Abstract
The objective of this study was to evaluate the effect of mixtures of the phosphate industry’s by-products and sewage sludge on some heavy metals (Pb, Zn and Cd) in the soil–plant system and the microbial load (bacteria, fungi and actinobacteria) in the soil. The experimental layout was a completely randomized design with ten treatments and four replications. The treatments consist of a combination of five substrates: phosphogypsum (PG), phosphate sludge (PS), sewage sludge (SS), phosphate waste rocks (PWR) and original mine topsoil (TS). Heavy metals analysis, phytoextraction efficiency (PEE) and bioconcentration factor (BCF) were carried out for three Ryegrass cuts. The microbial load of each treatment was determined at the end of the experiment. The results showed that the Pb, Zn and Cd contents of the treatments were well below the permissible limits given in the literature. The highest BCF and PEE were with treatment T4. Furthermore, bacteria, fungi and actinobacteria were significantly influenced by the different proportions of by-products used in the treatments, with the highest richness observed for the T4 treatment. For a successful reclamation of mine sites, it would be useful to determine the speciation of metals in the soil solution and the microbial genetic diversity.
1. Introduction
Morocco has a very long mining tradition which constitutes an important vector of economic and social development [1,2]. Mining activity is dominated by the extraction and beneficiation of rock phosphates. This country remains the largest producer of rock phosphate and has around two-thirds of the world’s total reserves [3,4,5]. The Cherifien Phosphates Office (abbreviated: OCP) is the phosphate industry responsible for phosphate-based fertilizer and the extraction of the raw phosphates from the Moroccan undergrounds thanks to open-pit sites whose production reaches approximately 26.6 million tons per year [6,7,8]. The intense mining activity related to phosphate beneficiation and fertilizer production generates large amounts of by-products such as phosphogypsum (PG), phosphate sludge (PS) and phosphate waste rock (PWR) [9,10,11].
Furthermore, the storage and handling of phosphogypsum and phosphate sludge are at the same time costly and a loss since they can be valorized in agriculture and other fields. In addition, sewage sludge is a by-product of sewage treatment plants [12]. While numerous studies have shown that sewage sludge was an effective mine soil amendment [13], it is nevertheless a problem in various countries due to its increasing volume and the impacts associated with its disposal. In 2015, Morocco produced more than 240,000 tons of sewage sludge, and it is estimated that more than 320,000 tons of sewage sludge will be produced from wastewater treatment plants in 2025 [14,15].
Engaged in mining soil rehabilitation and their difficult management in harsh pedo-climatic conditions, and concerned with the environmental footprint limitation, OCP, in collaboration with its partners, is looking for effective and efficient techniques for the valorization of these by-products through the revegetation of mining sites and other uses, including degraded soils. Indeed, phosphate industry by-products and sewage sludge contain various amounts of nitrogen, sulfur, calcium, phosphorous, micronutrients and large amounts of organic matter that can contribute to the success of revegetation [13,14,16,17,18]. Furthermore, the studies performed by [1] showed that a proportion of 65% of phosphogypsum enriched the substrate in phosphorus by improving the crop yield. The addition of 5% of sewage sludge contributed to a significant improvement of ryegrass aerial biomass. Topsoil can also be mixed with phosphate sludge and sewage sludge and used as an alternative substrate for the reclamation of the mined soils. According to several authors [5,19,20,21,22,23,24,25], these by-products may contain pollutants that must be evaluated and monitored to avoid any potential threat to the environment and ensure their safe use on a large scale. Contrary to organic molecules, which can be metabolized or destroyed, heavy metals are persistent and not biodegradable and could constitute a source of contamination to plants as well as other organisms in the trophic chain [13,14]. A study carried out by [26] revealed that cadmium (Cd), Zinc (Zn) and lead (Pb) were the heavy metals most likely to be found in phosphate mining by-products. Moreover, according to [14], several environmental issues are related to sewage sludge, depending on its composition, especially soil contaminants. Sewage sludges may contain a high level of heavy metals such as Cd, Zn and Pb, which at high levels become toxic and restricts their use as an amendment and fertilizer in agriculture, revegetation or reclamation of mining sites and degraded soils in general. Thus, these heavy metals, even at low concentrations, may constitute serious threats to living organisms through their ability to stimulate, aggregate properties and mutagenesis [27].
The soil microbial population plays a significant role in organic matter transformation and soil nutrient management [28]. According to [29], soil microbial biomass and microbial activity are indicators of soil maturity and health. Indeed, by-product nutrients stimulate microbial activity and microbial biomass by increasing populations of actinobacteria, bacteria and fungi. However, variations in environmental conditions may exert a specific population pressure, shifting to population and affecting the composition of microbial species [30]. In addition, heavy metals present in the by-products may affect a variety of microbial processes in soil, thereby affecting the nutrient cycling and the capacity to perform key ecological functions, such as the mineralization of organic compounds and synthesis of organic matter [31]. Thus, these by-products may not be effective in increasing the mobility and bioavailability of heavy metals. For these reasons, the use of by-products for the successful reclamation of large-scale mining sites requires adequate monitoring of heavy metal behavior in soil–plant systems along with the evaluation of microbial species and their diversity. Although the heavy metals and microbial species related to by-product use have been investigated [21,28,29,30,31,32,33,34,35,36,37,38,39], little information is available in the literature about the fate of heavy metals resulting from the mixing of by-products of the phosphate industry and sewage sludge. Furthermore, very few microbial studies have specifically investigated how these by-products mixing affect microbial species. This study is a continuation of studies carried out by [1] on the new approach for mining site reclamation by using phosphate industry by-products and sludge mixtures.
