Abstract
Understanding space use and movement behavior can benefit conservation and management of species by identifying areas of high importance. However, this can be challenging for highly mobile species, especially those which use a wide range of habitats across ontogeny. The Bahamas is hypothesized to be an important area for tiger sharks, but the utility of the area for this species within the broader western North Atlantic is not fully understood. Therefore, we assessed (1) whether the area near Bimini serves as an important pupping location for tiger sharks, (2) their level of residency and site fidelity to the area, and (3) regional dispersal across ontogeny. Frequent captures of young-of-year tiger sharks, as well as ultrasonography showing near-term and recently postpartum females supports the hypothesis that pupping occurs in the area. However, small juveniles had low overall recapture rates and sparse acoustic detections near Bimini, indicating they do not reside in the area for long or may suffer high natural mortality. Large juvenile and sexually mature tiger sharks had higher overall local residency, which increased during cooler water winter months. The probability of dispersal from Bimini increased for larger individuals. Repeated, long-term site fidelity was displayed by some mature females, with several returning to Bimini across multiple years. Satellite tracking showed that tiger sharks extensively used areas outside of The Bahamas, including traveling more than 12,000 km. Together, these results show that Bimini is an important area for tiger sharks, serving as a pupping ground, rather than a nursery ground, a finding which could be incorporated into future conservation and management efforts.
1. Introduction
Anthropogenic disturbances, such as overexploitation and habitat degradation, are leading to the loss of biodiversity and increasing extinction risk across multiple taxa of marine animals, including sharks and rays [1,2]. Given the significance of these risks, conservation and management efforts can be improved by incorporating a multifaceted approach [3]. However, these efforts are complicated for highly mobile species, especially those which spend considerable time moving between areas of jurisdiction or in ‘high-seas’ fishing zones [4]. Understanding regional movements can help to identify areas of high use and thereby inform conservation efforts [5] and improve marine spatial planning for essential habitats [6,7].
Identifying areas of high importance or essential habitats is particularly challenging for species with large areas of space use, and in these instances, efforts may need to focus upon greatest potential conservation benefit [8]. For example, areas used for reproduction can be especially important, as these areas have a disproportionately large influence on the early life history of elasmobranch species [9]. Some species use well-delineated pupping and nursery grounds for the first few years of their life, such as lemon sharks, Negaprion brevirostris [10], and sandbar sharks, Carcharhinus plumbeus [11]. However, oceanic species, such as blue sharks, Prionace glauca, may use large spatial areas during the juvenile stage, making the delineation of pupping or nursery grounds challenging [12]. In addition, habitat selection, space use, and movement behavior can change with ontogeny [11]. Therefore, studying movement patterns across extended periods of time and age classes can be used to more accurately ascertain the extent to which important areas, such as pupping and nursery grounds, are used by highly mobile species.
Monitoring pupping and nursery grounds and understanding dispersal from them can improve stock assessments, inform abundance surveys, and help maximize area-specific conservation efforts [3]. Heupel et al. [13] proposed guidelines for characterizing elasmobranch nursery grounds as areas in which three criteria are documented for newborn and young-of-year individuals; (1) there is higher relative abundance than elsewhere; (2) individuals remain in the area over extended periods of time; and (3) the area is used across multiple years or cohorts. In comparison, pupping grounds are differentiated from nursery grounds in that these areas meet criteria 1 and 3, but individuals disperse soon after birth and do not remain in the area for long [9]. These metrics have been used to improve the designation of pupping and nursery grounds for several species of elasmobranchs [14,15,16].
Tiger sharks, Galeocerdo cuvier (Péron & Lesueur, 1822), are large-bodied sharks, commonly found in tropical and temperate marine systems, with a circumglobal distribution [17]. Given their wide distribution, previous research has focused on their role as high-level predators in marine systems [18,19,20] and their movement ecology [21,22]. While traditionally considered nearshore species, data show they also frequently use oceanic areas [23]. In some instances, reliable food sources can be a primary driver of movements and the seasonal occurrence of tiger sharks [24,25]. However, movement patterns can vary significantly across individuals, even for those at the same location [22,26]. It is therefore hypothesized that biological factors, such as reproduction, may contribute to the seasonal occurrence [27] and movements of tiger sharks [26]. Understanding the drivers of movements can be informative for determining how regional connectivity and distribution are influenced by ocean and climate conditions [28].
Despite the previous research, significant data gaps still remain for a complete understanding of tiger shark life history, including assessing areas of reproductive importance, movement behavior during early life stages, and changes of movement with ontogeny [29]. Some tiger shark pupping areas have been identified in the nearshore waters along the southeastern United States, predominately along the Florida panhandle and in the ‘Charleston Bump’ area near the Georgia and South Carolina coasts [30]. However, this research was confined to USA territorial waters, and pupping locations throughout the remainder of the western North Atlantic region have not been assessed. As tiger sharks are known to readily move across multiple jurisdictions in the region [22,28], the investigation of other potential pupping or nursery grounds and the connectivity of these areas is important for future management efforts in the region.
The waters of The Bahamas are proposed to be frequently used by tiger sharks, including during gestation [27] and pupping is hypothesized to occur in the shallow waters of The Great Bahama Bank [31,32]. Over the past 35 years, young-of-year (YOY) tiger sharks have been frequently and consistently caught during fishery-independent longline surveys conducted by the Bimini Biological Field Station (BBFS) in the waters surrounding Bimini, The Bahamas [32]. However, the duration over which tiger sharks use this area after birth, how movement behavior changes across ontogeny, and the dispersal from this area to elsewhere in the region are unknown. Here, we use a combination of long-term mark-recapture surveys and biotelemetry to investigate (1) whether Bimini serves as a pupping or nursery ground, (2) the level of site fidelity and residency to the area across ontogeny, and (3) how dispersal from Bimini to the wider western North Atlantic varies across life stages for tiger sharks.
2. Methods
2.1. Study Location
Bimini, The Bahamas (25.73° N, −79.27° W), is a set of two mangrove-fringed islands positioned along the western edge of The Great Bahama Bank, 80 km east of Miami, FL, USA (Figure 1). The two islands are separated by a shallow water lagoon, with habitats surrounding the islands that include shallow-water sandflats and seagrass beds to the north, east, and south, with the western edge consisting of rocky reefs that slope to the deep waters of the Florida Straits. In 2011, the Commonwealth of The Bahamas established a ‘shark sanctuary’ within all waters of its Exclusive Economic Zone (EEZ), prohibiting the catching of sharks and sale of their products.
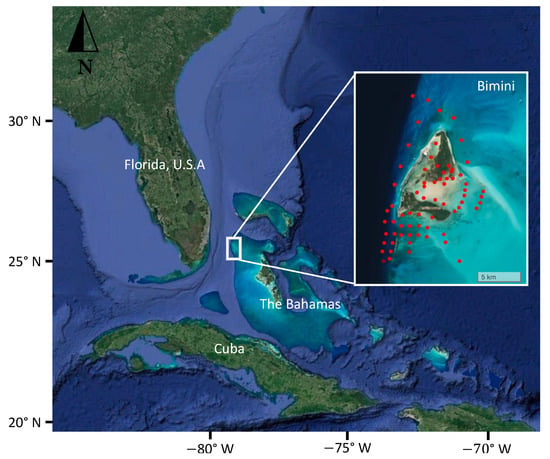
Figure 1.
