Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review
Abstract
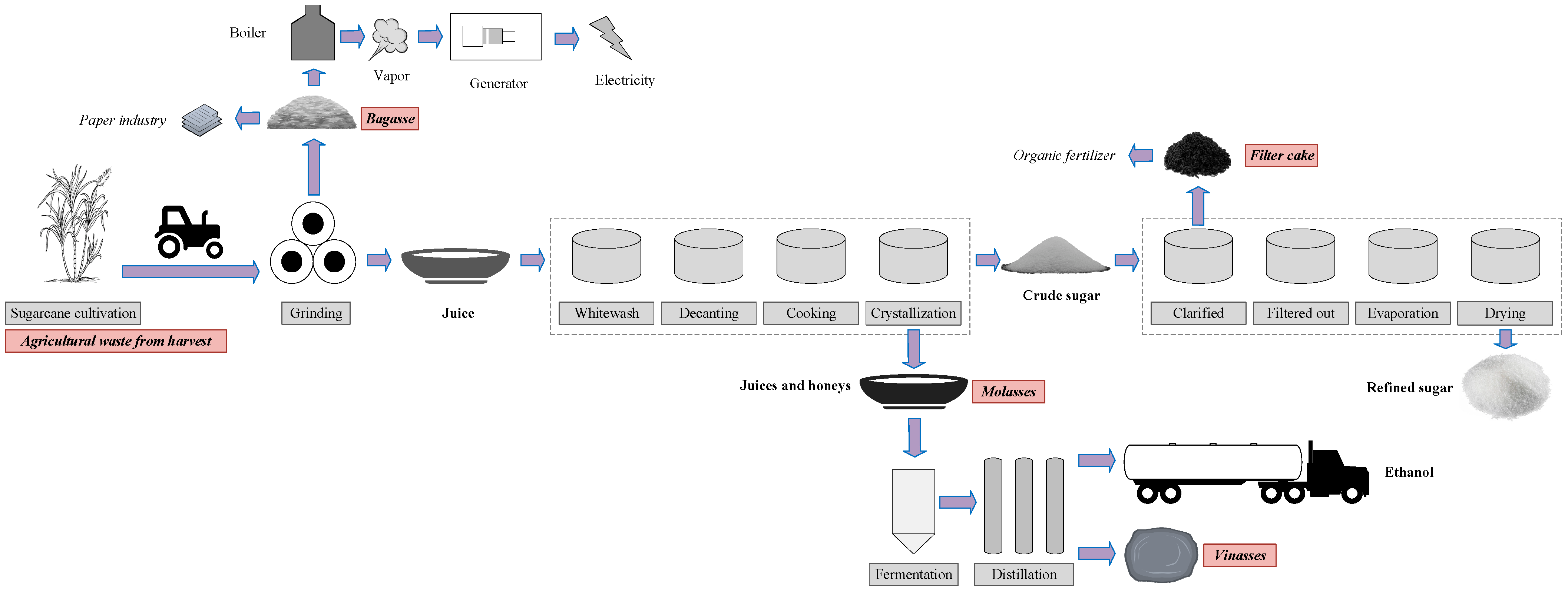
1. Introduction
2. Materials and Methods
3. Results
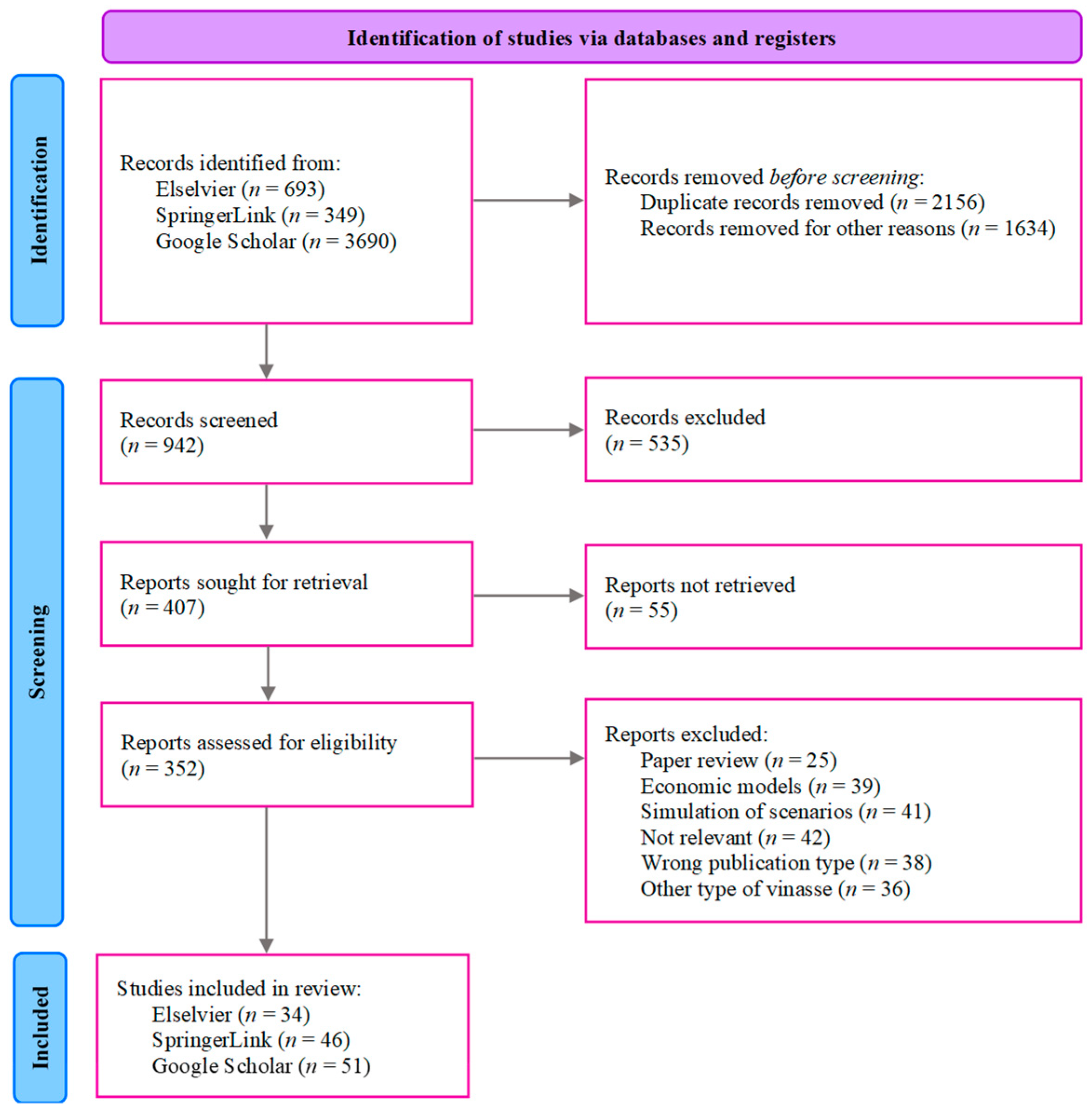
3.1. Search
3.2. Eligibility
3.3. Selection of Studies
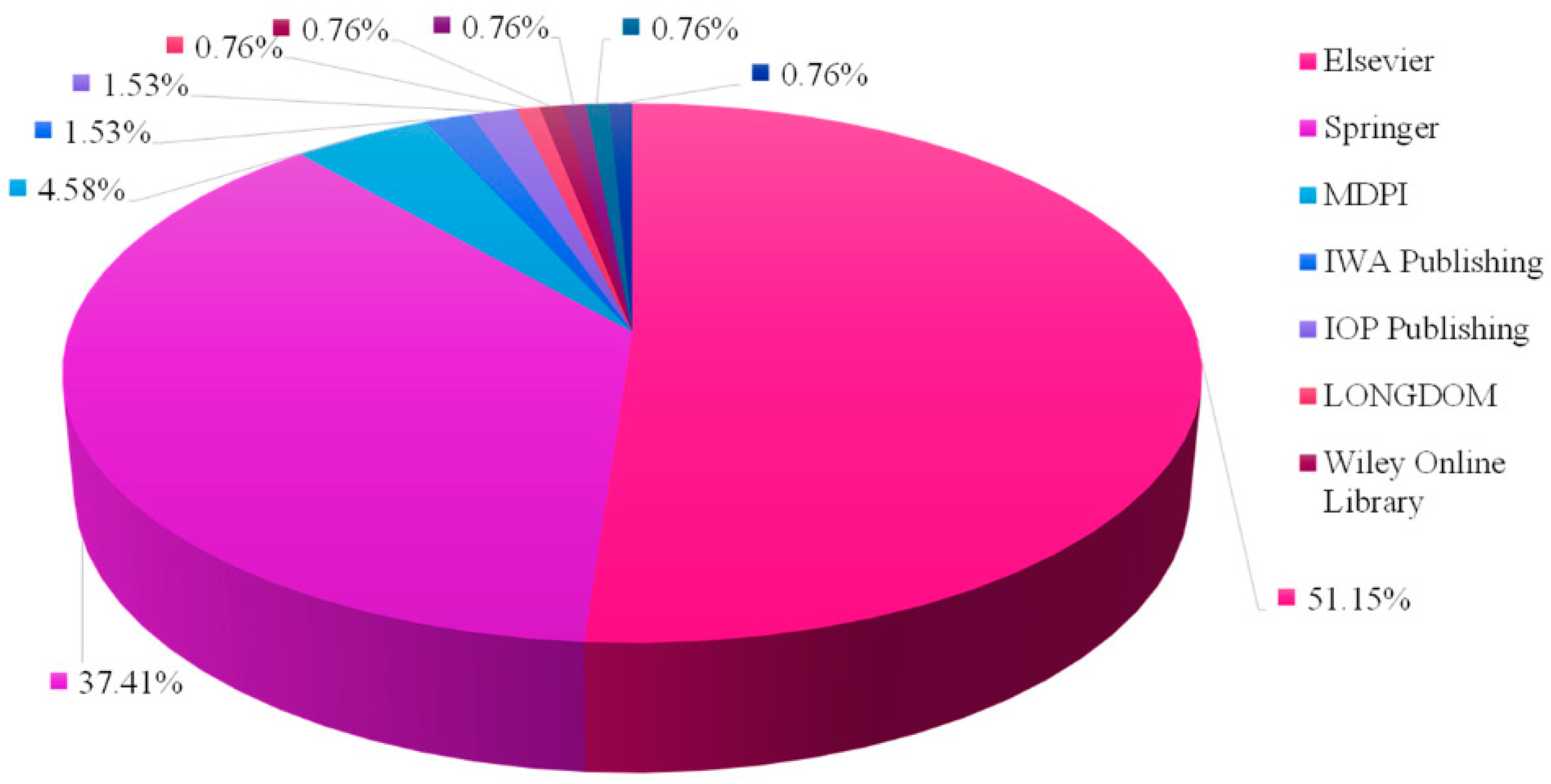
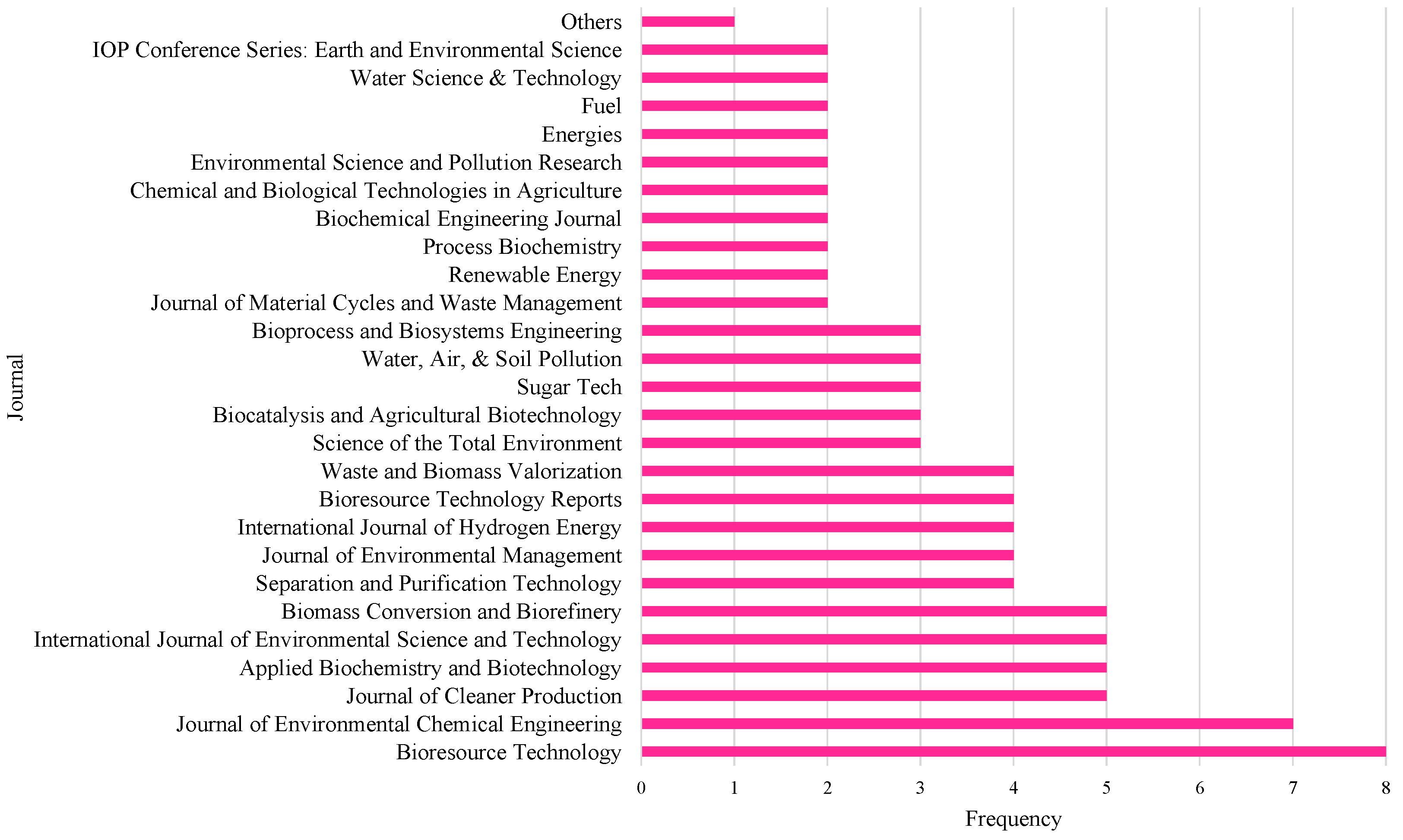
3.4. Study Characteristics
3.4.1. Geographical Distribution of Studies
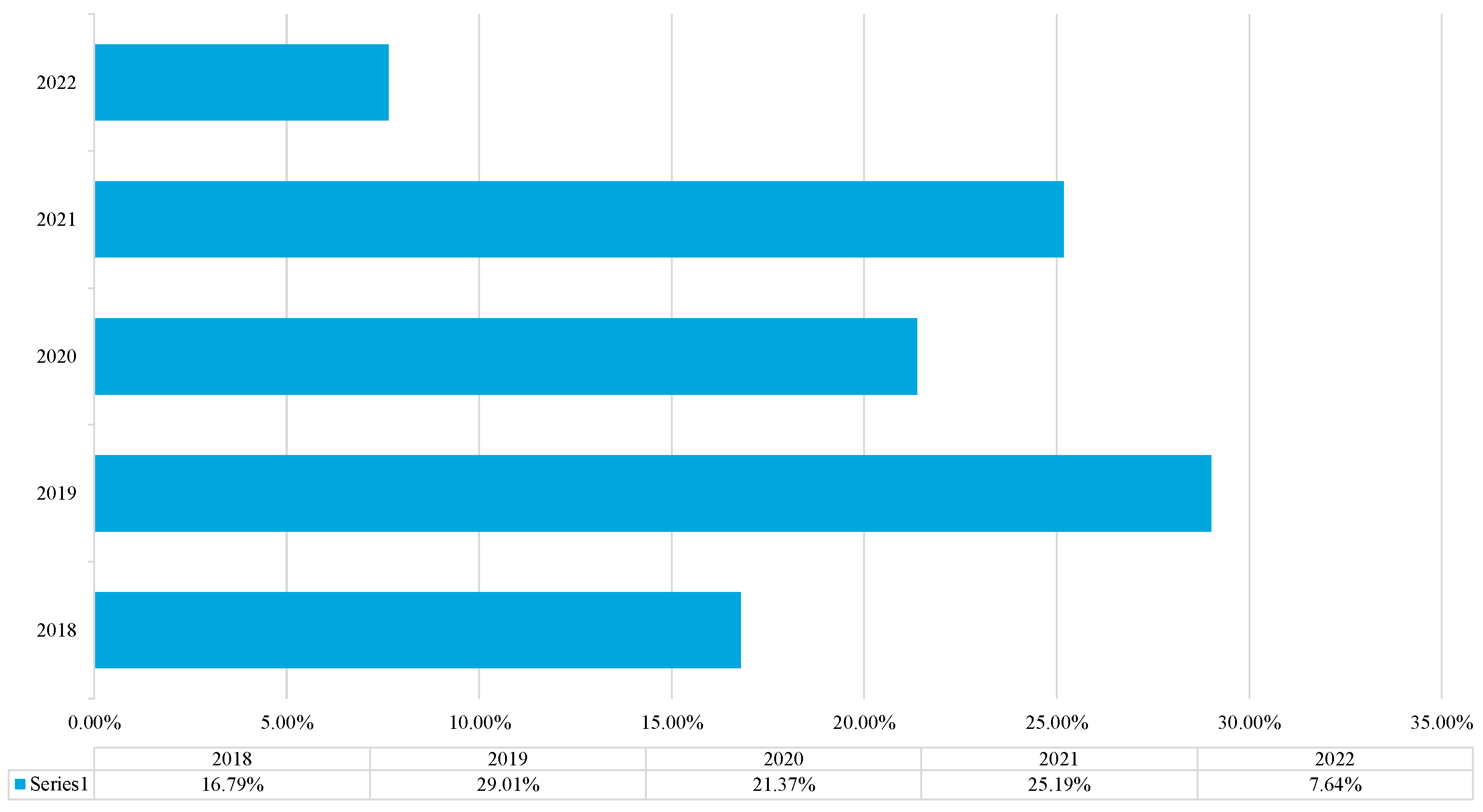
