Spatial Distribution and Invasion Potential of the Naturalized Downy Thorn Apple (Datura innoxia): Evidence from Classification and Ordination
Abstract
1. Introduction
2. Materials and Methods
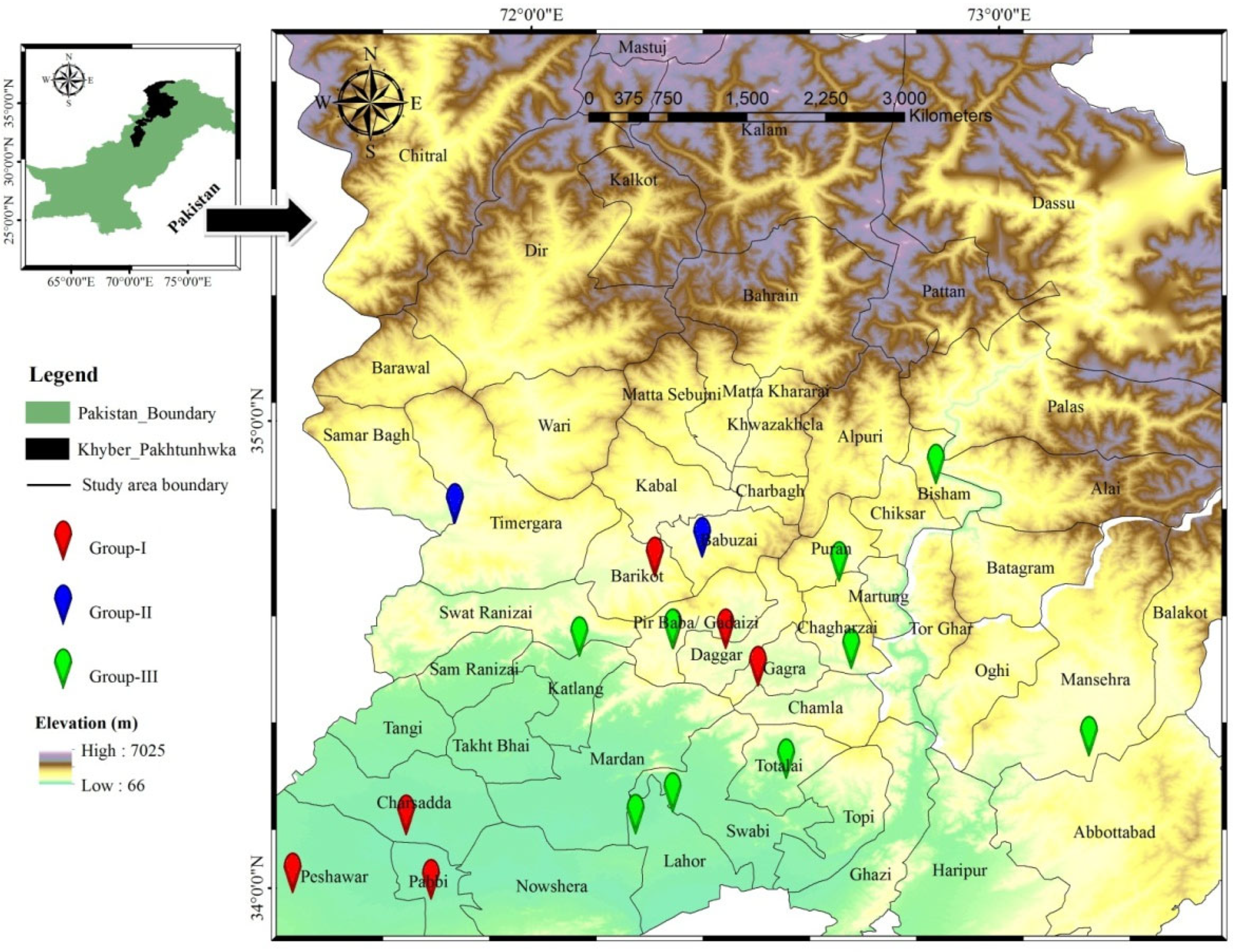
2.1. Study Area
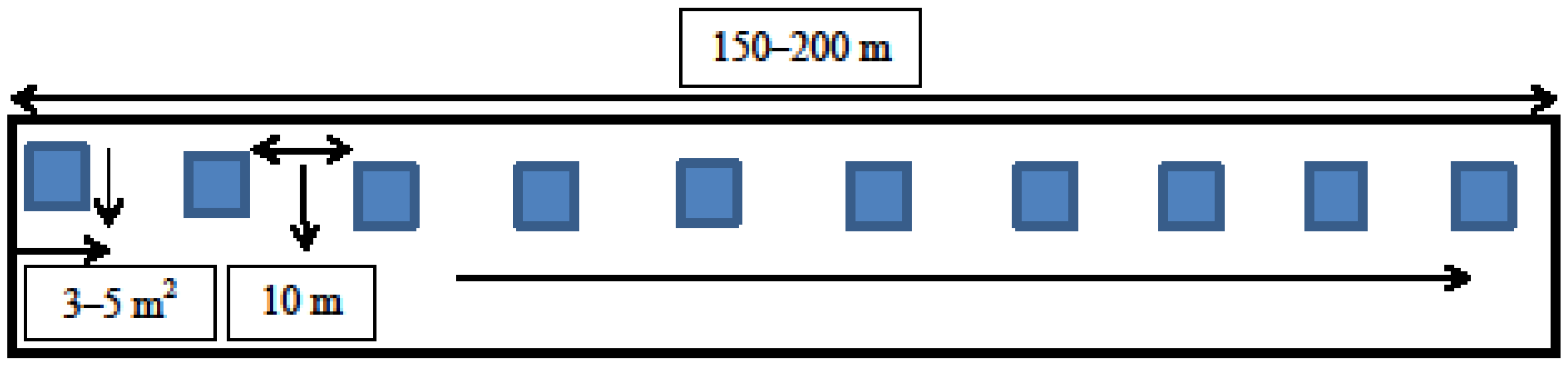
2.2. Field Studies of Invasive Species Distribution and Interspecies Associations
2.3. Assessment of Diversity Indices
2.4. Assessment of Environmental and Biotic Factors
2.5. Data Analyses
3. Results
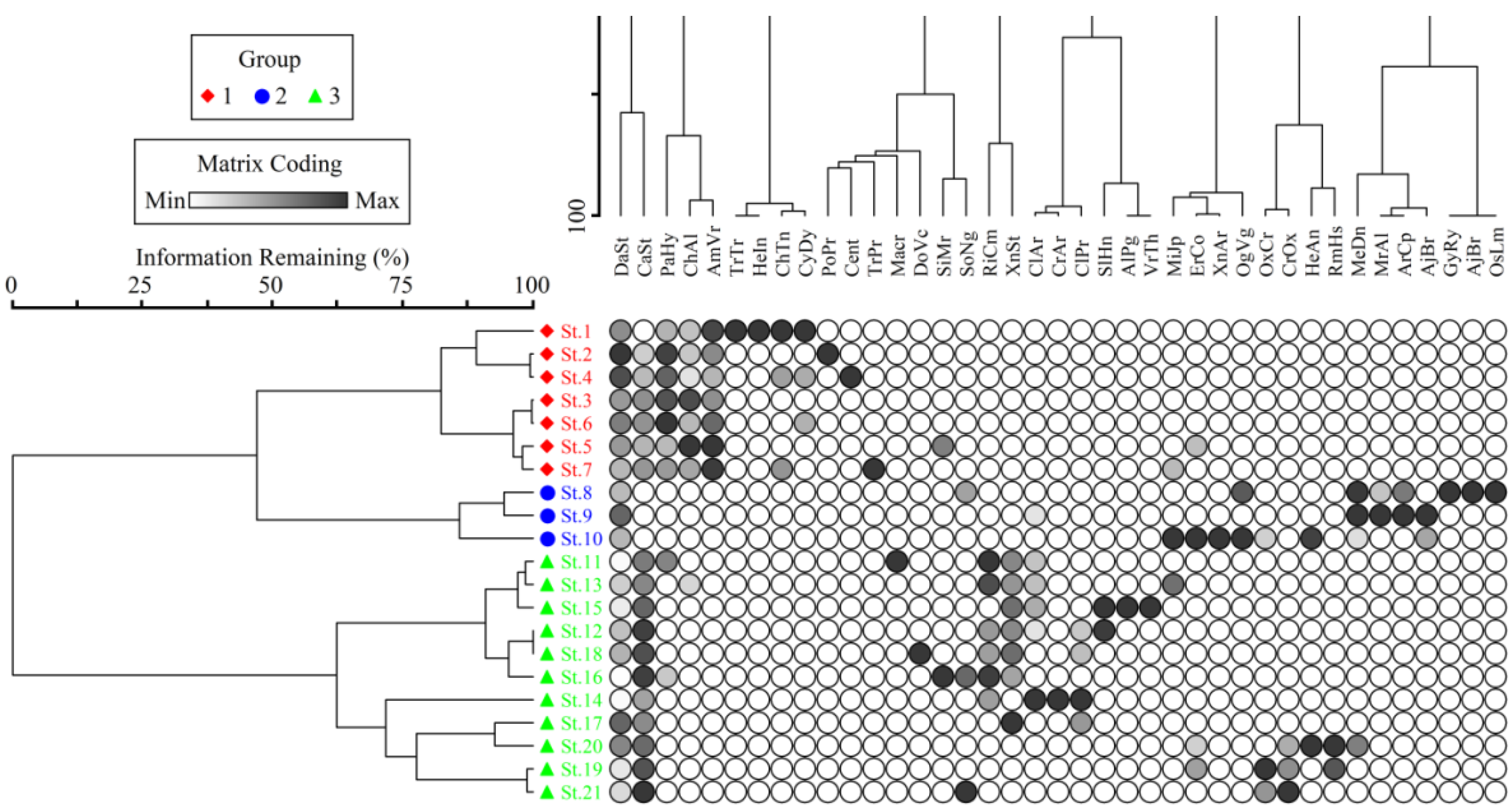
3.1. Floristic Composition
3.2. Vegetation Communities and Diversity Indices
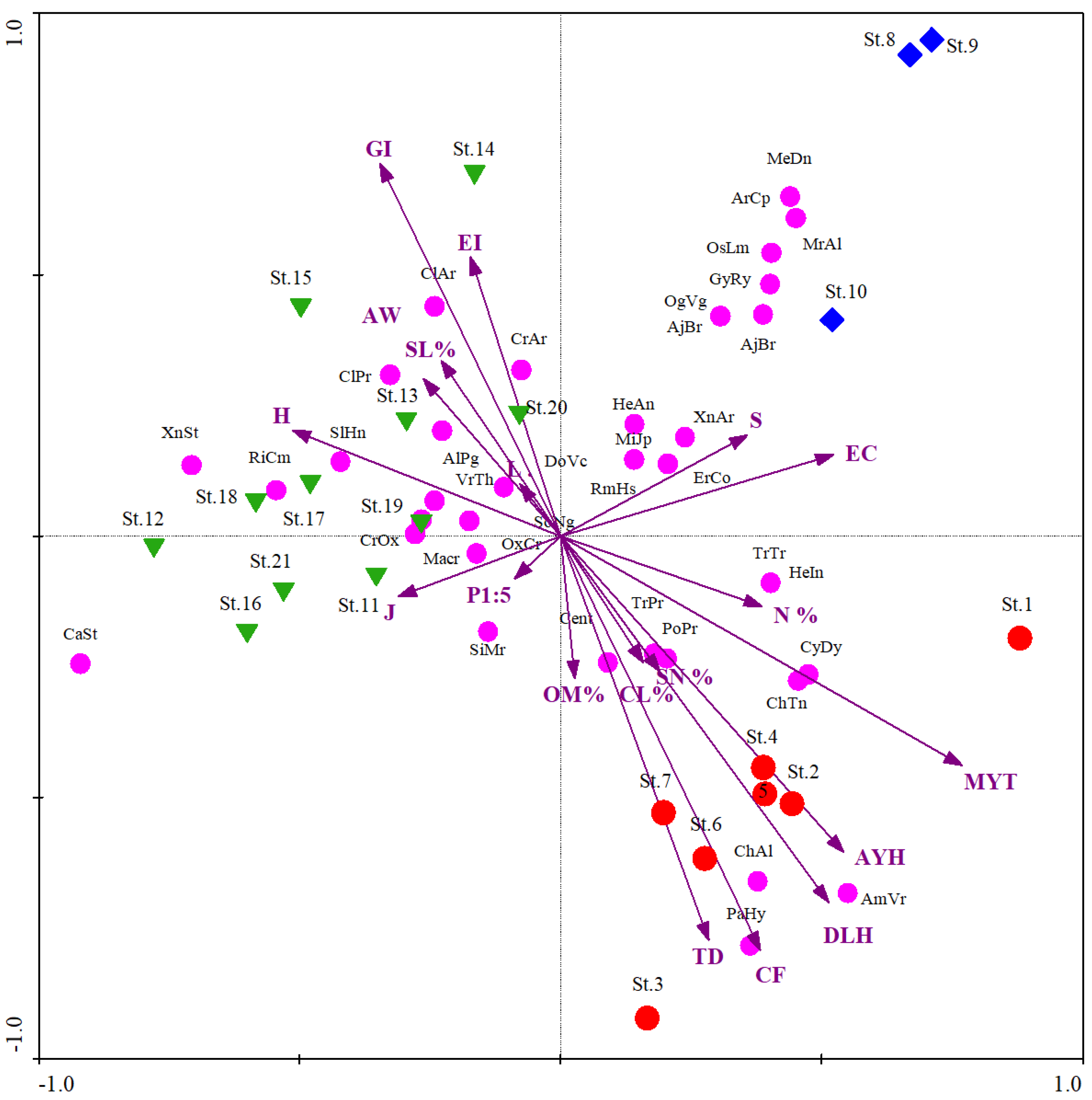
3.3. Relation with Biotic and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Erhardt, A.; Baur, B. Alterations of stepp like grasslands in Eastern Europe: A threat to regional biodiversity hotspots. Conserv. Biol. 2005, 19, 1606–1618. [Google Scholar] [CrossRef]
- Khan, N.; Bibi, K.; Ullah, R. Distribution pattern and ecological determinants of an invasive plant Parthenium hysterophorus L., in Malakand division of Pakistan. J. Mt. Sci. 2020, 17, 1670–1683. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Rahman, A. Comparison of invaded and non-invaded sites: A case study of rough cocklebur (Xanthium strumarium L.), an alien invasive species. Appl. Ecol. Environ. Res. 2021, 19, 1533–1548. [Google Scholar] [CrossRef]
- Stohlgren, T.; Rejmánek, M. No universal scale-dependent impacts of invasive species on native plant species richness. Biol.L. Lett. 2014, 10, 2013093. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Barnett, D.T.; Jarnevich, C.S.; Flather, C.; Kartesz, J. The myth of plant species saturation. Ecol. Lett. 2008, 11, 313–322. [Google Scholar] [CrossRef]
- Pauchard, A.; Shea, K. Integrating the study of non-native plant invasions across spatial scales. Biol. Invasions 2006, 8, 399–413. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot. 2011, 98, 539–548. [Google Scholar] [CrossRef]
- Price, E.P.F.; Spyreas, G.; Matthews, J.W. Biotic homogenization of regional wetland plant communities within short time-scales in the presence of an aggressive invader. J. Ecol. 2018, 106, 1180–1190. [Google Scholar] [CrossRef]
- Simberloff, D. The role of propagule pressure in biological invasions. Annual. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K. Which factor explains the life history of Xanthium strumarium L., an aggressive alien invasive plant species, along its altitudinal gradient? Plant Direct. 2022, 6, e375. [Google Scholar] [CrossRef]
- Mandal, G.; Joshi, S.P. The role of habitat types and soil physicochemical properties in the spread of a non-native shrub Lantana camara in the Doon valley, Western Himalaya, India. J. Environ. Geogh. 2014, 7, 31–42. [Google Scholar] [CrossRef]