Study location in Bimini, The Bahamas. Inset map shows acoustic receiver (Innovasea, VR2W) locations (red dots).
2.2. Capture Methods
Tiger sharks were caught and tagged during fisheries-independent surveys within approximately 15 km of Bimini from 1984 through 2020. Captures primarily occurred during monthly shallow-water (depth range 2–5 m) longline surveys [31,32], but some sharks were caught using drumline sets [33], polyball fishing [5], and deep-water horizontal longlines [34]. Captured sharks were secured alongside the research vessel and remained in the water while data were collected. Sex and three length measurements were recorded to the nearest 0.1 cm, i.e., from the tip of the snout to the precaudal notch (PCL), the fork of the tail (FL), and the tip of the tail (TL). Age classes were assigned based on a combination of Branstetter et al. [35], Kneebone et al. [36], and Driggers et al. [30]. Sharks measuring < 180 cm FL were classified as ‘small juvenile’, with those less than 110 cm further defined as YOY. Sharks measuring >180 cm FL, but not yet sexually mature, were classified as ‘large juvenile.’ Males were determined to be sexually mature based on the presence of fully calcified claspers capable of rotation at the base, which typically occurs at approximately 255 cm FL. Females > 265 cm FL were classified as mature [35]. From 2017 onward, the reproductive status of some mature females was determined by ultrasonography (Ibex Pro, E.I. Medical, Loveland, CO, USA). ‘Gravid’ tiger sharks were determined as those for which at least one pup was observed in utero, whereas ‘non-gravid’ tiger sharks were those for which no pups could be identified on the ultrasound.
2.3. Tagging
Upon capture, sharks were inspected for the presence of any identification tags indicating previous capture from our research group or others. If no tag was present, a new dart tag (National Oceanic and Atmospheric Administration (NOAA), Cooperative Shark Tagging Program (CSTP)) was deployed at the base of the first dorsal fin, with a stainless steel barbed dart secured into the musculature. Dart tags were only deployed for sharks deemed to be in good condition (i.e., showing no signs of impairment that would require an early release from the research vessel) and large enough (>~90 cm FL) that the dart could be safely implanted into the muscle. For recaptured sharks, the tag number was recorded. If a recapture occurred outside of the study location, the CSTP informed BBFS with available information about the recapture, including days at liberty, length of the shark upon recapture, and coordinates of recapture.
A combination of passive acoustic and satellite telemetry was used to assess movement of individual tiger sharks, with individuals opportunistically chosen based on availability during surveys. Acoustic tags were deployed by gently rolling the shark ventral side up in order to induce a temporary state of tonic immobility [37], and subsequently the acoustic transmitter (V16-6H-6X, 69 khz, 120 s average nominal delay, Vemco Innovasea, Halifax, NS, Canada) was surgically implanted into the body cavity. The small incision made for the tag implantation was closed using 2–3 sutures. Acoustic detections were recorded on an array of receivers (VR2W, Vemco Innovasea) surrounding Bimini. This array consisted of approximately 60 stations (Figure 1), but this number varied across the study duration as new stations were established and receivers were lost or damaged. The effective detection range (i.e., maximum distance at which 50% of emitted signals can still be detected by receiver stations) of acoustic receivers in the Bimini array was estimated to be 211 m on the bank (seagrass and sandflats) and 255 m in the reef habitat [5]. Acoustic receivers were retrieved, downloaded, cleaned of biofouling, batteries replaced, and redeployed at least once per year. The detection of acoustic-tagged individuals beyond Bimini was accomplished through data-sharing networks, including the Integrated Tracking of Aquatic Animals in the Gulf of Mexico (iTag), Florida Atlantic Coast Telemetry (FACT), and Ocean Tracking Network (OTN).
Smart Positioning and Temperature satellite tags (SPOT 6; Wildlife Computers, Redmond, WA, USA; Models SPOT 257, 258 and 364, with estimated battery lives of 1286, 300, and 300 days, respectively; 30 s repetition rate; 250 daily location uplink limit) were affixed to the first dorsal fin of some female tiger sharks. Similar to the acoustic tags, candidates were opportunistically chosen depending on tag availability, with priority given to pregnant individuals. Satellite transmitters attempted to transmit signals to the ARGOS satellite network when the shark’s dorsal fin breached the water surface. However, satellites are not always overhead, or the tag may not remain at the surface long enough to obtain an accurate location fix based upon the number and proximity of satellites within the transmission range, resulting in a range of location classes (LC) of 3, 2, 1, 0, A, B, or Z (ordered from the lowest error of 250 m to the highest error or location error) [22,38], with class ‘Z’ omitted from analysis.
2.4. Data Analysis
To examine residency through mark-recapture, the probability of a tiger shark with a dart tag being recaptured was modeled using logistic regression, with sex (categorical) and fork length (continuous) as fixed effects. Two models were fit, one for the probability of being recaptured near Bimini, and one for the probability of being recaptured in the CSTP database throughout the remainder of the region.
Data generated from the acoustic array were processed to remove any false detections, defined as a single detection within the array across a one-hour time frame [39], double detections occurring simultaneously on multiple receivers, and any detections within 24 h post tag deployment, to avoid bias of capture for movement behavior [5,7]. Acoustic detections occurring on the Bimini array from 9 March 2015 through 30 September 2020 were included in the analysis. Acoustic detections received from iTag, FACT and OTN by 1 May 2021 were included in the analysis.
To examine local residency near Bimini, a standardized monthly residency index was calculated for each individual as the number of days detected within the Bimini acoustic array divided by the number of days in the month [7]. Drivers of local residency were assessed using a zero-inflated generalized linear mixed-effect model (GLMM) with Poisson distribution, using the ‘glmmTMB’ package [40] in R (Version 4.0.3; R Core Team, Vienna, Austria). The number of days detected per month was the explanatory variable, with mean monthly water temperature (continuous), FL (continuous), and sex (categorical) included as fixed effects, and the transmitter ID was included as a random effect to account for individual variation in residency behavior [41]. Days in a month, or the number of days remaining in the first month post tagging, was used as an offset to balance the uneven number of days per months. To understand the biological drivers of regional movements, the probabilities of acoustic detections occurring outside The Bahamas were analyzed using a binomial logistic regression model with fork length and sex as predictors.
Tiger shark satellite locations were downloaded from the Argos Satellite System via the Wildlife Computers Data Portal. The ‘ArgosFilter’ package [42] in R was used to prepare satellite detections for movement analyses. Detections were first filtered to exclude any points with location class ‘Z’ as these indicate the location process failed and the location was not reliable. An estimated maximum sustained swim speed of 3 m s−1 was used based upon similar telemetry studies of tiger sharks in the region [22]. Subsequently, any detections that would require a greater swim speed from other reliable location points were deemed improbable and were removed. Uplinks from satellite tags were not evenly distributed across days, i.e., some days had several reported locations while others had none. Therefore, a track of the most probable locations was determined with a hierarchical, first-difference, correlated random-walk switching state-based model (hDCRWS) run within a Bayesian framework in the ‘BSAM’ package [43] in R following methods in Lea et al. [22]. This methodology accounted for location class, mean turning angle, and the estimated mean swim speed of the tiger shark to generate the most probable location points. To balance movements across days, rather than number of location points, a time-step of 12 h was used to produce two location estimates per day for each tiger shark track, even on days with no detections [22]. For instances of gaps in transmissions greater than 20 days, the track was split into sections [22].
