Land Use Land/Cover Change Reduces Woody Plant Diversity and Carbon Stocks in a Lowland Coastal Forest Ecosystem, Tanzania
Abstract
1. Introduction
2. Materials and Methods
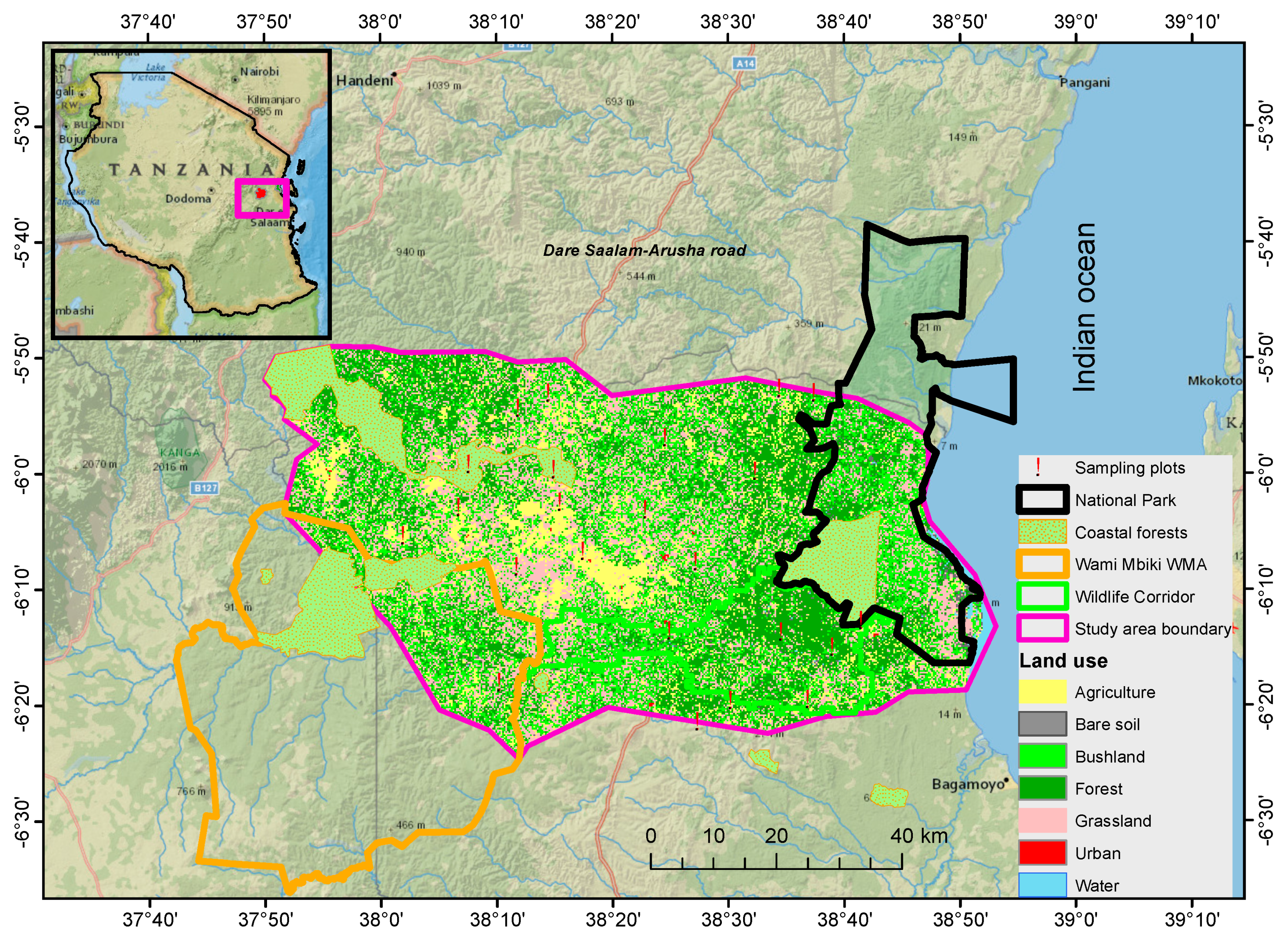
2.1. Study Area Description
2.2. Remote Sensing Image Classification and Predictive Model
2.3. Vegetation Assessment
2.4. Soil Sampling and Physico-Chemical Analyses
2.5. Woody Plant Species Diversity
2.6. Carbon Estimation
| C Pool | Allometric Equations | Mean C Density (t/ha) | ±SE | Reference |
|---|---|---|---|---|
| AGCwoody | 61.2 | 17.8 | [72,73] | |
| BGCwoody | 34.9 | 7.3 | [42,73] | |
| Cgrass | 2.5 | 0.1 | [56] | |
| SOC | 76.0 | 6.3 | [57,59,63] | |
| Ctotal | 172.1 | 31.4 |
2.7. Statistical Analysis
3. Results
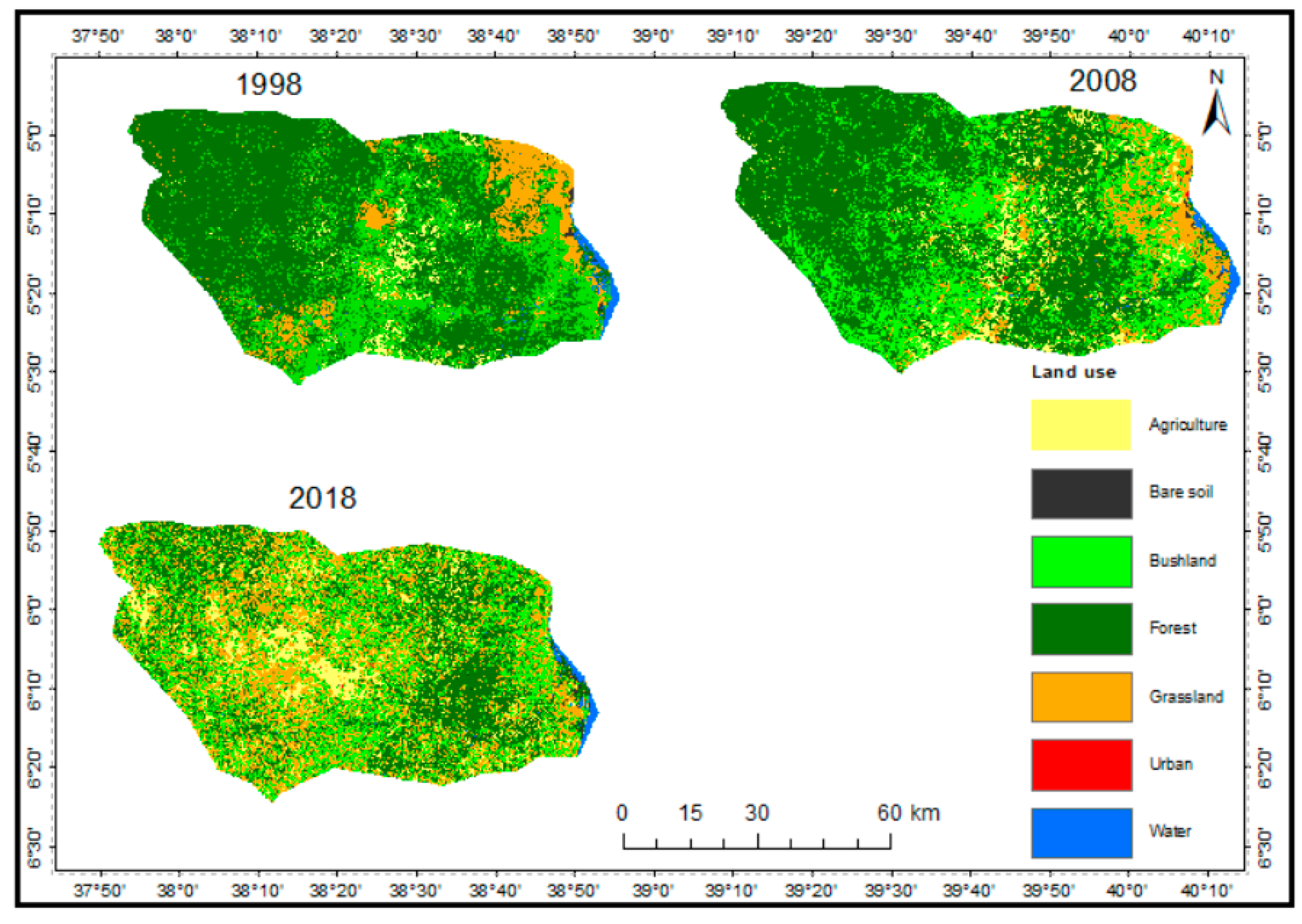
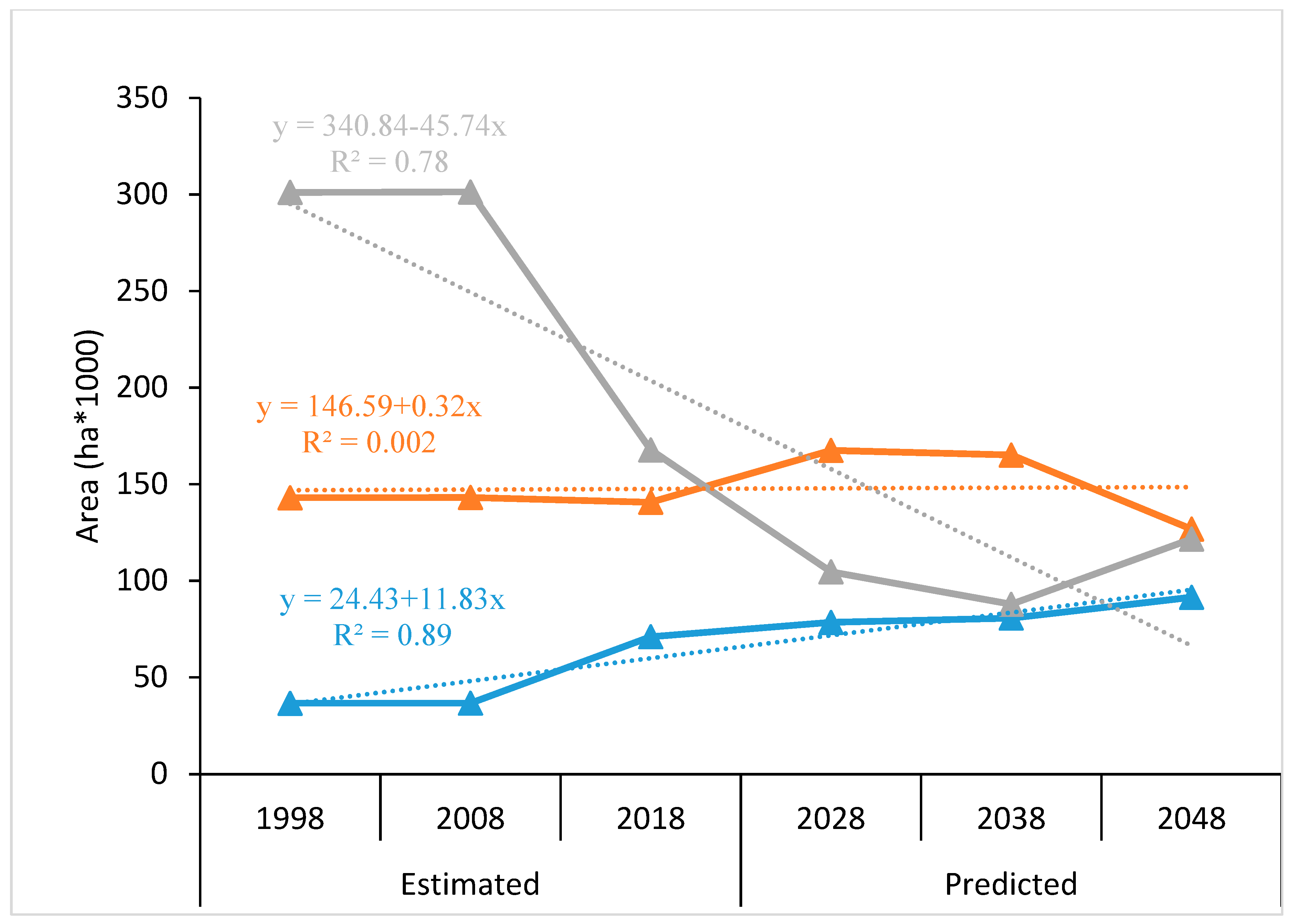
3.1. Land Cover Change in the LCF Ecosystem over the Last 20 Years
3.2. Plant Species Diversity and Composition
3.3. Carbon Stocks in Vegetation
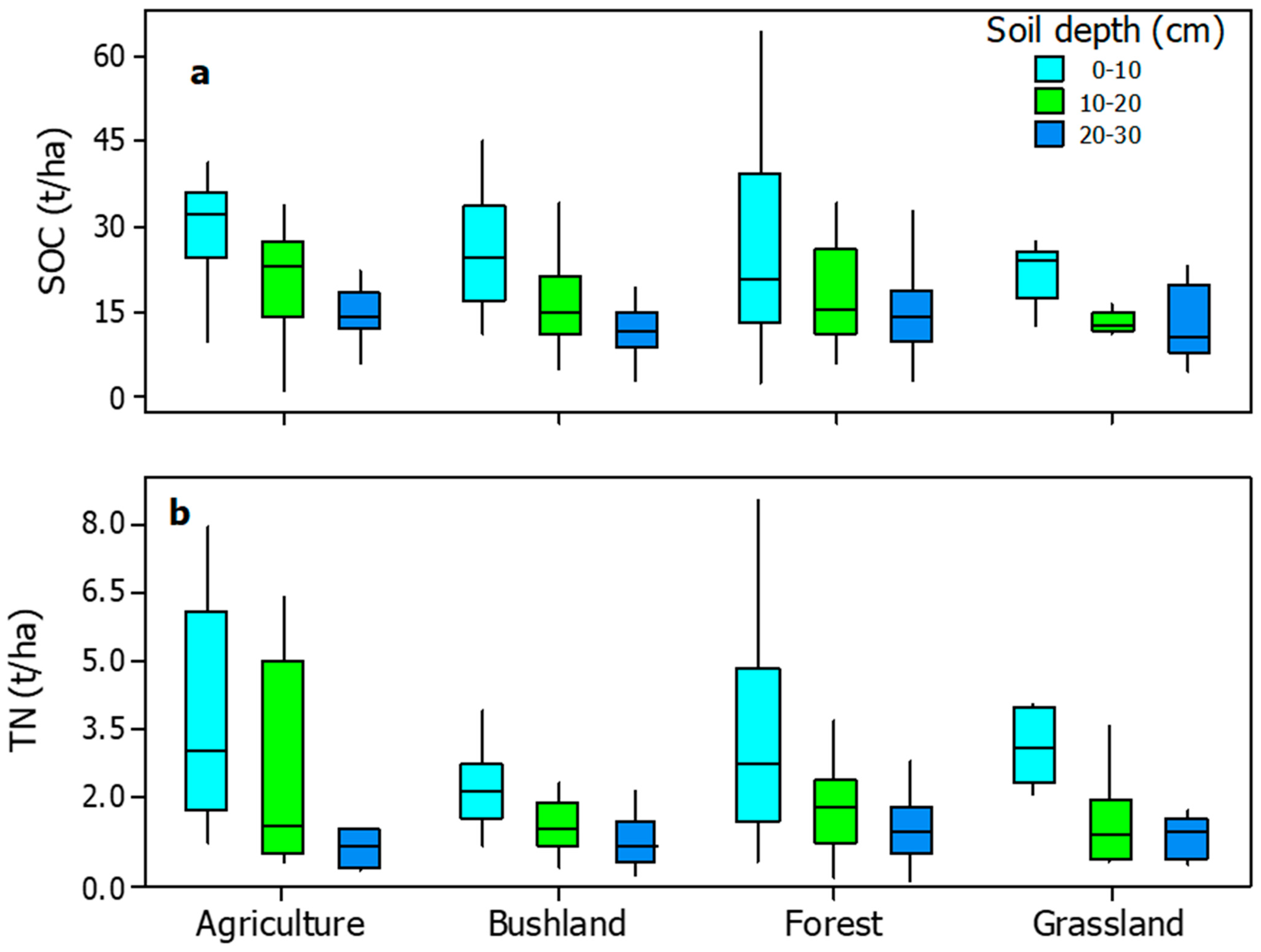
3.4. Soil Carbon (SOC) and Nitrogen (TN)
3.5. Overall Carbon Stocks
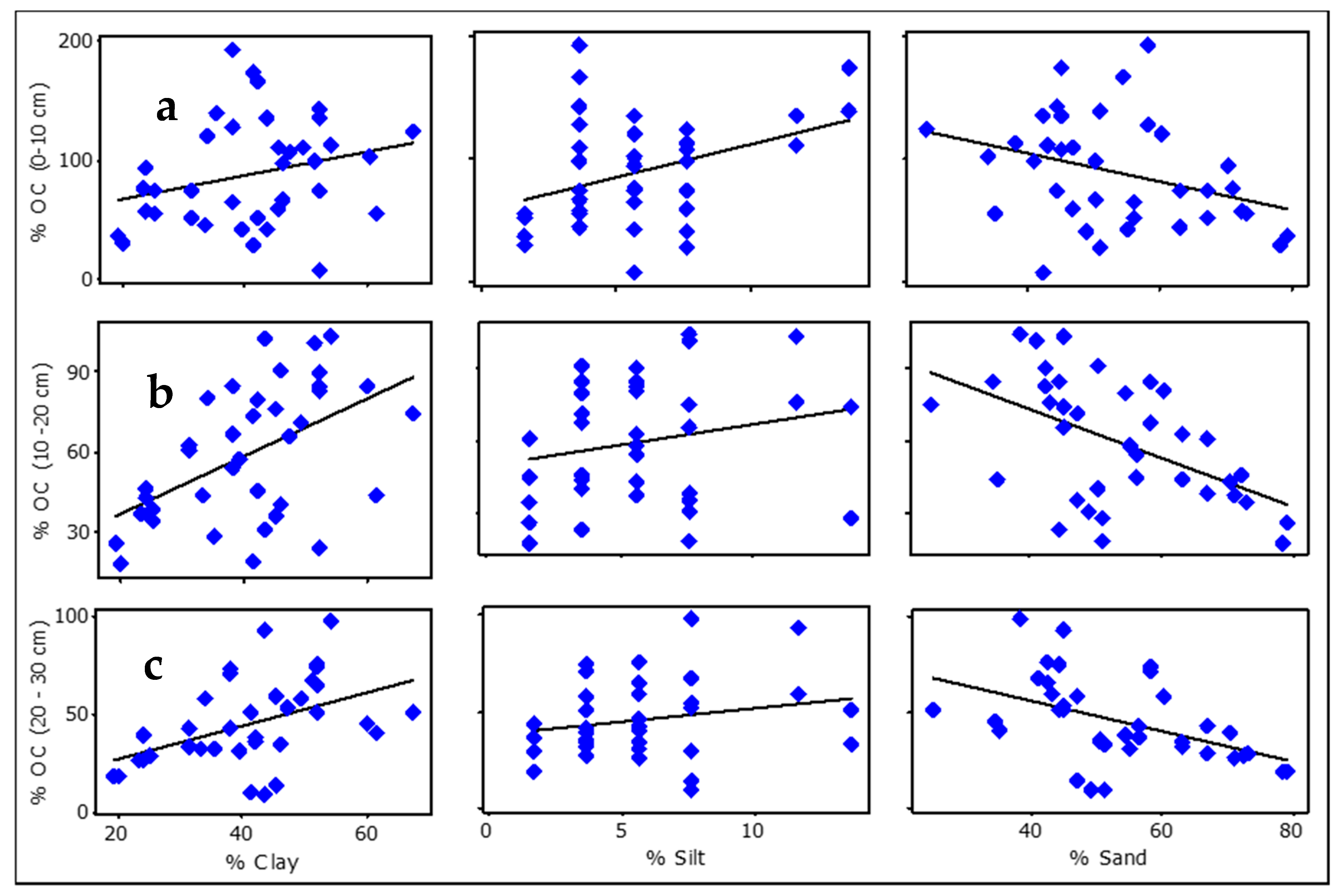
3.6. Relationship between Soil Organic Carbon and Soil Physico-Chemical Properties
4. Discussion
4.1. Forest Loss and Agricultural Expansion in the LCF-Ecosystem
4.2. Woody Plant Species and Composition across LULC Classes
4.3. Relationship between Plant AGC, Diversity and Composition
4.4. Tree Species Population Structure and AGC