- Chambers, D.C.; Perie, N.; de Blois, S. Challenges in modelling the abundance of 105 tree species in eastern North America using climate, edaphic, and topographic variables. For. Ecol. Manag. 2013, 291, 20–29. [Google Scholar] [CrossRef]
- Figueiredo, F.O.G.; Zuquim, G.; Tuomisto, H.; Moulatlet, G.M.; Balslev, H.; Costa, F.R.C. Beyond climate control on species range: The importance of soil data to predict the distribution of Amazonian plant species. J. Biogeogr. 2018, 45, 190–200. [Google Scholar] [CrossRef]
- Logan, J.A.; Regniere, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Marticorena, C. Patterns and Future Predictions. In Invasive Species in a Changing World; Springer: Berlin, Germany, 2000; p. 385. [Google Scholar]
- Mligo, C. Diversity and distribution pattern of riparian plant species in the Wami River system, Tanzania. J. Plant Ecol. 2017, 10, 259–270. [Google Scholar] [CrossRef][Green Version]
- Shrestha, U.B.; Shrestha, B.B. Climate change amplifies plant invasion hotspots in Nepal. Divers. Distrib. 2019, 25, 1599–1612. [Google Scholar] [CrossRef]
- Ahmad, M.; Uniyal, S.K.; Batish, D.R.; Singh, H.P.; Jaryan, V.; Rathee, S.; Kohli, R.K. Patterns of plant communities along vertical gradient in Dhauladhar Mountains in lesser Himalayas in North-Western India. Sci. Total Environ. 2020, 716, 136919. [Google Scholar] [CrossRef]
- Begum, H.A.; Hamayun, M.; Shad, N.; Khan, W.; Ahmad, J.; Khan, M.E.H.; Jones, D.A.; Ali, K. Effects of UV radiation on germination, growth, chlorophyll content, and fresh and dry weights of Brassica rapa L. and Eruca sativa L. Sarhad. J. Agric. 2021, 37, 1016–1024. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Pyšek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Chang. Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef]
- Dudley, S.A. Plasticity and the functional ecology of plants. In Phenotypic Plasticity: Functional and Conceptual Approaches; Oxford University Press: Oxford, UK, 2004; p. 2. [Google Scholar]
- Seastedt, T.R.; Oldfather, M.F. Climate Change, Ecosystem processes and biological diversity responses in high elevation communities. Climate 2021, 9, 87. [Google Scholar] [CrossRef]
- Kesarkar, P.; Khan, S. Ethnomedicinal plants used in the treatment of respiratory diseases around western Ghats region of Maharashtra, India. J. Glob. Biosci. 2021, 10, 8447–8457. [Google Scholar]
- Nayyar, M.S.; Hanif, M.A.; Mjaeed, M.I.; Ayub, M.A.; Rehman, R. Datura. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–216. [Google Scholar]
- Setshogo, M.P. A review of some medicinal and or hallucinogenic Solanaceous plants of Botswana: The genus Datura L. Int. J. Appl. Res. Nat. Prod. 2015, 1, 15–23. [Google Scholar]
- Drake, L.R.; Lin, S.; Rayson, G.D.; Jackson, P.J. Chemical modification and metal binding studies of Datura innoxia. Environ. Sci. Technol. 1995, 30, 110–114. [Google Scholar] [CrossRef]
