Optimizing Phosphorus Application Rate and the Mixed Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Can Improve the Phosphatase Activity and Organic Acid Content in Alfalfa Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Soil Sample Collection
2.4. Measurement Index and Method
2.4.1. Determination of Alkaline Phosphatase Activity (AKP) in the Soil
2.4.2. Determination of Organic Acid Content
2.4.3. pH Value
2.4.4. Soil Organic Matter (SOM)
2.4.5. Data Processing and Analysis
3. Results
3.1. The Changes of AKP Content in Soil under Different Treatments
3.2. The Changes of Organic Acid Content under Different Treatments
3.3. The pH Change Caused by Different Treatments in Soil
3.4. Change of Bacteria and Phosphorus Treatments on Soil Organic Matter
3.5. Correlation Analysis of Each Index
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Effects of Inoculating AMF and PSB on Phosphatase Activity in Soil under Different Phosphorus Applications
4.2. Effects of Inoculating AMF and PSB on Organic Acid Content under Different Phosphorus Applications
4.3. Effects of Inoculating AMF and PSB on pH Value and SOM Content in Soil under Different Phosphorus Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| AKP | Alkaline phosphatase activity |
| Bm | Bacillus megaterium |
| Fm | Funneliformis mosseae |
| PSB | Phosphate solubilizing bacteria |
| P | Phosphorus |
| SOM | Soil organic matter |
References
- Zhang, Q.; Liu, J.; Liu, X.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Optimizing water and phosphorus management to improve hay yield and water- and phosphorus-use efficiency in alfalfa under drip irrigation. Food Sci. Nutr. 2020, 8, 2406–2418. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, J.; Wan, L.; Zhou, Z.; Wang, Z.; Song, C.; Zhou, Y.; Cao, X. Mutual Dependence of Nitrogen and Phosphorus as Key Nutrient Elements: One Facilitates Dolichospermum flos-aquae to Overcome the Limitations of the Other. Environ. Sci. Technol. 2018, 52, 5653–5661. [Google Scholar] [CrossRef]