The objective of this study was to evaluate the effects of the mixture of the phosphate industry by-products and sewage sludge on some heavy metals (Pb, Zn and Cd) and the microbial load (bacteria, fungi and actinobacteria) in the soil–plant system.
2. Materials and Methods
2.1. Substrates Used
The by-products used for this study were phosphogypsum (PG), phosphate waste rock (PWR), sewage sludge (SS) and phosphate sludge (PS). The physicochemical characteristics of different substrates were already known (Table 1).

Table 1.
Physical-chemical properties of the substrates used.
PG was collected from the Jorf Lasfar phosphate industry complex of OCP, located at Jorf Lasfa, close to El Jadida, on the Morocco Atlantic coast.
PWR was sampled in the dumps located near the phosphate mine of the “Mining Operations of Gantour, OCP group”. Samples were obtained from a mixture of three random samples taken from the upper 20 to 30 cm on the site after rock phosphate extraction.
SS (completely dried) came from the Youssoufia wastewater treatment plant, and PS originate from the Youssoufia phosphate beneficiation complex.
All samples were sieved through a 2 mm mesh sieve before physicochemical analysis.
Topsoil (TS) used corresponds to topsoil gathered and put aside prior to mining. It was air dried before being stored in a cool place for reuse during the reclamation phase.
The samples from each by-product used in the present study were analyzed at the soil, plant and water laboratory of the Agriculture Innovation Transfer Technology Center (AITTC) of the University Mohammed 6 polytechnic (UM6P) at Ben Guérir, Morocco, in order to determine their main characteristics.
2.2. Experimental Layout
The pot experiment was conducted in a greenhouse at AITTC for 4 months using Italian ryegrass (Lolium multiflorum). The average temperatures under greenhouse conditions were 12 °C in the morning, 18 °C in the afternoon and 25 °C in the evening; 10 kg of each treatment, consisting of different by-product mixtures, were placed in pots. The experimental layout is a randomized block with five substrates of defined proportions, 10 treatments (T) and 4 replications (R) (Figure 1 and Table 2). Fertilizers were applied in treatments not receiving sludge as described by [1]. The plants in all treatments were irrigated with water from AITTC, whose physicochemical characteristics were already known (Table 3). Ryegrass was harvested and used to calculate bioconcentration factors (BCF) during the three cuts (1st cut at 36 days, then 2nd cut at 38 days after 1st cut and 3rd cut at 60 days after 2nd cut).
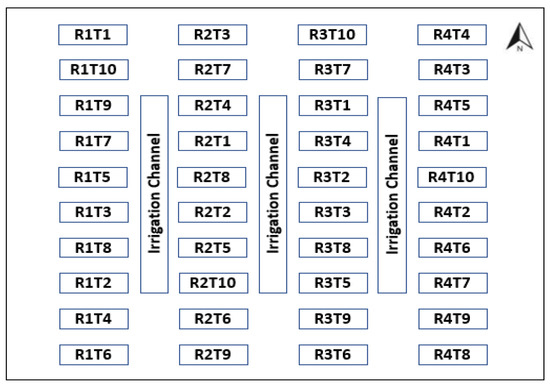
Figure 1.
Test layout.

Table 2.
Treatments used in the experiment [1].

Table 3.
Physicochemical parameters of irrigation water [1].
2.3. Soil and Plant Tissue Analysis
The physicochemical properties of the 10 treatments were determined using different methods. pH was determined in a 1:2.5 soil to distilled water ratio (NF ISO 10390). Organic carbon was determined using the Walkley–Black titration method (NF ISO 10694) [40]. Cation exchange capacity (CEC) was determined by cobalt-hexamine saturation (spectrometry) (NFX 31–130). Particle-size distribution was determined by means of the pipette Robinson (NF ISO 11377) [41]. The total Cd, Pb and Zn contents were measured by ICP-MS (Inductively Coupled Plasma-Mass Spectrometry) after digestion of the samples in HNO3-HCl. All these physicochemical properties were determined for the three cuts of Italian Ryegrass (1st cut at 36 days after seeding, then 2nd cut after 1st cut and 3rd cut at 60 days after 2nd cut).
The ability of the plant to absorb metals from the soil and transfer them from the roots was estimated by the bioconcentration factor (BCF), as described by [42,43]. Thus, the BCF index determined in this study is as follows:
where [M] is the total metal concentration (mg/kg).
BCF = [M]soil/[M]aerial parts,
According to [42,44], plants with BCF > 1 are suitable for phytoextraction and are qualified as a hyperaccumulator, while plants with BCF < 1 are suitable for the phytoimmobilization process.
The phytoextraction efficiency (PEE) by ryegrass under different treatments was calculated as suggested by [45]:
where [M] is the total metal concentration (mg/kg).
PEE (%) = ([M]in plant tissue × Plant Dry Weight per Pot/[M]soil × Soil Weight in Pot) × 100
For microbial assessment, the dilution plate count technique was performed using nutrient agar and potato dextrose agar for bacterial and fungal counts, respectively [34]. Furthermore, for actinobacteria, the dilution plate count technique was used as described by [46]. This microbial assessment was performed at the end of the experimentation.