- Khan, F.K. Pakistan Geography Economy and People, 4th ed.; Oxford University Press: Islamabad, Pakistan, 2015. [Google Scholar]
- Dawood, M. Spatio-statistical analysis of temperature fluctuation using Mann–Kendall and Sen’s slope approach. Clim. Dyn. 2017, 48, 783–797. [Google Scholar]
- Ali, A.; Khan, T.A.; Ahmad, S. Analysis of climate data of Khyber Pakhtunkhwa, Pakistan. Int. Res. J. Eng. Technol. 2018, 5, 4266–4282. [Google Scholar]
- Deo, R.C.; Şahin, M. Application of the artificial neural network model for prediction of monthly standardized precipitation and evapotranspiration index using hydrometeorological parameters and climate indices in eastern Australia. Atmos. Res. 2015, 161, 65–81. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Lone, G.R.; Kohli, R.K. Niche width analyses facilitate identification of high-risk endemic species at high altitudes in western Himalayas. Ecol. Indic. 2021, 126, 107653. [Google Scholar] [CrossRef]
- Morsdorf, F.; Mårell, A.; Koetz, B.; Cassagne, N.; Pimont, F.; Rigolot, E.; Allgöwer, B. Discrimination of vegetation strata in a multi-layered Mediterranean forest ecosystem using height and intensity information derived from airborne laser scanning. Remote Sens. Environ. 2010, 114, 1403–1415. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Martínez-Falcón, A.P.; Zurita, G.A.; Ortega-Martínez, I.J.; Moreno, C.E. Populations and assemblages living on the edge: Dung beetles responses to forests-pasture ecotones. Peer J. 2018, 6, e6148. [Google Scholar] [CrossRef]
- Nasir, E.; Ali, S.I. (Eds.) Flora of West Pakistan; Fakhri Press: Karachi, Pakistan, 1972. [Google Scholar]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.P. Flora Alpina: Atlas des 4.500 Plantes Vasculaires des Alpes Photographies en Couleurs de Konrad Lauber et Croquis d’André Michel; Belin Publisher: Paris, France, 2004. [Google Scholar]
- Maan, I.; Kaur, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Exotic avenue plantations turning foe: Invasive potential, distribution and impact of Broussonetia papyrifera in Chandigarh, India. Urban For. Urban Green. 2021, 59, 127010. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Appl. Sci. 2021, 11, 11704. [Google Scholar] [CrossRef]
- Reis, A.T.; Coelho, J.P.; Rucandio, I.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Thermo-desorption: A valid tool for mercury speciation in soils and sediments? Geoderma 2015, 237, 98–104. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science of America and American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Yadav, R.L.; Tomar, S.S.; Sharma, U.C. Output: Input ratios and apparent balances of N, P and K inputs in a rice-wheat system in North-West India. Exp. Agric. 2002, 38, 457–468. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.; Romberger, J.S.; Papendick, R.I. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Gangwar, D.P.; Baskar, M. Texture Determination of Soil by Hydrometer Method for Forensic Purpose; Central Forensic Science Laboratory: Chandigarh, India, 2019.
