The Influence of Plastic Barriers on Aerosol Infection Risk during Airport Security Checks
Abstract
:1. Introduction
2. Methods
2.1. Modeling of TSA Area
2.2. CFD Modeling
2.3. Aerosol Infection Risk Estimation
2.4. Local Ventilation Efficiencies
3. Results
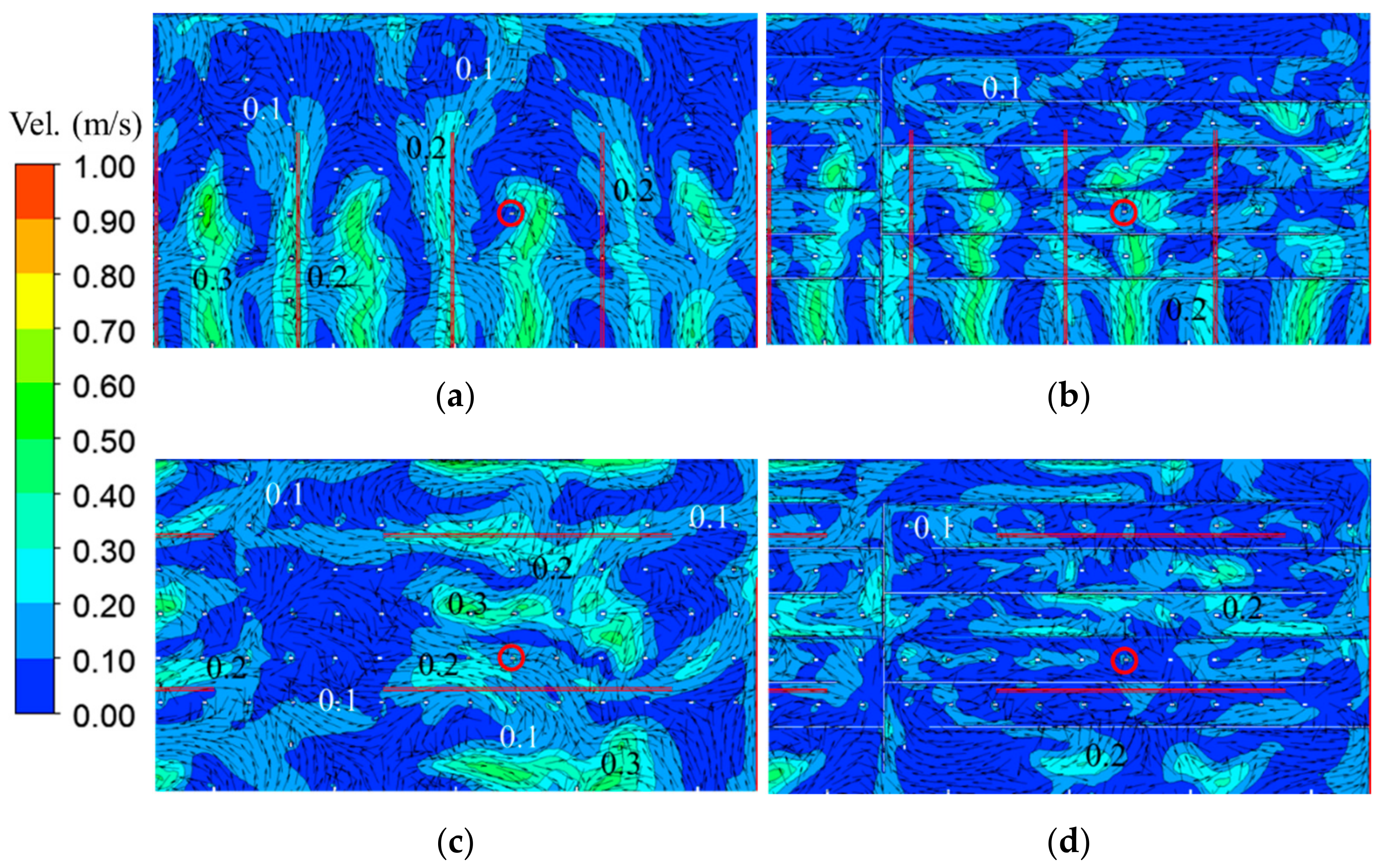
3.1. Velocity Distribution
3.2. Local Ventilation Efficiency
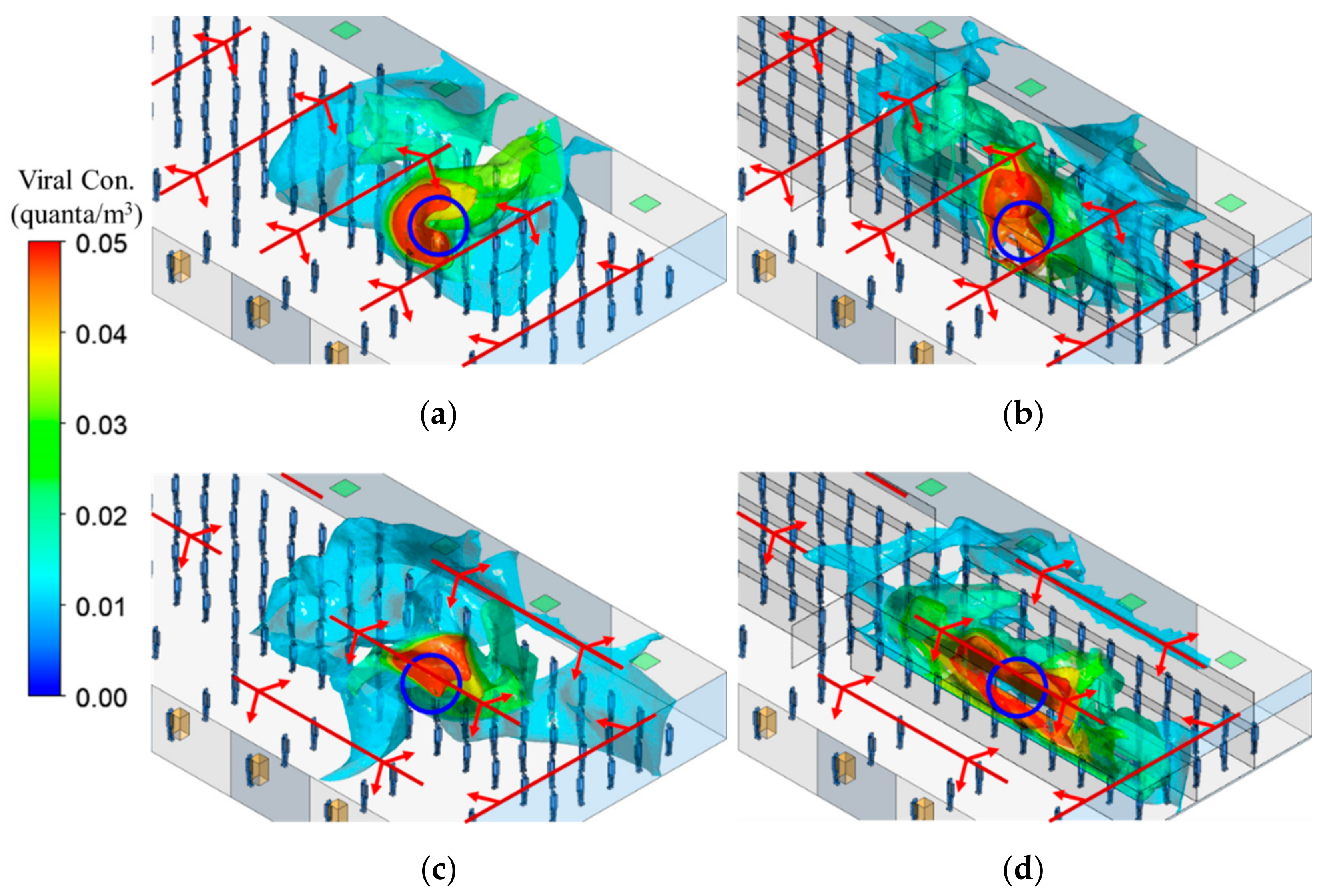