- Berg, W.K.; Lissbrant, S.; Cunningham, S.M.; Brouder, S.M.; Volenec, J.J. Phosphorus and potassium efects on taproot C and N reserve pools and long-term persistence of alfalfa (Medicago sativa L.). Plant Sci. 2018, 272, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kucey, R.M.N. Phosphate-Solubilizing Bacteria and Fungi in Various Cultivated and Virgin Alberta Soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial biofilms in nature: Unlocking their potential for agricultural applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Che, Y.; Wang, L.; Zhao, Z.; Zhang, Y.; Wei, L.; Xiao, Y. Rice straw biochar and phosphorus inputs have more positive effects on the yield and nutrient uptake of Lolium multiflorum than arbuscular mycorrhizal fungi in acidic Cd-contaminated soils. Chemosphere 2019, 235, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hui, J.F.; Sun, M.Y.; Liu, X.S.; Lu, W.H.; Ma, C.H.; Zhang, Q.B. Effects of phosphorus application level, inoculation of AMF and Phosphorus Solubilizing Bacteria on Alfalfa Yield and phosphorus utilization efficiency. J. Agric. Eng. 2020, 36, 142–149. [Google Scholar] [CrossRef]
- Bolan, N. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Javot, H.; Pumplin, N.; Harrison, M.J. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ. 2006, 30, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Louche, J.; Ali, M.A.; Hurteau, B.C. Efciency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol. Ecol. 2010, 73, 323–335. [Google Scholar] [PubMed] [Green Version]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Xie, X.; Weng, B.; Cai, B.; Dong, Y.; Yan, C. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth and nutrient uptake of Kandelia obovata (Sheue, Liu & Yong) seedlings in autoclaved soil. Appl. Soil Ecol. 2014, 75, 162–171. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y.; Liu, X.; Du, G. Spatio-temporal dynamics of arbuscular mycorrhizal fungi associated with glomalin-related soil protein and soil enzymes in different managed semiarid steppes. Mycorrhiza 2014, 24, 525–538. [Google Scholar] [CrossRef]
- Gomezjurado, M.E.G.; de Abreu, L.M.; Marra, L.M.; Pfenning, L.H.; Moreira, F.M.D.S. Phosphate Solubilization by Several Genera of Saprophytic fungi and Its Influence on Corn and Cowpea Growth. J. Plant Nutr. 2014, 38, 675–686. [Google Scholar] [CrossRef]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.-T.; Zeng, Q.-W.; Zhang, S.-C.; Liao, B.; Shu, W.-S.; Li, J.