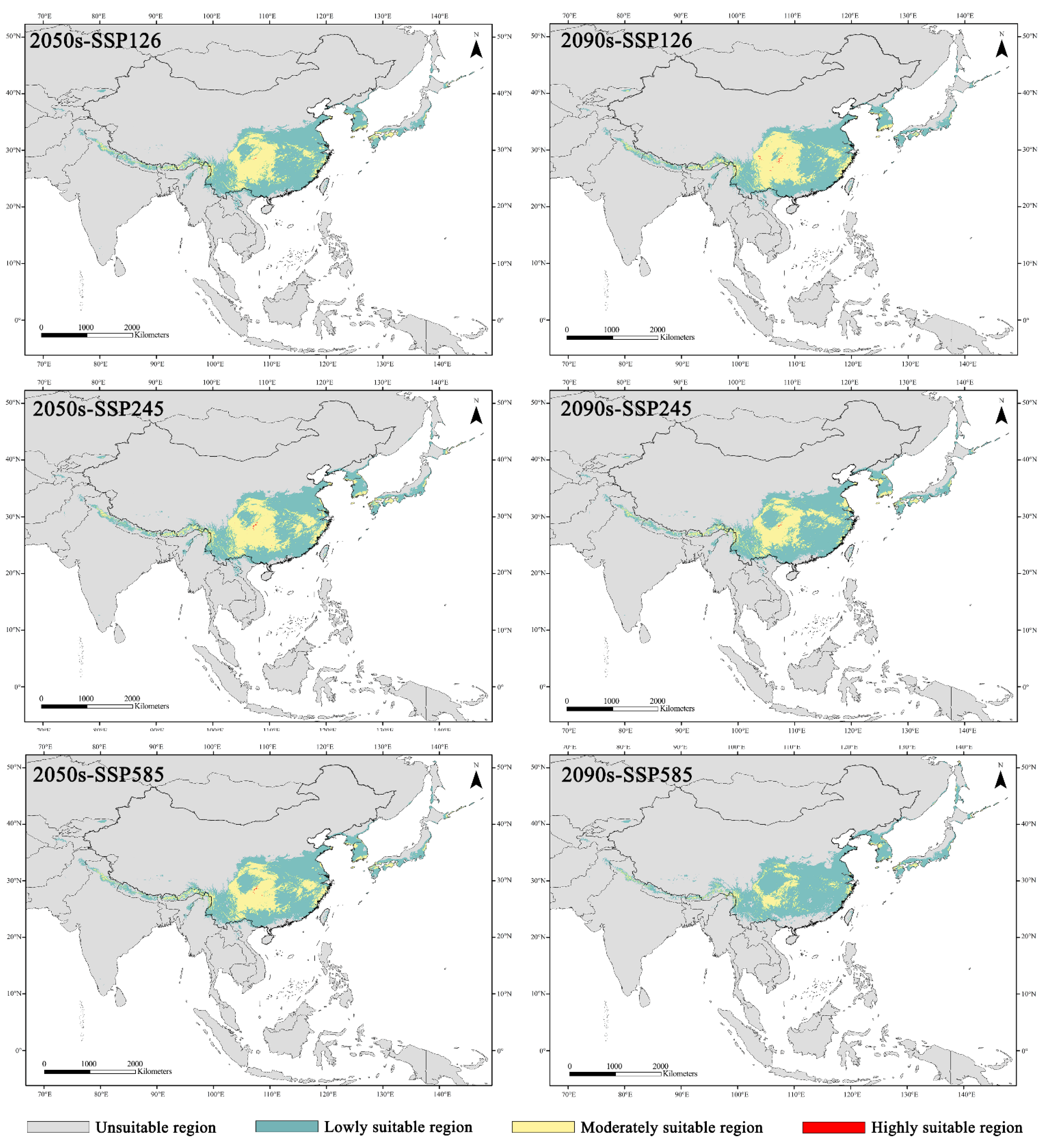
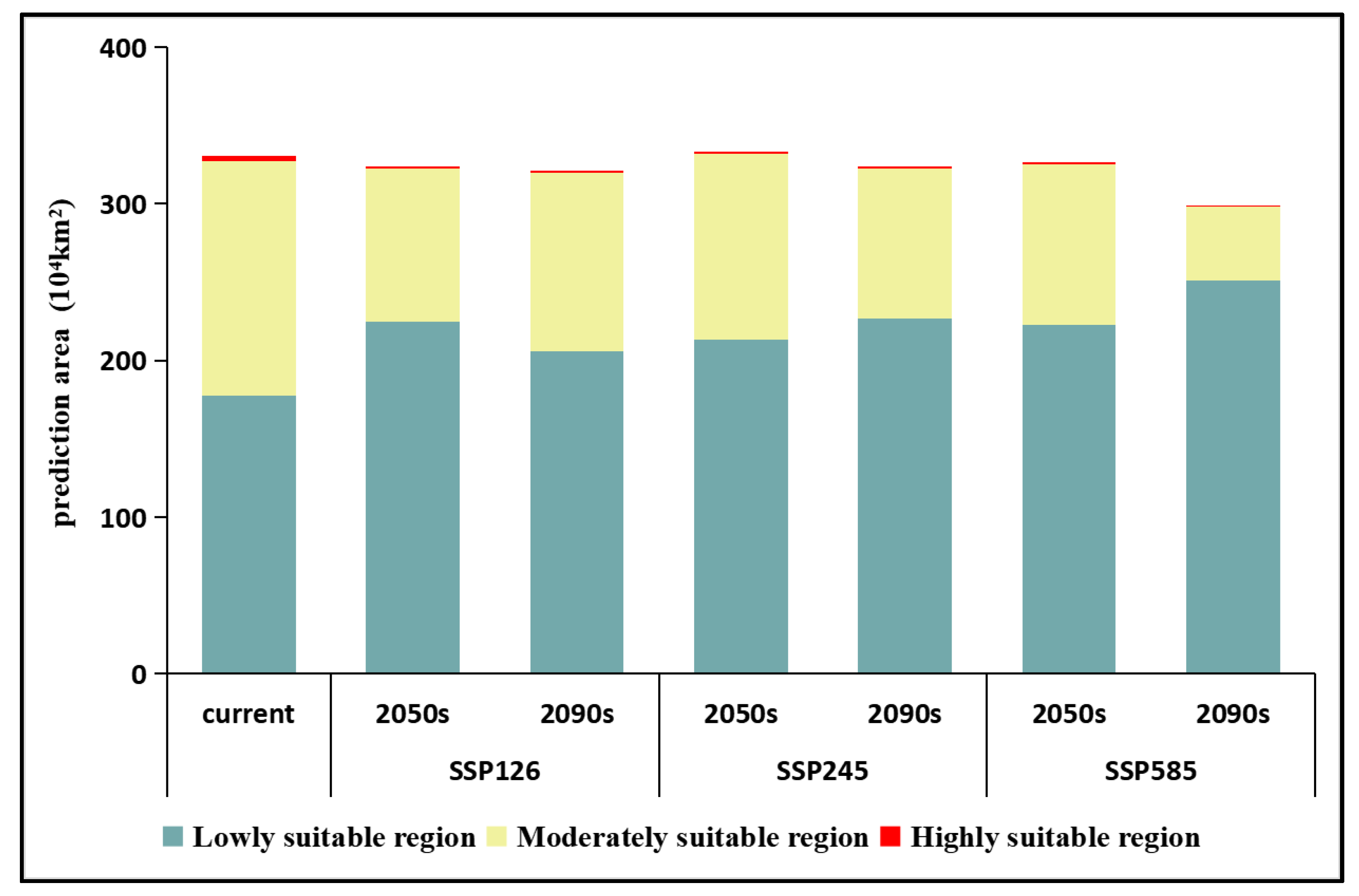
3.4. Potential Suitable Areas of Z. armatum under Future Climate Scenarios
4. Discussion
4.1. Important Climate Variables influencing Potential Distribution of Z. armatum
4.2. Future Change in the Potential Distribution of Z. armatum
4.3. Z. armatum Protective Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Rojas, M.; Samset, B.H.; Cobb, A.; Diongue Niang, P.; Edwards, S.; Emori, S.H.; Faria, E.; Hawkins, P.; Hope, P.; et al. Framing, Context, and Methods. In Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 147–286. [Google Scholar]
- Adeel, Z. Ecosystems and Human Well-Being: Desertification Synthesis: A Report of the Millennium Ecosystem Assessment; World Resources Institute: Washington, DC, USA, 2005; ISBN 978-1-56973-590-9. [Google Scholar]
- He, Q. Biotic interactions and ecosystem dynamics under global change: From theory to application. Chin. J. Plant Ecol. 2021, 45, 1075–1093. [Google Scholar] [CrossRef]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Alo, C.A.; Wang, G. Potential future changes of the terrestrial ecosystem based on climate projections by eight general circulation models: Future ecosystem changes. J. Geophys. Res. 2008, 113, G01004. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity: Biodiversity and climate change. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Evaluating bioclimate envelope models. Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Qiao, H.J.; Lin, C.T.; Ji, L.Q.; Jiang, Z.G. mMWeb—An Online Platform for Employing Multiple Ecological Niche Modeling Algorithms. PLoS ONE 2012, 7, e43327. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Ahmed, S.E.; McInerny, G.; O’Hara, K.; Harper, R.; Salido, L.; Emmott, S.; Joppa, L.N. Scientists and software—Surveying the species distribution modelling community. Divers. Distrib. 2015, 21, 258–267. [Google Scholar] [CrossRef]
