Production of Refractory Bricks through Combustion Synthesis from Metallurgical Wastes and the Thermo-Physical Properties of the Products
Abstract
:1. Introduction
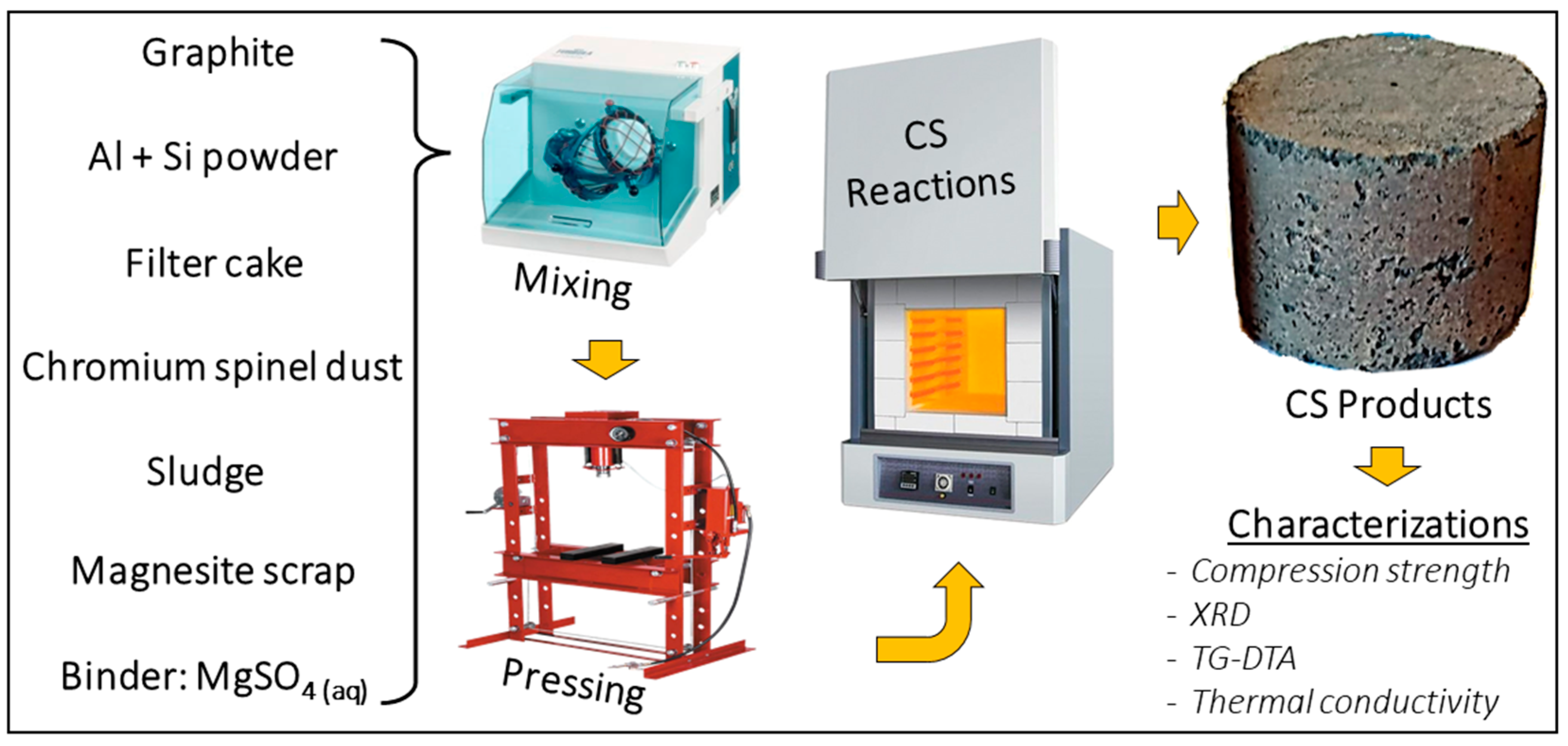
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. X-ray Diffraction Analysis
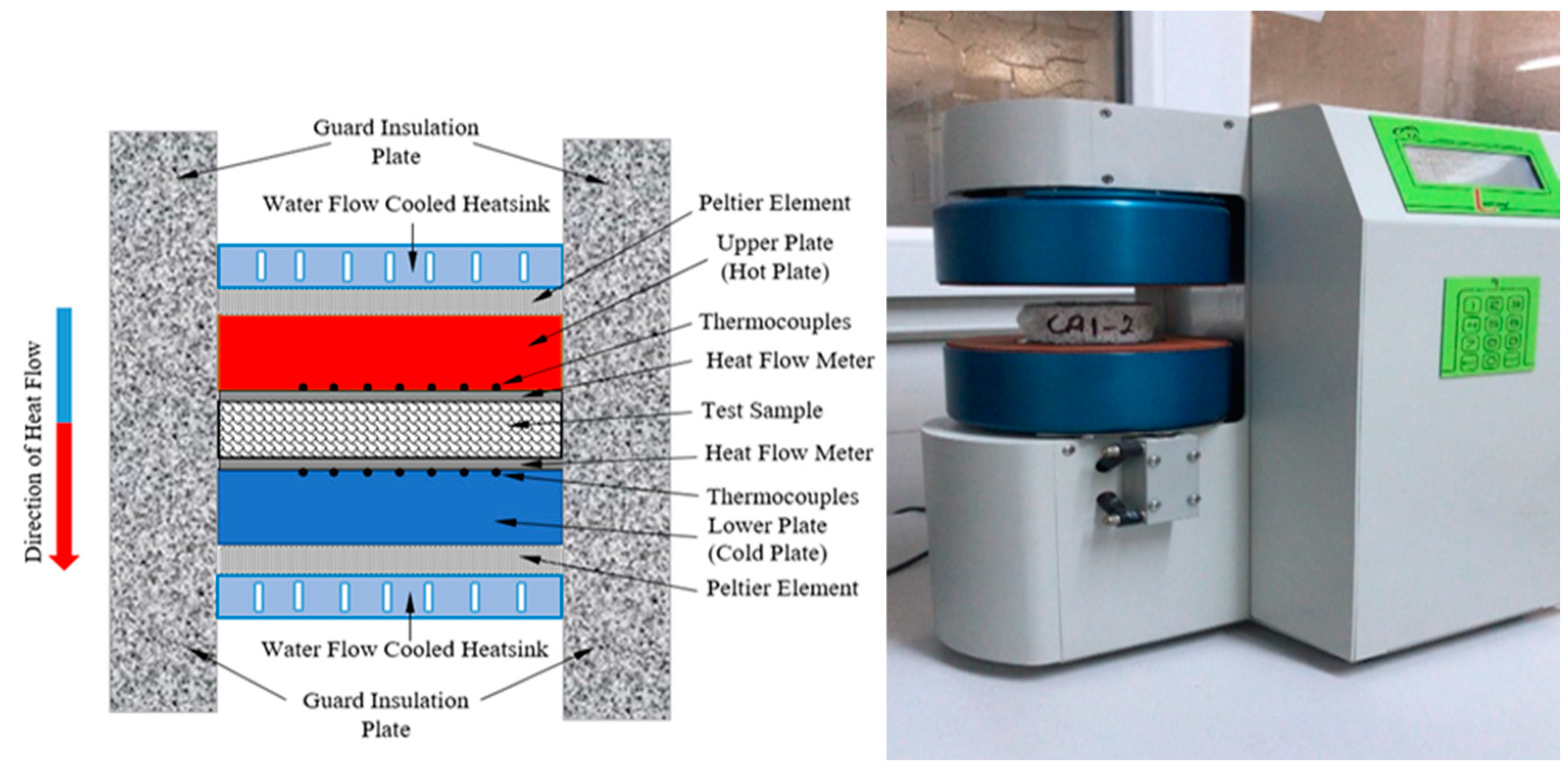
2.2.2. Thermal Conductivity Analysis
2.2.3. Thermochemical Simulation Analysis
2.2.4. Compression Strengths’ Tests
2.2.5. TG/DTA Analysis
3. Results and Discussion
3.1. X-ray Diffraction Analysis of Refractory Bricks based on Metallurgical Waste
3.2. Thermal Conductivity Analysis Results
3.3. Thermochemical Modeling Results
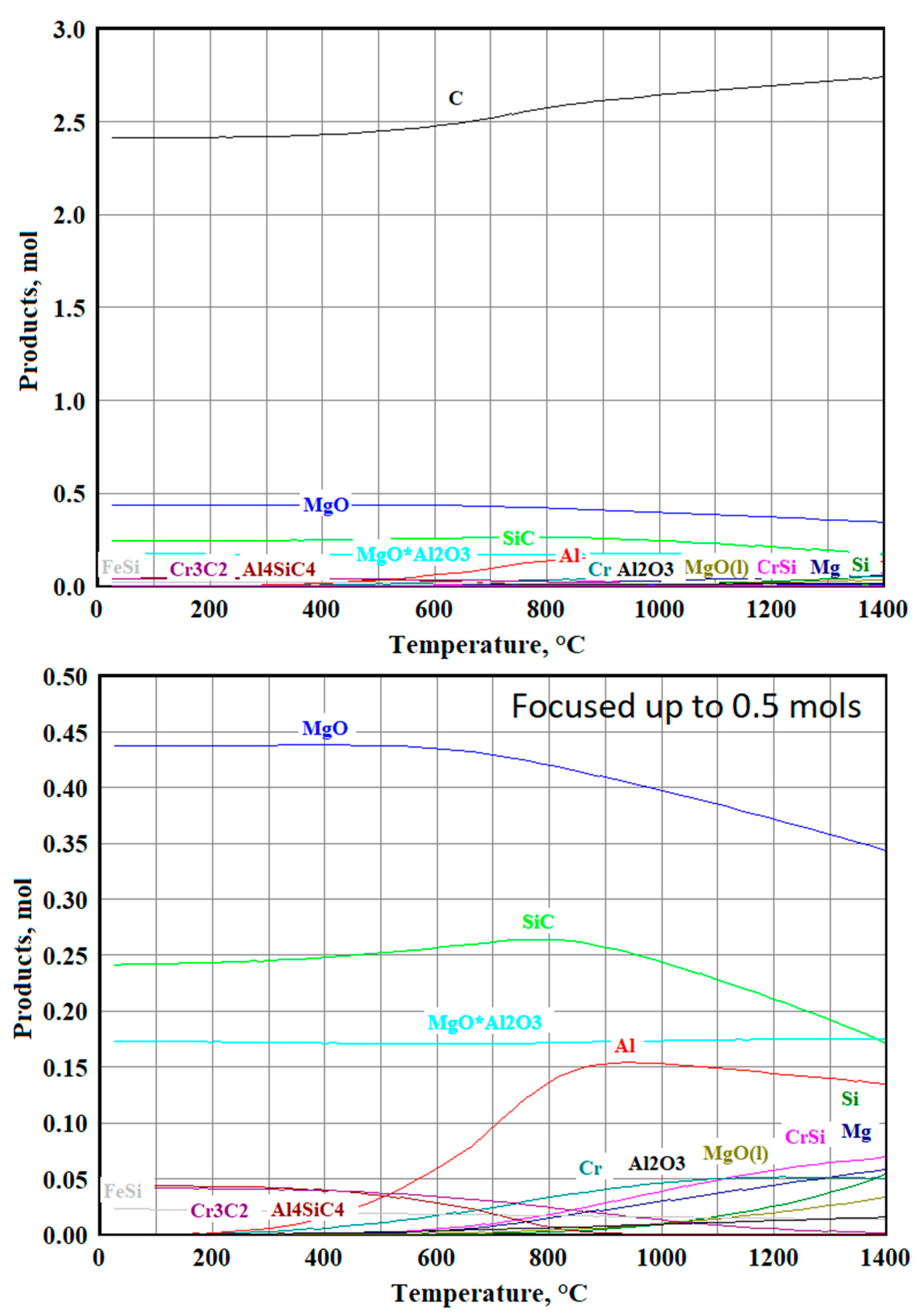