4.5. Impacts of Land Use Land Cover Change on Soil Organic Carbon Stocks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | 1998 | 2008 | 2018 | 1998–2008 | 2008–2018 | 1998–2008 | 2008–2018 | 1998–2008 | 2008–2018 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Land Cover | Area (ha) | Area (%) | Area (ha) | Area (%) | Area (ha) | Area (%) | Area (%) | Area (%) | ha/year | km2/year | (%/year) | (%/year) |
| Agriculture | 18,818 | 3.5 | 36,580 | 6.8 | 70,797 | 13.2 | 3.3 | 6.4 | −2 | −3422 | −9.4 | −9.4 |
| Bare soil | 776 | 0.2 | 2760 | 0.5 | 4364 | 0.8 | 0.4 | 0.3 | −198 | −160 | −25.6 | −5.8 |
| Bushland | 135,757 | 25.3 | 143,725 | 26.9 | 140,137 | 26.2 | 1.5 | −0.7 | −797 | 359 | −0.6 | 0.2 |
| Forest | 323,250 | 60.4 | 301,197 | 56.3 | 167,958 | 31.4 | −4.1 | −24.9 | 2205 | 13,324 | 0.7 | 4.4 |
| Grassland | 50,146 | 9.4 | 45,720 | 8.6 | 147,393 | 27.6 | −0.8 | 19 | 443 | −10,167 | 0.9 | −22.2 |
| Urban area | 66 | 0 | 213 | 0 | 336 | 0.1 | 0 | 0 | −15 | −12 | −22.3 | −5.8 |
| Water | 6235 | 1.2 | 4843 | 0.9 | 4046 | 0.8 | −0.3 | −0.2 | 139 | 80 | 2.2 | 1.6 |
| Total | 535,048 | 100 | 532,278 | 100 | 526,621 | 100.1 | ||||||
| Year: 1998 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year: 2008 | Agriculture | Bare Soil | Bushland | Forest | Grassland | Urban | Water | Total 2008 | Gross Gain |
| Agriculture | 18,555 | 1 | 9047 | 5788 | 3130 | 0 | 60 | 36,580 | 18,025 |
| Bare Soil | 75 | 218 | 717 | 619 | 989 | 0 | 142 | 2760 | 2541 |
| Bushland | 0 | 21 | 53,016 | 68,350 | 22,065 | 1 | 269 | 143,723 | 90,707 |
| Forest | 0 | 57 | 54,735 | 239,048 | 6885 | 2 | 472 | 301,199 | 62,151 |