- Guo, K.Q.; Jiang, X.L.; Xu, G.B. Potential suitable distribution area of Quercus lamellosa and the influence of climate change. Chin. J. Ecol. 2021, 40, 2563–2574. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martinez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.P.; Lees, D.C. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.Q.; Zhang, W.H.; Ye, X.Z.; Zhang, G.F.; Liu, B.; Ruan, S.N. Prediction of Potential Distribution Area and Analysis of Dominant Environmental Variables of Davidia involucrate Based on Maxent. J. Sichuan Arg. Univ. 2021, 39, 604–612. [Google Scholar] [CrossRef]
- Tan, Y.H.; Zhang, X.J.; Yuan, S.S.; Yu, J.H. Prediction of the ecological suitability of Gentiana macrophylla Pall. under scenarios of global climate change. Chin. J. Ecol. 2020, 39, 3766–3773. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.Y.; Ma, X.W.; Peng, H.B.; Shan, J.R. Modeling the current land suitability and future dynamics of global soybean cultivation under climate change scenarios. Field Crops Res. 2021, 263, 108069. [Google Scholar] [CrossRef]
- Brijwal, L.; Pandey, A.; Tamta, S. An overview on phytomedicinal approaches of Zanthoxylum armatum DC.: An important magical medicinal plant. J. Med. Plants Res. 2013, 7, 366–370. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1997; Volume 43, pp. 43–44. ISBN 978-7-03-005486-9. [Google Scholar]
- Patade, V.Y.; Kumar, K.; Gupta, A.K.; Grover, A.; Negi, P.S.; Dwivedi, S.K. Improvement in Seed Germination Through Pre-treatments in Timur (Zanthoxylum armatum DC.): A Plant with High Medicinal, Economical and Ecological Importance. Natl. Acad. Sci. Lett. 2020, 43, 295–297. [Google Scholar] [CrossRef]
- Ma, Y.; Fei, X.T.; Li, J.M.; Liu, Y.L.; Wei, A.Z. Effects of location, climate, soil conditions and plant species on levels of potentially toxic elements in Chinese Prickly Ash pericarps from the main cultivation regions in China. Chemosphere 2020, 244, 125501. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.X.; Wei, A.Z.; Feng, S.J.; Liu, Y.H. Research Progress on Chinese Prickly Ash. China Condiment 2016, 41, 149–156. [Google Scholar] [CrossRef]