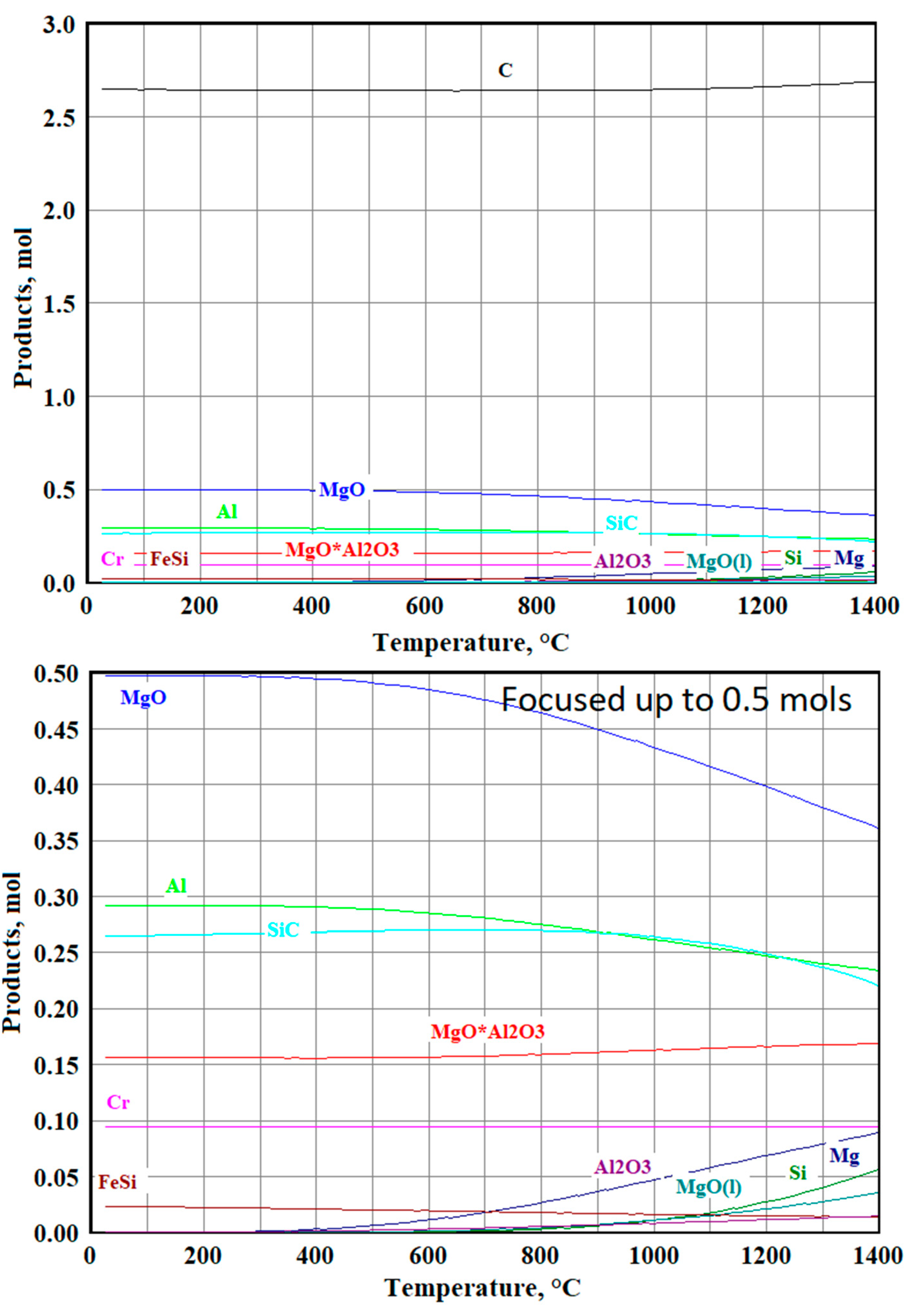
3.3.1. Modeling Results for the Sample №1
3.3.2. Modeling Results for the Sample №2
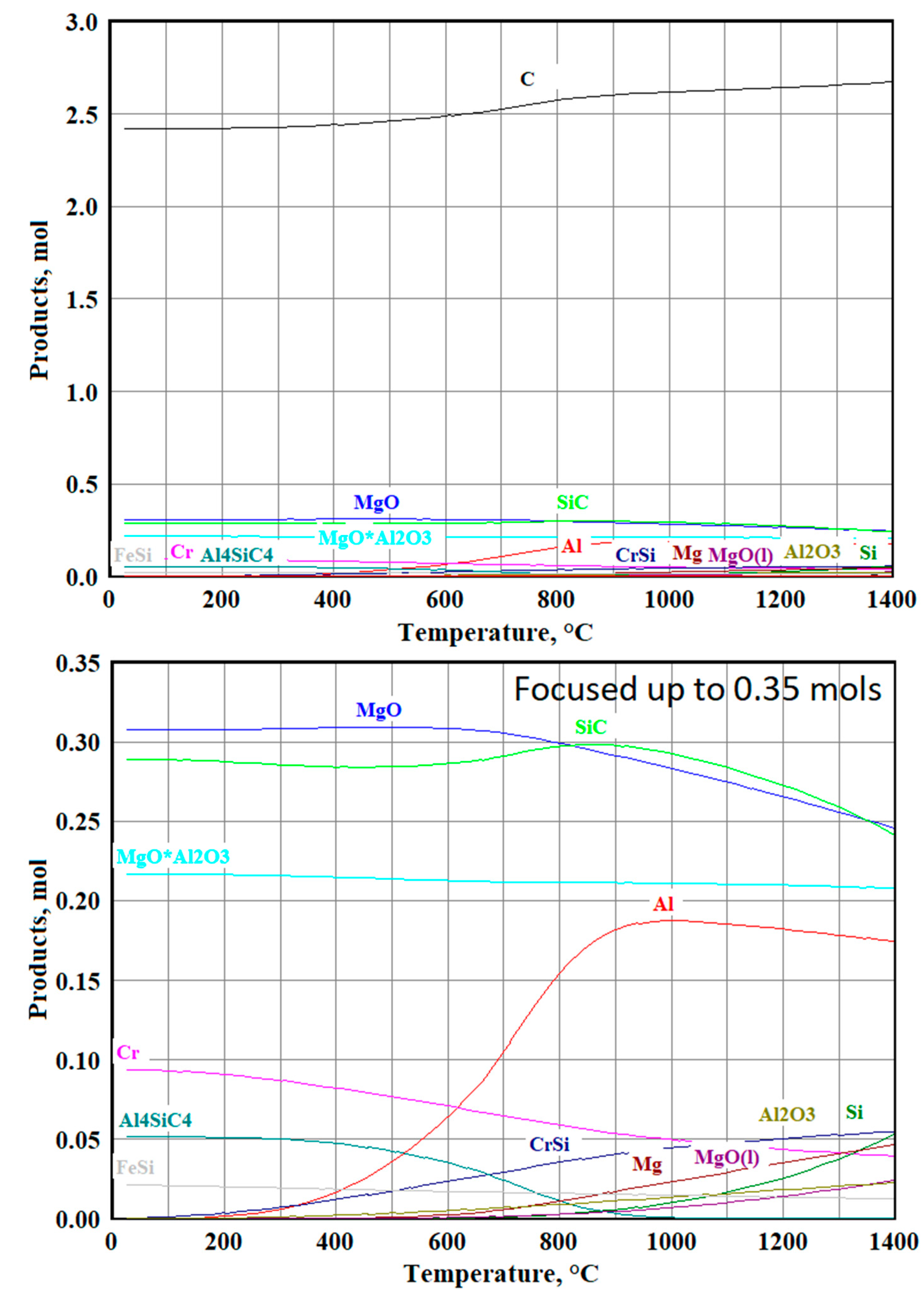
3.3.3. Modeling Results for the Sample №3
3.3.4. Modeling Results for the Sample №4
3.4. Compression Strengths’ Tests Results
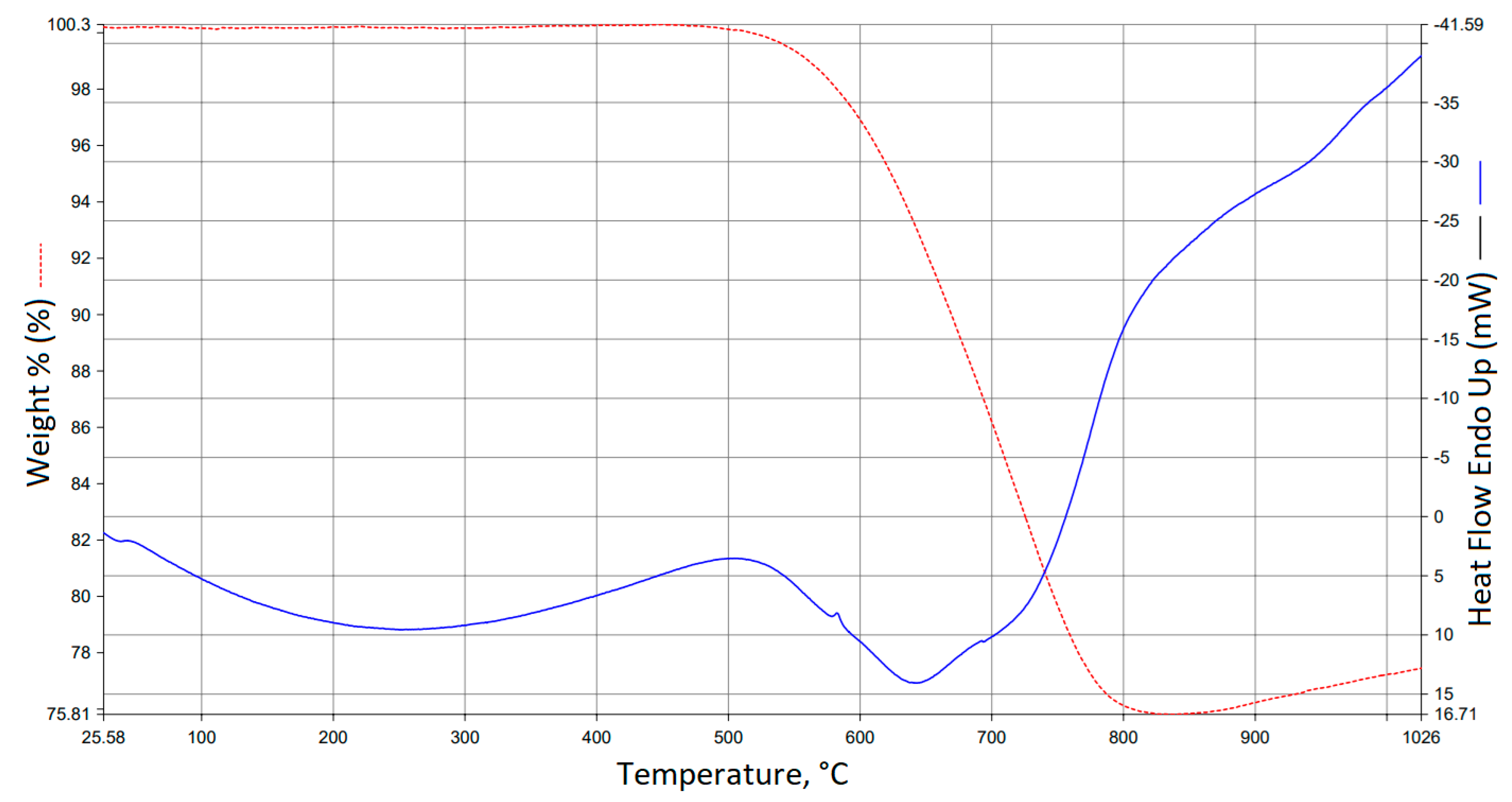
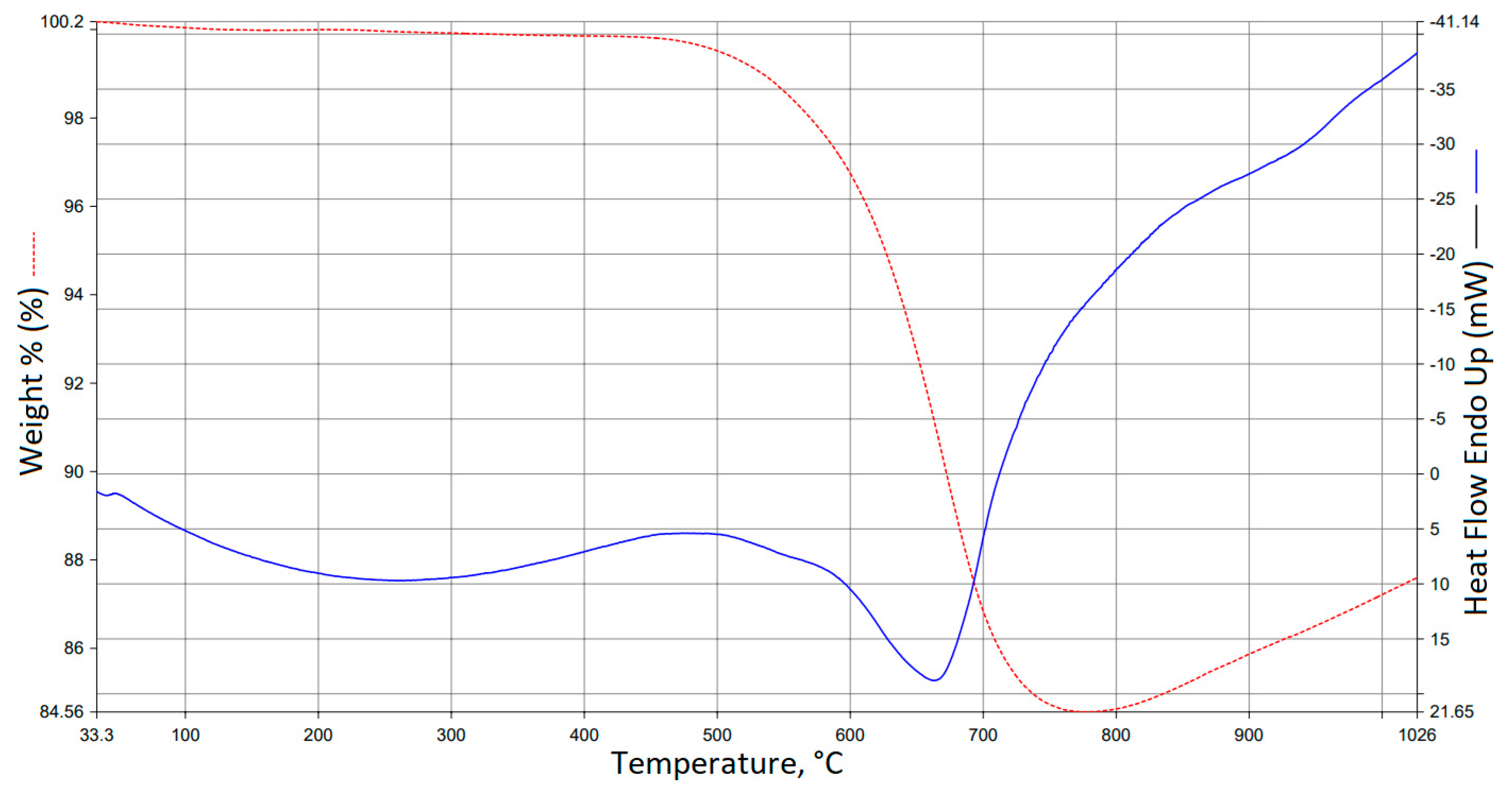
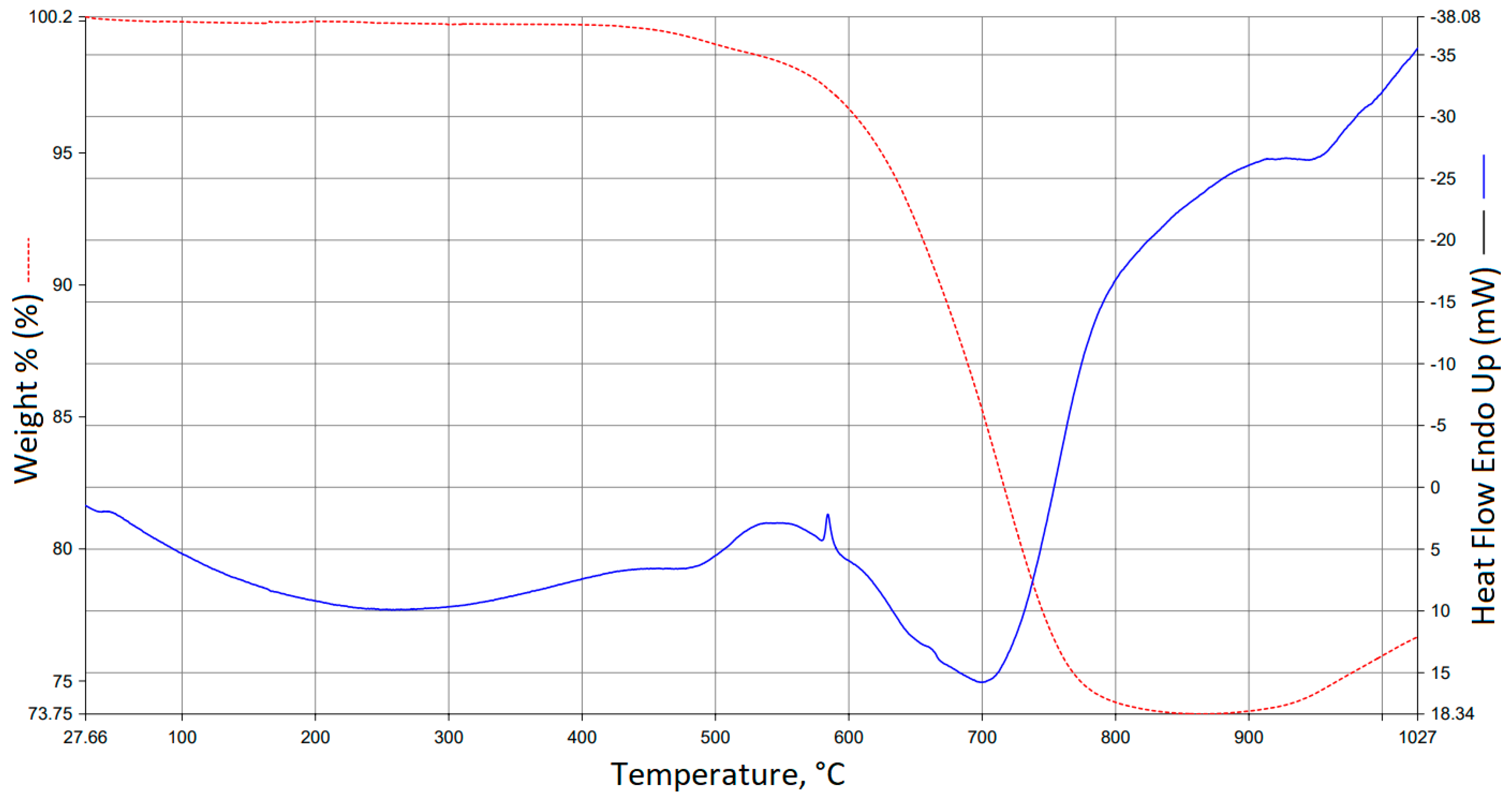
3.5. TG/DTA Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aleluia, J.; Ferrão, P. Assessing the costs of municipal solid waste treatment technologies in developing Asian countries. Waste Manag. 