| Grassland | 175 | 466 | 17,976 | 8978 | 16,860 | 0 | 1266 | 45,721 | 28,861 |
| Urban Area | 11 | 0 | 80 | 28 | 42 | 52 | 0 | 213 | 161 |
| Water | 2 | 12 | 205 | 422 | 175 | 0 | 4024 | 4841 | 817 |
| Total 1998 | 18,819 | 776 | 135,776 | 323,232 | 50,145 | 55 | 6234 | 535,036 | |
| Gross loss | 264 | 557 | 82,760 | 84,185 | 33,285 | 3 | 2209 | 203,263 | |
| Year 2018 | Year 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Agriculture | Bare Soil | Bushland | Forest | Grassland | Urban | Water | Total 2018 | Gross Gain | |
| Agriculture | 6561 | 807 | 18,746 | 40,563 | 4052 | 0 | 63 | 70,793 | 64,232 |
| Bare Soil | 171 | 126 | 774 | 1132 | 1702 | 0 | 458 | 4362 | 4236 |
| Bushland | 11,567 | 657 | 44,284 | 66,010 | 17,448 | 14 | 155 | 140,135 | 95,851 |
| Forest | 7495 | 291 | 35,839 | 116,704 | 7260 | 3 | 361 | 167,952 | 51,249 |
| Grassland | 10,718 | 793 | 44,025 | 76,670 | 15,068 | 0 | 132 | 147,407 | 132,339 |
| Urban Area | 56 | 16 | 19 | 18 | 30 | 196 | 1 | 336 | 140 |
| Water | 12 | 71 | 34 | 99 | 159 | 0 | 3672 | 4046 | 374 |
| Total 2008 | 36,580 | 2760 | 143,721 | 301,196 | 45,721 | 213 | 4842 | 535,032 | |
| Gross loss | 30,018 | 2634 | 99,438 | 184,492 | 30,652 | 17 | 1170 | 348,422 | |
References
- Phillips, J.; Ramirez, S.; Wayson, C.; Duque, A. Differences in carbon stocks along an elevational gradient in tropical mountain forests of Colombia. Biotropica 2019, 51, 490–499. [Google Scholar] [CrossRef]