3.4.2. Distribution of Studies over the Search Period
3.4.3. Scope of Studies
3.4.4. Technological Processes Used in the Treatment of Vinasse
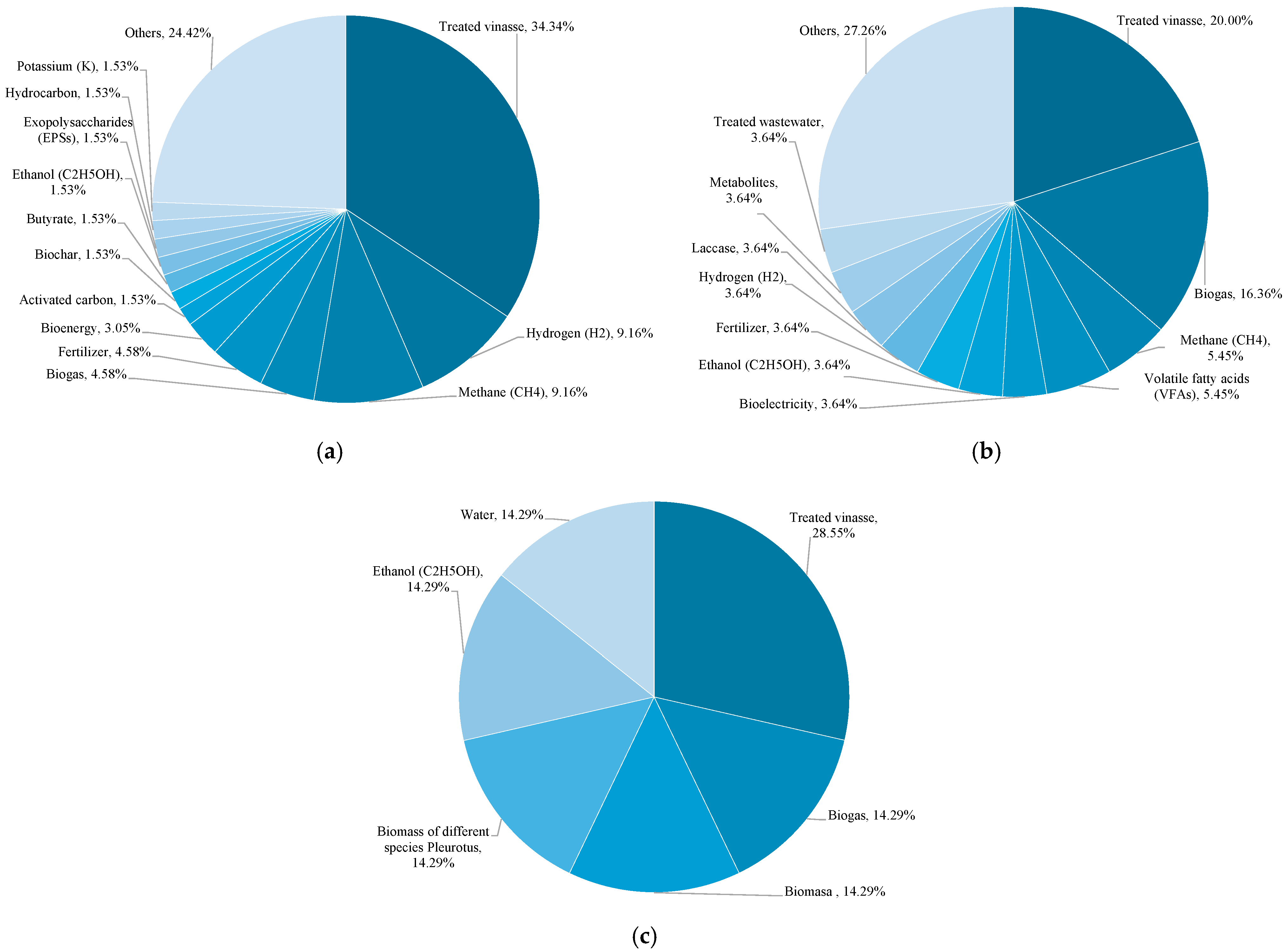
3.4.5. Recovered Bioproducts
3.4.6. Industrial Sectors
3.4.7. Applications of Vinasse in the Agricultural Sector
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- INEGI. Agriculture. Available online: https://www.inegi.org.mx/temas/agricultura/#Informacion_general (accessed on 11 May 2022).