To further investigate regional movements and the occupation of tiger sharks within the jurisdiction of The Bahamas Exclusive Economic Zone (EEZ), analyses were conducted at the individual and group levels. For individuals, locations were determined to be within or outside the EEZ bounds of The Bahamas based on the GPS coordinates of satellite detections overlaid with the ‘Natural-Earth’ shape file in R. The percentage of time spent within the EEZ was calculated as the number of daily location estimates within the EEZ bounds, divided by the total number of days the satellite tag was transmitting. Further, we used the ‘MOVE’ package [44] to perform a dynamic Brownian bridge movement model (dBBMM, [45]) to calculate utilization distributions (UDs) at the individual level. To avoid error overestimation by the model when tiger sharks were not detected, the variance was constrained (using the brownian.motion.variance.dyn function) each time an individual was absent for >24 h. The sizes of the 50% and 95% (core and extended areas, respectively) UDs were calculated using the volumeUD() function in the ‘fishtrack3d’ library [46]. Subsequently, all individual rasters were summed into one global raster to calculate group-level UDs. The obtained global raster was scaled so that the raster cells summed to 1 to allow for UD calculations (similarly to Van Zinnicq Bergmann et al., [7]). The contours of the group-level UDs were then plotted in relation to the EEZ. All statistical analyses were performed in the RStudio (2021.09.1; R Studio Team, Vienna, Austria) integrated development environment for R version 4.0.3 (R Core Team). Statistical significance was determined at an α = 0.05, and where appropriate, the mean and standard deviation are reported.
3. Results
3.1. Captures
From November 1984 to 30 September 2020, 926 individual tiger sharks (73.1% n = 677 females, 26.9% n = 249 male) for which accurate size, sex, and tag information was available were sampled near Bimini. All size classes were represented in captures for both sexes (overall mean FL = 172.8 cm ± 61.4). Females ranged from 67 to 334 cm FL (mean = 177.6 ± 60.8) and males from 55 to 319 cm FL (mean = 160.1 ± 61.3). Small juveniles (<180 cm FL) were the predominant life stage, representing 57.0% (n = 528) of the tiger sharks caught, and 18.1% (n = 168) of the tiger sharks were less than 110 cm FL, considered to be YOY [36]. Catches of all tiger shark age classes were distributed throughout the year, but there was an increase in the frequency of YOY individuals during the summer and early fall months (Figure 2). From 2017 to 2019, ultrasounds were conducted for nine mature females, resulting in seven confirmed pregnant, one recently postpartum (within a few weeks post pupping; personal communication Dr. Natalie MyIniczenko DVM Disney’s Animals, Science, and Environment), and one not pregnant. Ultrasounds of all mature females were not possible due to technical issues with the unit.
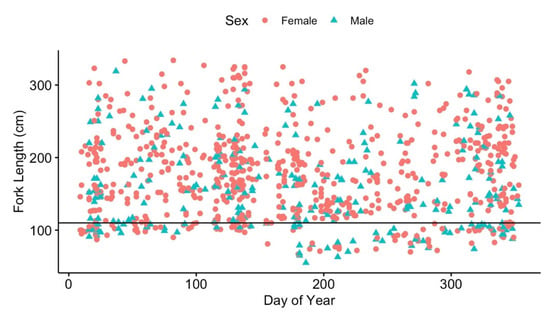
Figure 2.
Relationship between day of year and fork length for tiger sharks, Galeocerdo cuvier, captured near Bimini, The Bahamas. Red circles represent female sharks, and blue triangles represent male sharks. The horizontal black line is the designation of fork length for ‘young-of-year’ size class.
3.2. Local and Regional Recaptures
Of the captured tiger sharks, 789 were marked with a dart tag (590 females and 199 males), and 35 (4.4%) of these were later recaptured by BBFS near Bimini, two of which were recaptured twice. Of the Bimini recaptures, females were recaught more frequently (n = 30, 5.1% recapture rate) than males (n = 5, 2.5% recapture rate). The mean time at liberty between tagging and recapture near Bimini was 354.2 (±501.0) days, and the maximum time at liberty for a tiger shark was 2549 days (approximately seven years). The probability of recapture near Bimini was not significantly correlated with sex (p-value = 0.37) or FL at the time of tagging (p-value = 0.41). An additional 25 tiger sharks (21 females and 4 males; 3.2%) were reported through the CSTP as being recaptured away from the study site (Figure 3). For these tiger sharks, the time at liberty until recapture ranged from 66 to 2282 days (mean = 790 ± 666.1), with an average horizontal displacement of 978 km (±1029.8 km) and maximum of 3531 km. The probability of recapture away from Bimini was not significantly correlated with sex (p-value = 0.55) or FL at the time of tagging (p-value = 0.82).
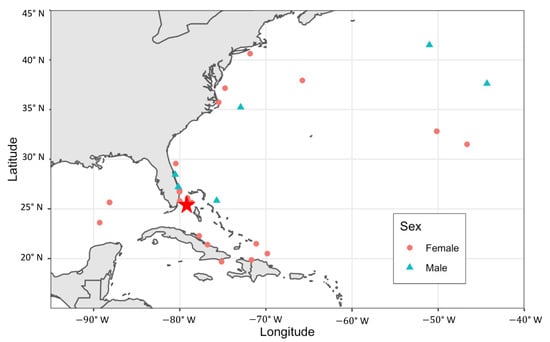
Figure 3.
Recapture locations of tiger sharks, Galeocerdo cuvier, originally captured and tagged in Bimini, The Bahamas (marked with a red star) with dart tags and reported through the National Marine Fisheries Service Cooperative Shark Tagging Program.
3.3. Local Residency
Sixty-four tiger sharks, 18 males and 46 females, were implanted with acoustic transmitters (Supplemental Table S1). At the time of tagging, the size of the tiger sharks ranged from 67 to 333 cm FL (mean = 182.9 ± 92.9 cm); 32 tiger sharks were classified as small juveniles, 11 as large juveniles, and 21 as sexually mature. A total of 11,285 acoustic detections were recorded across 51 stations in the Bimini array.
Overall, individual monthly residency indices within the Bimini array ranged from 0.00 to 0.14 (mean 0.01 ± 0.03). Small juvenile tiger sharks had a lower mean residency index (mean = 0.00 ± 0.001) when compared to large juvenile (mean = 0.02 ± 0.03) and mature (mean = 0.02 ± 0.04) tiger sharks (Figure 4). Nine of the twenty-one mature tiger sharks were detected in Bimini across two years, and three individuals were detected across four years (Figure 5). The results of the GLMM indicated that water temperature (p-value < 0.001) and fork length (p-value < 0.001) were significant predictors of the monthly residency index; however, sex was not (p-value = 0.71) (Table 1).
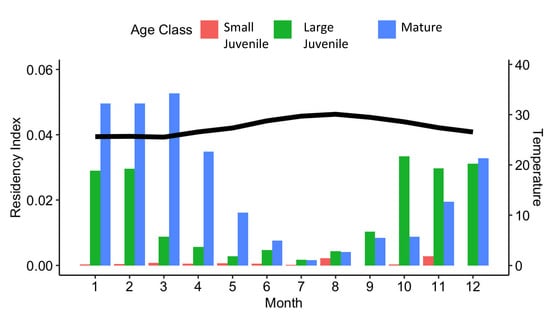
Figure 4.