- Häger, A.; Schwendenmann, L. Forest Carbon Sequestration and Global Change. In The Paradigm of Forests and the Survival of the Fittest; CRC Press: Boca Raton, FL, USA, 2018; pp. 39–86. ISBN 1315367173. [Google Scholar]
- Olorunfemi, I.E.; Olufayo, A.A.; Fasinmirin, J.T.; Komolafe, A.A. Dynamics of land use land cover and its impact on carbon stocks in Sub-Saharan Africa: An overview. Environ. Dev. Sustain. 2021, 1–37. [Google Scholar] [CrossRef]
- Kauppi, P.E.; Sandström, V.; Lipponen, A. Forest resources of nations in relation to human well-being. PLoS ONE 2018, 13, e0196248. [Google Scholar] [CrossRef] [PubMed]
- Massetti, A.; Gil, A. Mapping and assessing land cover/land use and aboveground carbon stocks rapid changes in small oceanic islands’ terrestrial ecosystems: A case study of Madeira Island, Portugal (2009–2011). Remote Sens. Environ. 2020, 239, 111625. [Google Scholar] [CrossRef]
- Ahrends, A.; Burgess, N.D.; Milledge, S.A.; Bulling, M.T.; Fisher, B.; Smart, J.C.; Clarke, G.P.; Mhoro, B.E.; Lewis, S.L. Predictable waves of sequential forest degradation and biodiversity loss spreading from an African city. Proc. Natl. Acad. Sci. USA 2010, 107, 14556–14561. [Google Scholar] [CrossRef]
- McNicol, I.M.; Ryan, C.M.; Dexter, K.G.; Ball, S.M.J.; Williams, M. Aboveground carbon storage and its links to stand structure, tree diversity and floristic composition in south-eastern Tanzania. Ecosystems 2018, 21, 740–754. [Google Scholar] [CrossRef]