- FAO. FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 11 May 2022).
- SADER. CONADESUCA 8th Statistical Report of the Agro-Industrial Sector of Sugar Cane in Mexico, Harvests 2011–2012/2020–2021. Available online: https://siiba.conadesuca.gob.mx/infocana/informe/informe.html (accessed on 13 May 2022).
- Moran-Salazar, R.G.; Sanchez-Lizarraga, A.L.; Rodriguez-Campos, J.; Davila-Vazquez, G.; Marino-Marmolejo, E.N.; Dendooven, L.; Contreras-Ramos, S.M. Utilization of Vinasses as Soil Amendment: Consequences and Perspectives. Springerplus 2016, 5, 1007. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Rodríguez, E.F.; Fukushima, N.A.; Palacios-Bereche, R.; Ensinas, A.V.; Nebra, S.A. Vinasse Concentration and Juice Evaporation System Integrated to the Conventional Ethanol Production Process from Sugarcane—Heat Integration and Impacts in Cogeneration System. Renew. Energy 2018, 115, 474–488. [Google Scholar] [CrossRef]
- Rabelo, S.C.; da Costa, A.C.; Vaz Rossel, C.E. Industrial Waste Recovery. In Sugarcane: Agricultural Production, Bioenergy and Ethanol; Academic Press: Cambridge, MA, USA, 2015; pp. 365–381. [Google Scholar] [CrossRef]
- da Silva, J.J.; da Silva, B.F.; Zanoni, M.V.B.; Stradiotto, N.R. Sample Preparation and Antibiotic Quantification in Vinasse Generated from Sugarcane Ethanol Fuel Production. J. Chromatogr. A 2022, 1666, 462833. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.C.; Trichez, D.; Sallet, L.P.; de Paula e Silva, F.C.; Almeida, J.R.M. Technological Advancements in 1G Ethanol Production and Recovery of By-Products Based on the Biorefinery Concept. In Advances in Sugarcane Biorefinery: Technologies, Commercialization, Policy Issues and Paradigm Shift for Bioethanol and By-Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 73–95. [Google Scholar] [CrossRef]
- Hakika, D.C.; Sarto, S.; Mindaryani, A.; Hidayat, M. Decreasing COD in Sugarcane Vinasse Using the Fenton Reaction: The Effect of Processing Parameters. Catalysts 2019, 9, 881. [Google Scholar] [CrossRef]
- Nakashima, R.N.; de Oliveira Junior, S. Comparative Exergy Assessment of Vinasse Disposal Alternatives: Concentration, Anaerobic Digestion and Fertirrigation. Renew. Energy 2020, 147, 1969–1978. [Google Scholar] [CrossRef]
- Silva, L.C.M.; Moreira, R.A.; Pinto, T.J.S.; Vanderlei, M.R.; Athayde, D.B.; Lopes, L.F.P.; Ogura, A.P.; Yoshii, M.P.C.; Freitas, J.S.; Montagner, C.C.; et al. Lethal and Sublethal Toxicity of Pesticides and Vinasse Used in Sugarcane Cultivation to Ceriodaphnia Silvestrii (Crustacea: Cladocera). Aquat. Toxicol. 2021, 241, 106017. [Google Scholar] [CrossRef]
- de Jesus, G.C.; Gaspar Bastos, R.; Altenhofen da Silva, M. Production and Characterization of Alginate Beads for Growth of Immobilized Desmodesmus Subspicatus and Its Potential to Remove Potassium, Carbon and Nitrogen from Sugarcane Vinasse. Biocatal. Agric. Biotechnol. 2019, 22, 101438. [Google Scholar] [CrossRef]
- Tamashiro, J.R.; Kinoshita, A.; Pereira Silva, L.H.; Friol Guedes de Paiva, F.; Antunes, P.A.; Simões, R.D. Compressive Resistance of Concrete Produced with Recycled Concrete Aggregate and Sugarcane Vinasse Waste-Water. Clean. Eng. Technol. 2022, 6, 100362. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; Lourenço, V.A.; Filho, P.B.; dos Santos, G.B.; Przybyla, G. National Potential Production of Methane and Electrical Energy from Sugarcane Vinasse in Brazil: A Thermo-Economic Analysis. J. Environ. Chem. Eng. 2020, 8, 103422. [Google Scholar] [CrossRef]
- Marafon, A.C.; Salomon, K.R.; Amorim, E.L.C.; Peiter, F.S. Use of Sugarcane Vinasse to Biogas, Bioenergy, and Biofertilizer Production. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 179–194. [Google Scholar] [CrossRef]
- Coelho, M.P.M.; Correia, J.E.; Vasques, L.I.; de Castro Marcato, A.C.; de Andrade Guedes, T.; Soto, M.A.; Basso, J.B.; Kiang, C.; Fontanetti, C.S. Toxicity Evaluation of Leached of Sugarcane Vinasse: Histopathology and Immunostaining of Cellular Stress Protein. Ecotoxicol. Environ. Saf. 2018, 165, 367–375. [Google Scholar] [CrossRef]
- Velásquez-Riaño, M.; Carvajal-Arias, C.E.; Rojas-Prieto, N.L.; Ausecha-García, S.A.; Vera-Díaz, M.Á.; Meneses-Sánchez, J.S.; Villa-Restrepo, A.F. Evaluation of a Mixed Simultaneous Vinasse Degradation Treatment Using Komagataeibacter Kakiaceti GM5 and Trametes Versicolor DSM 3086. Ecotoxicol. Environ. Saf. 2018, 164, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, M.; Kiani Deh Kiani, M.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Melchor, G.I.; Salgado-García, S.; Palma, D.; Lagunes-Espinoza, L.; Castelán-Estrada, M.; Ruiz Rosado, O. Vinasse and Filter Cake Compost as Nutrient Source for Sugarcane in a Molic Gleysol of Chiapas, México. Interciencia 2008, 33, 855–860. [Google Scholar]
- FIRA. FIRA Boletín Informativo: Producción Sostenible de Caña de Azúcar en México; 2010. Available online: www.fira.gob.mx (accessed on 22 May 2022).
- Pati, D.; Lorusso, L.N. How to Write a Systematic Review of the Literature. HERD Health Environ. Res. Des. J. 2017, 11, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Montiel, A.; Montalvo, N.; Fernández, G.; García, L.E.; Bautista, H.; Sandoval, L.C. Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review; OSF: Misantla, Mexico, 2022. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Morton, S.; Berg, A.; Levit, L.; Eden, J. Finding What Works in Health Care: Standards for Systematic Reviews; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Elsevier. Available online: https://www.elsevier.com/es-mx (accessed on 18 March 2022).
- Springer. Nature Springer. Available online: https://link.springer.com/ (accessed on 23 March 2022).
- Google. Google Scholar. Available online: https://scholar.google.com/ (accessed on 28 March 2022).