Mean monthly residency index of small juvenile (red bars), large juvenile (green bars), and mature (blue bars) tiger sharks, Galeocerdo cuvier, in Bimini, The Bahamas. The black line represents the mean monthly water temperature.

Figure 5.
Abacus plots for acoustic detections of tiger sharks, Galeocerdo cuvier, tagged in Bimini, The Bahamas. Panels correspond to age classes: (A) small juveniles, (B) large juveniles, and (C) adults.

Table 1.
Results of zero-inflated generalized linear mixed model results for monthly residency of tiger sharks, Galeocerdo cuvier, in Bimini, The Bahamas. Fork length, water temperature, and sex were predictors. The * indicates statistical significance.
3.4. Regional Movements
Regional movements were obtained from 1610 detections at 104 stations in cooperative arrays. Three of the 32 small juveniles (9.4%), six of the 11 large juvenile (54.5%), and seven of the 21 mature tiger sharks (33.3%) were detected on acoustic arrays outside The Bahamas (Figure 5 and Figure 6). The results of logistic regression showed that the probability of acoustic detections outside Bimini and The Bahamas was significantly correlated with sex (p-value < 0.001) and fork length at the time of tagging (p-value = 0.03), with females and larger tiger sharks more likely to be detected on other arrays throughout the region (Figure 6). Detections on cooperative arrays were primarily along the southeastern USA and the Gulf of Mexico coasts (Figure 6). Of those that dispersed from The Bahamas, none of the three juveniles (0%), three of the six large juveniles (50%), and three of the seven mature (42.9%) tiger sharks later returned to Bimini. For example, mature female tiger shark #16741 departed the Bimini area in February 2017 and was then detected off the coast of South Carolina during May and June of the same year, a distance of ~750 km. She returned to the northern Bahamas in January 2018 and back near Bimini in January 2020. Similarly, mature female #17559 departed Bimini on 7 August 2018 and was then detected off the North Carolina coast on 7 September 2018, a distance of ~1000 km. She returned to the Bimini area during March 2019 and again during January 2020.

Figure 6.
Lines represent stylized movements between acoustic detections on cooperative receiver arrays throughout the southeastern United States of America and The Bahamas for (A) small juvenile, (B) large juvenile, and (C) mature tiger sharks, Galeocerdo cuvier.
Movements were obtained from SPOT tags deployed on eight tiger sharks, six of which also had an acoustic transmitter implanted. SPOT tag models 258 and 364 (estimated battery life of 300 days) had a combined average transmission duration of 105.3 (±64.7) days, whereas model 257 (estimated battery life of 1286 days) had an average transmission duration of 322 (±229.3). SPOT-tagged tiger sharks spent an average of 33.0% (±25.5) of this tracking duration within the EEZ of The Bahamas (Table 2). Utilization distributions indicated a core use area surrounding Bimini and the western Bahamas. However, tiger sharks also spent considerable time in the vicinity of the southeastern USA, Cuba, and Hispaniola (Figure 7). Mean 50% UD encompassed 33,287.5 (±36,186.2) km2 and mean 95% UD encompassed 431,825 (±586,583.8) km2.

Table 2.
Summary information for tiger sharks, Galeocerdo cuvier, tagged with Smart Positioning and Temperature (SPOT) satellite tags near Bimini, The Bahamas. Utilization distribution (UD) represents the 50% and 95% levels of area (km2) occupied. ‘Time (%) in EEZ’ was calculated as the percentage of days at liberty spent within The Bahamas Exclusive Economic Zone (EEZ) based on daily location estimates. The ‘SPOT model’ shows the transmitter model number used for that individual.
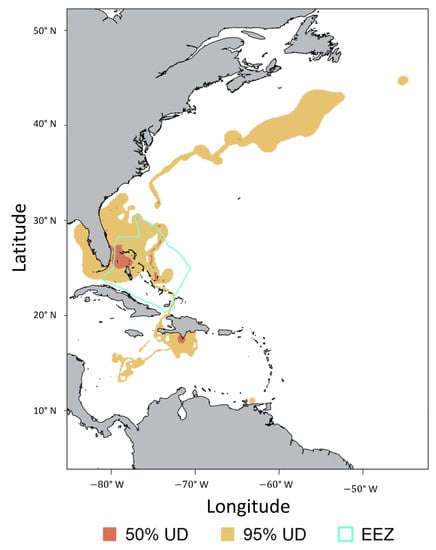
Figure 7.
Utilization distribution comprising all tiger sharks, Galeocerdo cuvier, tagged with SPOT transmitters near Bimini, The Bahamas. The Bahamas Exclusive Economic Zone (‘EEZ’) is denoted by the teal line.
4. Discussion
4.1. Overview
The findings in this study provide new insight about the duration to which the area near Bimini, The Bahamas, is used across ontogeny for tiger sharks and their dispersal in the western North Atlantic Ocean. All age classes, from young-of-year (YOY) to sexually mature, were captured, including several females being confirmed near-term or recently post-partum. Residency within the immediate area surrounding Bimini was predominately driven by size and water temperature, with small juvenile tiger sharks rarely recaptured and only detected over short durations. However, small juveniles were also infrequently detected by cooperative acoustic arrays in the region, potentially indicting limited regional dispersal of this age class or high natural mortality. Larger juveniles and mature tiger sharks were monitored around Bimini over longer durations, with increased residency during the cool water winter months. Large juvenile and mature tiger sharks were also tracked more frequently throughout the broader western North Atlantic region. Some larger tiger sharks also returned to Bimini after prolonged absences, including annual returns of up to five years, providing evidence for multi-year fidelity to this area. Taken together, these findings show the area is important for tiger sharks, including as a pupping ground.
4.2. Evidence for a Pupping Location
Two lines of evidence, documentation of near-term gravid and recently postpartum females and frequent catches of YOY, point to the area around Bimini being a pupping location for tiger sharks. Ultrasonography results support pupping occurring near Bimini during the late spring and summer. Given the two-year reproductive cycle and estimated 15 month gestation period of tiger sharks [47], it was expected that approximately 50% of the mature females would be at some stage of pregnancy at any one point in time. For example, in the waters of Tiger Beach, The Bahamas, Sulikowski et al. [27] found that 23 of the 40 (57.5%) mature female tiger sharks were pregnant and also estimated a late spring or summer pupping season. In our study, eight of the nine mature females (88%) were pregnant or recently postpartum (within days or weeks). However, mechanical failures with the ultrasound prevented a more robust assessment of all mature female sharks, and future research should focus on this to incorporate seasonality of reproduction across multiple locations in the region.
Our catch data support previous research conducted near Bimini, in which the tiger shark mean size of capture was correlated with the month of the year, and YOY tiger sharks caught during the presumed pupping season of July to September [31]. The size composition of tiger sharks caught near Bimini was consistent from 1984 to 2019 [32], indicating this area has potentially been continually used as a pupping location over multiple decades. Higher relative abundance is one of the criteria for distinguishing important pupping and nursery areas [13], but comparing between different surveys can be a challenge due to differences in sampling methods across research groups. However, comparisons of studies can at least provide some context within the region. For example, a fishery-independent longline survey conducted in the eastern Bahamas had a mean fork length for tiger sharks of 248.65 cm, compared to 172.8 cm in our study, potentially suggesting a higher relative frequency of smaller tiger sharks near Bimini. The shallow water bank habitat sampled in our study is a commonly used habitat for juvenile tiger sharks [48], likely contributing to the smaller mean size.