2017, 69, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Mourato, S. Cost-Benefit Analysis and the Environment; OECD: Paris, France, 2015. [Google Scholar]
- Balasubramanian, M. Economics of Solid Waste Management: A Review. In Strategies of Sustainable Solid Waste Management; Saleh, H.M., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-559-6. [Google Scholar]
- Rajendran, K.; Varma, V.S.; Mahapatra, D.M.; Kondusamy, D. Economics of Solid Waste Management. Waste to Wealth; Springer: Singapore, 2018; pp. 259–275. [Google Scholar]
- Staden, Y.; Beukes, J.P.; van Zyl, P.; du Toit, J.S.; Dawson, N.F. Characterization and liberation of chromium from fine ferrochrome waste materials. Miner. Eng. 2014, 56, 112–120. [Google Scholar] [CrossRef]
- Dolinskii, V.A.; Nikitin, L.D.; Domnin, K.I. Recycling wastes in sinter and hot-metal production. Steel Transl. 2010, 40, 712–716. [Google Scholar] [CrossRef]
- Menshov, P.V.; Khlupin, Y.V.; Makarovskikh, A.V. Ash and Slag Waste as a Secondary Raw Material. Procedia Chem. 2014, 10, 184–191. [Google Scholar] [CrossRef]
- Özkan, A.; Günkaya, Z.; Tok, G.; Karacasulu, L.; Metesoy, M.; Banar, M.; Kara, A. Life Cycle Assessment and Life Cycle Cost Analysis of Magnesia Spinel Brick Production. Sustainability 2016, 8, 662. [Google Scholar] [CrossRef]
- Yamaguchi, A. Self-repairing function in the carbon-containing refractory. Int. J. Appl. Ceram. Technol. 2007, 4, 490–495. [Google Scholar] [CrossRef]