2.4. Statistical Analysis
Statistical analysis of heavy metal contents and microbial load were based on the ANOVA test. The calculated means and standard deviations were also reported. A significant ANOVA at p ≤ 0.05 indicates the treatments are significantly different. Tukey’s post-hoc multiple mean comparison test was used to test significant differences between treatments at p ≤ 0.05. A Pearson’s correlation matrix among different parameters (physical, chemical and microbiological) was constructed. Principal component analysis (PCA) was performed using the FactoMineR package in R software. It was used to establish the correlation matrix between heavy metals from the soil, the abundance of soil microorganisms and the physico-chemical parameters. All computations were performed using the statistical software R, version 4.0.5 and Excel 2016.
3. Results and Discussion
3.1. Physicochemical Properties and Heavy Metals of Treatments
Physicochemical properties and heavy metals of the ten treatments showed that the textures were sandy silt for T1, T3, T4, T5 and T7 treatments and sandy silt clay for T6, T8, T9 and T10 treatments (Table 4). The texture was different from the T2, which was clay. The pH of the mixture related to different treatments varied from slightly alkaline (T2, T3, T4, T5, T6, T7, T8, T9 and T10) to alkaline (T1). According to [47,48], alkaline soils with pH between 7.1 and 8.1 have a lower risk of heavy metal leaching and bioavailability to plants. The organic matter concentrations were between 0.32% and 3.83%. The lowest values were recorded for treatment T6. The highest value was observed for treatment T10. CEC varied between 30.90 and 49.26 cmol(+)/kg. These values were high compared to those obtained by [49] (9.8–22.60 cmol(+)/kg) during their study in Tunisia on the transfer of lead, zinc and cadmium from mine tailings to wheat. According to [50], high CEC values have the potential for immobilizing heavy metals.

Table 4.
Physicochemical properties of the different treatments.
Pb, Zn and Cd contents during three ryegrass cuts showed that Pb, Zn and Cd contents were highest in all treatment substrates and lowest in the aerial parts (Table 5 and Table 6). Our results are in agreement with those obtained by [49], who found higher contents of Pb, Zn and Cd in the tailings when compared to those of wheat grown on the same tailing. Zn is an essential micronutrient for plants. The Zn contents of the different by-product mixtures of the present study varied between 22 and 80 mg/kg. According to [51], the maximum allowable concentration (MAC) of Zn in agricultural soils proposed by the European Union is 150–300 mg/kg. Cadmium (Cd) and lead (Pb) are two toxic heavy metals to humans and other living organisms when their level is high in the soil, but their contents in soil are generally low [43,44,45,46,47,48,49,50,51,52]. The Cd and Pb soil contents determined in the present study ranged from 0.2 to 1.79 mg/kg and 0.2 to 1.7 mg/kg, respectively. These values are lower than the MAC proposed by European Union for Cd (1–3 mg/kg) and Pb (50–300 mg/kg) in agricultural soils. Furthermore, Zn (0.238–2.136 mg/kg), Pb (0.001–0.026 mg/kg) and Cd (0.001–0.069 mg/kg) contents in plant aerial parts are lower than the Plant Leaf Toxicity Limits (PLTL) (5–30; 30–300 and 100–400 mg/kg for Cd, Pb and Zn, respectively). Thus, the by-products of the phosphate industry and the sewage sludge used in this study neither generate pollution nor constitute a hazard to the environment and human health. These findings are in agreement with a previous study carried out by [53] demonstrated that there is no significant contaminant generation from the phosphate limestone by-products. These by-products can therefore be used for the reclamation of mining sites as well as for agriculture.

Table 5.
Pb, Zn, Cd contents of the ryegrass aerial parts during the three cuts compared to Plant Leaf Toxicity Limits (PLTL).

Table 6.
Soil Pb, Zn, Cd contents at three cuts compared to allowable limits in agricultural soils.
3.2. Bioconcentration Factors (BCF) and Phytoextraction Efficiency (PEE) under Different Treatments
Generally, bioconcentration factor (BCF) values depend on the degree of soil contamination, crop species and soil condition. Thus, it is difficult to accurately measure BCF values that correspond to each condition [54]. However, when the BCF value exceeds 1, plant species are considered to exhibit potential removal for the concerned element [32]. The average BCF of Cd, Pb and Zn varied between 0.0036 and 0.2335, 0.0058 and 0.204 and 0.0059 and 0.0557, respectively. Bioconcentration factors (BCF) of heavy metals showed the relatively highest BCF values (0.2335, 0.204 and 0.0557 for Cd, Pb and Zn, respectively) with T4 (65% PG, 30% PS and 5% SS) (Table 7). This observation corroborated results from [55] that showed that the addition of PG (40 and 80%) caused an accumulation of Cd and Zn in the roots of tomato seedlings. Furthermore, the estimated BCF values for the heavy metals Cd, Pb and Zn for the ten treatments were lower than 1, with values between <0.001 mg/kg and 0.2335 mg/kg. Thus, BCF < 1 indicated that ryegrass was suitable for phytoimmobilization with T4 as the best treatment. This is corroborated by reports [42,44], according to which plants with BCF < 1 are suitable for phytoimmobilization. The Phytoimmobilization capacity of ryegrass has been demonstrated by [55] during their study on two lead and zinc smelters in northern France (Metaleurop Nord, Noyelles-Godault and Nyrstar, Auby). This phytoimmobilization is possible due to the symbiosis between the rhizosphere of the plant and the microbial population. Indeed, plants can alter rhizosphere microbial populations and increase the immobilization of contaminants. This could minimize exposure to contaminants and toxicity to the plant [56]. In addition, the BCF values of Zn (<0.001–0.329 mg/kg) and Cd (<0.001–0.329 mg/kg) were lower or within the range of US-EPA standards. BCF values for Pb (<0.001–0.329 mg/kg) have not yet been regulated by US-EPA.