- Queiroga, J.A.; Souza, D.F.; Nunes, E.H.M.; Silva, A.F.R.; Amaral, M.C.S.; Ciminelli, V.S.T.; Vasconcelos, W.L. Preparation of Alumina Tubular Membranes for Treating Sugarcane Vinasse Obtained in Ethanol Production. Sep. Purif. Technol. 2018, 190, 195–201. [Google Scholar] [CrossRef]
- Martínez, E.A.; dos Santos, J.F.; Araujo, G.S.; de Souza, S.M.A.; de Cássia Lacerda Brambilla Rodrigues, R.; Canettieri, E.V. Production of Single Cell Protein (SCP) from Vinasse. In Fungal Biorefineries; Kumar, S., Dheeran, P., Taherzadeh, M., Khanal, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 215–238. ISBN 978-3-319-90379-8. [Google Scholar]
- da Silva, D.R.; Crespi, M.S.; Ribeiro, C.A.; Capela, J.M.V. Thermal Decomposition Kinetics of Sugarcane Mills Wastes. J. Therm. Anal. Calorim. 2018, 131, 811–822. [Google Scholar] [CrossRef]
- da Silva, I.A.; de Lima, S.T.; Siqueira, M.R.; da Veiga, M.A.M.S.; Reginatto, V. Landfill Leachate Enhances Fermentative Hydrogen Production from Glucose and Sugarcane Processing Derivatives. J. Mater. Cycles Waste Manag. 2018, 20, 777–786. [Google Scholar] [CrossRef]
- del Gobbo, L.M.; Colin, V.L. Fungal Technology Applied to Distillery Effluent Treatment. In Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Prasad, R., Aranda, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 185–197. ISBN 978-3-030-02369-0. [Google Scholar]
- Chuppa-Tostain, G.; Hoarau, J.; Watson, M.; Adelard, L.; Shum Cheong Sing, A.; Caro, Y.; Grondin, I.; Bourven, I.; Francois, J.-M.; Girbal-Neuhauser, E.; et al. Production of Aspergillus Niger Biomass on Sugarcane Distillery Wastewater: Physiological Aspects and Potential for Biodiesel Production. Fungal Biol. Biotechnol. 2018, 5, 1. [Google Scholar] [CrossRef]
- Saglam, N.; Yesilada, O.; Saglam, S.; Apohan, E.; Sam, M.; Ilk, S.; Emul, E.; Gurel, E. Bioremediation Applications with Fungi. In Mycoremediation and Environmental Sustainability: Volume 2; Prasad, R., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–37. ISBN 978-3-319-77386-5. [Google Scholar]
- Fuess, L.T.; Garcia, M.L.; Zaiat, M. Seasonal Characterization of Sugarcane Vinasse: Assessing Environmental Impacts from Fertirrigation and the Bioenergy Recovery Potential through Biodigestion. Sci. Total Environ. 2018, 634, 29–40. [Google Scholar] [CrossRef]
- Chingono, K.E.; Sanganyado, E.; Bere, E.; Yalala, B. Adsorption of Sugarcane Vinasse Effluent on Bagasse Fly Ash: A Parametric and Kinetic Study. J. Environ. Manag. 2018, 224, 182–190. [Google Scholar] [CrossRef]
- del Nery, V.; Alves, I.; Zamariolli Damianovic, M.H.R.; Pires, E.C. Hydraulic and Organic Rates Applied to Pilot Scale UASB Reactor for Sugar Cane Vinasse Degradation and Biogas Generation. Biomass Bioenergy 2018, 119, 411–417. [Google Scholar] [CrossRef]
- Ahmed, P.M.; Pajot, H.F.; de Figueroa, L.I.C.; Gusils, C.H. Sustainable Bioremediation of Sugarcane Vinasse Using Autochthonous Macrofungi. J. Environ. Chem. Eng. 2018, 6, 5177–5185. [Google Scholar] [CrossRef]
- Fuess, L.T.; Klein, B.C.; Chagas, M.F.; Alves Ferreira Rezende, M.C.; Garcia, M.L.; Bonomi, A.; Zaiat, M. Diversifying the Technological Strategies for Recovering Bioenergy from the Two-Phase Anaerobic Digestion of Sugarcane Vinasse: An Integrated Techno-Economic and Environmental Approach. Renew. Energy 2018, 122, 674–687. [Google Scholar] [CrossRef]
- Vaquerizo, F.R.; Cruz-Salomon, A.; Valdovinos, E.R.; Pola- Albores, F.; Lagunas-Rivera, S.; Meza- Gordillo, R.; Ruiz Valdiviezo, V.M.; Simuta Champo, R.; Moreira- Acosta, J. Anaerobic Treatment of Vinasse from Sugarcane Ethanol Production in Expanded Granular Sludge Bed Bioreactor. J. Chem. Eng. Process Technol. 2018, 9, 375. [Google Scholar] [CrossRef]
- López, I.; Borzacconi, L.; Passeggi, M. Anaerobic Treatment of Sugar Cane Vinasse: Treatability and Real-Scale Operation. J. Chem. Technol. Biotechnol. 2018, 93, 1320–1327. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; de Alencar Neves, T.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Start-up Phase of a Two-Stage Anaerobic Co-Digestion Process: Hydrogen and Methane Production from Food Waste and Vinasse from Ethanol Industry. Biofuel Res. J. 2018, 5, 813–820. [Google Scholar] [CrossRef]
- Pinto, M.P.M.; Mudhoo, A.; de Alencar Neves, T.; Berni, M.D.; Forster-Carneiro, T. Co–Digestion of Coffee Residues and Sugarcane Vinasse for Biohythane Generation. J. Environ. Chem. Eng. 2018, 6, 146–155. [Google Scholar] [CrossRef]
- Fuess, L.T.; Ferraz, A.D.N.; Machado, C.B.; Zaiat, M. Temporal Dynamics and Metabolic Correlation between Lactate-Producing and Hydrogen-Producing Bacteria in Sugarcane Vinasse Dark Fermentation: The Key Role of Lactate. Bioresour. Technol. 2018, 247, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.R.; Silva, E.L. Continuous Hydrogen Production from Cofermentation of Sugarcane Vinasse and Cheese Whey in a Thermophilic Anaerobic Fluidized Bed Reactor. Int. J. Hydrog. Energy 2018, 43, 13081–13089. [Google Scholar] [CrossRef]
- Vilar, D.S.; Carvalho, G.O.; Pupo, M.M.S.; Aguiar, M.M.; Torres, N.H.; Américo, J.H.P.; Cavalcanti, E.B.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Leite, M.S.; et al. Vinasse Degradation Using Pleurotus Sajor-Caju in a Combined Biological—Electrochemical Oxidation Treatment. Sep. Purif. Technol. 2018, 192, 287–296. [Google Scholar] [CrossRef]
- Kazak, O.; Eker, Y.R.; Bingol, H.; Tor, A. Preparation of Chemically-Activated High Surface Area Carbon from Waste Vinasse and Its Efficiency as Adsorbent Material. J. Mol. Liq. 2018, 272, 189–197. [Google Scholar] [CrossRef]
- Sydney, E.B.; Novak, A.C.; Rosa, D.; Pedroni Medeiros, A.B.; Brar, S.K.; Larroche, C.; Soccol, C.R. Screening and Bioprospecting of Anaerobic Consortia for Biohydrogen and Volatile Fatty Acid Production in a Vinasse Based Medium through Dark Fermentation. Process Biochem. 2018, 67, 1–7. [Google Scholar] [CrossRef]
- Santana Junior, A.E.; Duda, R.M.; de Oliveira, R.A. Improving the Energy Balance of Ethanol Industry with Methane Production from Vinasse and Molasses in Two-Stage Anaerobic Reactors. J. Clean. Prod. 2019, 238, 117577. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Lelis, J.; Alves-Ferreira, J.; Carvalho, F. Treatment of Vinasse from Sugarcane Ethanol Industry: H2SO4, NaOH and Ca(OH)2 Precipitations, FeCl3 Coagulation-Flocculation and Atmospheric CO2 Carbonation. J. Environ. Chem. Eng. 2019, 7, 103203. [Google Scholar] [CrossRef]
- Campanhol, B.S.; Silveira, G.C.; Castro, M.C.; Ceccato-Antonini, S.R.; Bastos, R.G. Effect of the Nutrient Solution in the Microbial Production of Citric Acid from Sugarcane Bagasse and Vinasse. Biocatal. Agric. Biotechnol. 2019, 19, 101147. [Google Scholar] [CrossRef]
- Santos, P.S.; Zaiat, M.; Oller do Nascimento, C.A.; Fuess, L.T. Does Sugarcane Vinasse Composition Variability Affect the Bioenergy Yield in Anaerobic Systems? A Dual Kinetic-Energetic Assessment. J. Clean. Prod. 2019, 240, 118005. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Canettieri, E.V.; Souza, S.M.A.; Rodrigues, R.C.L.B.; Martínez, E.A. Treatment of Sugarcane Vinasse from Cachaça Production for the Obtainment of Candida Utilis CCT 3469 Biomass. Biochem. Eng. J. 2019, 148, 131–137. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; de Aragão, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) Production by Cupriavidus Necator from Sugarcane Vinasse and Molasses as Mixed Substrate. Process Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Sousa, S.P.; Lovato, G.; Albanez, R.; Ratusznei, S.M.; Rodrigues, J.A.D. Improvement of Sugarcane Stillage (Vinasse) Anaerobic Digestion with Cheese Whey as Its Co-Substrate: Achieving High Methane Productivity and Yield. Appl. Biochem. Biotechnol. 2019, 189, 987–1006. [Google Scholar] [CrossRef]
- Silva, G.A.; Ferreira, S.L.; de Souza, G.R.; da Silva, J.A.; Pagliuso, J.D. Utilization of a New Approach for the Potassium Concentration of Sugarcane Vinasse by Reverse Osmosis: Case Study. Int. J. Environ. Sci. Technol. 2019, 16, 6441–6446. [Google Scholar] [CrossRef]
- Vaz, F.L.; de Souza, R.d.F.R.; Dutra, E.D.; Alencar, B.R.A.; Vidal, E.E. Valorization of Sugar-Ethanol Industry Waste Vinasse for Increased Second-Generation Ethanol Production Using Spathaspora Passalidarum Yeast Strains. Sugar Tech 2019, 21, 312–319. [Google Scholar] [CrossRef]
- Gallucci, A.D.; Natera, M.; Moreira, L.A.; Nardi, K.T.; Altarugio, L.M.; de Mira, A.B.; de Almeida, R.F.; Otto, R. Nitrogen-Enriched Vinasse as a Means of Supplying Nitrogen to Sugarcane Fields: Testing the Effectiveness of N Source and Application Rate. Sugar Tech 2019, 21, 20–28. [Google Scholar] [CrossRef]
- Lovato, G.; Batista, L.P.P.; Preite, M.B.; Yamashiro, J.N.; Becker, A.L.S.; Vidal, M.F.G.; Pezini, N.; Albanez, R.; Ratusznei, S.M.; Rodrigues, J.A.D. Viability of Using Glycerin as a Co-Substrate in Anaerobic Digestion of Sugarcane Stillage (Vinasse): Effect of Diversified Operational Strategies. Appl. Biochem. Biotechnol. 2019, 188, 720–740. [Google Scholar] [CrossRef]