-T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Saxena, J.; Rawat, J.; Sanwal, P. Enhancement of Growth and Yield of Glycine max Plants with Inoculation of Phosphate Solubilizing Fungus Aspergillus niger K7 and Biochar Amendment in Soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2334–2347. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Li, Y.B.; Tan, X.P.; He, W.; Xie, W.; Megharaj, M.; Wei, G.H. Effect of arsenate contamination on free, immobilized and soil alkaline phosphatases: Activity, kinetics and thermodynamics. Eur. J. Soil Sci. 2016, 68, 126–135. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Wei, M.; Zeng, G.; Hou, S.; Li, D.; Xu, H. Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 2020, 239, 124706. [Google Scholar] [CrossRef]
- Liu, B.; Schieber, J.; Mastalerz, M.; Teng, J. Organic matter content and type variation in the sequence stratigraphic context of the Upper Devonian New Albany Shale, Illinois Basin. Sediment. Geol. 2019, 383, 101–120. [Google Scholar] [CrossRef]
- Chatterjee, S. A New Coefficient of Correlation. J. Am. Stat. Assoc. 2020, 116, 2009–2022. [Google Scholar] [CrossRef]
- Shaw, A.N.; Cleveland, C.C. The effects of temperature on soil phosphorus availability and phosphatase enzyme activities: A cross-ecosystem study from the tropics to the Arctic. Biogeochemistry 2020, 151, 113–125. [Google Scholar] [CrossRef]
- Ghorchiani, M.; Etesami, H.; Alikhani, H.A. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018, 258, 59–70. [Google Scholar] [CrossRef]
- Amaya-Carpio, L.; Davies, F.; Fox, T.; He, C. Arbuscular mycorrhizal fungi and organic fertilizer influence photosynthesis, root phosphatase activity, nutrition, and growth of Ipomoea carnea ssp. fistulosa. Photosynthetica 2009, 47, 1–10. [Google Scholar] [CrossRef]
- Abdelfattah, G.M.; Asrar, A.; Al-Amri, S.M.; Abdel-Salam, E. Influence of arbuscular mycorrhiza and phosphorus fertilization on the gas exchange, growth and phosphatase activity of soybean (Glycine max L.) plants. Photosynthetica 2014, 52, 581–588. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, R.; Kertesz, M.A.; Zhang, F.; Feng, G. Arbuscular mycorrhizal fungal hyphae mediating acidification can promote phytate mineralization in the hyphosphere of maize (Zea mays L.). Soil Biol. Biochem. 