- Mligo, C. Anthropogenic disturbance on the vegetation in Makurunge woodland, Bagamoyo district, Tanzania. Tanzan. J. Sci. 2011, 37, 95–108. [Google Scholar]
- Leach, K.; Montgomery, W.I.; Reid, N. Modelling the influence of biotic factors on species distribution patterns. Ecol. Model. 2016, 337, 96–106. [Google Scholar] [CrossRef]
- Hailu, H. Analysis of vegetation phytosociological characteristics and soil physico-chemical conditions in Harishin Rangelands of Eastern Ethiopia. Land 2017, 6, 4. [Google Scholar] [CrossRef]
- McCune, B. Influence of noisy environmental data on canonical correspondence analysis. Ecology 1997, 78, 2617–2623. [Google Scholar] [CrossRef]
- Khan, N.; Ali, F.; Ali, K.; Shaukat, S. Composition, structure and regeneration dynamcis of Olea ferruginea Royle forests from Hindikush ranges of Pakistan. J. Mt. Sci. 2015, 12, 647–658. [Google Scholar]
- Ullah, R.; Khan, N.; Hewitt, N.; Ali, K.; Jones, D.A.; Khan, M.E.H. Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L. Sustainability 2022, 14, 7141. [Google Scholar] [CrossRef]
- Seifu, A.; Seboka, N.; Misganaw, M.; Bekele, T.; Merawi, E.; Ayenew, A.; Faris, G. Impact of invasive alien plant, Xanthium strumarium, on species diversity and composition of invaded plant communities in Borena Zone, Ethiopia. Biodivers. Int. J. 2017, 1, 00004. [Google Scholar] [CrossRef]
- Qureshi, H.; Anwar, T.; Arshad, M.; Osunkoya, O.O.; Adkins, S.W. Impacts of Xanthium strumarium L. invasion on vascular plant diversity in Pothwar Region (Pakistan). Ann. Di Bot. 2019, 9, 73–82. [Google Scholar]
- Iqbal, M.; Iram, A.; Liu, M.C.; Feng, Y.L. Competitive Approach of Invasive Cocklebur (Xanthium Strumarium) with Native Weed Species Diversity in Northeast China. PloS ONE 2020. [Google Scholar] [CrossRef]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Tappeiner, J.; Zasada, J.; Ryan, P.; Newton, M. Salmonberry clonal and population structure: The basis for a persistent cover. Ecology 1991, 72, 609–618. [Google Scholar] [CrossRef]
- Timsina, B.; Shrestha, B.B.; Rokaya, M.B.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grassland communities in Nepal. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, R.; Ali, K.; Jones, D.A.; Khan, M.E.H. Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan. Agriculture 2022, 12, 687. [Google Scholar] [CrossRef]
- Dietz, H.; Edwards, P.J. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 2006, 87, 1359–1367. [Google Scholar] [CrossRef]