Table 7.
Bioconcentration factor (BCF) of Zn, Cd and Pb for each aerial part of ryegrass under different treatments.
On the other hand, the average phytoextraction efficiency (PEE) of Cd, Pb and Zn from ryegrass ranged from 0.31 to 2.11%, 0.20 to 1.83% and 0.43 to 2.01%, respectively (Table 8). PEE of heavy metals showed the highest values with T4 (2.11, 1.83 and 2.01 for Cd, Pb and Zn, respectively). This finding indicated that the mixing of by-products could increase both the uptake and transport of Cd, Pb and Zn from the soil environment to ryegrass biomass, particularly in its roots tissue and its phytoextraction efficiency (PEE).

Table 8.
Phytoextraction Efficiency (PEE) of Zn, Cd and Pb for different treatments.
The optimal treatment (T4) composed of 65%PG, 30% PS and 5% SS could favor the successful phytoimmobilization and phytoextraction of heavy metals during the reclamation process.
Furthermore, the variations of heavy metals observed between the different treatments could be explained by the nature of the metals. According to [42], this variation would be due to the nature of the metal, which could be attributed to the form of the metal in the rhizosphere of the substrate. Thus, it appeared that, depending on the nature of the heavy metal in the contaminated soil, ryegrass could have different phytoremediation processes (phytoimmobilization and phytoextraction) regardless of the treatment. However, these different processes seem to depend on the nature and mobile form of the metal in the rhizosphere. In fact, several studies showed the phytoremediation capacity of ryegrass [56,57,58,59,60]. However, this study suggested that ryegrass may have a different phytoremediation process which is probably related to the function of the rhizosphere in the substrate and the form in which the metal can be taken up. Metal speciation will help us better understand the different processes.
3.3. Effects of the Different Treatments on the Microbial Load
The total microflora counts showed that bacteria, fungi and actinobacteria were significantly (p < 0.05) influenced by the variation of the different proportions of substrates used in the treatments T1, T2, T3, T4, T8, T9 and T10 (Figure 2). A high actinobacteria population was observed for most of the treatments (T1, T3, T4, T5, T8, T9 and T10), with the maximum value obtained for treatment T4 (65% PG, 5% SS and 30 % PS) with 281 CFU g−1 of soil. In addition, the highest values of bacteria and fungi were obtained for treatment T4. However, the lowest values of bacteria and fungi were obtained for treatment T6, which is a treatment without SS and PS. Thus, the percentages of PG, PS and SS used in the T4 treatment stimulated microbial activity, as evidenced by increased populations of actinobacteria, bacteria and fungi. These findings corroborate those of [30], who pointed out that by-products application stimulates microbial activity. Furthermore, the abundance of actinobacteria can be explained by their ability to evolve and thrive in soils under both optimal and less optimal conditions [60,61]. According to [62], factors that probably contribute to the evolutionary success of actinobacteria in diverse soil habitats are the ability to utilize a wide variety of carbon sources and to generally subsist on low amounts of resources, their capacity to form spores and thereby survive inhospitable conditions in the soil and the ability to produce secondary metabolites with antibiotic properties. In addition, the effect of the substrates through their physico-chemical composition has strongly affected the microbial diversity either by contribution, stimulation or inhibition. According to [1], treatment T4 was rich in N, P and K with high biomass. Thus, the high BCF and PEE values observed in the T4 treatment could be explained by the microbial activity that improved the conditions for environmental adaptability. The mixing of by-products has led to the development of microbial populations, which cause many physico-chemical changes in the mixture. These changes could influence the distribution of metals through the release of heavy metals during the mineralization of organic matter or the solubilization of metals through the decrease of pH, the biosorption of metals by the microbial biomass or the complexation of metals with newly formed humic substances (HS) or other factors [63]. However, a metagenomic study would help to understand these mechanisms.
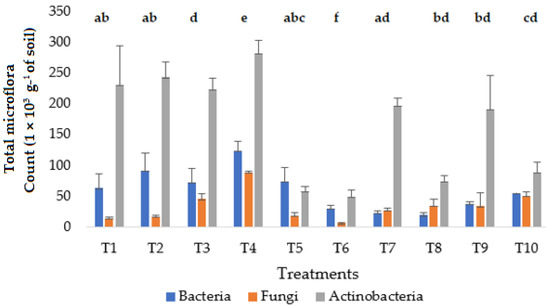
Figure 2.
Impact of the treatments on the total microflora after final harvest. (Error bar represents standard deviation of three replicates; bars with same letters are not significant at p < 0.05).