2013, 65, 69–74. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; He, X.-H. Differences of hyphal and soil phosphatase activities in drought-stressed mycorrhizal trifoliate orange (Poncirus trifoliata) seedlings. Sci. Hortic. 2011, 129, 294–298. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef]
- De Oliveira, I.F.; Simeone, M.L.F.; de Guimarães, C.C.; Garcia, N.S.; Schaffert, R.E.; de Sousa, S.M. Sorgoleone concentration influences mycorrhizal colonization in sorghum. Mycorrhiza 2020, 31, 259–264. [Google Scholar] [CrossRef]
- Aoki, M.; Fujii, K.; Kitayama, K. Environmental Control of Root Exudation of Low-Molecular Weight Organic Acids in Tropical Rainforests. Ecosystems 2012, 15, 1194–1203. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative Stress and Antioxidant Responses in Young Leaves of Mulberry Plants Grown Under Nitrogen, Phosphorus or Potassium Deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Chen, L.-S.; Tang, N.; Jiang, H.-X.; Yang, L.-T.; Li, Q.; Smith, B.R. Changes in organic acid metabolism differ between roots and leaves of Citrus grandis in response to phosphorus and aluminum interactions. J. Plant Physiol. 2009, 166, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Yamakawa, T.; Ikeda, M.; Ikeda, M.; Ishizuka, J.J. Effects of root exudates of Rj2Rj3- and Rj4- genotype soybeans on growth and chemotaxis of Bradyrhizobium japonicum. Soil Sci Plant Nutr. 1996, 42, 413–417. [Google Scholar] [CrossRef]
- Bavaresco, L.; Colla, R.; Fogher, C. Different responses to root infection with endophytic microorganisms of Vitis vinifera L. cv. pinot blanc grown on calcareous soil. J. Plant Nutr. 2000, 23, 1107–1116. [Google Scholar] [CrossRef]
- Kumar, A.; Dwivedi, G.K.; Tewari, S.; Paul, J.; Anand, R.; Kumar, N.; Kumar, P.; Singh, H.; Kaushal, R. Carbon Mineralization and Inorganic Nitrogen Pools under Terminalia chebula Retz.-Based Agroforestry System in Himalayan Foothills, India. For. Sci. 2020, 66, 634–643. [Google Scholar] [CrossRef]
- Matse, D.T.; Huang, C.-H.; Huang, Y.-M.; Yen, M.-Y. Nitrogen uptake and growth of white clover inoculated with indigenous and exotic Rhizobium strains. J. Plant Nutr. 2020, 43, 2013–2027. [Google Scholar] [CrossRef]