- Duguma, L.A.; Atela, J.; Minang, P.A.; Ayana, A.N.; Gizachew, B.; Nzyoka, J.M.; Bernard, F. Deforestation and forest degradation as an environmental behavior: Unpacking realities shaping community actions. Land 2019, 8, 26. [Google Scholar] [CrossRef]
- Teucher, M.; Schmitt, C.B.; Wiese, A.; Apfelbeck, B.; Maghenda, M.; Pellikka, P.; Lens, L.; Habel, J.C. Behind the fog: Forest degradation despite logging bans in an East African cloud forest. Glob. Ecol. Conserv. 2020, 22, e01024. [Google Scholar] [CrossRef]
- Kashaigili, J.; Mdemu, M.V.; Nduganda, A.R.; Mbilinyi, B.P. Integrated assessment of forest cover change and above-ground carbon stock in Pugu and Kazimzubwi forest reserves, Tanzania. Adv. Remote Sens. 2013, 2. [Google Scholar] [CrossRef]
- Houghton, R.A.; Goodale, C.L. Effects of land-use change on the carbon balance of terrestrial ecosystems. Ecosyst. Land Use Chang. 2004, 153, 85–98. [Google Scholar]
- Lopez-Toledo, L.; Ibarra-Manríquez, G.; Burslem, D.F.R.P.; Martínez-Salas, E.; Pineda-García, F.; Martínez-Ramos, M. Protecting a single endangered species and meeting multiple conservation goals: An approach with Guaiacum sanctum in Yucatan Peninsula, Mexico. Divers. Distrib. 2012, 18, 575–587. [Google Scholar] [CrossRef]
- Díaz, S.; Hector, A.; Wardle, D.A. Biodiversity in forest carbon sequestration initiatives: Not just a side benefit. Curr. Opin. Environ. Sustain. 2009, 1, 55–60. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Lopez-Gonzalez, G.; Sonké, B.; Affum-Baffoe, K.; Baker, T.R.; Ojo, L.O.; Phillips, O.L.; Reitsma, J.M.; White, L.; Comiskey, J.A.; et al. Increasing carbon storage in intact African tropical forests. Nature 2009, 457, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.P.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef]