- Akishev, A.K.; Fomenko, S.M.; Tolendiuly, S. Effect of Refractory Thermal Stresses and Parameters on Development of the Internal Temperature Field. Refract. Ind. Ceram. 2020, 60, 561–565. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P. Historical retrospective of SHS: An autoreview. Int. J. Self-Propagating High-Temp. Synth. 2008, 17, 242. [Google Scholar] [CrossRef]
- Bugdayci, M.; Alkan, M.; Turan, A.; Yücel, O. Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction. High Temp. Mater. Processes 2018, 37, 889–898. [Google Scholar] [CrossRef]
- Wojciech, K.; Slawomir, D. Transition metal impurities in carbon-based materials: Pitfalls, artifacts, and deleterious effects. Carbon 2020, 168, 748–845. [Google Scholar]
- Thethwayo, B.M.; Steenkamp, J.D. A review of carbon-based refractory materials and their applications. J. South. Afr. Inst. Min. Metall. 2020, 120, 641–650. [Google Scholar] [CrossRef]
- Fomenko, S.M.; Akishev, A.; Tolendiuly, S. Thermal flows influence on the change of temperature stresses in surface and inner layers of refractories. Mater. Today Proc. 2020, 33, 1853–1858. [Google Scholar] [CrossRef]
- Fomenko, S.M.; Akishev, A.; Tolendiuly, S.; Almagambetov, M.; Yeskendirov, R. The technology for producing refractory products based metallurgical production waste. AIP Conf. Proc. 2021, 2380, 40003. [Google Scholar]
- Ruuska, A.; Häkkinen, T. Material Efficiency of Building Construction. Buildings 2014, 4, 266–294. [Google Scholar] [CrossRef]
- Ilutiu-Varvara, D.-A.; Aciu, C. Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability 2022, 14, 5488. [Google Scholar] [CrossRef]
- Büyükkaya, K.; Güler, B.; Koru, M. Investigation of the Thermal and Mechanical Properties of Organic Waste Reinforced Polyester Composites. Iran J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 757–766. [Google Scholar] [CrossRef]
- Available online: https://www.tainstruments.com/fox-50/ (accessed on 1 September 2022).