- Cao, J.; Huang, Y.; Wang, C. Rhizosphere interactions between earthworms (Eisenia fetida) and arbuscular mycorrhizal fungus (Funneliformis mosseae) promote utilization efficiency of phytate phosphorus in maize. Appl. Soil Ecol. 2015, 94, 30–39. [Google Scholar] [CrossRef]
- Yu, X.-J.; Chen, Q.; Shi, W.-C.; Gao, Z.; Sun, X.; Dong, J.-J.; Li, J.; Wang, H.-T.; Gao, J.-G.; Liu, Z.-G.; et al. Interactions between phosphorus availability and microbes in a wheat–maize double cropping system: A reduced fertilization scheme. J. Integr. Agric. 2022, 21, 840–854. [Google Scholar] [CrossRef]
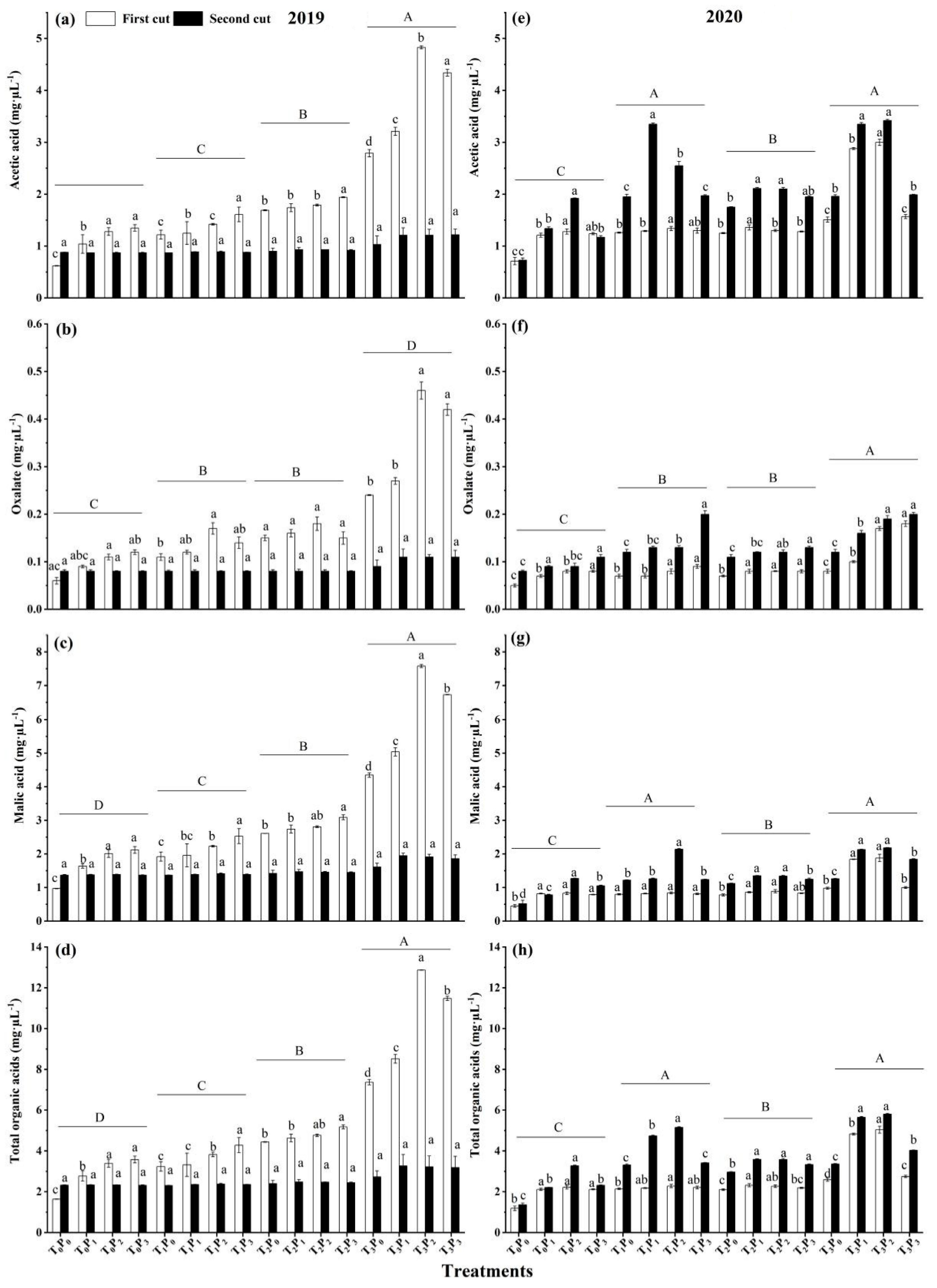


 and ■ represent P0, P1, P2, and P3, respectively.
and ■ represent P0, P1, P2, and P3, respectively.
 and ■ represent P0, P1, P2, and P3, respectively.
and ■ represent P0, P1, P2, and P3, respectively.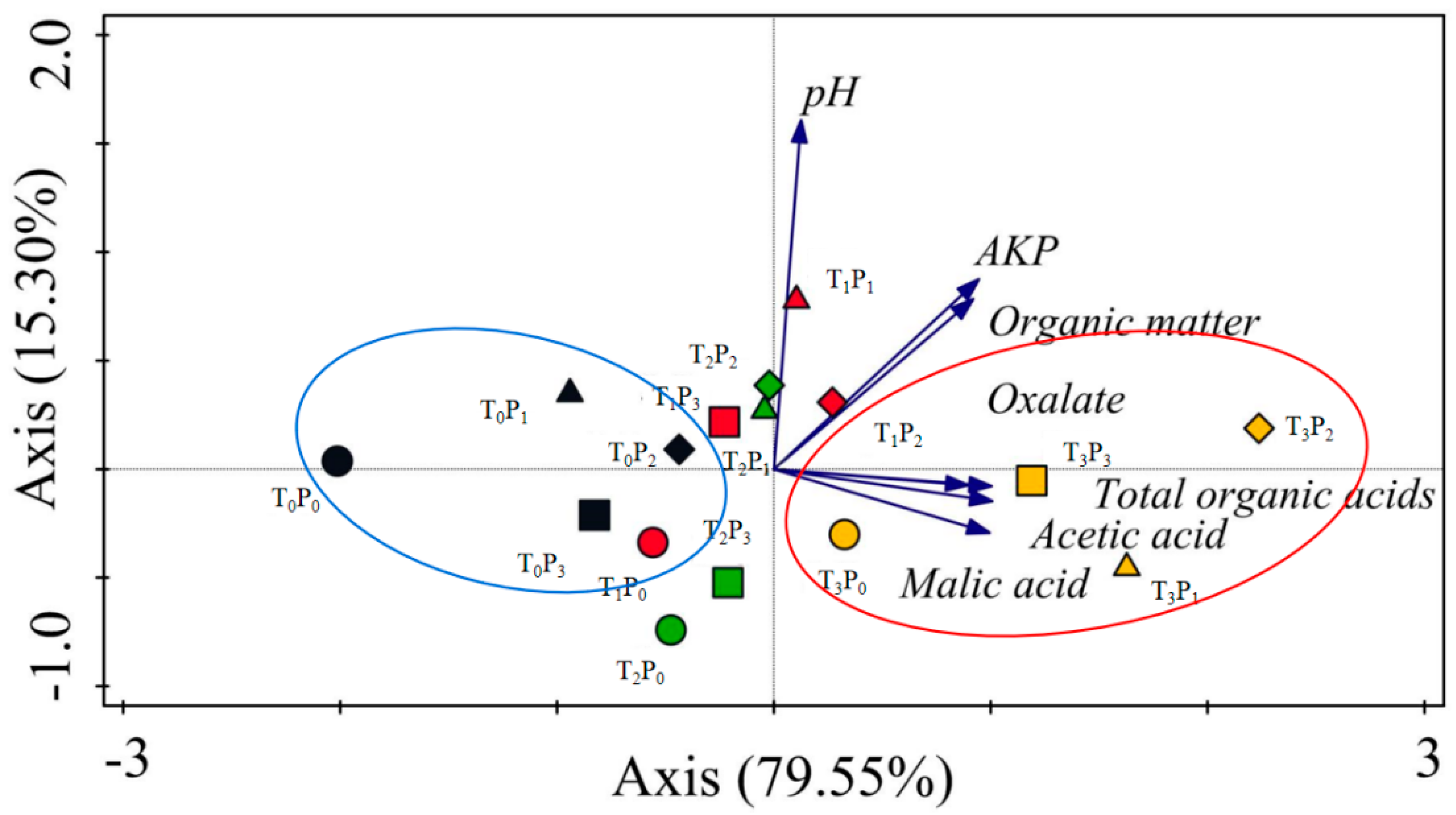
| Treatments | Funneliformis mosseae (Fm, T1) | Bacillus megaterium (Bm, T2) | Double Inoculation (Fm × Bm, T3) | No Inoculation Bacteria (CK, T0) |
|---|---|---|---|---|
| 0 mg kg−1 (P0) | T1P0 | T2P0 | T3P0 | T0P0 |
| 50 mg kg−1 (P1) | T1P1 | T2P1 | T3P1 | T0P1 |
| 100 mg kg−1 (P2) | T1P2 | T2P2 | T3P2 | T0P2 |
| 150 mg kg−1 (P3) | T1P3 | T2P3 | T3P3 | T0P3 |
| Bulk Density/ (g cm−3) | Alkaline-N/ (mg kg−1) | Organic Matter/ (g kg−1) | Available Phosphorus/ (mg kg−1) | Total Phosphorus/ (g kg−1) | Available K/ (mg kg−1) |
|---|---|---|---|---|---|
| 1.48 | 72.6 | 24.28 | 18.17 | 0.21 | 135.6 |
| Number | Treatments | NH4H2PO4 (mg pot−1) (Containing N 12.2%) | CN2H4O (mg pot−1) (Containing N 46%) | Funneliformis mosseae (g pot−1) | Bacillus megaterium (mL pot−1) |
|---|---|---|---|---|---|
| 1 | T0P0 | 0 | 105.3 | 0 | 0 |
| 2 | T0P1 | 35.1 | 72.9 | 0 | 0 |
| 3 | T0P2 | 72.9 | 35.1 | 0 | 0 |
| 4 | T0P3 | 105.3 | 0 | 0 | 0 |
| 5 | T1P0 | 0 | 105.3 | 10 | 0 |
| 6 | T1P1 | 35.1 | 72.9 | 10 | 0 |
| 7 | T1P2 | 72.9 | 35.1 | 10 | 0 |
| 8 | T1P3 | 105.3 | 0 | 10 | 0 |
| 9 | T2P0 | 0 | 105.3 | 0 | 10 |
| 10 | T2P1 | 35.1 | 72.9 | 0 | 10 |
| 11 | T2P2 | 72.9 | 35.1 | 0 | 10 |
| 12 | T2P3 | 105.3 | 0 | 0 | 10 |
| 13 | T3P0 | 0 | 105.3 | 5 | 5 |
| 14 | T3P1 | 35.1 | 72.9 | 5 | 5 |
| 15 | T3P2 | 72.9 | 35.1 | 5 | 5 |
| 16 | T3P3 | 105.3 | 0 | 5 | 5 |