4.3. Residency and Site Fidelity
The residency and site fidelity of tiger sharks were demonstrated through both mark-recapture and telemetry. However, the overall recapture rate near Bimini was low, suggesting many tiger sharks may be moving out of the area. The local recapture rate of 4.7% was lower than the 7.3% recapture rate for tiger sharks tagged in the USA over the past 50 years [49]. This may be a result of shark fishing and surveys occurring at considerably higher frequencies in the USA than in The Bahamas, thereby increasing opportunity for recaptures. This is supported by the shorter mean time at liberty for tiger sharks tagged in the USA (mean 365 days) [49] compared to those tagged near Bimini (mean 790 days).
There was low residency and longevity of small juvenile tiger sharks near Bimini, indicating limited long-term fidelity of this age class to the area. It is possible that small juvenile tiger sharks are using the broader Great Bahama Bank area, which provides ample key habitats for tiger sharks, such as seagrass, sandflat, and nearshore waters [50], but this vast area has relatively little acoustic receiver coverage compared to the nearshore waters of the USA. Hence, the lack of acoustic coverage could partially explain the infrequent acoustic detections in the area. The overall low detections, limited residency within the array, and short longevity of small juvenile tiger sharks may also be indicative of high natural mortality at this early life stage. In the western North Atlantic, the annual mortality rate was estimated at 61% and 73% for YOY and age 1 tiger sharks, respectively [30], and this high mortality could limit the ability to monitor young tiger sharks across long durations.
Large juvenile and mature tiger sharks had a higher residency index and longevity than small juveniles, potentially due to higher survival or biological drivers such as foraging and reproduction. Multi-year fidelity of tiger sharks in The Bahamas was previously documented at a provisioning dive site, Tiger Beach, Grand Bahama [51,52]. At this location, the seasonal residency of mature female tiger sharks was hypothesized to be partly driven by reproductive cycle, with mature females utilizing the waters of The Bahamas during gestation [27]. Mature female tiger sharks had the highest residency to Bimini during the cooler water winter months, before departing in late spring, potentially following parturition. Despite the estimated 2-year reproductive cycle, some females were present in Bimini in consecutive years. Therefore, reproduction alone may not be driving seasonal residency, as some tiger sharks were using the waters around Bimini presumably during both ‘pregnant’ and ‘recovery’ years. This is similar to what has been documented for great hammerhead sharks near Bimini, in which females had annual fidelity to the area [5].
4.4. Regional Movements and Connectivity
Multiple sets of data, including mark-recaptures, acoustic detections, and satellite telemetry, provided evidence of connectivity throughout the western North Atlantic region. The NOAA CSTP provided initial evidence of large-scale dispersal throughout the region. Tiger sharks had horizontal displacements of more than 3500 km, including to the northern reaches of their range (41° N), which corroborates the oceanic distribution of this species [23]. These mark-recaptures are also consistent with the overall results of the NOAA CSTP program, showing widespread dispersal of tiger sharks throughout the western North Atlantic Ocean [28,49].
Large juvenile and mature tiger sharks were more likely to be detected throughout the coastal areas of the southeastern USA and the Gulf of Mexico. While there were few instances of small juveniles being detected outside The Bahamas, the ability for extensive movement at an early life stage was evident by individual #14746, a 117 cm FL (145 cm TL) female detected off the coast of Delaware, USA, 103 days after tagging, covering a distance of ~1500 km. The greater number of detections and higher degree of connectivity for larger sharks are not surprising given their higher natural survival and presumed greater ability to transit offshore habitats [50], such as those separating The Bahamas from the USA and other areas in the region. However, only one of the five large juvenile and mature male tiger sharks (312 cm TL mature male) were later detected outside The Bahamas. The absence of detections of other males is not confirmation they did not disperse to other areas, as previous research shows male tiger sharks extensively use offshore areas [22,50,53], which would be beyond the range of most acoustic receiver arrays.
Large juvenile and mature tiger sharks were also more likely to return to Bimini following movements outside The Bahamas. This type of fidelity following large-scale movements has been documented for tiger sharks in both the Atlantic [22] and Pacific Oceans [26,54], and may be a common behavior for this species. However, there were still extended temporal data gaps for these mature individuals tagged near Bimini. The Great Bahama Bank and area surrounding Bimini presumably provide diverse and productive habitats (i.e., reef, seagrass, mangroves), with ample resources to support large tiger sharks. It is therefore unclear if prolonged absences in acoustic detections were indicative of dispersal from Bimini or of space use beyond the limited range of the Bimini array.
The extended range of satellite-tagged tiger sharks includes a large amount space use outside The Bahamas EEZ, which corroborates findings from other research projects in the region showing tiger sharks readily crossing jurisdictions [51,53]. This is especially important to consider given Bimini’s close proximity to waters under the jurisdiction of other countries, such as the USA (~40 km) and Cuba (~250 km), which have different management and harvest regulations for sharks. The northern movements observed for satellite-tagged tiger sharks in this study were similar to those tracked by Lea et. al. [22], who proposed tiger sharks were drawn to the area of the North Atlantic due to high biological productivity, including marine turtles, or for reproduction. Tiger sharks previously tagged in the northern Bahamas displayed northward movements to approximately 42° N [28], while our study shows space use to at least 45° N. Residency time in the extreme northern range was brief, and it is not clear the frequency to which this area is used by the species over multiple years. Therefore, future research should incorporate analysis of oceanic factors such as sea surface temperature and seasonal warm water gyres from the Gulf Stream as potential drivers for these northern movements. Understanding the drivers of this expansive space use, including the northern bounds, may be important as ocean and climatic conditions continue to change in the future [28].
4.5. Notable Individual Movements
Several tagged tiger sharks were tracked returning to Bimini following prolonged absences and extensive movements in the region. For example, PTT ID 174072 (acoustic ID 17556), a mature female, was caught and tagged near Bimini on 10 May 2018. Tracking data showed that she departed the area on 27 April 2019 and although satellite transmissions were sparse in the following months, she was detected off the northern coast of Venezuela during June 2019. She soon began to move northward and was detected off the coast of Nova Scotia, Canada, by August 2019. The satellite tag ceased reliable transmissions on 5 September 2019, but the acoustic transmitter detections recorded her in Bimini on 7 April 2020; completing a round trip journey of more than 12,000 km, across a 344-day span (Figure 8). Female tiger shark PTT ID 174073 (acoustic ID 12726) was tagged near Bimini on 26 June 2018. Nearly immediately, she moved northward, reaching the area off the east coast of Nova Scotia two months later, then moving southward during September 2018, a trip of 4500 km across 97 days. The satellite tag ceased transmissions near Bermuda, but she was detected on the Bimini acoustic array from February through May 2019 (Figure 8).
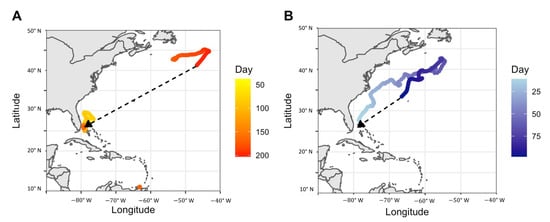
Figure 8.
Location estimates of tiger sharks, Galeocerdo cuvier (A) PTT ID 174072 and (B) PTT 174073 generated from Bayesian State-Space Movement Model with ‘Day’ at liberty since tagging. The dashed line represents the shortest potential tiger shark path determined through acoustic telemetry post-satellite tag ceasing transmissions.