- Gordon, C.E.; Bendall, E.R.; Stares, M.G.; Collins, L.; Bradstock, R.A. Aboveground carbon sequestration in dry temperate forests varies with climate not fire regime. Glob. Chang. Biol. 2018, 24, 4280–4292. [Google Scholar] [CrossRef]
- Yousefi, S.; Khatami, R.; Mountrakis, G.; Mirzaee, S.; Pourghasemi, H.R.; Tazeh, M. Accuracy assessment of land cover/land use classifiers in dry and humid areas of Iran. Environ. Monit. Assess. 2015, 187, 641. [Google Scholar] [CrossRef]
- Noulèkoun, F.; Birhane, E.; Mensah, S.; Kassa, H.; Berhe, A.; Gebremichael, Z.M.; Adem, N.M.; Seyoum, Y.; Mengistu, T.; Lemma, B. Structural diversity consistently mediates species richness effects on aboveground carbon along altitudinal gradients in northern Ethiopian grazing exclosures. Sci. Total Environ. 2021, 776, 145838. [Google Scholar] [CrossRef]
- Asase, A.; Asitoakor, B.K.; Ekpe, P.K. Linkages between tree diversity and carbon stocks in unlogged and logged West African tropical forests. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2012, 8, 217–230. [Google Scholar] [CrossRef]
- Younge, A.; Negussie, G.; Burgess, N. Eastern Africa Coastal Forest Programme. In Proceedings of the Regional Workshop Report, Nairobi, Kenya, 4–7 February 2002; pp. 4–7. [Google Scholar]
- Sheil, D. Tanzanian coastal forests–unique, threatened, and overlooked. Oryx 1992, 26, 107–114. [Google Scholar] [CrossRef]