3.4. Relationship between Soil Microbial Load and Physico-Chemical Properties
In order to verify whether the relative abundance of the soil microbial load is influenced by the different physico-chemical properties (heavy metals in the soil, P, N, K, etc.), a principal component analysis (PCA) and Pearson correlation were performed on these data (Figure 3 and Table 9). The inertia of the first dimensions showed if there were strong relationships between variables and suggests the number of dimensions that should be studied (Figure 4). The first two dimensions of analysis expressed 47.55% of the variance. These two dimensions were sufficient to explain the interactions between the different variables. Each variable is represented by a dimension (Dim). The experience showed that most of the PCA information is usually provided by these two-dimension axes, Dim1 and Dim 2. They define the Dim1 × Dim2 principal plane that we have chosen for our analysis. The correlation between the variables showed a significant correlation between pH × P (−0.90) and Bacteria × Fungi (0.77). There is also a lower degree of correlation between variables such as: P × Pb (−0.58); Fungi × Actinobacteria (−0.49); Cd × Zn (−0.45); Bacteria × Actinobacteria (−0.42); K × Zn (−0.42); pH × Pb (0.60); N × K (0.46); N × Pb (0.41); OM × N (0.39); Zn × Fungi (0.34); Zn × Bacteria (0.31). These different correlations reflected the influence of each physico-chemical property on the other ones and on the soil microbial load. A similar result was obtained by [64,65], where the effects of microbial communities varied significantly between them and on the physicochemical properties of mining soils. However, this influence could contribute to the successful rehabilitation of mine sites. This supports the understanding of different mechanisms to appreciate the relationship between soil, plant and microorganisms.
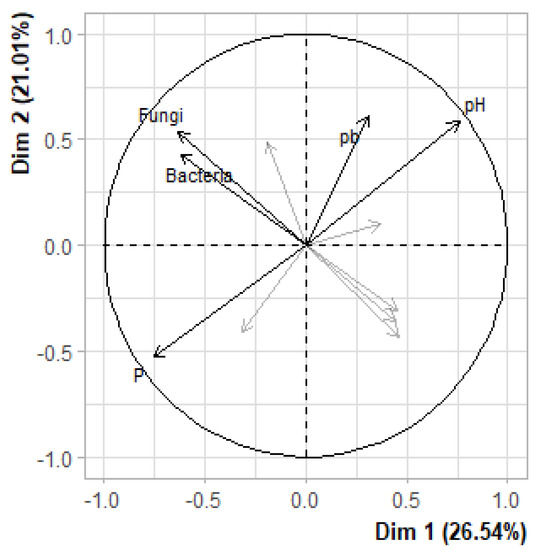
Figure 3.
Principle component analysis (PCA) showing the correlation between heavy metals from soil and plant and microorganisms. The labeled variables are those the best shown on the plane.

Table 9.
Pearson correlation coefficient between soil microbial load and the different physico-chemical properties.
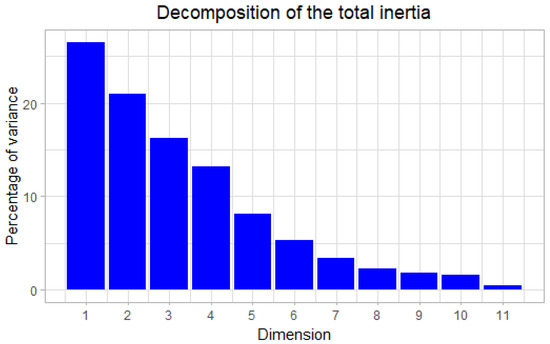
Figure 4.
Decomposition of the total inertia.
4. Conclusions
The use of by-products for the successful reclamation of large-scale mining sites proposes adequate monitoring of heavy metal behavior in soil–plant systems and knowledge of microbial diversity. In this case, the results of this study confirmed that the Pb, Zn and Cd contents of the ten treatments were well below the permissible limits given in the literature. Thus, the by-products of the phosphate industry and the sewage sludge used in this study do not generate pollution nor constitute a hazard to the environment and human health. These by-products could therefore be used for the reclamation of mining sites as well as for agriculture.
Furthermore, the averages BCF and PEE revealed that ryegrass was suitable for phytoimmobilization and phytoextraction. This phytoimmobilization capacity of ryegrass was possible due to the symbiosis between the rhizosphere and the microbial population. In addition, BCF and PEE showed that the highest values were obtained under treatment T4. Thus, the optimal treatment (T4) composed of 65%PG, 30% PS and 5% SS could promote the successful phytoimmobilization and phytoextraction of heavy metals during the reclamation process. Similarly, the evaluation of the microbial population showed a higher richness in actinobacteria with the T4 treatment. Thus, mixing by-products could stimulate microbial activity.
It would be useful to determine the speciation of metals in the soil solution and microbial genetic diversity in order to successfully reclaim mine sites through the valorization of phosphate industry by-products and sewage sludge.
Author Contributions
Conceptualization, Y.K.D.G. and K.E.M.; methodology, Y.K.D.G., Y.B., A.S. and H.J.A.A.; software, Y.K.D.G.; validation, L.M., Y.O., M.H., M.E.G. and K.E.M.; formal analysis, Y.K.D.G. and A.S.; investigation, Y.K.D.G., Y.B., H.J.A.A. and M.H.; resources, M.H., Y.O. and K.E.M.; data curation, M.H. and K.E.M.; writing—original draft preparation, Y.K.D.G. and H.M.; writing—review and editing, Y.K.D.G.; visualization, L.M., Y.O., M.H., A.A.-n. and K.E.M.; supervision, L.M., M.H., Y.O. and K.E.M.; project administration, K.E.M.; funding acquisition, K.E.M. and M.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Gantour OCP phosphate production site of Ben Guerir, Morocco, under the “specific agreement OCP-UM6P no. RE02”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be provided upon request from the corresponding author.