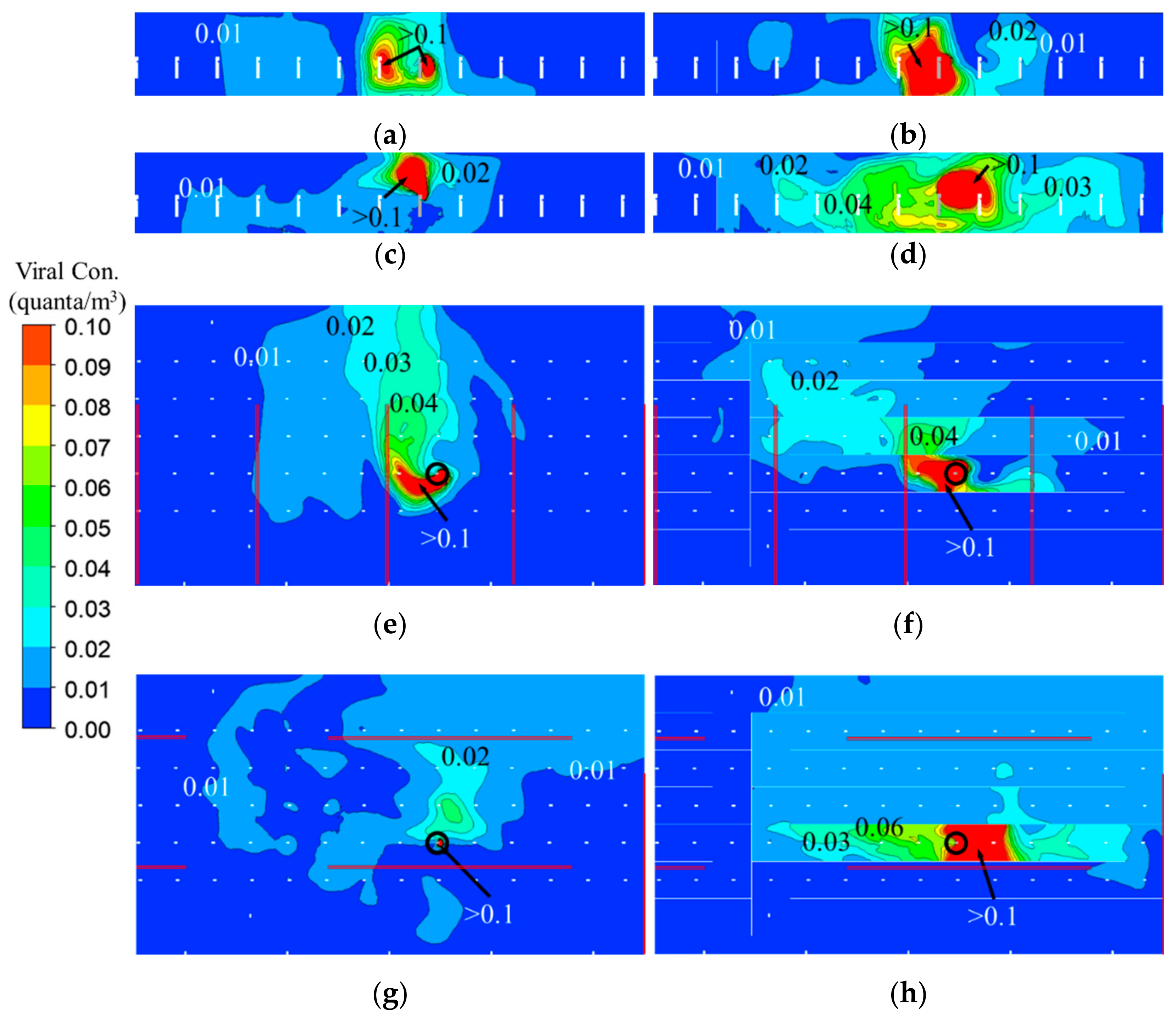
3.3. Viral Bioaerosol (Quanta) Distribution
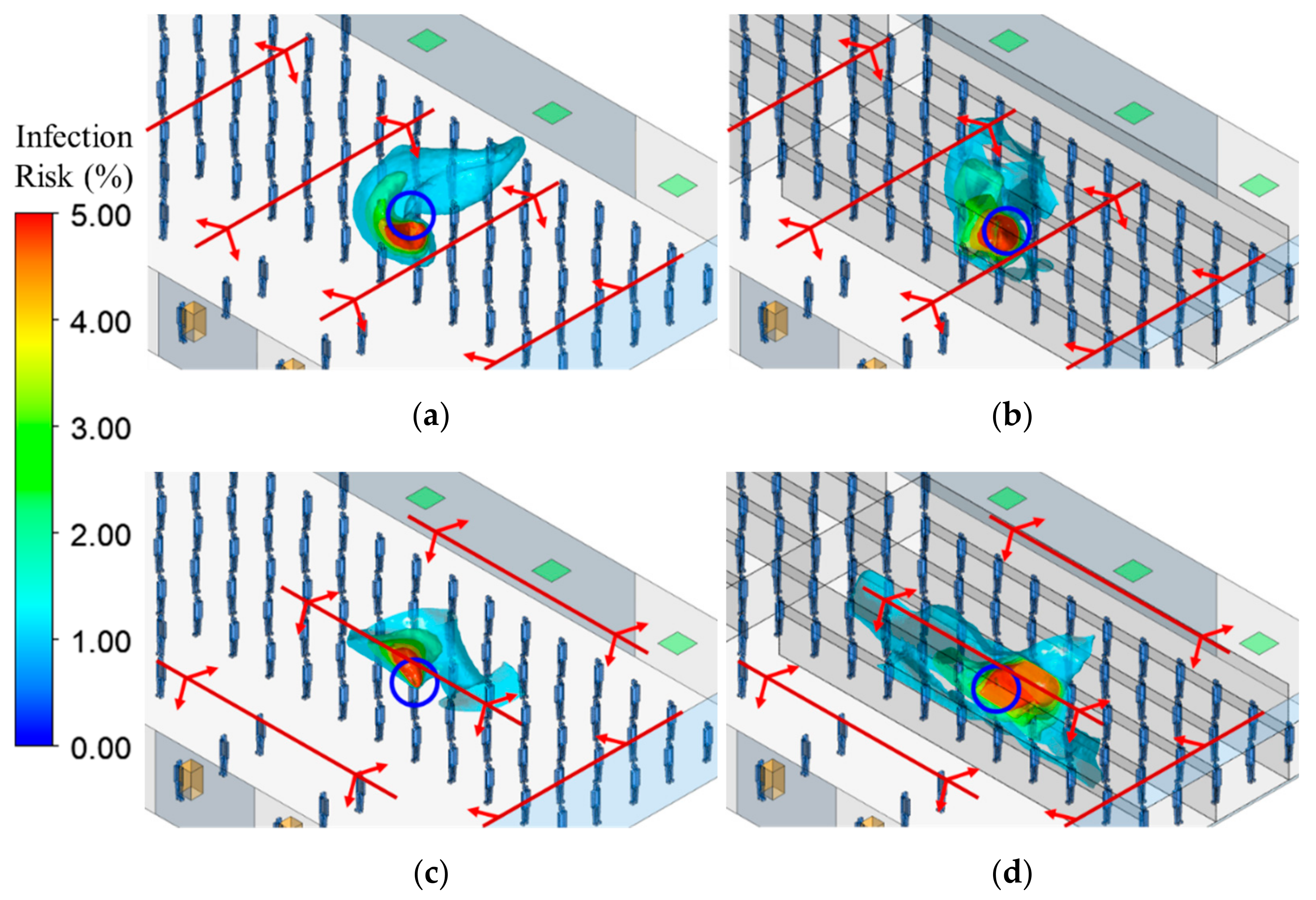
3.4. Infection Risk
4. Discussion
5. Conclusions
- (1)
- Plastic barriers block air movement between two canyons created by barriers, while at the same time they support air movement along the canyon. Therefore, using plastic barriers can completely change air movement between the index case and other passengers, both in direction and speed. Apparently, the plastic barriers were positioned to impede air movement by the existing ventilation in Case 2 and hence locally concentrated viral aerosols in the canyon.
- (2)
- In Case 1, using plastic barriers only made small differences in the volumes with high viral aerosol concentrations in the breathing zone of TSA. In Case 2, using plastic barriers led to the volume increase of 40%, 1.7 times, and 108.6 times for the region with viral aerosol concentration to be >0.01 quanta/m3, >0.02 quanta/m3, and >0.05 quanta/m3, respectively. Consistent with the results of flow field, in Case 2, using plastic barriers significantly increased the volume of high viral aerosol concentrations in the breathing zone.