- Polo, A.; Cappitelli, F.; Villa, F.; Pinzari, F. Biological invasion in the indoor environment: The spread of Eurotium halophilicum on library materials. Int. Biodeterior. Biodegrad. 2017, 118, 34–44. [Google Scholar] [CrossRef]
- Geiger, E.L.; McPherson, G.R. Response of semi-desert grasslands invaded by non-native grasses to altered disturbance regimes. J. Biogeogr. 2005, 32, 895–902. [Google Scholar] [CrossRef]
- Chhogyel, N.; Kumar, L.; Bajgai, Y. Invasion status and impacts of parthenium weed (Parthenium hysterophorus) in West-Central region of Bhutan. Biol. Invasions 2021, 23, 2763–2779. [Google Scholar] [CrossRef]
- Sjödin, N.E.; Bengtsson, J.; Ekbom, B. The influence of grazing intensity and landscape composition on the diversity and abundance of flower-visiting insects. J. Appl. Ecol. 2008, 45, 763–772. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Oduor, A.M.; Long, H.; Fandohan, A.B.; Liu, J.; Yu, X. An invasive plant provides refuge to native plant species in an intensely grazed ecosystem. Biol. Invasions 2018, 20, 2745–2751. [Google Scholar] [CrossRef]
- Eaton, W.D.; Farrell, R.E. Catabolic and genetic microbial indices, and levels of nitrate, ammonium and organic carbon in soil from the black locust (Robinia pseudo-acacia) and tulip poplar (Liriodendron tulipifera) trees in a Pennsylvania forest. Biol. Fertil. Soils 2004, 39, 209–214. [Google Scholar] [CrossRef]
- Dong, L.J.; He, W.M. The relative contributions of climate, soil, diversity and interactions to leaf trait variation and spectrum of invasive Solidago canadensis. BMC Ecol. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Sardans, J.; Alonso, R.; Janssens, I.A.; Carnicer, J.; Vereseglou, S.; Rillig, M.C.; Penuelas, J. Foliar and soil concentrations and stoichiometry of nitrogen and phosphorous across E uropean Pinus sylvestris forests: Relationships with climate, N deposition and tree growth. Funct. Ecol. 2016, 30, 676–689. [Google Scholar] [CrossRef]
- Vasquez, E.; Sheley, R.; Svejcar, T. Creating invasion resistant soils via nitrogen management. Invasive Plant Sci. Manag. 2008, 1, 304–314. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Cowie, B.W.; Strathie, L.W.; Goodall, J.M.; Venter, N.; Witkowski, E.T.; Byrne, M.J. Does host plant quality constrain the performance of the Parthenium beetle Zygogramma bicolorata? Biol. Control 2019, 139, 104078. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, diversity, and invasion: Implications for conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef]
- Hester, A.J.; Hobbs, R.J. Influence of fire and soil nutrients on native and non-native annuals at remnant vegetation edges in the Western Australian wheatbelt. J. Veg. Sci. 1992, 3, 101–108. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Akinsanmi, O.A.; Lim, L.S.; Perrett, C.; Callander, J.; Dhileepan, K. Parthenium hysterophorus L. (Asteraceae) invasion had limited impact on major soil nutrients and enzyme activity: Is the null effect real or reflects data insensitivity? Plant Soil 2017, 420, 177–194. [Google Scholar] [CrossRef]