| Treat-ments | Rhizosphere Soil AKP in 2019 | Non-Rhizosphere Soil AKP in 2019 | Rhizosphere Soil AKP in 2020 | Non-Rhizosphere Soil AKP in 2020 | ||||
|---|---|---|---|---|---|---|---|---|
| First Cut | Second Cut | First Cut | Second Cut | First Cut | Second Cut | First Cut | Second Cut | |
| T0P0 | 2.76 ± 0.04 Cc | 6.03 ± 0.09 Cd | 3.32 ± 0.06 Cc | 6.70 ± 0.09 Bb | 1.84 ± 0.01 Cd | 1.08 ± 0.03 Dd | 1.09 ± 0.02 Dc | 1.11 ± 0.04 Dd |
| T0P1 | 3.18 ± 0.08 Ba | 6.62 ± 0.08 Bc | 3.63 ± 0.05 Bb | 7.11 ± 0.03 Aa | 2.16 ± 0.02 Dc | 2.11 ± 0.05 Da | 1.86 ± 0.06 Da | 1.88 ± 0.08 Db |
| T0P2 | 2.93 ± 0.04 Bbc | 6.81 ± 0.03 Ca | 3.77 ± 0.05 Aa | 7.13 ± 0.16 Aa | 2.34 ± 0.01 Da | 1.84 ± 0.03 Db | 1.80 ± 0.03 Dab | 2.59 ± 0.07 Da |
| T0P3 | 2.84 ± 0.06 Cb | 6.53 ± 0.06 Bb | 3.27 ± 0.09 Bc | 6.88 ± 0.3 Bab | 2.23 ± 0.02 Cb | 1.49 ± 0.06 Dc | 1.72 ± 0.03 Bb | 1.65 ± 0.01 Dc |
| T1P0 | 3.18 ± 0.02 Ab | 6.29 ± 0.04 Bb | 3.72 ± 0.04 Ab | 7.19 ± 0.16 Aa | 2.17 ± 0.07 Bd | 1.87 ± 0.05 Bc | 1.51 ± 0.05 Bc | 2.15 ± 0.07 Bd |
| T1P1 | 3.20 ± 0.04 Bb | 6.32 ± 0.12 Cb | 3.84 ± 0.06 Aa | 7.21 ± 0.18 Aa | 2.84 ± 0.03 Ba | 2.97 ± 0.03 Bb | 2.37 ± 0.08 Ca | 5.21 ± 0.05 Aa |
| T1P2 | 3.33 ± 0.08 Aa | 7.19 ± 0.01 Ba | 3.35 ± 0.08 Bc | 7.13 ± 0.09 Aa | 2.46 ± 0.01 Cb | 3.72 ± 0.06 Ba | 2.31 ± 0.09 Ca | 3.31 ± 0.06 Bb |
| T1P3 | 3.23 ± 0.01 Ab | 7.17 ± 0.01 Aa | 3.34 ± 0.05 Bc | 6.89 ± 0.17 Bb | 2.26 ± 0.08 Cc | 3.01 ± 0.06 Bb | 1.79 ± 0.04 Bb | 2.87 ± 0.04 Bc |
| T2P0 | 3.06 ± 0.04 Bbc | 6.44 ± 0.13 ABb | 3.19 ± 0.02 Db | 6.91 ± 0.15 Ba | 2.10 ± 0.06 Bd | 1.72 ± 0.04 Cc | 1.36 ± 0.01 Cd | 1.54 ± 0.03 Cd |
| T2P1 | 3.30 ± 0.06 Aa | 6.63 ± 0.08 Ba | 3.29 ± 0.04 Ca | 7.07 ± 0.08 Aa | 2.62 ± 0.02 Cb | 2.32 ± 0.03 Ca | 2.65 ± 0.02 Bb | 3.10 ± 0.04 Ca |
| T2P2 | 3.09 ± 0.05 Bb | 6.36 ± 0.11 Db | 3.38 ± 0.03 Ba | 6.85 ± 0.25 Ba | 2.69 ± 0.02 Ba | 2.29 ± 0.02 Ca | 3.25 ± 0.06 Ba | 2.90 ± 0.02 Cb |
| T2P3 | 2.97 ± 0.06 Bc | 5.22 ± 0.10 Cc | 3.31 ± 0.06 Ba | 6.83 ± 0.17 Ba | 2.34 ± 0.03 Bc | 1.83 ± 0.02 Cb | 1.77 ± 0.01 Bc | 2.21 ± 0.08 Cc |
| T3P0 | 3.10 ± 0.03 ABb | 6.60 ± 0.02 Ab | 3.52 ± 0.06 Bd | 6.77 ± 0.02 Bb | 2.26 ± 0.01 Ad | 2.27 ± 0.06 Ad | 3.47 ± 0.05 Ac | 3.04 ± 0.06 Ad |
| T3P1 | 3.36 ± 0.06 Aa | 6.83 ± 0.17 Ac | 3.71 ± 0.08 Bc | 7.09 ± 0.08 Aa | 2.93 ± 0.06 Ab | 3.47 ± 0.07 Ab | 3.51 ± 0.11 Ac | 4.56 ± 0.07 Bb |