- (3)
- The volume with high infection risk in breathing zone is also greatly impacted by the relative spatial relationship between plastic barriers and air diffusers. Importantly, without plastic barriers and with diffuser distribution present in Case 2, the high-risk volume was approximately zero in the first 25 min, so infection control is effective with this ventilation system. However, the use of plastic barriers increased the risk for the passenger, resulting in the volume of 8.7 m3 for 1% risk region, and 1 m3 for 5% risk region, at 25 min. Meanwhile, in Case 1, the volume was <1 m3 for 1% risk region and <0.7 m3 for 5% risk region, in the first 25 min. Therefore, in the first 25 min, using plastic barriers made a small difference in risk for Case 1, but in Case 2, this difference was much more significant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 1 July 2022).
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, December 1–8, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Peebles, A. CDC Pares Omicron View to a Still-Dominant 59% of U.S. Cases (1). Available online: https://news.bloomberglaw.com/coronavirus/cdc-pares-omicron-estimate-to-a-still-dominant-59-of-u-s-cases (accessed on 31 December 2021).
- Slifka, M.K.; Gao, L. Is Presymptomatic Spread a Major Contributor to COVID-19 Transmission? Nat. Med. 2020, 26, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef] [PubMed]
- Schoen, L.J. Guidence for Building Operations During the COVID-19 Pandemic. ASHRAE J. 2020, 62, 72–74. [Google Scholar]
- Kim, Y.-I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.-H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The Airborne Lifetime of Small Speech Droplets and Their Potential Importance in SARS-CoV-2 Transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 11875–11877. [Google Scholar] [CrossRef]
- Fineberg, H.V.; Council, N.R. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (April 1, 2020); The National Academies Press NRC: Washington, DC, USA; The National Academies Council: Washington, DC, USA, 2020. [Google Scholar]
- Kwon, K.-S.; Park, J.-I.; Park, Y.J.; Jung, D.-M.; Ryu, K.-W.; Lee, J.-H. Evidence of Long-Distance Droplet Transmission of SARS-CoV-2 by Direct Air Flow in a Restaurant in Korea. J. Korean Med. Sci. 2020, 35, e415. [Google Scholar] [CrossRef]
- Shen, Y.; Li, C.; Dong, H.; Wang, Z.; Martinez, L.; Sun, Z.; Handel, A.; Chen, Z.; Chen, E.; Ebell, M.H.; et al. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Intern. Med. 2020, 180, 1665–1671. [Google Scholar] [CrossRef]
- Azimi, P.; Keshavarz, Z.; Laurent, J.G.C.; Stephens, B.; Allen, J.G. Mechanistic Transmission Modeling of COVID-19 on the Diamond Princess Cruise Ship Demonstrates the Importance of Aerosol Transmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2015482118. [Google Scholar] [CrossRef]
- Hwang, S.E.; Chang, J.H.; Oh, B.; Heo, J. Possible Aerosol Transmission of COVID-19 Associated with an Outbreak in an Apartment in Seoul, South Korea, 2020. Int. J. Infect. Dis. 2021, 104, 73–76. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S.; et al. Probable Airborne Transmission of SARS-CoV-2 in a Poorly Ventilated Restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- WHO. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 9 February 2022).
- CDC Science Brief: SARS-CoV-2 and Potential Airborne Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html (accessed on 17 March 2021).