- Shea, J.M.; Wagnon, P.; Immerzeel, W.W.; Biron, R.; Brun, F.; Pellicciotti, F. A comparative high-altitude meteorological analysis from three catchments in the Nepalese Himalaya. Int. J. Water Resour. Dev. 2015, 31, 174–200. [Google Scholar] [CrossRef]
- Hou, Q.Q.; Chen, B.M.; Peng, S.L.; Chen, L.Y. Effects of extreme temperature on seedling establishment of non-native invasive plants. Biol. Invasions 2014, 16, 2049–2061. [Google Scholar] [CrossRef]
- Kadam, R.M.; Dhavle, S.D.; Allapure, R.B.; Jadhav, B.S. Evolution of phenological plasticity in Parthenium hysterophorus in response to air pollution stress and unordered environmental variation. Asian J. Environ. Sci. 2009, 3, 131–133. [Google Scholar]
- Eskelinen, A.; Harrison, S. Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition. Ecology 2014, 95, 682–692. [Google Scholar] [CrossRef]
- Datta, A.; Kühn, I.; Ahmad, M.; Michalski, S.; Auge, H. Processes affecting altitudinal distribution of invasive Ageratina adenophora in western Himalaya: The role of local adaptation and the importance of different life-cycle stages. PloS ONE 2017, 12, e0187708. [Google Scholar]
- Gonzalo Turpin, H.; Hazard, L. Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia. J. Ecol. 2009, 97, 742–751. [Google Scholar] [CrossRef]
- Vasseur, F.; Exposito-Alonso, M.; Ayala-Garay, O.J.; Wang, G.; Enquist, B.J.; Vile, D.; Weigel, D. Adaptive diversification of growth allometry in the plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2018, 115, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Kapler, E.J.; Thompson, J.R.; Widrlechner, M.P. Assessing stakeholder perspectives on invasive plants to inform risk analysis. Invasive Plant Sci. Manag. 2012, 5, 194–208. [Google Scholar] [CrossRef]
- Raghubanshi, A.S.; Rai, L.C.; Gaur, J.P.; Singh, J.S. Invasive Alien species and biodiversity in India. Curr. Sci. 2005, 88, 539–540. [Google Scholar]
- Dickie, I.A.; Bennett, B.M.; Burrows, L.E.; Nuñez, M.A.; Peltzer, D.A.; Porté, A.; Richardson, D.M.; Rejmánek, M.; Rundel, P.W.; van Wilgen, B.W. Conflicting values: Ecosystem services and invasive tree management. Biol. Invasions 2014, 16, 705–719. [Google Scholar] [CrossRef]

| Botanical Names | Acronyms | Group I | Group II | Group III |
|---|---|---|---|---|
| Datura innoxia Mill. | Dain | 45.48 ± 2.45 | 41.28 ± 3.98 | 34.33 ± 2.16 |
| Ajuga bracteosa Wall. ex Benth. | Ajbr | * | 1.82 ± 1.82 | * |
| Alternanthera pungens Kunth | Alpg | * | * | 0.53 ± 0.53 |
| Amaranthus viridis L. | Amvr | 6.96 ± 0.8 | * | * |