Acknowledgments
Authors would like to greatly acknowledge the OCP Gantour production site of Ben Guerir for the financial support under the Specific Agreement RE02. The AITTC Soil, Water and Plant Analysis Laboratory and the AITTC experimental farm for providing the facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guéablé, Y.K.D.; Bezrhoud, Y.; Moulay, H.; Moughli, L.; Hafidi, M.; El Gharouss, M.; El Mejahed, K. New Approach for Mining Site Reclamation Using Alternative Substrate Based on Phosphate Industry By-Product and Sludge Mixture. Sustainability 2021, 13, 10751. [Google Scholar] [CrossRef]
- Boutaleb, F.; Boutaleb, N.; Bahlaouan, B.; Deblij, S.; El Antri, S. Effect of Phosphate Mine Tailings from Morocco on the Mechanical Properties of Ceramic Tiles. Int. J. Eng. Res. 2020, 9, 140–145. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M.; Ait Babram, M.; Mansori, M.; Favotto, C.; Hakkou, R. Phosphate Sludge-Based Ceramics: Microstructure and Effects of Processing Factors. J. Build. Eng. 2017, 11, 48–55. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 10–14. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M.; Vitola, L.; Alami, J.; Hakkou, R. Recycling of Phosphate Mine Tailings for the Production of Geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- Anjjar, A.; Driouch, Y.; Benjelloun, F.; Ait Hmeid, H.; El Alami, A. The Phosphate Series of Benguerir (Maastrichtian Ypresian, Morocco): Mineralogy and Mine Planning. J. Mater. Environ. Sci. 2020, 11, 574–583. [Google Scholar]
- El Kiram, N.; Jaffal, M.; Kchikach, A.; El Azzab, D.; El Ghorfi, M.; Khadiri, O.; Jourani, E.S.; Manar, A.; Nahim, M. Phosphatic Series under Plio-Quaternary Cover of Tadla Plain, Morocco: Gravity and Seismic Data. Comptes Rendus Geosci. 2019, 351, 420–429. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M.; Hakkou, R. Phosphogypsum recycling: New horizons for a more sustainable road material application. J. Build. Eng. 2020, 30, 101267. [Google Scholar] [CrossRef]
- Gabardi, S.E.L.; Chliyeh, M.; Ouazzani Touhami, A.; Modafar, C.E.L.; Filalimaltouf, A.; Abed, S.E.L.; Ibnsouda, K.S.; Soumia, A.; Moukhli, A.; Benkirane, R.; et al. Diversity of mycorrhizal fungi arbuscular at phosphates sludge, khouribga region (Morocco). Plant Arch. 2019, 19, 2233–2241. [Google Scholar]
- Loutou, M.; Taha, Y.; Benzaazoua, M.; Daafi, Y.; Hakkou, R. Valorization of Clay By-Product from Moroccan Phosphate Mines for the Production of Fired Bricks. J. Clean. Prod. 2019, 229, 169–179. [Google Scholar] [CrossRef]
- Ammar, E.; Maury, H.; Morin, L.; Sghir, A. Environmental, Economic, and Ethical Assessment of the Treated Wastewater and Sewage Sludge Valorization in Agriculture. Handb. Environ. Chem. 2021, 103, 49–78. [Google Scholar]
- Sheoran, V.; Sheoran, A. Reclamation of abandoned mine land. J. Min. Metall. A Min. 2009, 45, 13–32. [Google Scholar]
- Mohamed, A.; Elbaghdadi, M.; Rais, J.; Barakat, A.; Ennaji, W.; Karroum, L.A.; Oumenskou, H.; Ouigmane, A.; Mechadi, M.; Salahddine, D. Characteristics of Sewage Sludge Produced from Wastewater Treatment Plant in the Moroccan City Khouribga. Desalinat. Water Treat. 2018, 112, 179–185. [Google Scholar]
- El Fels, L. Suivi Physico-Chimique, Microbiologique et Écotoxicologique du Compostage de Boues de Step Mélangées à Des Déchets de Palmier: Validation de Nouveaux Indices de Maturité. Ph.D. Thesis, University of Toulouse, Toulouse, France, 2014; p. 295. [Google Scholar]
- Grobelak, A.; Placek, A.; Grosser, A.; Singh, B.R.; Almås, Å.R.; Napora, A.; Kacprzak, M. Effects of Single Sewage Sludge Application on Soil Phytoremediation. J. Clean. Prod. 2017, 155, 189–197. [Google Scholar] [CrossRef]
- Wijesekara, H.; Bolan, N.S.; Kumarathilaka, P.; Geekiyanage, N.; Kunhikrishnan, A.; Seshadri, B.; Saint, C.; Surapaneni, A.; Vithanage, M. Biosolids Enhance Mine Site Rehabilitation and Revegetation. Environ. Mater. Waste 2016, 138, 45–71. [Google Scholar]
- Mays, D.A.; Mortvedt, J.J. Crop Response to Soil Applications of Phosphogypsum. J. Environ. Qual. 1986, 15, 78–81. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Machraoui, S.; Purushotham, M.M.; Naregundi, K.; Labidi, S. Assessment of Radionuclide Transfer Factors and Transfer Coefficients near Phosphate Industrial Areas of South Tunisia. Environ. Sci. Pollut. Res. 2019, 26, 28341–28351. [Google Scholar] [CrossRef]
- Al-Hwaiti, S.M.; AL-Khashman, O.A.; Al-Shaweesh, M.; Almohtasib, A.H. Potentially Utiliations of Jordan Phosphogypqum: A review. Int. J. Curr. Res. 2019, 11, 3258–3262. [Google Scholar]