- Kim, J.; Choe, Y.J.; Lee, J.; Park, Y.J.; Park, O.; Han, M.S.; Kim, J.-H.; Choi, E.H. Role of Children in Household Transmission of COVID-19. Arch. Dis. Child. 2020, 106, 709–711. [Google Scholar] [CrossRef]
- Marshall, K.; Vahey, G.M.; McDonald, E.; Tate, J.E.; Herlihy, R.; Midgley, C.M.; Kawasaki, B.; Killerby, M.E.; Alden, N.B.; Staples, J.E.; et al. Exposures Before Issuance of Stay-at-Home Orders Among Persons with Laboratory-Confirmed COVID-19—Colorado, March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 847–849. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, C.; Song, L.; Wang, X.; Zhou, Y.; Fei, C.; Liu, H. Aerosol Transmission, an Indispensable Route of COVID-19 Spread: Case Study of a Department-Store Cluster. Front. Environ. Sci. Eng. 2020, 15, 46. [Google Scholar] [CrossRef]
- Wang, L.; Duan, Y.; Zhang, W.; Liang, J.; Xu, J.; Zhang, Y.; Wu, C.; Xu, Y.; Li, H. Epidemiologic and Clinical Characteristics of 26 Cases of COVID-19 Arising from Patient-to-Patient Transmission in Liaocheng, China. Clin. Epidemiol. 2020, 12, 387–391. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.-M.; Yi, S.; Lee, S.; Na, B.-J.; Kim, C.B.; Kim, J.-I.; Kim, H.S.; Kim, Y.B.; Park, Y.; et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg. Infect. Dis. 2020, 26, 1666–1670. [Google Scholar] [CrossRef]
- The APHI S&T Team. Aviation Public Health Initiative: Assessment of Risks of SARS-CoV-2 Transmission during Air Travel and Non-Pharmaceutical Interventions to Reduce Risk—Phase Two Report: Curb-to-Curb Travel through Airports; Havard, T.H., Ed.; Chan School of Public Health: Boston, MA, USA, 2021. [Google Scholar]
- Eykelbosh, A. A Rapid Review of the Use of Physical Barriers in Non-Clinical Settings and COVID-19 Transmission; National Collaborating Centre for Environmental Health: Vancouver, BC, USA, 2021; p. 16. [Google Scholar]
- Rooney, C.M.; McIntyre, J.; Ritchie, L.; Wilcox, M.H. Evidence Review of Physical Distancing and Partition Screens to Reduce Healthcare Acquired SARS-CoV-2. Infect. Prev. Pract. 2021, 3, 100144. [Google Scholar] [CrossRef]
- Zhu, S.; Demokritou, P.; Spengler, J. Experimental and Numerical Investigation of Micro-Environmental Conditions in Public Transportation Buses. Build. Environ. 2010, 45, 2077–2088. [Google Scholar] [CrossRef]
- Zhu, S.; Srebric, J.; Spengler, J.D.; Demokritou, P. An Advanced Numerical Model for the Assessment of Airborne Transmission of Influenza in Bus Microenvironments. Build. Environ. 2012, 47, 67–75. [Google Scholar] [CrossRef]
- ANSYS. Ansys Fluent V19.2 ANSYS Fluent Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2018. [Google Scholar]
- Nishi, Y. Human and Thermal environment. In Mechanism of Comfortable Thermal Environment; The Society of Heating, Air-Conditioning and Sanitary Engineers of Japan: Tokyo, Japan, 2005; p. 30. [Google Scholar]
- Wells, W.F. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections. J. Am. Med. Assoc. 1955, 159, 90. [Google Scholar] [CrossRef]
- Holmberg, S.; Li, Y. Modelling of the Indoor Environment—Particle Dispersion and Deposition. Indoor Air 1998, 8, 113–122. [Google Scholar] [CrossRef]
- Pichurov, G.; Srebric, J.; Zhu, S.; Vincent, R.L.; Brickner, P.W.; Rudnick, S.N. A Validated Numerical Investigation of the Ceiling Fan’s Role in the Upper-Room UVGI Efficacy. Build. Environ. 2015, 86, 109–119. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of Airborne Viral Emission: Quanta Emission Rate of SARS-CoV-2 for Infection Risk Assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef]
- Riley, E.C.; Murphy, G.; Riley, R.L. Airborne Spread of Measles in a Suburban Elementary School. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef]
- Sandberg, M.; Sjöberg, M. The Use of Moments for Assessing Air Quality in Ventilated Rooms. Build. Environ. 1983, 18, 181–197. [Google Scholar] [CrossRef]
- Chanteloup, V.; Mirade, P.-S. Computational Fluid Dynamics (CFD) Modelling of Local Mean Age of Air Distribution in Forced-Ventilation Food Plants. J. Food Eng. 2009, 90, 90–103. [Google Scholar] [CrossRef]
- Ning, M.; Mengjie, S.; Mingyin, C.; Dongmei, P.; Shiming, D. Computational Fluid Dynamics (CFD) Modelling of Air Flow Field, Mean Age of Air and CO2 Distributions inside a Bedroom with Different Heights of Conditioned Air Supply Outlet. Appl. Energy 2016, 164, 906–915. [Google Scholar] [CrossRef]
- Kato, S.; Murakami, S.; Kobayashi, H. New Scales for Evaluating Ventilation Efficiency as Affected by Supply and Exhaust Opening Based on Spatial Distribution of Contaminant. In Proceedings of the ISRACVE’92, Tokyo, Japan, 22–24 July 1992; pp. 177–186. [Google Scholar]
- Davidson, L.; Olsson, E. Calculation of Age and Local Purging Flow Rate in Rooms. Build. Environ. 1987, 22, 111–127. [Google Scholar] [CrossRef]
- Liu, Z.; Li, R.; Wu, Y.; Ju, R.; Gao, N. Numerical Study on the Effect of Diner Divider on the Airborne Transmission of Diseases in Canteens. Energy Build. 2021, 248, 111171. [Google Scholar] [CrossRef]
- Ye, J.; Ai, Z.; Zhang, C. A New Possible Route of Airborne Transmission Caused by the Use of a Physical Partition. J. Build. Eng. 2021, 44, 103420. [Google Scholar] [CrossRef]
- Ham, S. Prevention of Exposure and Dispersion of COVID-19 Using Air Purifiers: Challenges and Concerns. Epidemiol. Health 2020, 42, e2020027. [Google Scholar] [CrossRef] [PubMed]
- CDC Omicron Variant: What You Need to Know. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html (accessed on 27 December 2021).