- Morais, D.V.; Bastos, R.G. Phycocyanin Production by Aphanothece Microscopica Nägeli in Synthetic Medium Supplemented with Sugarcane Vinasse. Appl. Biochem. Biotechnol. 2019, 187, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ventorino, V.; Nicolaus, B.; di Donato, P.; Pagliano, G.; Poli, A.; Robertiello, A.; Iavarone, V.; Pepe, O. Bioprospecting of Exopolysaccharide-Producing Bacteria from Different Natural Ecosystems for Biopolymer Synthesis from Vinasse. Chem. Biol. Technol. Agric. 2019, 6, 18. [Google Scholar] [CrossRef]
- Lovato, G.; Albanez, R.; Triveloni, M.; Ratusznei, S.M.; Rodrigues, J.A.D. Methane Production by Co-Digesting Vinasse and Whey in an AnSBBR: Effect of Mixture Ratio and Feed Strategy. Appl. Biochem. Biotechnol. 2019, 187, 28–46. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Simões, M.F.; Santos, J.G.; Peixoto, L.; Martins, C.R.; Silva, B.P.; Neto, A.O.; Brito, A.G.; Maiorano, A.E. Application of Microbial Fuel Cell Technology for Vinasse Treatment and Bioelectricity Generation. Biotechnol. Lett. 2019, 41, 107–114. [Google Scholar] [CrossRef]
- dos Reis, K.C.; Coimbra, J.M.; Duarte, W.F.; Schwan, R.F.; Silva, C.F. Biological Treatment of Vinasse with Yeast and Simultaneous Production of Single-Cell Protein for Feed Supplementation. Int. J. Environ. Sci. Technol. 2019, 16, 763–774. [Google Scholar] [CrossRef]
- de Oliveira, G.H.D.; Niz, M.Y.K.; Zaiat, M.; Rodrigues, J.A.D. Effects of the Organic Loading Rate on Polyhydroxyalkanoate Production from Sugarcane Stillage by Mixed Microbial Cultures. Appl. Biochem. Biotechnol. 2019, 189, 1039–1055. [Google Scholar] [CrossRef]
- Santos, D.R.; Cunha, O.d.M.; Bisinoti, M.C.; Ferreira, O.P.; Moreira, A.B.; Melo, C.A. Hydrochars Produced with By-Products from the Sucroenergetic Industry: A Study of Extractor Solutions on Nutrient and Organic Carbon Release. Environ. Sci. Pollut. Res. 2019, 26, 9137–9145. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.K.F.; de Castro, H.F. Vinasse Treatment within the Sugarcane-Ethanol Industry Using Ozone Combined with Anaerobic and Aerobic Microbial Processes. Environments 2019, 6, 5. [Google Scholar] [CrossRef]
- Montalvo, G.E.B.; Thomaz-Soccol, V.; Vandenberghe, L.P.S.; Carvalho, J.C.; Faulds, C.B.; Bertrand, E.; Prado, M.R.M.; Bonatto, S.J.R.; Soccol, C.R. Arthrospira Maxima OF15 Biomass Cultivation at Laboratory and Pilot Scale from Sugarcane Vinasse for Potential Biological New Peptides Production. Bioresour. Technol. 2019, 273, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Niz, M.Y.K.; Etchelet, I.; Fuentes, L.; Etchebehere, C.; Zaiat, M. Extreme Thermophilic Condition: An Alternative for Long-Term Biohydrogen Production from Sugarcane Vinasse. Int. J. Hydrog. Energy 2019, 44, 22876–22887. [Google Scholar] [CrossRef]
- de Souza Moraes, B.; Mary dos Santos, G.; Palladino Delforno, T.; Tadeu Fuess, L.; José da Silva, A. Enriched Microbial Consortia for Dark Fermentation of Sugarcane Vinasse towards Value-Added Short-Chain Organic Acids and Alcohol Production. J. Biosci. Bioeng. 2019, 127, 594–601. [Google Scholar] [CrossRef]
- Fuess, L.T.; Zaiat, M.; do Nascimento, C.A.O. Novel Insights on the Versatility of Biohydrogen Production from Sugarcane Vinasse via Thermophilic Dark Fermentation: Impacts of PH-Driven Operating Strategies on Acidogenesis Metabolite Profiles. Bioresour. Technol. 2019, 286, 121379. [Google Scholar] [CrossRef]
- Rodrigues Reis, C.E.; Furtado Carvalho, A.K.; Bento, H.B.S.; de Castro, H.F. Integration of Microbial Biodiesel and Bioethanol Industries through Utilization of Vinasse as Substrate for Oleaginous Fungi. Bioresour. Technol. Rep. 2019, 6, 46–53. [Google Scholar] [CrossRef]
- del Gobbo, L.M.; Villegas, L.B.; Colin, V.L. The Potential Application of an Autochthonous Fungus from the Northwest of Argentina for Treatment of Sugarcane Vinasse. J. Hazard. Mater. 2019, 365, 820–826. [Google Scholar] [CrossRef]
- Dirbeba, M.J.; Aho, A.; DeMartini, N.; Brink, A.; Mattsson, I.; Hupa, L.; Hupa, M. Fast Pyrolysis of Dried Sugar Cane Vinasse at 400 and 500 °C: Product Distribution and Yield. Energy Fuels 2019, 33, 1236–1247. [Google Scholar] [CrossRef]
- de Castro Marcato, A.C.; de Souza, C.P.; de Paiva, A.B.; Eismann, C.E.; Navarro, F.F.; Camargo, A.F.M.; Menegário, A.A.; Fontanetti, C.S. Hybrid Treatment System for Remediation of Sugarcane Vinasse. Sci. Total Environ. 2019, 659, 115–121. [Google Scholar] [CrossRef]
- Devia-Orjuela, J.S.; Alvarez-Pugliese, C.E.; Donneys-Victoria, D.; Marriaga Cabrales, N.; Barba Ho, L.E.; Brém, B.; Sauciuc, A.; Gál, E.; Espin, D.; Schichtel, M.; et al. Evaluation of Press Mud, Vinasse Powder and Extraction Sludge with Ethanol in a Pyrolysis Process. Energies 2019, 12, 4145. [Google Scholar] [CrossRef]
- Bettani, S.R.; de Oliveira Ragazzo, G.; Leal Santos, N.; Kieckbusch, T.G.; Gaspar Bastos, R.; Soares, M.R.; Altenhofen da Silva, M. Sugarcane Vinasse and Microalgal Biomass in the Production of Pectin Particles as an Alternative Soil Fertilizer. Carbohydr. Polym. 2019, 203, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella Vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- Barros, L.B.M.; Andrade, L.H.; Drewes, J.E.; Amaral, M.C.S. Investigation of Electrodialysis Configurations for Vinasse Desalting and Potassium Recovery. Sep. Purif. Technol. 2019, 229, 115797. [Google Scholar] [CrossRef]
- Karchiyappan, T.; Delcolle, R.D.; Goncalves, G.L.; Vareschini, D.T.; Gimenes, M.L. Treatment of Vinasse Liquid from Sugarcane Industry Using Electro-Coagulation/Flocculation Followed by Ultra Filtration. Pol. J. Chem. Technol. 2019, 21, 40–47. [Google Scholar] [CrossRef]
- Albuquerque, J.N.; Ratusznei, S.M.; Rodrigues, J.A.D. Biomethane Production by Thermophilic Co-Digestion of Sugarcane Vinasse and Whey in an AnSBBR: Effects of Composition, Organic Load, Feed Strategy and Temperature. J. Environ. Manag. 2019, 251, 109606. [Google Scholar] [CrossRef]
- Crisafulli, R.; de Lino Amorim, F.M.; de Oliveira Marcionilio, S.M.L.; Mendes Cunha, W.; Brenda, B.R.; Dias, J.A.; Linares, J.J. Electrochemistry for Biofuels Waste Valorization: Vinasse as a Reducing Agent for Pt/C and Its Application to the Electrolysis of Glycerin and Vinasse. Electrochem. Commun. 2019, 102, 25–30. [Google Scholar] [CrossRef]
- le Phuong, T.; Besson, M. Catalytic Wet Air Oxidation Using Supported Pt and Ru Catalysts for Treatment of Distillery Wastewater (Cognac and Sugarcane Vinasses). Energies 2019, 12, 3974. [Google Scholar] [CrossRef]
- Adarme, O.F.H.; Baêta, B.E.L.; Filho, J.B.G.; Gurgel, L.V.A.; de Aquino, S.F. Use of Anaerobic Co-Digestion as an Alternative to Add Value to Sugarcane Biorefinery Wastes. Bioresour. Technol. 2019, 287, 121443. [Google Scholar] [CrossRef]
- Madaleno, L.L.; de Barros, V.G.; Kesserling, M.A.; Teixeira, J.R.; Duda, R.M.; de Oliveira, R.A. The Recycling of Biodigested Vinasse in an Upflow Anaerobic Sludge Blanket Reactor Is a Feasible Approach for the Conservation of Freshwater in the Biofuel Ethanol Industry. J. Clean. Prod. 2020, 262, 121196. [Google Scholar] [CrossRef]
- Magalhães, N.C.; Silva, A.F.R.; Cunha, P.V.M.; Drewes, J.E.; Amaral, M.C.S. Role of Nanofiltration or Reverse Osmosis Integrated to Ultrafiltration-Anaerobic Membrane Bioreactor Treating Vinasse for the Conservation of Water and Nutrients in the Ethanol Industry. J. Water Process Eng. 2020, 36, 101338. [Google Scholar] [CrossRef]
- Santos, N.L.; de Oliveira Ragazzo, G.; Cerri, B.C.; Soares, M.R.; Kieckbusch, T.G.; da Silva, M.A. Physicochemical Properties of Konjac Glucomannan/Alginate Films Enriched with Sugarcane Vinasse Intended for Mulching Applications. Int. J. Biol. Macromol. 2020, 165, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Romanholo Ferreira, L.F.; Torres, N.H.; de Armas, R.D.; Fernandes, C.D.; Vilar, D.d.S.; Aguiar, M.M.; Pompeu, G.B.; Monteiro, R.T.R.; Iqbal, H.M.N.; Bilal, M.; et al. Fungal Lignin-Modifying Enzymes Induced by Vinasse Mycodegradation and Its Relationship with Oxidative Stress. Biocatal. Agric. Biotechnol. 2020, 27, 101691. [Google Scholar] [CrossRef]
- Junior, J.A.; Vieira, Y.A.; Cruz, I.A.; da Silva Vilar, D.; Aguiar, M.M.; Torres, N.H.; Bharagava, R.N.; Lima, Á.S.; de Souza, R.L.; Romanholo Ferreira, L.F. Sequential Degradation of Raw Vinasse by a Laccase Enzyme Producing Fungus Pleurotus Sajor-Caju and Its ATPS Purification. Biotechnol. Rep. 2020, 25, e00411. [Google Scholar] [CrossRef] [PubMed]
- Eder, A.S.; Magrini, F.E.; Spengler, A.; da Silva, J.T.; Beal, L.L.; Paesi, S. Comparison of Hydrogen and Volatile Fatty Acid Production by Bacillus Cereus, Enterococcus Faecalis and Enterobacter Aerogenes Singly, in Co-Cultures or in the Bioaugmentation of Microbial Consortium from Sugarcane Vinasse. Environ. Technol. Innov. 2020, 18, 100638. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Valle, G.F.; Bento, H.B.S.; Carvalho, A.K.F.; Alves, T.M.; de Castro, H.F. Sugarcane By-Products within the Biodiesel Production Chain: Vinasse and Molasses as Feedstock for Oleaginous Fungi and Conversion to Ethyl Esters. Fuel 2020, 277, 118064. [Google Scholar] [CrossRef]