- Su, J. Investigation and Analysis Report of Chinese Prickly Ash Industry. Nongjiakeji 2021, 1, 21–25. [Google Scholar]
- Phuyal, N.; Jha, P.K.; Prasad Raturi, P.; Rajbhandary, S. Zanthoxylum armatum DC.: Current knowledge, gaps and opportunities in Nepal. J. Ethnopharmacol. 2019, 229, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.S.; Butola, J.S.; Sharma, A. Assessment of diversity, distribution, conservation status and preparation of management plan for medicinal plants in the catchment area of parbati hydroelectric project stage—III in Northwestern Himalaya. J. Mt. Sci. 2007, 4, 034–056. [Google Scholar] [CrossRef]
- Feng, S.J.; Liu, Z.S.; Hu, Y.; Tian, J.Y.; Yang, T.X.; Wei, A.Z. Genomic analysis reveals the genetic diversity, population structure, evolutionary History and relationships of Chinese pepper. Hortic. Res. 2020, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, T.; Liu, Z.; Chen, L.; Hou, N.; Wang, Y.; Wei, A. Genetic Diversity and Relationships of Wild and Cultivated Zanthoxylum Germplasms Based on Sequence-Related Amplified Polymorphism (SRAP) Markers. Genet. Resour. Crop Evol. 2015, 62, 1193–1204. [Google Scholar] [CrossRef]
- Nooreen, Z.; Tandon, S.; Yadav, N.P.; Kumar, P.; Xuan, T.D.; Ahmad, A. Zanthoxylum: A Review of its Traditional Uses, Naturally Occurring Constituents and Pharmacological Properties. Curr. Org. Chem. 2019, 23, 1307–1341. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum Bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. IJMS 2017, 18, 2172. [Google Scholar] [CrossRef]
- Zhou, T.J.; Zou, L.W.; Chen, X.L. Commentary on the Coupled Model Intercomparison Project Phase 6 (CMIP6). Clim. Chang. Res. 2019, 15, 445–456. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Klein Goldewijk, K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365–372. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhuo, Z.H.; Wang, R.L.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e00691. [Google Scholar] [CrossRef]