The movements and reproductive cycle of these two sharks correspond with the hypothesis that at least some tiger sharks reside in Bimini during and immediately following gestation and pupping, but that they may mate elsewhere. Shark 174072 was recently post-partum at the time of tagging and therefore resided around Bimini during ‘recovery’ time following pupping. Her northward movements the following summer corresponded to the proposed mating season, which may occur during seasonal food source aggregations. Shark 174073 was determined to be non-gravid at the time of capture and immediately moved northward before returning to Bimini the following spring, while presumably pregnant. We acknowledge these are limited observations, but they may provide first insights into some drivers of movement and long-term site fidelity, including the possible reproductive fidelity of tiger sharks. Reproductive fidelity, and in some cases even natal philopatry, has been documented in other shark species, such as lemon sharks near Bimini [55]. Future research could include genetic analysis of offspring to investigate repeated pupping in the area across multiple years.
5. Conclusions
Considering the evidence of near-term and recently post-partum females, the frequent capture of YOY pups, but low residency of small juveniles in the area, we suggest that the area surrounding Bimini is an important pupping area for tiger sharks. However, based on the available data, this area does not qualify as a nursery area, and it is unclear if traditional ‘nursery’ areas exist for this highly mobile species. It is more probable that juvenile tiger sharks use expansive nearshore waters, similar to those of the dusky shark, Carcharhinus obscurus [8]. Further research, including standardized surveys (i.e., gear construction similar to other surveys in the region), would be required to put the relative abundance of YOY into perspective with other pupping grounds documented in the USA. This is particularly important given the significant connectivity for tiger sharks between The Bahamas, the southeastern USA, and the overall western North Atlantic region. Telemetry also showed that large juvenile and mature female tiger sharks have large areas of space use, frequently crossing international jurisdictions. However, following these large-scale movements, female tiger sharks also showed multi-year fidelity to the area around Bimini. Taken together, our data suggest the area surrounding Bimini, and the broader Great Bahama Bank, is important for tiger sharks in the western North Atlantic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su141610017/s1, Figure S1: Ultrasound images from tiger sharks Galeocerdo cuvier sampled near Bimini, The Bahamas. (A) Recently postpartum female with distention and folds in uterine wall, suggesting recent pregnancy and pupping. (B) A near-term pup with noticeable cranium and jaw structure; Table S1: Tag identification, fork length cm (FL), total length cm (TL), and ultrasonography results for tiger sharks Galeocerdo cuvier sampled near Bimini, The Bahamas.
Author Contributions
Conceptualization, M.J.S., A.C.S., S.H.G. and T.L.G.; methodology M.J.S., A.C.S., F.D., M.P.M.v.Z.B., V.H., S.H.G., T.L.G.; formal analysis M.J.S., A.C.S., F.D.; investigation M.J.S., A.C.S., F.D., M.P.M.v.Z.B., V.H., S.H.G., T.L.G.; resources M.J.S., S.H.G., T.L.G.; data curation M.J.S., F.D., M.P.M.v.Z.B., V.H., S.H.G., T.L.G.; writing—original draft preparation M.J.S. and A.C.S.; writing—review and editing M.J.S., A.C.S., F.D., M.P.M.v.Z.B., V.H., T.L.G.; supervision M.J.S., A.C.S., S.H.G., T.L.G.; project administration M.J.S., S.H.G., T.L.G.; funding acquisition M.J.S., S.H.G., T.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through from Bimini Biological Field Station Foundation, Save Our Seas Foundation (Grants #260 and #436), Hai-Stiftung Shark Foundation, Rock The Ocean Foundation, and Ocean Tracking Network.
Institutional Review Board Statement
Surveys were carried out under various research protocols granted by The Bahamas Department of Marine Resources to Dr. Samuel Gruber, University of Miami, and Bimini Biological Field Station Foundation, permit MA&M/FIS/17B. Longline survey methodology, tiger shark capture, data collection, and tagging were conducted in accordance with University of Alaska Fairbanks Institutional Animal Care and Use Committee protocol number 923356-3.
Acknowledgments
We are grateful to the Save Our Seas Foundation for the continued support of research grants for the duration of the study and to the Hai-Stiftung Shark Foundation for the continued support of the acoustic telemetry project in both Bimini and Florida, USA. We are also grateful for the support of Rock The Ocean Foundation provides to BBFSF, including this project. The large scope of this project, in both time and effort, would not have been possible without the tremendous effort of all Bimini Shark Lab volunteers, students, and staff. We are extremely grateful for their assistance with longline surveys, tagging efforts, and maintenance of the acoustic array. We are grateful for the guidance of Natalie MyIniczenko with reproductive analysis and to E.I. Medical for the generous sponsorship of an ultrasound unit. The regional movement analysis would not have been possible without the cooperative acoustic telemetry arrays, and we are grateful for all involved with these efforts. Special thanks go to all involved with the NMFS Cooperative Shark Tagging program data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E.; et al. Global Status and Conservation Potential of Reef Sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing Drives over One-Third of All Sharks and Rays toward a Global Extinction Crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef] [PubMed]
- Hays, G.C.; Bailey, H.; Bograd, S.J.; Bowen, W.D.; Campagna, C.; Carmichael, R.H.; Casale, P.; Chiaradia, A.; Costa, D.P.; Cuevas, E.; et al. Translating Marine Animal Tracking Data into Conservation Policy and Management. Trends Ecol. Evol. 2019, 34, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, N.; Humphries, N.E.; Couto, A.; Vedor, M.; Da Costa, I.; Sequeira, A.M.; Mucientes, G.; Santos, A.M.; Abascal, F.J.; Abercrombie, D.L.; et al. Global Spatial Risk Assessment of Sharks under the Footprint of Fisheries. Nature 2019, 572, 461–466. [Google Scholar] [CrossRef]