- Cerri, B.C.; Borelli, L.M.; Stelutti, I.M.; Soares, M.R.; da Silva, M.A. Evaluation of New Environmental Friendly Particulate Soil Fertilizers Based on Agroindustry Wastes Biopolymers and Sugarcane Vinasse. Waste Manag. 2020, 108, 144–153. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Magalhães, N.C.; Cunha, P.V.M.; Amaral, M.C.S.; Koch, K. Influence of COD/SO42− Ratio on Vinasse Treatment Performance by Two-Stage Anaerobic Membrane Bioreactor. J. Environ. Manag. 2020, 259, 110034. [Google Scholar] [CrossRef]
- Fuess, L.T.; dos Santos, G.M.; Delforno, T.P.; de Souza Moraes, B.; da Silva, A.J. Biochemical Butyrate Production via Dark Fermentation as an Energetically Efficient Alternative Management Approach for Vinasse in Sugarcane Biorefineries. Renew. Energy 2020, 158, 3–12. [Google Scholar] [CrossRef]
- Laranja, M.J.; da Silva, R.C.J.; Bisinoti, M.C.; Moreira, A.B.; Ferreira, O.P.; Melo, C.A. Semivolatile Organic Compounds in the Products from Hydrothermal Carbonisation of Sugar Cane Bagasse and Vinasse by Gas Chromatography-Mass Spectrometry. Bioresour. Technol. Rep. 2020, 12, 100594. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Furtado, T.P.B.; da Silva, S.C.; Lange, L.C.; Amaral, M.C.S. Vinasse Treatment Using Hybrid Tannin-Based Coagulation-Microfiltration-Nanofiltration Processes: Potential Energy Recovery, Technical and Economic Feasibility Assessment. Sep. Purif. Technol. 2020, 248, 117152. [Google Scholar] [CrossRef]
- Barros, L.B.M.; Brasil, Y.L.; Silva, A.F.R.; Andrade, L.H.; Amaral, M.C.S. Potassium Recovery from Vinasse by Integrated Electrodialysis—Precipitation Process: Effect of the Electrolyte Solutions. J. Environ. Chem. Eng. 2020, 8, 104238. [Google Scholar] [CrossRef]
- Sica, P.; Carvalho, R.; Das, K.C.; Baptista, A.S. Biogas and Biofertilizer from Vinasse: Making Sugarcane Ethanol Even More Sustainable. J. Mater. Cycles Waste Manag. 2020, 22, 1427–1433. [Google Scholar] [CrossRef]
- Rego, G.C.; Ferreira, T.B.; Ramos, L.R.; de Menezes, C.A.; Soares, L.A.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Bioconversion of Pretreated Sugarcane Vinasse into Hydrogen: New Perspectives to Solve One of the Greatest Issues of the Sugarcane Biorefinery. Biomass Convers. Biorefinery 2020, 1–15. [Google Scholar] [CrossRef]
- Sacchi, G.D.; de Souza Leite, L.; Reali, M.A.P.; Bichara, A.; Seleghim, M.H.R. Coagulation and Microfiltration Application for Sugarcane Vinasse Clarification. Water Air Soil Pollut. 2020, 231, 571. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Hartanto, D.; Rohman, H.A.; Mitamaytawati; Qudus, N.; Daniyanto. Valorization of Sugarcane-Based Bioethanol Industry Waste (Vinasse) to Organic Fertilizer. In Valorisation of Agro-industrial Residues—Volume II: Non-Biological Approaches; Zakaria, Z.A., Aguilar, C.N., Kusumaningtyas, R.D., Binod, P., Eds.; Springer: Cham, Switzerland, 2020; pp. 203–223. ISBN 978-3-030-39208-6. [Google Scholar]
- Iltchenco, J.; Almeida, L.G.; Beal, L.L.; Marconatto, L.; dos Anjos Borges, L.G.; Giongo, A.; Paesi, S. Microbial Consortia Composition on the Production of Methane from Sugarcane Vinasse. Biomass Convers. Biorefinery 2020, 10, 299–309. [Google Scholar] [CrossRef]
- Kamali, N.; Mehrabadi, A.R.; Mirabi, M.; Zahed, M.A. Synthesis of Vinasse-Dolomite Nanocomposite Biochar via a Novel Developed Functionalization Method to Recover Phosphate as a Potential Fertilizer Substitute. Front. Environ. Sci. Eng. 2020, 14, 70. [Google Scholar] [CrossRef]
- Contreras-Contreras, J.A.; Bernal-González, M.; Solís-Fuentes, J.A.; del Carmen Durán-Domínguez-de-Bazúa, M. Polyphenols from Sugarcane Vinasses, Quantification, and Removal Using Activated Carbon After Biochemical Treatment in Laboratory-Scale Thermophilic Upflow Anaerobic Sludge Blanket Reactors. Water Air Soil Pollut. 2020, 231, 401. [Google Scholar] [CrossRef]
- Behravan, H.R.; Voroney, P.; Khorassani, R.; Fotovat, A.; Moezei, A.A.; Taghavi, M. Chemical and Spectroscopic Characterization of Humic Acids Extracted from Filter Cake Using Different Basic Solutions. Sugar Tech 2020, 22, 311–318. [Google Scholar] [CrossRef]
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; de Almeida, P.Z.; Teixeira de Moraes Polizeli, M. Trametes Versicolor Laccase Production Using Agricultural Wastes: A Comparative Study in Erlenmeyer Flasks, Bioreactor and Tray. Bioprocess Biosyst. Eng. 2020, 43, 507–514. [Google Scholar] [CrossRef]
- Rulli, M.M.; Villegas, L.B.; Colin, V.L. Treatment of Sugarcane Vinasse Using an Autochthonous Fungus from the Northwest of Argentina and Its Potential Application in Fertigation Practices. J. Environ. Chem. Eng. 2020, 8, 104371. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Spessato, L.; Melo, S.A.R.; Bedin, K.C.; Zhang, T.; Asefa, T.; Silva, T.L.; Almeida, V.C. Sugarcane Vinasse-Derived Nanoporous N-S-Doped Carbon Material Decorated with Co: A New and Efficient Multifunctional Electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 9669–9682. [Google Scholar] [CrossRef]
- Nagarajan, S.; Ranade, V.V. Pre-Treatment of Distillery Spent Wash (Vinasse) with Vortex Based Cavitation and Its Influence on Biogas Generation. Bioresour. Technol. Rep. 2020, 11, 100480. [Google Scholar] [CrossRef]
- Aragão, M.S.; Menezes, D.B.; Ramos, L.C.; Oliveira, H.S.; Bharagava, R.N.; Romanholo Ferreira, L.F.; Teixeira, J.A.; Ruzene, D.S.; Silva, D.P. Mycoremediation of Vinasse by Surface Response Methodology and Preliminary Studies in Air-Lift Bioreactors. Chemosphere 2020, 244, 125432. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, E.; Godoy, R.F.B.; Radomski, F.A.D.; Crisigiovanni, E.L.; Branco, K.B.Z.F.; Arroyo, P.A. Chlorella Vulgaris Growth in Different Biodigested Vinasse Concentrations: Biomass, Pigments and Final Composition. Water Sci. Technol. 2020, 82, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Chuppa-Tostain, G.; Tan, M.; Adelard, L.; Shum-Cheong-Sing, A.; François, J.-M.; Caro, Y.; Petit, T. Evaluation of Filamentous Fungi and Yeasts for the Biodegradation of Sugarcane Distillery Wastewater. Microorganisms 2020, 8, 1588. [Google Scholar] [CrossRef]
- Soto, M.F.; Diaz, C.A.; Zapata, A.M.; Higuita, J.C. BOD and COD Removal in Vinasses from Sugarcane Alcoholic Distillation by Chlorella Vulgaris: Environmental Evaluation. Biochem. Eng. J. 2021, 176, 108191. [Google Scholar] [CrossRef]
- Borges, A.d.V.; Fuess, L.T.; Alves, I.; Takeda, P.Y.; Damianovic, M.H.R.Z. Co-Digesting Sugarcane Vinasse and Distilled Glycerol to Enhance Bioenergy Generation in Biofuel-Producing Plants. Energy Convers. Manag. 2021, 250, 114897. [Google Scholar] [CrossRef]
- Eng Sánchez, F.; Tadeu Fuess, L.; Soares Cavalcante, G.; Ângela Talarico Adorno, M.; Zaiat, M. Value-Added Soluble Metabolite Production from Sugarcane Vinasse within the Carboxylate Platform: An Application of the Anaerobic Biorefinery beyond Biogas Production. Fuel 2021, 286, 119378. [Google Scholar] [CrossRef]
- Piffer, M.A.; Zaiat, M.; do Nascimento, C.A.O.; Fuess, L.T. Dynamics of Sulfate Reduction in the Thermophilic Dark Fermentation of Sugarcane Vinasse: A Biohydrogen-Independent Approach Targeting Enhanced Bioenergy Production. J. Environ. Chem. Eng. 2021, 9, 105956. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Tümen, F. Potassium Struvite (Slow Release Fertilizer) and Activated Carbon Production: Resource Recovery from Vinasse and Grape Marc Organic Waste Using Thermal Processing. Process Saf. Environ. Prot. 2021, 147, 1077–1087. [Google Scholar] [CrossRef]
- Montoya, G.; Gutierrez, M.I.; Giraldo, J.D.; Jaramillo, L.D.; Ruiz-Sandoval, J.; Orozco, S.; Orozco, F.; Ward, J.; Rojas, G.; Villegas-Torres, M.F. Sustainable Sugarcane Vinasse Biorefinement for Trans-Aconitic Acid-Based Biopolymer Synthesis and Bioenergy Generation. Bioresour. Technol. Rep. 2021, 15, 100786. [Google Scholar] [CrossRef]
- Chai, A.; Wong, Y.S.; Ong, S.A.; Aminah Lutpi, N.; Sam, S.T.; Kee, W.C.; Ng, H.H. Haldane-Andrews Substrate Inhibition Kinetics for Pilot Scale Thermophilic Anaerobic Degradation of Sugarcane Vinasse. Bioresour. Technol. 2021, 336, 125319. [Google Scholar] [CrossRef] [PubMed]
- Buller, L.S.; Romero, C.W.d.S.; Lamparelli, R.A.C.; Ferreira, S.F.; Bortoleto, A.P.; Mussatto, S.I.; Forster-Carneiro, T. A Spatially Explicit Assessment of Sugarcane Vinasse as a Sustainable By-Product. Sci. Total Environ. 2021, 765, 142717. [Google Scholar] [CrossRef]
- Bernal, A.P.; de Menezes, C.A.; Silva, E.L. A New Side-Looking at the Dark Fermentation of Sugarcane Vinasse: Improving the Carboxylates Production in Mesophilic EGSB by Selection of the Hydraulic Retention Time and Substrate Concentration. Int. J. Hydrog. Energy 2021, 46, 12758–12770. [Google Scholar] [CrossRef]
- Nogueira, E.W.; Gouvêa de Godoi, L.A.; Marques Yabuki, L.N.; Brucha, G.; Zamariolli Damianovic, M.H.R. Sulfate and Metal Removal from Acid Mine Drainage Using Sugarcane Vinasse as Electron Donor: Performance and Microbial Community of the down-Flow Structured-Bed Bioreactor. Bioresour. Technol. 2021, 330, 124968. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Song, C.; Liu, L.; Gong, W.; Ding, Y.; Yao, J. Efficient Removal of Cationic Dyes via Activated Carbon with Ultrahigh Specific Surface Derived from Vinasse Wastes. Bioresour. Technol. 2021, 322, 124540. [Google Scholar] [CrossRef]