- Liu, K.; Nie, G.G.; Zhang, S. Study on the Spatiotemporal Evolution of Temperature and Precipitation in China from 1951 to 2018. Adv. Earth. Sci. 2020, 35, 1113–1126. [Google Scholar] [CrossRef]
- Hu, M.; Ye, M.; Guo, Y. The Flower Bud Morphological Differentiation and Bud Structure of Zanthoxylum armatum DC. J. Nucl. Agr. Sci. 2019, 33, 1423–1431. [Google Scholar]
- Ou, W.F.; Wei, A.Z.; Yang, T.X.; Li, L.X.; Feng, S.J.; Hou, N. Observation and Simulation of the Growth Rules of One-year-old Zanthorylum bungeanum Seedlings from Three Provenances. J. Northwest For. Univ. 2016, 31, 121–125. [Google Scholar] [CrossRef]
- Zhao, C. Study on Photosynthetic Characteristics and Low Temperature Stress Physiology of Zanthoxylum armatum; Sichuan Agricultural University: Chengdu, China, 2018. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, M. Research Status on the Taxonomic and Component of Green Zanthoxylum bungeanum Maxim. North. Hortic. 2010, 14, 199–203. [Google Scholar]
- Ma, L.; Yan, H.W.; He, Y.; Zhang, Q.; Liu, B. Vegetation changes in south Himalayas areas based on remote sensing monitoring during 2001–2015. Arid Land Geogr. 2017, 40, 405–414. [Google Scholar]
- Yang, Y.; Dai, X.G.; Wang, P. Projection of Asian Precipitation for the Coming 30 Years and Its Bias Correction. Chin. J. Atmos. Sci. 2022, 46, 40–54. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Li, Y.H.; Xu, Y. Aridity Change Characteristics over Northern Region of China under RCPs Scenario. Plateau Meteorol. 2016, 35, 94–106. [Google Scholar] [CrossRef]
- Wu, J.G.; Lv, J.J. Potential effect of climate change on the distribution and range of arid regions. Res. Environ. Sci. 2009, 2, 199–206. [Google Scholar] [CrossRef]
- Wang, Y.H.; Pei, S.J.; Xu, J.C. Sustainable management of medicinal plant resourcesin China: Literature review and implications. Resour. Sci. 2002, 24, 81–88. [Google Scholar]
- Meilleur, B.A.; Hodgkin, T. In situ conservation of crop wild relatives: Status and trends. Biodivers. Conserv. 2004, 13, 663–684. [Google Scholar] [CrossRef]