- Omicron, Delta, Alpha, and More: What to Know About the Coronavirus Variants. Available online: https://www.yalemedicine.org/news/covid-19-variants-of-concern-omicron (accessed on 27 December 2021).
- Gu, H.; Krishnan, P.; Ng, D.Y.M.; Chang, L.D.J.; Liu, G.Y.Z.; Cheng, S.S.M.; Hui, M.M.Y.; Fan, M.C.Y.; Wan, J.H.L.; Lau, L.H.K.; et al. Probable Transmission of SARS-CoV-2 Omicron Variant in Quarantine Hotel, Hong Kong, China, November 2021. Emerg. Infect. Dis. 2021, 28, 460–462. [Google Scholar] [CrossRef] [PubMed]
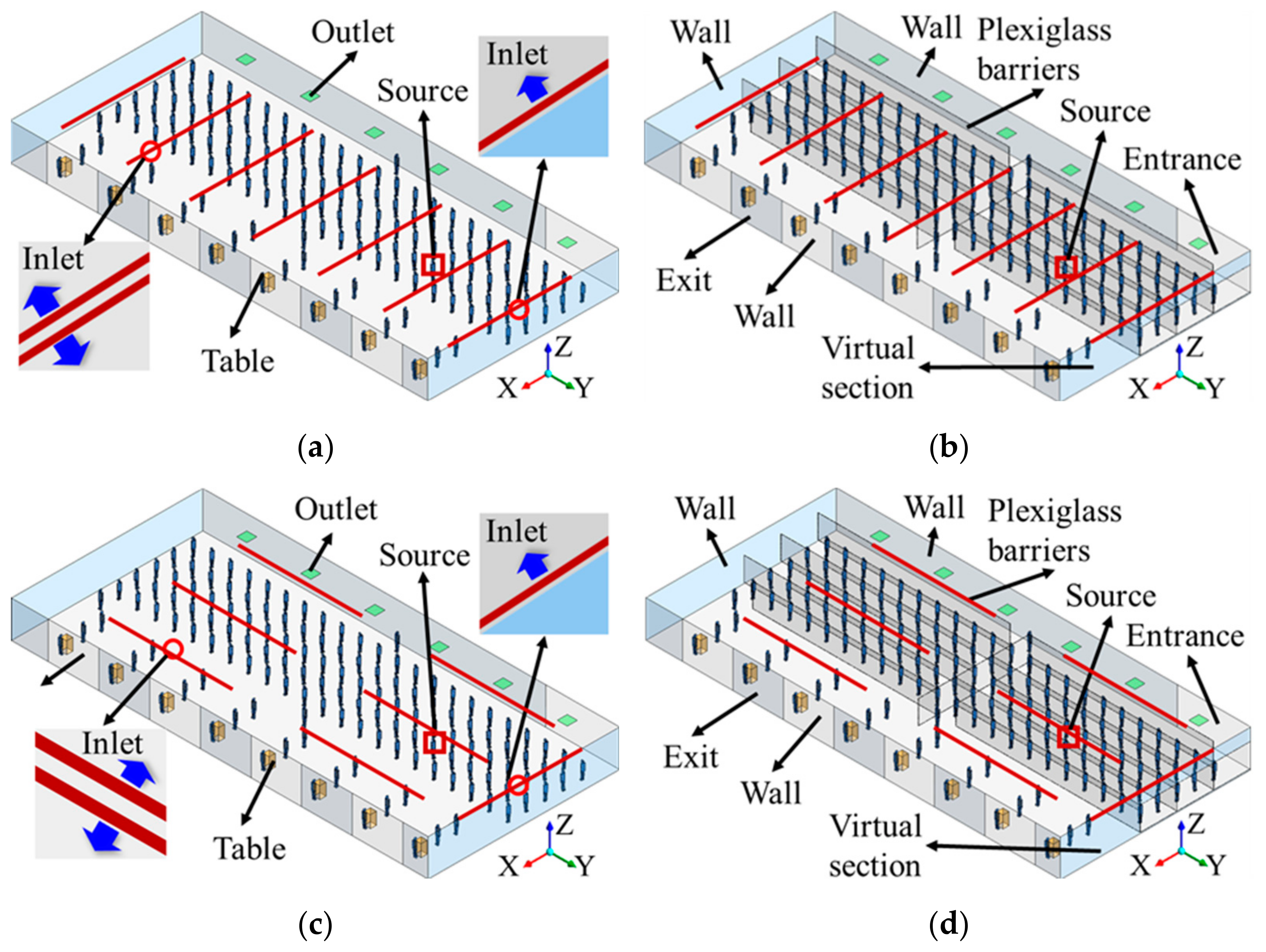


| Inlet | Size: 12 m × 0.025 m (13); airflow rate: 3.4 m3/s; velocity: 3.3 m/s at horizontal direction, 0.9 m/s downward to the floor; temperature: 17 °C |
| Outlet | Size: 1 m × 1 m (6), free-slip |
| Ceiling | No-slip, 7.53 W/m2 for heat release from lighting |
| Floor | No-slip, 12.35 W/m2 for the radiant heat release from human bodies and heat release from equipment |
| Wall | No-slip, adiabatic |
| Entrance, exit, and virtual section | Symmetric |
| Human body surface | No-slip, 23.84 W/m2 for convective heat release |
| Source body’s mouth | Area: 3 cm2, velocity: 1.33 m/s, temperature: 34 °C; quanta generation rate: 100 quanta/h |
| Cases | 1-1 | 1-2 | 2-1 | 2-2 |
|---|---|---|---|---|
| MRLA (s) | 464 | 445 | 603 | 663 |