- Ramos, L.R.; Lovato, G.; Rodrigues, J.A.D.; Silva, E.L. Anaerobic Digestion of Vinasse in Fluidized Bed Reactors: Process Robustness between Two-Stage Thermophilic-Thermophilic and Thermophilic-Mesophilic Systems. J. Clean. Prod. 2021, 314, 128066. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Junior, A.D.N.F.; Franco, T.T.; Moraes, B.S. Operational and Biochemical Aspects of Co-Digestion (Co-AD) from Sugarcane Vinasse, Filter Cake, and Deacetylation Liquor. Appl. Microbiol. Biotechnol. 2021, 105, 8969–8987. [Google Scholar] [CrossRef]
- Niz, M.Y.K.; Fuentes, L.; Etchebehere, C.; Zaiat, M. Sugarcane Vinasse Extreme Thermophilic Digestion: A Glimpse on Biogas Free Management. Bioprocess Biosyst. Eng. 2021, 44, 1405–1421. [Google Scholar] [CrossRef]
- Magrini, F.E.; de Almeida, G.M.; da Maia Soares, D.; Fuentes, L.; Ecthebehere, C.; Beal, L.L.; da Silveira, M.M.; Paesi, S. Effect of Different Heat Treatments of Inoculum on the Production of Hydrogen and Volatile Fatty Acids by Dark Fermentation of Sugarcane Vinasse. Biomass Convers. Biorefinery 2021, 11, 2443–2456. [Google Scholar] [CrossRef]
- Callejas, C.; López, I.; Bovio-Winkler, P.; Etchebehere, C.; Borzacconi, L. Temporal Analysis of the Microbiota Involved in the Anaerobic Degradation of Sugarcane Vinasse in a Full-Scale Methanogenic UASB Reactor. Biomass Convers. Biorefinery 2021, 12, 3887–3897. [Google Scholar] [CrossRef]
- Magrini, F.E.; de Almeida, G.M.; da Maia Soares, D.; dos Anjos Borges, L.G.; Marconatto, L.; Giongo, A.; Paesi, S. Variation of the Prokaryotic and Eukaryotic Communities After Distinct Methods of Thermal Pretreatment of the Inoculum in Hydrogen-Production Reactors from Sugarcane Vinasse. Curr. Microbiol. 2021, 78, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- da Conceição, V.M.; Pozzi, E.; Sakamoto, I.K.; Motteran, F.; Pires, E.C. Effect of Organic Loading Rate on the Microbial Community in Anaerobic Chambered Reactor Processing Ethanol Distillery Vinasse. Water Air Soil Pollut. 2021, 232, 495. [Google Scholar] [CrossRef]
- Brito Codato, C.; Gaspar Bastos, R.; Ceccato-Antonini, S.R. Sequential Process of Solid-State Cultivation with Fungal Consortium and Ethanol Fermentation by Saccharomyces Cerevisiae from Sugarcane Bagasse. Bioprocess Biosyst. Eng. 2021, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Volpi, M.P.C.; Brenelli, L.B.; Mockaitis, G.; Rabelo, S.C.; Franco, T.T.; Moraes, B.S. Use of Lignocellulosic Residue from Second-Generation Ethanol Production to Enhance Methane Production Through Co-Digestion. BioEnergy Res. 2022, 15, 602–616. [Google Scholar] [CrossRef]
- Tasic, M.B.; de Jesus Bonon, A.; Rocha Barbosa Schiavon, M.I.; Colling Klein, B.; Veljković, V.B.; Maciel Filho, R. Cultivation of Chlamydomonas Reinhardtii in Anaerobically Digested Vinasse for Bioethanol Production. Waste Biomass Valorization 2021, 12, 857–865. [Google Scholar] [CrossRef]
- Karouach, F.; Bakraoui, M.; Zguani, A.; Hammadi, A.; el Bari, H. Co-Digestion of Industrial Recycled Pulp and Paper Sludge with Vinasse Wastewater: Experimental and Theoretical Study. Int. J. Environ. Sci. Technol. 2021, 18, 3651–3664. [Google Scholar] [CrossRef]
- Bastos, A.V.S.; Teixeira, M.B.; Soares, F.A.L.; da Silva, E.C.; dos Santos, L.N.S.; Gomes, F.H.F. Immediate and Residual Effects of Mineral and Organomineral Nitrogen Sources Associated with Concentrated Vinasse on Maize. J. Soil Sci. Plant Nutr. 2021, 21, 1382–1396. [Google Scholar] [CrossRef]
- Raza, Q.-U.-A.; Bashir, M.A.; Rehim, A.; Raza, H.M.A. Role of Sugarcane Industrial Byproducts on Soil Physicochemical Properties and Metal Accumulation in Rice. Environ. Sci. Pollut. Res. 2021, 29, 24726–24736. [Google Scholar] [CrossRef]
- Fregolente, L.G.; dos Santos, J.V.; Mazzati, F.S.; Miguel, T.B.A.R.; Miguel, E.d.C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Hydrochar from Sugarcane Industry By-Products: Assessment of Its Potential Use as a Soil Conditioner by Germination and Growth of Maize. Chem. Biol. Technol. Agric. 2021, 8, 16. [Google Scholar] [CrossRef]
- Ferreiro, O.B.; Kronemberger, F.A.; Borges, C.P. Sugarcane Stillage Treatment Using Direct Contact Membrane Distillation. Waste Biomass Valorization 2021, 12, 3987–3999. [Google Scholar] [CrossRef]
- Iltchenco, J.; Peruzzo, V.; Eva Magrini, F.; Marconatto, L.; Paula Torres, A.; Luiz Beal, L.; Paesi, S. Microbiota Profile in Mesophilic Biodigestion of Sugarcane Vinasse in Batch Reactors. Water Sci. Technol. 2021, 84, 2028–2039. [Google Scholar] [CrossRef]
- Kiani Deh Kiani, M.; Parsaee, M.; Mahdavifar, Z. Biogas Production from Sugarcane Vinasse at Mesophilic and Thermophilic Temperatures by Static Granular Bed Reactor (SGBR). Sustain. Energy Technol. Assess. 2021, 48, 101569. [Google Scholar] [CrossRef]
- Caillet, H.; Adelard, L. Start-Up Strategy and Process Performance of Semi-Continuous Anaerobic Digestion of Raw Sugarcane Vinasse. Waste Biomass Valorization 2021, 12, 185–198. [Google Scholar] [CrossRef]
- Rulli, M.M.; Villegas, L.B.; Barcia, C.S.; Colin, V.L. Bioconversion of Sugarcane Vinasse into Fungal Biomass Protein and Its Potential Use in Fish Farming. J. Environ. Chem. Eng. 2021, 9, 106136. [Google Scholar] [CrossRef]
- Widyastuti, N.; Hidayat, M.; Purnomo, C.W. Enhanced Biogas Production from Sugarcane Vinasse Using Electro-Fenton as Pre-Treatment Method. IOP Conf. Ser. Earth Environ. Sci. 2021, 830, 012079. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. Effects of Cultivation Conditions on Chlorella Vulgaris and Desmodesmus Sp. Grown in Sugarcane Agro-Industry Residues. Bioresour. Technol. 2021, 342, 125949. [Google Scholar] [CrossRef]
- Heredia Falconí, J.H.; Soares, J.; Rocha, D.N.; Gomes Marçal Vieira Vaz, M.; Martins, M.A. Strain Screening and Ozone Pretreatment for Algae Farming in Wastewaters from Sugarcane Ethanol Biorefinery. J. Clean. Prod. 2021, 282, 124522. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Karimi, K.; Taherzadeh, M.J. Valorization of Vinasse and Whey to Protein and Biogas through an Environmental Fungi-Based Biorefinery. J. Environ. Manag. 2022, 303, 114138. [Google Scholar] [CrossRef]
- Kee, W.-C.; Wong, Y.-S.; Ong, S.-A.; Lutpi, N.A.; Sam, S.-T.; Chai, A.; Eng, K.-M. Photocatalytic Degradation of Sugarcane Vinasse Using ZnO Photocatalyst: Operating Parameters, Kinetic Studies, Phytotoxicity Assessments, and Reusability. Int. J. Environ. Res. 2022, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Rabelo, C.A.B.S.; Camargo, F.P.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Optimization of Key Factors Affecting Hydrogen and Ethanol Production from Xylose by Thermoanaerobacterium Calidifontis VCS1 Isolated from Vinasse Treatment Sludge. Waste Biomass Valorization 2022, 13, 1897–1912. [Google Scholar] [CrossRef]
- Aguiar, M.M.; Wadt, L.C.; Vilar, D.S.; Hernández-Macedo, M.L.; Kumar, V.; Monteiro, R.T.R.; Mulla, S.I.; Bharagava, R.N.; Iqbal, H.M.N.; Bilal, M.; et al. Vinasse Bio-Valorization for Enhancement of Pleurotus Biomass Productivity: Chemical Characterization and Carbohydrate Analysis. Biomass Convers. Biorefinery 2022, 1–10. [Google Scholar] [CrossRef]
- Candido, C.; Cardoso, L.G.; Lombardi, A.T. Bioprospecting and Selection of Tolerant Strains and Productive Analyses of Microalgae Grown in Vinasse. Braz. J. Microbiol. 2022, 53, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, L.N.M.; Queluz, J.G.T.; Garcia, M.L. Assessment of Phase Distribution and Removal of Metals in Anaerobic Digesters. Int. J. Environ. Sci. Technol. 2022, 19, 463–474. [Google Scholar] [CrossRef]
- Martinez-Silveira, A.; Garmendia, G.; Rufo, C.; Vero, S. Production of Microbial Oils by the Oleaginous Yeast Rhodotorula Graminis S1/2R in a Medium Based on Agro-Industrial by-Products. World J. Microbiol. Biotechnol. 2022, 38, 46. [Google Scholar] [CrossRef]
- Kee, W.-C.; Wong, Y.-S.; Ong, S.-A.; Lutpi, N.A.; Sam, S.-T.; Chai, A.; Ng, H.-H. Insights into Modified Sequencing Batch Reactor for the Treatment of Sugarcane Vinasse: Role of Recirculation Process. Int. J. Environ. Sci. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Hakika, D.C.; Sarto, S.; Mindaryani, A.; Hidayat, M. Influence of Fenton Pretreatment on Anaerobic Digestion of Sugarcane Vinasse: Effect of H2O2 Dosage. IOP Conf. Ser. Earth Environ. Sci. 2022, 963, 012009. [Google Scholar] [CrossRef]
- Travassos, J.A.; Godoy, O.; Conceição, J.; Pires, L.; Araújo, C.M. Uso de Vinaza En El Proceso de Sacarificacion de Biomasa Lignocelulosica. Patent No. 2013011093, 14 April 2014. [Google Scholar]
- Revah, S.; Pierre, C.; Domenech, F. Proceso de Enriquecimiento de Residuos Lignocelulosicos Con Proteinas de Levadura. MXPA06006420A, 8 August 2008. [Google Scholar]
- Domenech, F.; Revah-Moissev, S.; Christen, P. Procedimiento de Enriquecimiento de Residuos Lignocelulosicos En Proteinas de Levadura. Patent No. 44/9575, 3 March 2008. [Google Scholar]