- Midhat, L.; Ouazzani, N.; Esshaimi, M.; Ouhammou, A.; Mandi, L. Assessment of Heavy Metals Accumulation by Spontaneous Vegetation: Screening for New Accumulator Plant Species Grown in Kettara Mine-Marrakech, Southern Morocco. Int. J. Phytoremed. 2017, 19, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, S.; Abril, J.M.; Mas, J.L.; Periáñez, R.; Polvillo, O.; Delgado, A.; Quintero, J.M. Transfer of Cd, Pb, Ra and U from phosphogypsum amended soils to tomato plants. Water. Air. Soil Pollut. 2009, 203, 65–77. [Google Scholar] [CrossRef]
- Miller, W.P.; Sumner, M.E. Agricultural and Industrial Uses of By-Product Gypsums. ACS Symp. Ser. 1997, 668, 226–239. [Google Scholar]
- Arocena, J.M.; Dudas, M.J.; Poulsen, L.; Rutherford, P.M. Weathering of Clay Minerals Induced by Fluoride-Containing Solutions from Phosphogypsum By-Product. Can. J. Soil Sci. 1995, 75, 219–226. [Google Scholar] [CrossRef]
- Kassir, L.N.; Lartiges, B.; Ouaini, N. Effects of Fertilizer Industry Emissions on Local Soil Contamination: A Case Study of a Phosphate Plant on the East Mediterranean Coast. Environ. Technol. 2012, 33, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Es-Said, A.; Nafai, H.; Hamdaoui, L.E.; Bouhaouss, A.; Bchitou, R. Adsorptivity and Selectivity of Heavy Metals Cd(Ii), Cu(Ii), and Zn(Ii) toward Phosphogypsum. Desalinat. Water Treat. 2020, 197, 291–299. [Google Scholar] [CrossRef]
- Nayak, S.; Mishra, C.S.K.; Guru, B.C.; Rath, M. Effect of Phosphogypsum Amendment on Soil Physico-Chemical Properties, Microbial Load and Enzyme Activities. J. Environ. Biol. 2011, 32, 613–617. [Google Scholar]
- Machulla, G.; Bruns, M.A.; Scow, K.M. Microbial Properties of Mine Spoil Materials in the Initial Stages of Soil Development. Soil Sci. Soc. Am. J. 2005, 69, 1069–1077. [Google Scholar] [CrossRef]
- Press, C.M.; Mahaffee, W.F.; Kloepper, J.W.; Edwards, J.H.; Edwards, J.H. Organic By-Product Effects on Soil Chemical Properties and Microbial Communities. Compost. Sci. Util. 1996, 4, 70–80. [Google Scholar] [CrossRef]
- Harchi, J. Abandoned Mines in South of Morocco: Impact on Physicochemical and Biological Properties of Soils and on Heavy Metals Uptake by Plants. Master Sci. Tech. Biotechnol. 2020, 37, 22–25. [Google Scholar]
- Kandeler, E.; Tscherko, D.; Bruce, K.D.; Stemmer, M.; Hobbs, P.J.; Bardgett, R.D.; Amelung, W. Structure and Function of the Soil Microbial Community in Microhabitats of a Heavy Metal Polluted Soil. Biol. Fertil. Soils 2000, 32, 390–400. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Trace Elements and Fluoride in Phosphogypsum Leachates. Environ. Technol. 1995, 16, 343–354. [Google Scholar] [CrossRef]
- Jalali, J.; Gaudin, P.; Capiaux, H.; Ammar, E.; Lebeau, T. Fate and Transport of Metal Trace Elements from Phosphogypsum Piles in Tunisia and Their Impact on Soil Bacteria and Wild Plants. Ecotoxicol. Environ. Saf. 2019, 174, 12–25. [Google Scholar] [CrossRef]
- Anning, A.K.; Akoto, R. Assisted Phytoremediation of Heavy Metal Contaminated Soil from a Mined Site with Typha Latifolia and Chrysopogon Zizanioides. Ecotoxicol. Environ. Saf. 2018, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, P.; Samkumar, R.A.; Satheesh, V.; Subashchandrabose, S.R.; Ramadass, K.; Naidu, R.; Venkateswarlu, K.; Megharaj, M. Microbes from mined sites: Harnessing their potential for reclamation of derelict mine sites. Environ. Pollut. 2017, 230, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, Mechanism and Biotechnology of Phosphate Solubilising Microorganism in Mangrove—A Review. Biocatal. Agric. Biotechnol. 2014, 97, 110. [Google Scholar] [CrossRef]
- Mishra, C.S.K.; Nayak, S.; Guru, B.C.; Rath, M. Environmental Impact and Management of Wastes from Phosphate Fertilizer Plants. J. Ind. Pollut. Control 2010, 26, 57–60. [Google Scholar]
- Montero, A.; Tojo, Y.; Matsuo, T.; Matsuto, T.; Yamada, M.; Asakura, H.; Ono, Y. Gypsum and Organic Matter Distribution in a Mixed Construction and Demolition Waste Sorting Process and Their Possible Removal from Outputs. J. Hazard. Mater. 2010, 175, 747–753. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods of Soil Analysis, Part 3. Chem. Methods 1982, 5, 961–1010. [Google Scholar]
- Thomas, G.W. Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties. ASA Monogr. Number 1982, 9, 159–165. [Google Scholar]
- Bongoua-Devisme, A.J.; Akotto, O.F.; Guéty, T.; Kouakou, S.A.A.E.; Ndoye, F.; Diouf, D. Enhancement of Phytoremediation Efficiency of Acacia Mangium Using Earthworms in Metal-Contaminated Soil in Bonoua, Ivory Coast. Afr. J. Biotechnol. 2019, 18, 622–631. [Google Scholar] [CrossRef]