| Artemisia capillaris Thunb. | Arcp | * | 4.70 ± 2.49 | * |
| Calendula arvensis L. | Clar | * | 1.40 ± 1.40 | 6.14 ± 2.90 |
| Calotropis procera (Aiton) W.T.Aiton | Clpr | * | * | 2.55 ± 1.28 |
| Cannabis sativa L. | Cast | 12.11 ± 2.5 | * | 25.24 ± 1.7 |
| Carthamus oxycantha M.Bieb. | Crox | * | * | 3.09 ± 1.74 |
| Centaurea cyanus L. | Cent | 0.94 ± 0.94 | * | * |
| Chenopodium album L. | Chal | 9.76 ± 2.46 | 3.41 ± 2.05 | 0.36 ± 0.36 |
| Chrozophora tinctoria (L.) Raf. | Chtn | 2.98 ± 1.5 | * | * |
| Cirsium arvense (L.) Scop. | Crar | * | * | 0.64 ± 0.64 |
| Cornus macrophylla Wall. | Coma | * | * | 0.28 ± 0.28 |
| Cynodon dactylon (L.) Pers. | Cydy | 1.94 ± 1.07 | * | * |
| Dodonaea viscosa Jacq. | Dovc | * | * | 0.48 ± 0.48 |
| Eryngium caeruleum M.Bieb. | Erco | 0.60 ± 0.60 | 4.35 ± 4.35 | * |
| Gymnosporia royleana Wall. ex M.A.Lawson | Gyry | * | 2.41 ± 2.41 | * |
| Heliotropium indicum L. | Hein | 0.6 ± 0.6 | * | * |
| Medicago denticulata Willd. | Medn | * | 13.92 ± 5.34 | 1.13 ± 1.13 |
| Mirabilis jalapa L. | Mijp | 0.61 ± 0.61 | 4.18 ± 4.18 | 0.82 ± 0.82 |
| Morus alba L. | Mral | * | 4.99 ± 3.42 | * |
| Origanum vulgare L. | Ogvg | * | 3.84 ± 1.94 | * |
| Otostegia limbata (Benth.) Bioss. | Oslm | * | 4.61 ± 4.61 | * |
| Oxalis corniculata L. | Oxcr | * | 1.59 ± 1.59 | 2.86 ± 2.02 |
| Parthenium hysterophorus L. | Pahy | 13.35 ± 2 | * | 1.57 ± 1.14 |
| Persicaria maculosa Gray | Prmu | 0.96 ± 0.96 | * | * |
| Ricinus communis L. | Ricm | 6.09 ± 1.58 | * | 4.43 ± 1.41 |
| Rumex hastatus D. Don | Rmhs | * | * | 0.94 ± 0.63 |
| Salvia moorcroftiana Wall. ex Benth. | Slmc | * | * | 0.82 ± 0.55 |
| Silybum marianum (L.) Geartn. | Simr | 0.79 ± 0.79 | * | 0.79 ± 0.79 |
| Solanum nigrum L. | Song | * | 1.02 ± 1.02 | 1.06 ± 0.71 |
| Trianthema portulacastrum L. | Trpr | 1.08 ± 1.08 | * | - |
| Tribulus terrestris L. | Trtr | 1.73 ± 1.73 | * | * |
| Verbascum thapsus L. | Vrth | * | * | 0.340.34 |
| Verbesina encelioides (Cav.) Benth. and Hook.f. ex A.Gray | Hean | * | 0.97 ± 0.97 | 0.28 ± 0.28 |
| Xanthium strumarium L. | Xnst | * | * | 10.44 ± 2.7 |
| Zanthoxylum armatum DC. | Xnar | * | 5.46 ± 5.46 | * |
| Variable | Group I | Group II | Group III | F-Value | p-Value |
|---|---|---|---|---|---|
| Species richness (S’) | 16 | 16 | 22 | ||
| Shannon–Wiener index (H’) | 1.43 ± 0.07 a | 1.46 ± 0.17 a | 1.81 ± 0.05 b | 3.31 | 0.05 |
| Pielou’s evenness index (E) | 0.76 ± 0.03 a | 0.70 ± 0.04 a | 0.79 ± 0.02 a | 1.57 | 0.23 |
| Erosion intensity (EI) | 13.57 ± 0.53 a | 20 ± 1.45 a | 45.43 ± 1.42 b | 16.16 | 0.00002 |
| Cultivated fields (CF) | 55 ± 1.37 a | 31.56 ± 0.95 b | 20.54 ± 0.76 b | 37.30 | 3.96 × 10–7 |
| Grazing intensity (GI) | 18.57 ± 0.34 a | 38.42 ± 1.54 b | 79.43 ± 0.67 c | 174.27 | 1.66 × 10–12 |
| Traffic density (TD) | 64.28 ± 0.87 a | 48.33 ± 3.65 a | 31.82 ± 1.11 b | 20.97 | 1.98 × 10–5 |