- Martínez, I. Procedimiento Para Obtener Un Producto Sólido Fertilizante y Biocombustible a Partir de Vinazas de Caña de Azúcar y Producto Sólido Fertilizante y Biocombustible Obtenido Mediante Dicho Procedimiento. WO2012069665A1, 31 May 2012. [Google Scholar]
- Martínez Garmendia, I. Method for Obtaining a Solid Fertilizing and Biocombustible Product from Sugar-Cane Vinasse and Solid Fertilizing and Biocombustible Product Obtained by Means of Said Method. WO2012069665A1, 31 May 2014. [Google Scholar]
- Martinez, I. Method for Obtaining a Solid Fertilizer and Biofuel Product from Sugarcane Vinasses and Solid Fertilizer and Biofuel Product Obtained by Means of Said Method. U.S. Patent US20130312471A1, 28 November 2013. [Google Scholar]
- Mantelatto, P.E.; Boscariol, F.C.; Gurgel, M.N.; Cesar, A.R.; Ciambell, J.R. Proceso Para La Produccion de Un Fertilizante Organo-Mineral, Que Comprende Los Subproductos Vinaza y Torta de Filtro Resultantes de La Fabricacion de Alcohol y Azucar a Partir de Caña de Azúcar. CO6310991A2, 22 August 2011. [Google Scholar]
- Zapata, L.G.; Zarate, G.A.; Zapata, J.V. Proceso Para La Produccion de Fertilizante Organico Mineral Granulado a Base de Vinaza Concentrada y Planta Para Su Producción. CO5980165A1, 28 November 2008. [Google Scholar]
- Melo, C.D.A.; Bisinoti, M.C.; Ferreira, O.P.; Moreira, A.B.; Santana, R.L.; Soares Junior, F.H. Method for Producing Organo-Mineral Fertilizer by Hydrothermal Carbonisation of Sugarcane Vinasse and Bagasse and Thus Obtained Product. WO2017132742A1, 10 August 2017. [Google Scholar]
- Silva, S.; Arbelaez, F.J. Proceso Para La Produccion de Abonos Organicos y El Producto Obtenido. WO2005009924A1, 3 February 2005. [Google Scholar]
- Energia e Adubos Ltda.; Biomassa Comercio de Raoesenergia e Adubos Ltda. Biomassa Comercio de Raoes Proceso Para Recuperacion Del Agua Industrial, Desintoxicacion y Secado de Vinaza a Traves de Micronizacion y Formulacion de Abono Organico Mineral a Partir de Vinaza. Patent No. 085011, 24 July 2013. [Google Scholar]
- Reina, J.E. Proceso de Tratamiento de Vinaza Para Generación de Producto No Contaminante Con Propiedades Nutritivas y Desinfectantes Del Suelo y La Planta. Patent No. 20160002191, 21 September 2016. [Google Scholar]
- Benso, A. Composición Bioestimulante a Base de Taninos y/o Sus Derivados, Para El Tratamiento de Las Plantas Cultivadas y/o Partes de Los Mismos. AR106139A1, 13 December 2017. [Google Scholar]
- Lewis, B. Improved Method for Preparing Concrete Admixture by Using Vinasse Prepared by Distilling Sugarcane Molasses. CN102020431A, 20 April 2011. [Google Scholar]
- Zaiqian, W.; Wenwei, L.; Weiwen, H. Method for Producing Sugarcane Fruit Vinegar Stock Solution by Utilizing Sugarcane Rum Vinasse Liquid. CN106047635A, 26 October 2016. [Google Scholar]
- Zengchao, R.; Yanbing, W.; Jinhe, L.; Daxing, P. Novel Saccharification-Fermentation Flavor Fish Sauce and Preparation Method Thereof. CN102793140B, 7 August 2012. [Google Scholar]
- Tracey, A.; Kannar, D. Polyphenol Compositions and Sugars Including Vinasse and/or Digestate and Methods of Their Preparation. WO2021133255A1, 1 July 2021. [Google Scholar]
- Leilei, W.; Qun, Z.; Fan, W. Method for Producing Feed by Fermenting Sugarcane Juice. Patent No. 113273641, 20 August 2021. [Google Scholar]
- Agudelo, E. Procedimiento Para Obtener Melaza a Partir de Vinaza. Patent No. 5970146, 31 October 2008. [Google Scholar]
- Reginato, M.A. Proceso de Tratamiento de Los Efluentes Industriales Denominados Vinazas y El Tandem de Filtrado Que Utiliza Dicho Proceso 2012. Patent No. 082417, 5 December 2012. [Google Scholar]
- Dolivar, N. Carbon Fixation Method for Reducing the Turbidity of Sugarcane Vinasse and Thus Processed Vinasse. WO2013056328A1, 25 April 2013. [Google Scholar]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane Vinasse as Organo-Mineral Fertilizers Feedstock: Opportunities and Environmental Risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef]


| Parameter | Specification |
|---|---|
| Search Period | January 2018 to February 2022 |
| Databases and Repositories | Elsevier, SpringerLink, and Google Scholar |
| Search Terms | “vinasse” AND “ethanol” OR “alcohol” AND “sugarcane” |
| Document Type | Except review articles |
| Discipline | No restriction |
| Subdiscipline | No restriction |
| Language | English |
| Country | No restriction |
| Type of Review | Peer-review |
| Scope and Limitations | PICO strategy |
| Selection Process | It was made by A.M.-R., N.M.-R., G.F.-L., and L.E.G.-S., verified by H.B.-S. and L.C.S.-H. |
| PICO | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Ethanol vinasse from sugarcane | Ethanol vinasse from non-sugarcane products, e.g., corn, agave |
| Intervention | Sugarcane ethanol vinasse treatment applications that reduce its negative impact on the environment, and applications of this vinasse as an input in the generation of bioproducts that use its properties as a product and-or process improver | Simulation models of ethanol production systems, which report estimated scenarios of sugarcane vinasse use; economic analysis models developed from the generation of scenarios |
| Comparator | Performance of vinasse as a process and-or product improver | None |
| Outcome | Potentiality of the uses and applications of treated vinasse or as an input in the development of processes-products | Adverse effects of sugarcane ethanol vinasse on the ecosystem, e.g., soil, flora, and fauna |
| Study | Process | Technology ** |
|---|---|---|
| [9,28,74,76,79,82,83,87,97,103,109,117,138] | Physical | Membranes, Fenton reaction, pyrolysis, electrodialysis (ED), catalytic wet air oxidation (CWAO),hydrodynamic cavitation, and others |
| [13,30,36,47,50,54,57,58,66,68,95,108,123,131,137,147] | Chemical | Thermolysis, adsorption by bagasse fly ash, hydrothermal carbonization (HTC), hydrolysis, photocatalytic degradation using zinc oxide (ZnO), and others |
| [12,17,29,31,32,33,34,35,37,38,39,40,41,42,43,44,45,48,49,51,52,53,55,59,60,61,62,63,64,65,67,69,70,71,72,73,77,78,81,84,85,88,89,90,91,93,94,98,99,101,102,106,107,110,111,112,113,114,115,116,119,120,121,122,124,125,126,127,128,129,130,132,133,134,135,139,140,141,142,144,145,146,148,149,151,152,153] | Biological | Cultivation, fermentation, anaerobic digestion (AD), anaerobic co-digestion (AcD), dark fermentation (DF), and others |
| [46,56,75,80,86,92,96,100,104,105,118,136,143,150,154] | Others * | Inverse osmosis (IO), nanofiltration (NF)/reverse osmosis (RO)/ultrafiltration (UF)/two-stage anaerobic membrane bioreactor (2S-AnMBR), microfiltration (MF)/nanofiltration (NF), coagulation/cross-flow microfiltration, electro-Fenton, and others |
| Study | Sector Industrial | Application |
|---|---|---|
| [56,58,66,73,74,75,77,79,92,97,101,103,107,117,135,136,137,144] | Agriculture | Soil remediation, crop improver (e.g., corn, tomato, rice), fertigation in sugar cane, fertilizer, among others |
| [31,33,35,37,39,40,41,42,43,44,45,48,49,52,55,59,62,63,69,71,72,76,81,82,84,85,86,90,91,94,96,98,99,102,109,112,114,116,119,120,121,124,125,126,127,128,129,130,132,134,139,140,141,143,148,152,154] | Bioenergy | Biofuels in the form of biofuels (biodiesel, bioethanol, and biogas or biomass), among others |
| [87] | Bioplastics | Biodegradable plastics |
| [34] | Biosensors | Measurement of biological or chemical parameters |
| [38,51,54,57,60,61,65,68,70,78,89,95,106,108,111,115,131,133] | Biotechnology | Pharmaceutical, biomedicine, food, cosmetics, and packaging, among others |
| [118] | Chemical | Chemistry |
| [13] | Construction | Concrete |
| [29,64,146,149] | Food | Food and Animal feed |
| [142] | Pisciculture | Fish feed |
| [9,12,17,28,30,32,36,46,47,50,53,67,80,83,88,93,100,104,105,110,113,122,123,138,145,147,150,151,153] | Waste and wastewater management | Wastewater remediation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel-Rosales, A.; Montalvo-Romero, N.; García-Santamaría, L.E.; Sandoval-Herazo, L.C.; Bautista-Santos, H.; Fernández-Lambert, G. Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review. Sustainability 2022, 14, 11635. https://doi.org/10.3390/su141811635
Montiel-Rosales A, Montalvo-Romero N, García-Santamaría LE, Sandoval-Herazo LC, Bautista-Santos H, Fernández-Lambert G. Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review. Sustainability. 2022; 14(18):11635. https://doi.org/10.3390/su141811635
Chicago/Turabian StyleMontiel-Rosales, Aarón, Nayeli Montalvo-Romero, Luis Enrique García-Santamaría, Luis Carlos Sandoval-Herazo, Horacio Bautista-Santos, and Gregorio Fernández-Lambert. 2022. "Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review" Sustainability 14, no. 18: 11635. https://doi.org/10.3390/su141811635
APA StyleMontiel-Rosales, A., Montalvo-Romero, N., García-Santamaría, L. E., Sandoval-Herazo, L. C., Bautista-Santos, H., & Fernández-Lambert, G. (2022). Post-Industrial Use of Sugarcane Ethanol Vinasse: A Systematic Review. Sustainability, 14(18), 11635. https://doi.org/10.3390/su141811635