- Buscaroli, A. An Overview of Indexes to Evaluate Terrestrial Plants for Phytoremediation Purposes (Review). Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Majid, N.M.; Islam, M.M.; Mathew, L. Heavy metal uptake and translocation by mangium (Acacia mangium) from 636 sewage sludge contaminated soil. Aust. J. Crop Sci. 2012, 6, 1228–1235. [Google Scholar]
- Yang, Y.; Ge, Y.; Zeng, H.; Zhou, X.; Peng, L.; Zeng, Q. Phytoextraction of Cadmium-Contaminated Soil and Potential of Regenerated Tobacco Biomass for Recovery of Cadmium. Sci. Rep. 2017, 7, 7210. [Google Scholar] [CrossRef] [PubMed]
- Ouhdouch, Y.; Barakate, M.; Finance, C. Actinomycetes of Moroccan Habitats: Isolation and Screening for Antifungal Activities. Eur. J. Soil Biol. 2001, 37, 69–74. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Bielecka, A.; Rylko, E.; Bojanowska, I.; Ochrona, S.I.Z. Zawartość Pierwiastków Metalicznych w Glebach i Warzywach z Ogrodów Działkowych Gdańska i Okolic (Contents of Metals in Soils and Vegetables from Gdansk and Straszyn Allotments). Environ. Prot. Nat. Resour. 2009, 40, 209–216. [Google Scholar]
- Boussen, S.; Soubrand, M.; Bril, H.; Ouerfelli, K.; Abdeljaouad, S. Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 2013, 192, 227–236. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.Q.; Wang, D.F. Immobilization of Heavy Metals in Sewage Sludge during Land Application Process in China: A Review. Sustainability 2017, 9, 2–19. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants: An Overview. J. Environ. Sci. Health Part A 1999, 34, 58. [Google Scholar]
- Aprile, A.; Sabella, E.; Francia, E.; Milc, J.; Ronga, D.; Pecchioni, N.; Ferrari, E.; Luvisi, A.; Vergine, M.; Bellis, L.D. Combined effect of Cadmium and Lead on Durum Wheat. Int. J. Mol. Sci. 2019, 20, 5891. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Nzioka, A.M.; Kim, Y.J.; Sakong, J. Evaluation of Bioconcentration Factors of Metals and Non-Metals in Crops and Soil from Abandoned Mines in Korea. Contemp. Probl. Ecol. 2017, 10, 583–590. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Valorization of Phosphate Waste Rocks and Sludge from the Moroccan Phosphate Mines: Challenges and Perspectives. Procedia Eng. 2016, 138, 110–118. [Google Scholar] [CrossRef]
- Waterlot, C.; Hechelski, M. Benefits of Ryegrass on Multicontaminated Soils Part 1, Effects of Fertilizers on Bioavailability and Accumulation of Metals. Sustainability 2019, 11, 5093. [Google Scholar] [CrossRef]
- Kirk, J.L.; Klironomos, J.N.; Lee, H.; Trevors, J.T. The Effects of Perennial Ryegrass and Alfalfa on Microbial Abundance and Diversity in Petroleum Contaminated Soil. Environ. Pollut. 2005, 133, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.C.; Chuan, H.G.; Tian, Y.T.; Xiao, W.Z.; Yi, R.X.; Yu, H.S.; Ke, F. Responses of Ryegrass (Lolium perenne L.) Grown in Mudflats to Sewage Sludge Amendment. J. Integr. Agric. 2014, 13, 426–433. [Google Scholar] [CrossRef]
- Lou, Y.; Lou, Y.; Luo, H.; Hu, T.; Li, H.; Fu, J. Toxic Effects, Uptake, and Translocation of Cd and Pb in Perennial Ryegrass. Ecotoxicology 2013, 22, 207–214. [Google Scholar] [CrossRef]
- Wang, Y.P.; Shi, J.Y.; Wang, H.; Lin, Q.; Chen, X.C.; Chen, Y.X. The Influence of Soil Heavy Metals Pollution on Soil Microbial Biomass, Enzyme Activity, and Community Composition near a Copper Smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef]
- Yadav, A.N.; Yadav, N. Stress-Adaptive Microbes for Plant Growth Promotion and Alleviation of Drought Stress in Plants. Acta Sci. Agric. 2018, 2, 85–88. [Google Scholar]
- Ranjan, V.; Sen, P.; Kumar, D. Dump Slope Stabilisation through Revegetation in Iron Ore Mines in Bonai Iron Ore Range: A Review. Int. J. Min. Miner. Eng. 2017, 8, 334–351. [Google Scholar] [CrossRef]
- Kaltenpoth, M. Actinobacteria as Mutualists: General Healthcare for Insects? Trends Microbiol. 2009, 17, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.C. Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 2005, 59, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Santos, S.S.; Vestergård, M.; González, A.M.M.; Ma, L.; Feng, Y.; Yang, X. A Field Study Reveals Links between Hyperaccumulating Sedum Plants-Associated Bacterial Communities and Cd/Zn Uptake and Translocation. Sci. Total Environ. 2022, 805, 150400. [Google Scholar] [CrossRef] [PubMed]
- Praeg, N.; Seeber, J.; Leitinger, G.; Tasser, E.; Newesely, C.; Tappeiner, U.; Illmer, P. The Role of Land Management and Elevation in Shaping Soil Microbial Communities: Insights from the Central European Alps. Soil Biol. Biochem. 2020, 150, 107951. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).