| Parameter | Groups | F-Value | p-Value | ||
|---|---|---|---|---|---|
| I | II | III | |||
| Elev. | 427 ± 73.9 a | 798.9 ± 31 b | 1151.66 ± 34 c | 10.03 | 0.006 |
| AA | 157.14 ± 31 | 177.18 ± 34 | 121.66 ± 86.2 | 0.32 | 0.73 |
| CL% | 29.66 ± 4.9 | 21.37 ± 2.05 | 20.88 ± 3.9 | 1.88 | 0.18 |
| SL% | 28.67 ± 3 a | 48.28 ± 5.7 b | 53.46 ± 14 b | 3.42 | 0.054 |
| SN% | 41.66 ± 5.4 | 30.34 ± 4.9 | 25.65 ± 10.5 | 0.12 | 0.88 |
| pH (1:5) | 6.7 ± 0.1 | 6.75 ± 0.1 | 6.7 ± 0.2 | 0.07 | 0.92 |
| OM% | 1.71 ± 0.24 a | 1.16 ± 0.23 a | 0.51 ± 0.11 b | 2.93 | 0.079 |
| OC% | 0.95 ± 0.19 a | 0.67 ± 0.13 a | 0.24 ± 0.1 b | 3.61 | 0.05 |
| L% | 7.57 ± 0.8 | 9.27 ± 1.5 | 11.28 ± 1.6 | 0.93 | 0.41 |
| N | 0.081 ± 0.019 a | 0.03 ± 0.009 b | 0.24 ± 0.1 c | 8.70 | 0.00 |
| P | 4.99 ± 0.46 | 4.85 ± 0.4 | 4.70 ± 0.83 | 0.11 | 0.90 |
| K | 88.15 ± 3.3 | 110.54 ± 13 | 111.66 ± 17 | 0.72 | 0.50 |
| EC | 375.11 ± 43 | 292.45 ± 23 | 388.66 ± 78 | 2.35 | 0.12 |
| WP | 0.175 ± 0.02 | 0.13 ± 0.008 | 0.13 ± 0.01 | 0.72 | 0.50 |
| FC | 0.30 ± 0.02 | 0.28 ± 0.01 | 0.28 ± 0.005 | 2.35 | 0.12 |
| BD | 1.36 ± 0.03 | 1.38 ± 0.01 | 1.37 ± 0.01 | 0.19 | 0.83 |
| SP | 0.48 ± 0.03 | 0.47 ± 0.005 | 0.47 ± 0.006 | 0.19 | 0.83 |
| AW | 0.12 ± 0.006 a | 0.15 ± 0.005 b | 0.15 ± 0.01 b | 3.24 | 0.06 |
| MYT | 19.27 ± 0.1 | 17.63 ± 0.25 | 14.9 ± 0.61 | 21.55 | 1.66 × 10–5 |
| AYH | 66.23 ± 0.21 | 54.3 ± 1.34 | 49.64 ± 0.64 | 19.18 | 3.45 × 10–5 |
| DLH | 12.05 ± 0.002 | 11.43 ± 0.21 | 11.371 ± 0.02 | 28.03 | 2.9 × 10–6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, K.; Khan, N.; Ullah, R.; Gul, A.; Khan, M.E.H.; Jones, D.A.; Ashraf, A. Spatial Distribution and Invasion Potential of the Naturalized Downy Thorn Apple (Datura innoxia): Evidence from Classification and Ordination. Sustainability 2022, 14, 10214. https://doi.org/10.3390/su141610214
Ali K, Khan N, Ullah R, Gul A, Khan MEH, Jones DA, Ashraf A. Spatial Distribution and Invasion Potential of the Naturalized Downy Thorn Apple (Datura innoxia): Evidence from Classification and Ordination. Sustainability. 2022; 14(16):10214. https://doi.org/10.3390/su141610214
Chicago/Turabian StyleAli, Kishwar, Nasrullah Khan, Rafi Ullah, Asma Gul, Muhammad Ezaz Hasan Khan, David Aaron Jones, and Azad Ashraf. 2022. "Spatial Distribution and Invasion Potential of the Naturalized Downy Thorn Apple (Datura innoxia): Evidence from Classification and Ordination" Sustainability 14, no. 16: 10214. https://doi.org/10.3390/su141610214
APA StyleAli, K., Khan, N., Ullah, R., Gul, A., Khan, M. E. H., Jones, D. A., & Ashraf, A. (2022). Spatial Distribution and Invasion Potential of the Naturalized Downy Thorn Apple (Datura innoxia): Evidence from Classification and Ordination. Sustainability, 14(16), 10214. https://doi.org/10.3390/su141610214