- Azeria, E.T.; Sanmartín, I.; Ås, S.; Carlson, A.; Burgess, N. Biogeographic patterns of the East African coastal forest vertebrate fauna. Biodivers. Conserv. 2007, 16, 883–912. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G. Habitat loss and extinction in the hotspots of biodiversity. J. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Mariki, S.B. Successes, threats, and factors influencing the performance of a community-based wildlife management approach: The case of Wami Mbiki WMA, Tanzania. In Wildlife Management-Failures, Successes and Prospects; Kideghesho, J.R., Rija, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Ntukey, L.T.; Munishi, L.K.; Kohi, E.; Treydte, A.C. Land Use/Cover Change Reduces Elephant Habitat Suitability in the Wami Mbiki–Saadani Wildlife Corridor, Tanzania. Land 2022, 11, 307. [Google Scholar] [CrossRef]
- Kideghesho, J.R. Realities on deforestation in Tanzania—Trends, drivers, implications and the way forward. Precious For. Earth 2015, 21–47. [Google Scholar] [CrossRef]
- Jochum, M.; Fischer, M.; Isbell, F.; Roscher, C.; Van der Plas, F.; Boch, S.; Boenisch, G.; Buchmann, N.; Catford, J.A.; Cavender-Bares, J. The results of biodiversity–ecosystem functioning experiments are realistic. Nat. Ecol. Evol. 2020, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Debonnet, G.; Nindi, S. Technical Study on Land Use and Tenure Options and Status of Wildlife Corridors in Tanzania: An Input to the Preparation of Corridor; USAID: Washington, DC, USA, 2017.