| Green Components | FC | S | MS | Cr-S-Rich | Cr-S-Poor |
|---|---|---|---|---|---|
| Mg2SiO4 | 62.7 | 65.0 | - | 30.5 | 25.2 |
| MgCr2O4 | 30.5 | - | - | 59.5 | 20.0 |
| MgAl2O4 | - | 20.9 | - | - | - |
| FeMgAlSiO2 | - | - | - | - | 7.5 |
| MgO | - | 12.6 | 90.55 | 2.5 | 43.5 |
| SiO2 | 2.6 | 1.5 | 2.05 | 2.5 | 2.3 |
| Al2O3 | - | - | 3.8 | - | - |
| TiO2 | - | - | 1.0 | - | - |
| CaCO3 | - | - | - | 3.5 | - |
| CaO | - | - | 1.8 | - | - |
| Fe2O3 | - | - | 0.8 | - | - |
| Fe | 4.2 | - | - | 1.5 | 1.5 |
| № | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Components | ||||
| Grained graphite (0.5–2 mm) | 20 | 30 | 30 | 30 |
| Milled graphite (<100 µm) | 5 | 5 | 5 | 5 |
| Al powder | 18 | 14 | 16 | 16 |
| Si powder | 4 | 4 | 4 | 4 |
| FC | 26.5 | 23.5 | 29.67 | 29.67 |
| Cr-S-rich | 26.5 | - | - | - |
| Cr-S-poor | - | 23.5 | - | - |
| S | - | - | 15.33 | - |
| MS | - | - | - | 15.33 |
| № | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Chemical Phases, wt. % | ||||
| MgAl2O4 | 38.3 | 69.3 | 76.4 | 71.7 |
| C | 27.6 | 16.6 | - | 3.1 |
| SiC | 15.9 | 7.4 | 10.7 | 11.3 |
| Al4.59Si1.41O9.7 | 9.4 | - | - | - |
| Cr7C3 | 4.8 | - | - | - |
| Al2Ca3(SiO4)3 | 3.9 | - | - | - |
| MgCr2O4 | - | 3.6 | - | - |
| Ca(Mg0.93Fe0.07)SiO4 | - | - | 5.9 | 9.7 |
| Cr | - | - | 4.0 | 4.2 |
| Si | - | 3.1 | 3.0 | - |
| Sample № | Thermal Conductivity Constant (W/mK) | Heat Flux (W/m2) |
|---|---|---|
| 1 | 0.841 | 267 |
| 2 | 0.929 | 309 |
| 3 | 1.020 | 308 |
| 4 | 0.511 | 160 |
| Sample № | Fm (N) | Sm (MPa) | em (%) |
|---|---|---|---|
| 1 | 21,796.4 | 15.8 | 6.63 |
| 2 | 21,811.19 | 15.7 | 4.34 |
| 3 | 21,758.02 | 15.7 | 9.89 |
| 4 | 3805.322 | 2.7 | 5.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, S.; Tolendiuly, S.; Turan, A.; Akishev, A. Production of Refractory Bricks through Combustion Synthesis from Metallurgical Wastes and the Thermo-Physical Properties of the Products. Sustainability 2022, 14, 11439. https://doi.org/10.3390/su141811439
Fomenko S, Tolendiuly S, Turan A, Akishev A. Production of Refractory Bricks through Combustion Synthesis from Metallurgical Wastes and the Thermo-Physical Properties of the Products. Sustainability. 2022; 14(18):11439. https://doi.org/10.3390/su141811439
Chicago/Turabian StyleFomenko, Sergey, Sanat Tolendiuly, Ahmet Turan, and Adil Akishev. 2022. "Production of Refractory Bricks through Combustion Synthesis from Metallurgical Wastes and the Thermo-Physical Properties of the Products" Sustainability 14, no. 18: 11439. https://doi.org/10.3390/su141811439
APA StyleFomenko, S., Tolendiuly, S., Turan, A., & Akishev, A. (2022). Production of Refractory Bricks through Combustion Synthesis from Metallurgical Wastes and the Thermo-Physical Properties of the Products. Sustainability, 14(18), 11439. https://doi.org/10.3390/su141811439