- Guttridge, T.L.; Bergmann, M.P.M.V.Z.; Bolte, C.; Howey, L.A.; Finger, J.S.; Kessel, S.T.; Brooks, J.L.; Winram, W.; Bond, M.E.; Jordan, L.K.B.; et al. Philopatry and Regional Connectivity of the Great Hammerhead Shark, Sphyrna Mokarran in the U.S. and Bahamas. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Lennox, R.J.; Engler-Palma, C.; Kowarski, K.; Filous, A.; Whitlock, R.; Cooke, S.J.; Auger-Méthé, M. Optimizing Marine Spatial Plans with Animal Tracking Data. Can. J. Fish. Aquat. Sci. 2019, 76, 497–509. [Google Scholar] [CrossRef]
- Bergmann, M.P.v.Z.; Guttridge, T.L.; Smukall, M.J.; Adams, V.M.; Bond, M.E.; Burke, P.J.; Fuentes, M.M.; Heinrich, D.D.; Huveneers, C.; Gruber, S.H.; et al. Using Movement Models and Systematic Conservation Planning to Inform Marine Protected Area Design for a Multi-Species Predator Community. Biol. Conserv. 2022, 266, 109469. [Google Scholar] [CrossRef]
- Bangley, C.W.; Curtis, T.H.; Secor, D.H.; Latour, R.J.; Ogburn, M.B. Identifying Important Juvenile Dusky Shark Habitat in the Northwest Atlantic Ocean Using Acoustic Telemetry and Spatial Modeling. Mar. Coast. Fish. 2020, 12, 348–363. [Google Scholar] [CrossRef]
- Heupel, M.R.; Kanno, S.; Martins, A.; Simpfendorfer, C.A. Advances in Understanding the Roles and Benefits of Nursery Areas for Elasmobranch Populations. Mar. Freshw. Res. 2019, 70, 897–907. [Google Scholar] [CrossRef]
- Chapman, D.D.; Babcock, E.A.; Gruber, S.H.; Dibattista, J.D.; Franks, B.R.; Kessel, S.A.; Guttridge, T.; Pikitch, E.K.; Feldheim, K.A. Long-Term Natal Site-Fidelity by Immature Lemon Sharks Negaprion brevirostris at a Subtropical Island. Mol. Ecol. 2009, 18, 3500–3507. [Google Scholar] [CrossRef]
- Grubbs, R.D.; Musick, J.A.; Conrath, C.L.; Romine, J.G. Long-Term Movements, Migration, and Temporal Delineation of a Summer Nursery for Juvenile Sandbar Sharks in the Chesapeake Bay Region. Am. Fish. Soc. Symp. 2007, 50, 87–107. [Google Scholar]
- Vandeperre, F.; Aires-Da-Silva, A.; Fontes, J.; Santos, M.; Santos, R.S.; Afonso, P. Movements of Blue Sharks Prionace Glauca across Their Life History. PLoS ONE 2014, 9, e103538. [Google Scholar] [CrossRef] [PubMed]
- Heupel, M.; Carlson, J.K.; Simpfendorfer, C.A. Shark Nursery Areas: Concepts, Definition, Characterization and Assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Froeschke, J.; Stunz, G.; Sterba-Boatwright, B.; Wildhaber, M. An Empirical Test of the ‘Shark Nursery Area Concept’ in Texas Bays Using a Long-Term Fisheries-Independent Data Set. Aquat. Biol. 2010, 11, 65–76. [Google Scholar] [CrossRef]
- Hollensead, L.; Grubbs, R.; Carlson, J.; Bethea, D. Assessing Residency Time and Habitat Use of Juvenile Smalltooth Sawfish Using Acoustic Monitoring in a Nursery Habitat. Endanger. Species Res. 2018, 37, 119–131. [Google Scholar] [CrossRef]
- Macdonald, C.; Jerome, J.; Pankow, C.; Perni, N.; Black, K.; Shiffman, D.; Wester, J. First Identification of Probable Nursery Habitat for Critically Endangered Great Hammerhead Sphyrna mokarran on the Atlantic Coast of the United States. Conserv. Sci. Pract. 2021, 3, e418. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Simpfendorfer, C.C. Tiger Shark: Galeocerdo cuvier. The IUCN Red List of Threatened Species 8235. 2019. Available online: http://www.iucnredlist.org/details/39378/0 (accessed on 25 April 2022).
- Heithaus, M.; Dill, L.; Marshall, G.; Buhleier, B. Habitat Use and Foraging Behavior of Tiger Sharks Galeocerdo cuvier in a Seagrass Ecosystem. Mar. Biol. 2002, 140, 237–248. [Google Scholar] [CrossRef]
- Burkholder, D.A.; Heithaus, M.R.; Fourqurean, J.; Wirsing, A.; Dill, L.M. Patterns of Top-down Control in a Seagrass Ecosystem: Could a Roving Apex Predator Induce a Behaviour-Mediated Trophic Cascade? J. Anim. Ecol. 2013, 82, 1192–1202. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Thums, M.; Heithaus, M.R.; Barnett, A.; Abrantes, K.; Holmes, B.J.; Zamora, L.M.; Frisch, A.J.; Pepperell, J.G.; Burkholder, D.; et al. The Trophic Role of a Large Marine Predator, the Tiger Shark Galeocerdo cuvier. Sci. Rep. 2017, 7, 7641. [Google Scholar] [CrossRef]
- Meyer, C.; Clark, T.; Papastamatiou, Y.; Whitney, N.; Holland, K. Long-Term Movement Patterns of Tiger Sharks Galeocerdo cuvier in Hawaii. Mar. Ecol. Prog. Ser. 2009, 381, 223–235. [Google Scholar] [CrossRef]
- Lea, J.S.E.; Wetherbee, B.M.; Queiroz, N.; Burnie, N.; Aming, C.; Sousa, L.L.; Mucientes, G.R.; Humphries, N.; Harvey, G.M.; Sims, D.; et al. Repeated, Long-Distance Migrations by a Philopatric Predator Targeting Highly Contrasting Ecosystems. Sci. Rep. 2015, 5, 11202. [Google Scholar] [CrossRef] [PubMed]
- Domingo, A.; Coelho, R.; Cortes, E.; Garcia-Cortes, B.; Mas, F.; Mejuto, J.; Miller, P.; Ramos-Cartelle, A.; Santos, M.N.; Yokawa, K. Is the Tiger Shark Galeocerdo cuvier a Coastal Species? Expanding Its Distribution Range in the Atlantic Ocean Using at-Sea Observer Data. J. Fish. Biol. 2016, 88, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.G.; Wetherbee, B.M.; Meyer, C.G. Using Acoustic Telemetry Monitoring Techniques to Quantity Movement Patterns and Site Fidelity of Sharks and Giant Trevally around French Frigate Shoals and Midway Atoll. Atoll Res. Bull. 2006, 543, 281–303. [Google Scholar]
- Fitzpatrick, R.; Thums, M.; Bell, I.; Meekan, M.G.; Stevens, J.D.; Barnett, A. A Comparison of the Seasonal Movements of Tiger Sharks and Green Turtles Provides Insight into Their Predator-Prey Relationship. PLoS ONE 2012, 7, e051927. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Meyer, C.G.; Carvalho, F.; Dale, J.J.; Hutchinson, M.R.; Holland, K.N. Telemetry and Random-Walk Models Reveal Complex Patterns of Partial Migration in a Large Marine Predator. Ecology 2013, 94, 2595–2606. [Google Scholar] [CrossRef]
- Sulikowski, J.; Wheeler, C.; Gallagher, A.; Prohaska, B.; Langan, J.; Hammerschlag, N. Seasonal and Life-Stage Variation in the Reproductive Ecology of a Marine Apex Predator, the Tiger Shark Galeocerdo cuvier, at a Protected Female-Dominated Site. Aquat. Biol. 2016, 24, 175–184. [Google Scholar] [CrossRef]
- Hammerschlag, N.; McDonnell, L.H.; Rider, M.J.; Street, G.M.; Hazen, E.L.; Natanson, L.J.; McCandless, C.T.; Boudreau, M.R.; Gallagher, A.J.; Pinsky, M.L.; et al. Ocean Warming Alters the Distributional Range, Migratory Timing, and Spatial Protections of an Apex Predator, the Tiger Shark (Galeocerdo cuvier). Glob. Chang. Biol. 2022, 28, 1990–2005. [Google Scholar] [CrossRef]