- Ebert, A.W.; Engels, J.M.M. Plant Biodiversity and Genetic Resources Matter! Plants 2020, 9, 1706. [Google Scholar] [CrossRef]
- Zhang, X.W.; Jiang, Y.M.; Bi, Y.; Liu, X.L.; Li, X.; Sun, T.; Chen, H.Y.; Li, J. Identification of potential distribution area for Hippophae rhamnoides subsp. sinensis by the MaxEnt model. Acta. Ecologica. Sin. 2022, 42, 1420–1428. [Google Scholar]

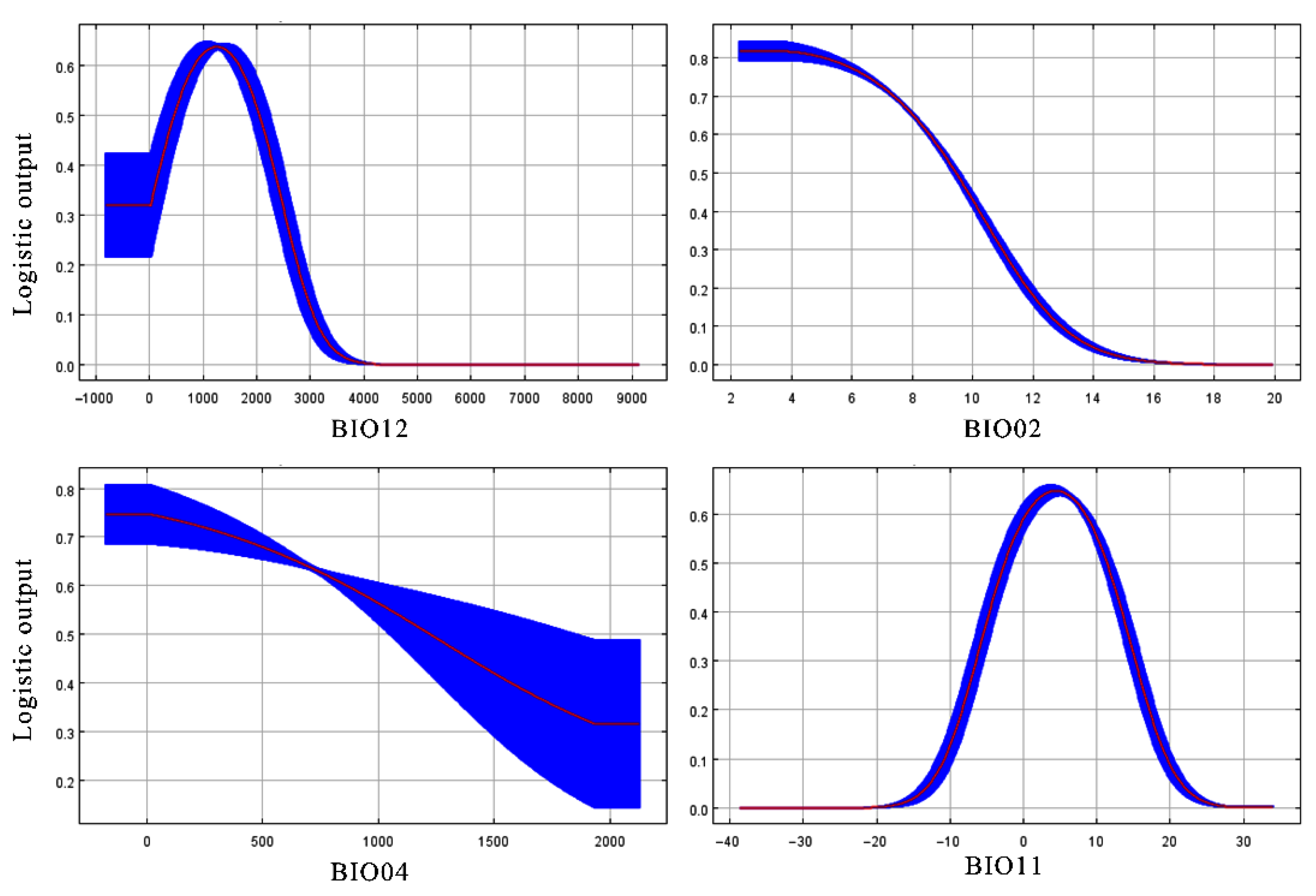

| Climate Factor | Description | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| BIO02 | Mean diurnal temperature range (Mean of monthly (max temp-min temp)) (°C × 10) | 43.9 | 21.5 |
| BIO11 | Mean temperature of coldest quarter (°C × 10) | 38.6 | 34.9 |
| BIO04 | Temperature seasonality (standard deviation × 100) | 12.5 | 5.4 |
| BIO14 | Precipitation of driest month (mm) | 2.2 | 7.1 |
| BIO12 | Annual precipitation (mm) | 1.4 | 19 |
| BIO08 | Mean temperature of wettest quarter (°C × 10) | 0.6 | 2.6 |
| BIO15 | Precipitation seasonality (coefficient of variation) (mm) | 0.4 | 7.9 |
| BIO18 | Precipitation of warmest quarter (mm) | 0.3 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, P.; Liu, Y.; Sui, M.; Ou, J. Prediction of Potential Habitats of Zanthoxylum armatum DC. and Their Changes under Climate Change. Sustainability 2022, 14, 12422. https://doi.org/10.3390/su141912422
Tian P, Liu Y, Sui M, Ou J. Prediction of Potential Habitats of Zanthoxylum armatum DC. and Their Changes under Climate Change. Sustainability. 2022; 14(19):12422. https://doi.org/10.3390/su141912422
Chicago/Turabian StyleTian, Pingping, Yifu Liu, Mingzhen Sui, and Jing Ou. 2022. "Prediction of Potential Habitats of Zanthoxylum armatum DC. and Their Changes under Climate Change" Sustainability 14, no. 19: 12422. https://doi.org/10.3390/su141912422
APA StyleTian, P., Liu, Y., Sui, M., & Ou, J. (2022). Prediction of Potential Habitats of Zanthoxylum armatum DC. and Their Changes under Climate Change. Sustainability, 14(19), 12422. https://doi.org/10.3390/su141912422