| T3P2 | 3.33 ± 0.07 Aa | 7.89 ± 0.20 Aa | 3.83 ± 0.02 Ab | 7.25 ± 0.04 Aa | 5.00 ± 0.01 Aa | 3.99 ± 0.05 Aa | 5.29 ± 0.13 Aa | 5.43 ± 0.05 Aa |
| T3P3 | 3.14 ± 0.09 Ab | 7.09 ± 0.10 Ab | 4.22 ± 0.02 Aa | 7.22 ± 0.06 Aa | 2.76 ± 0.05 Ac | 3.08 ± 0.04 Ac | 4.92 ± 0.06 Ab | 3.27 ± 0.12 Ac |
| T | ** | ** | ** | ** | ** | ** | ** | ** |
| P | ** | ** | ** | ** | ** | ** | ** | ** |
| T × P | ** | ** | ** | ** | ** | ** | ** | ** |
| Index | AKP | Malic Acid | Oxalate | Acetic Acid | Total Organic Acids | pH Value |
|---|---|---|---|---|---|---|
| Malic acid | 0.562 ** | |||||
| Oxalate | 0.769 ** | 0.579 ** | ||||
| Acetic acid | 0.394 * | 0.180 | 0.670 ** | |||
| Total organic acids | 0.577 ** | 0.984 ** | 0.670 ** | 0.200 | ||
| pH value | 0.627 ** | 0.094 | −0.620 | 0.576 ** | 0.147 | |
| Organic matter | 0.936 * | 0.613 ** | 0.832 ** | 0.647 ** | 0.612 ** | 0.618 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Liu, J.; Liu, X.; Ma, C.; Zhang, Q. Optimizing Phosphorus Application Rate and the Mixed Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Can Improve the Phosphatase Activity and Organic Acid Content in Alfalfa Soil. Sustainability 2022, 14, 11342. https://doi.org/10.3390/su141811342
An X, Liu J, Liu X, Ma C, Zhang Q. Optimizing Phosphorus Application Rate and the Mixed Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Can Improve the Phosphatase Activity and Organic Acid Content in Alfalfa Soil. Sustainability. 2022; 14(18):11342. https://doi.org/10.3390/su141811342
Chicago/Turabian StyleAn, Xiaoxia, Junying Liu, Xuanshuai Liu, Chunhui Ma, and Qianbing Zhang. 2022. "Optimizing Phosphorus Application Rate and the Mixed Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Can Improve the Phosphatase Activity and Organic Acid Content in Alfalfa Soil" Sustainability 14, no. 18: 11342. https://doi.org/10.3390/su141811342
APA StyleAn, X., Liu, J., Liu, X., Ma, C., & Zhang, Q. (2022). Optimizing Phosphorus Application Rate and the Mixed Inoculation of Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria Can Improve the Phosphatase Activity and Organic Acid Content in Alfalfa Soil. Sustainability, 14(18), 11342. https://doi.org/10.3390/su141811342