| Ts (s) | 623 | 595 | 587 | 603 |
| Cases | 1-1 | 1-2 | 2-1 | 2-2 |
|---|---|---|---|---|
| >0.01 quanta/m3 | 71.41 m3 | 71.67 m3 | 89.12 m3 | 125.04 m3 |
| >0.02 quanta/m3 | 27.33 m3 | 24.51 m3 | 8.01 m3 | 21.89 m3 |
| >0.05 quanta/m3 | 3.37 m3 | 3.80 m3 | 0.07 m3 | 7.36 m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Lin, T.; Spengler, J.D.; Cedeño Laurent, J.G.; Srebric, J. The Influence of Plastic Barriers on Aerosol Infection Risk during Airport Security Checks. Sustainability 2022, 14, 11281. https://doi.org/10.3390/su141811281
Zhu S, Lin T, Spengler JD, Cedeño Laurent JG, Srebric J. The Influence of Plastic Barriers on Aerosol Infection Risk during Airport Security Checks. Sustainability. 2022; 14(18):11281. https://doi.org/10.3390/su141811281
Chicago/Turabian StyleZhu, Shengwei, Tong Lin, John D. Spengler, Jose Guillermo Cedeño Laurent, and Jelena Srebric. 2022. "The Influence of Plastic Barriers on Aerosol Infection Risk during Airport Security Checks" Sustainability 14, no. 18: 11281. https://doi.org/10.3390/su141811281
APA StyleZhu, S., Lin, T., Spengler, J. D., Cedeño Laurent, J. G., & Srebric, J. (2022). The Influence of Plastic Barriers on Aerosol Infection Risk during Airport Security Checks. Sustainability, 14(18), 11281. https://doi.org/10.3390/su141811281