- Holland, K.N.; Anderson, J.M.; Coffey, D.M.; Holmes, B.J.; Meyer, C.G.; Royer, M.A. A Perspective on Future Tiger Shark Research. Front. Mar. Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Driggers, W.B., III; Ingram, G.W., Jr.; Grace, M.A.; Gledhill, C.T.; Henwood, T.A.; Horton, C.N.; Jones, C.M. Pupping Areas and Mortality Rates of Young Tiger Sharks Galeocerdo cuvier in the Western North Atlantic Ocean. Aquat. Biol. 2008, 2, 161–170. [Google Scholar] [CrossRef]
- Hansell, A.C.; Kessel, S.T.; Brewster, L.R.; Cadrin, S.X.; Gruber, S.H.; Skomal, G.B.; Guttridge, T.L. Local Indicators of Abundance and Demographics for the Coastal Shark Assemblage of Bimini, Bahamas. Fish. Res. 2018, 197, 34–44. [Google Scholar] [CrossRef]
- Smukall, M.J.; Carlson, J.; Kessel, S.T.; Guttridge, T.L.; Dhellemmes, F.; Seitz, A.C.; Gruber, S. Thirty-five Years of Tiger Shark Galeocerdo cuvier Relative Abundance near Bimini, The Bahamas, and the Southeastern United States with a Comparison across Jurisdictional Bounds. J. Fish. Biol. 2022, 101, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.J.; Wagner, D.N.; Irschick, D.J.; Hammerschlag, N. Body Condition Predicts Energy Stores in Apex Predatory Sharks. Conserv. Physiol. 2014, 2, cou022. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.J.; Brooks, A.M.; Williams, S.; Jordan, L.K.; Abercrombie, D.; Chapman, D.D.; Howey-Jordan, L.A.; Grubbs, R.D. First Description of Deep-Water Elasmobranch Assemblages in the Exuma Sound, The Bahamas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 115, 81–91. [Google Scholar] [CrossRef]
- Branstetter, S.; Musick, J.A.; Colvocoresses, J.A. A Comparison of the Age and Growth of the Tiger Shark, Galeocerdo cuvier, From Off Virginia and From the Northwestern Gulf of Mexico. Fish. Bull. 1987, 85, 269–279. [Google Scholar]
- Kneebone, J.; Natanson, L.J.; Andrews, A.H.; Howell, W.H. Using Bomb Radiocarbon Analyses to Validate Age and Growth Estimates for the Tiger Shark, Galeocerdo cuvier, in the Western North Atlantic. Mar. Biol. 2008, 154, 423–434. [Google Scholar] [CrossRef]
- Kessel, S.T.; Hussey, N.E. Tonic immobility as an anesthetic for elasmobranchs during surgical implantation procedures. Can. J. Fish. Aqua. 2015, 72, 1–5. [Google Scholar]
- Hays, G.; Åkesson, S.; Godley, B.; Luschi, P.; Santidrian, P. The Implications of Location Accuracy for the Interpretation of Satellite-Tracking Data. Anim. Behav. 2001, 61, 1035–1040. [Google Scholar] [CrossRef]
- Kessel, S.T.; Cooke, S.; Heupel, M.; Hussey, N.E.; Simpfendorfer, C.A.; Vagle, S.; Fisk, A.T. A Review of Detection Range Testing in Aquatic Passive Acoustic Telemetry Studies. Rev. Fish. Biol. Fish. 2013, 24, 199–218. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Rider, M.J.; McDonnell, L.H.; Hammerschlag, N. Multi-Year Movements of Adult and Subadult Bull Sharks Carcharhinus Leucas: Philopatry, Connectivity, and Environmental Influences. Aquat. Ecol. 2021, 55, 559–577. [Google Scholar] [CrossRef]
- Freitas, C.; Lydersen, C.; Fedak, M.A.; Kovacs, K.M. A simple new algorithm to filter marine mammal Argos locations. Mar. Mammal Sci. 2008, 24, 315–325. [Google Scholar] [CrossRef]
- Jonsen, I.D.; Flemming, J.M.; Myers, R.A. Robust state-space modeling of animal movement data. Ecology 2005, 86, 2874–2880. [Google Scholar] [CrossRef]
- Kranstauber, B.; Smolla, M.; Scharf, A.K. Move: Visualizing and Analyzing Animal Tracking Data. R Package Version 3.2.2. 2019. Available online: http://cran.r.project.org/package=move (accessed on 17 June 2022).
- Kranstauber, B.; Kays, R.; Lapoint, S.D.; Wikelski, M.; Safi, K. A dynamic Brownian bridge movement model to estimate utilization distributions for heterogenous animal movements. J. Anim. Ecol. 2012, 81, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Aspillaga, E.; Arlinghaus, R.; Martorell-Barcelo, M.; Follana-Berna, G.; Lana, A.; Campos-Candela, A.; Alos, J. Performance of a novel system for high-resolution tracking of marine fish societies. Anim. Biotelemetry 2021, 9, 1. [Google Scholar] [CrossRef]
- Whitney, N.M.; Crow, G.L. Reproductive Biology of the Tiger Shark Galeocerdo cuvier in Hawaii. Mar. Biol. 2007, 151, 63–70. [Google Scholar] [CrossRef]
- Afonso, A.S.; Hazin, F.H.V. Vertical Movement Patterns and Ontogenetic Niche Expansion in the Tiger Shark, Galeocerdo cuvier. PLoS ONE 2015, 10, e116720. [Google Scholar] [CrossRef]
- Kohler, N.E.; Turner, P.A. Distributions and Movements of Atlantic Shark Species: A 52-Year Retrospective Atlas of Mark and Recapture Data. Mar. Fish. Rev. 2019, 81, 1–93. [Google Scholar] [CrossRef]
- Ajemian, M.J.; Drymon, J.M.; Hammerschlag, N.; Wells, R.J.D.; Street, G.; Falterman, B.; McKinney, J.A.; Iii, W.B.D.; Hoffmayer, E.R.; Fischer, C.; et al. Movement Patterns and Habitat Use of Tiger Sharks Galeocerdo cuvier across Ontogeny in the Gulf of Mexico. PLoS ONE 2020, 15, e234868. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Gallagher, A.J.; Wester, J.; Luo, J.; Ault, J.S. Don’t Bite the Hand That Feeds: Assessing Ecological Impacts of Provisioning Ecotourism on an Apex Marine Predator. Funct. Ecol. 2012, 26, 567–576. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Gutowsky, L.; Gallagher, A.; Matich, P.; Cooke, S. Diel Habitat Use Patterns of a Marine Apex Predator Tiger Shark, Galeocerdo cuvier at a High Use Area Exposed to Dive Tourism. J. Exp. Mar. Biol. Ecol. 2017, 495, 24–34. [Google Scholar] [CrossRef]
- Lea, J.S.; Wetherbee, B.M.; Sousa, L.L.; Aming, C.; Burnie, N.; E Humphries, N.; Queiroz, N.; Harvey, G.M.; Sims, D.W.; Shivji, M.S. Ontogenetic Partial Migration Is Associated with Environmental Drivers and Influences Fisheries Interactions in a Marine Predator. ICES J. Mar. Sci. 2018, 75, 1383–1392. [Google Scholar] [CrossRef]
- Werry, J.M.; Planes, S.; Berumen, M.L.; Lee, K.A.; Braun, C.D.; Clua, E. Reef-Fidelity and Migration of Tiger Sharks, Galeocerdo cuvier, across the Coral Sea. PLoS ONE 2014, 9, e083249. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, K.A.; Gruber, S.H.; Dibattista, J.D.; Babcock, E.A.; Kessel, S.T.; Hendry, A.P.; Pikitch, E.K.; Ashley, M.V.; Chapman, D.D. Two Decades of Genetic Profiling Yield First Evidence of Natal Philopatry and Long-Term Fidelity to Parturition Sites in Sharks. Mol. Ecol. 2013, 23, 110–117. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).