- Nobert, J.; Jeremiah, J. Hydrological response of watershed systems to land use/cover change. a case of Wami River Basin. Open Hydrol. J. 2012, 6, 78–87. [Google Scholar] [CrossRef]
- Riggio, J.; Caro, T. Structural connectivity at a national scale: Wildlife corridors in Tanzania. PLoS ONE 2017, 12, e0187407. [Google Scholar] [CrossRef]
- Riggio, J.; Mbwilo, F.; Van de Perre, F.; Caro, T. The forgotten link between northern and southern Tanzania. Afr. J. Ecol. 2018, 56, 1012–1016. [Google Scholar] [CrossRef]
- Kikoti, A. Where Are the Conservation Corridors for Elephants in Saadani National Park and the Lower Wami-Ruvu River Basin of Eastern Tanzania? Summary Report of Elephant Collaring Operation; Coastal Resources Cwnter, University of Rhodes Island: Narragansett, RI, USA, 2010. [Google Scholar]
- Shelestov, A.; Lavreniuk, M.; Kussul, N.; Novikov, A.; Skakun, S. Exploring Google Earth Engine Platform for Big Data Processing: Classification of Multi-Temporal Satellite Imagery for Crop Mapping. Front. Earth Sci. 2017, 5, 17. [Google Scholar] [CrossRef]
- Thieme, A.; Yadav, S.; Oddo, P.C.; Fitz, J.M.; McCartney, S.; King, L.; Keppler, J.; McCarty, G.W.; Hively, W.D. Using NASA Earth observations and Google Earth Engine to map winter cover crop conservation performance in the Chesapeake Bay watershed. Remote Sens. Environ. 2020, 248, 111943. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Chatterjee, N.; Das, K. An integrated GIS approach to analyze the impact of land use change and land cover alteration on ground water potential level: A study in Kangsabati Basin, India. Groundw. Sustain. Dev. 2020, 11, 100399. [Google Scholar] [CrossRef]
- Bhattacharya, R.K.; Das Chatterjee, N.; Das, K. Land use and Land Cover change and its resultant erosion susceptible level: An appraisal using RUSLE and Logistic Regression in a tropical plateau basin of West Bengal, India. Environ. Dev. Sustain. 2021, 23, 1411–1446. [Google Scholar] [CrossRef]
- Buğday, E.; Erkan Buğday, S. Modelling and simulating land use/cover change using the artificial neural network from remote sensing data. Cerne 2019, 25, 246–254. [Google Scholar] [CrossRef]
- El-Tantawi, A.M.; Bao, A.; Chang, C.; Liu, Y. Monitoring and predicting land use/cover changes in the Aksu-Tarim River Basin, Xinjiang-China (1990–2030). Environ. Monit. Assess. 2019, 191, 1–18. [Google Scholar] [CrossRef]
- Guidingan, M.L.G.; Sanou, C.L.; Ragatoa, D.S.; Fafa, C.O.; Mishra, V.N. Assessing land use/land cover dynamic and its impact in Benin Republic using land change model and CCI-LC products. Earth Syst. Environ. 2019, 3, 127–137. [Google Scholar] [CrossRef]
- Talukdar, S.; Singha, P.; Mahato, S.; Pal, S.; Liou, Y.-A.; Rahman, A. Land-use land-cover classification by machine learning classifiers for satellite observations—A review. Remote Sens. 2020, 12, 1135. [Google Scholar] [CrossRef]
- Malimbwi, R.E.; Zahabu, E. NAFORMA Process and Biophysical Results. 2014. Available online: file:///C:/Users/MDPI/Downloads/NAFORMA%20PROCESS%20AND%20BIOPHYSICAL%20RESULT.pdf (accessed on 4 June 2022).
- Malimbwi Fundamentals of Forest Mensuration. A Compend. Sokoine Univ. Agric. Morogoro Tanzan. 1997. Available online: http://www.suaire.sua.ac.tz/handle/123456789/698 (accessed on 13 April 2021).
- Shirima, D.D.; Munishi, P.K.T.; Lewis, S.L.; Burgess, N.D.; Marshall, A.R.; Balmford, A.; Swetnam, R.D.; Zahabu, E.M. Carbon storage, structure and composition of miombo woodlands in Tanzania’s Eastern Arc Mountains. Afr. J. Ecol. 2011, 49, 332–342. [Google Scholar] [CrossRef]
- Swai, G.; Ndangalasi, H.J.; Munishi, P.K.T.; Shirima, D.D. Carbon stocks of Hanang forest, Tanzania: An implication for climate mitigation. J. Ecol. Nat. Environ. 2014, 6, 90–98. [Google Scholar] [CrossRef][Green Version]
- Burgess, N.D.; Clarke, G.P.; Rodgers, W.A. Coastal forests of eastern Africa: Status, endemism patterns and their potential causes. Biol. J. Linn. Soc. 1998, 64, 337–367. [Google Scholar] [CrossRef]
- Cuni-Sanchez, A.; Sullivan, M.J.P.; Platts, P.J.; Lewis, S.L.; Marchant, R.; Imani, G.; Hubau, W.; Abiem, I.; Adhikari, H.; Albrecht, T.; et al. High aboveground carbon stock of African tropical montane forests. Nature 2021, 596, 536–542. [Google Scholar] [CrossRef]
- Van Wyk, B. Field Guide to Trees of Southern Africa; Penguin Random House South Africa: Cape Town, South Africa, 2013; ISBN 1775841049. [Google Scholar]
- Burgess, N.D.; Clarke, G.P. Coastal Forests of Eastern Africa; Burgess, N.D., Clarke, G.P., Eds.; IUCN-The World Conservation Union, Publications Services Unit: Cambridge, UK, 2000; ISBN 2831704367. [Google Scholar]
- Dharani, N. Field Guide to Common Trees & Shrubs of East Africa; Penguin Random House South Africa: Cape Town, South Africa, 2011; ISBN 1431701386. [Google Scholar]
- Begossi, A. Use of ecological methods in ethnobotany: Diversity indices. Econ. Bot. 1996, 50, 280. [Google Scholar] [CrossRef]
- Pearson, T.; Walker, S.; Brown, S. Sourcebook for Land Use, Land-Use Change and Forestry Projects; World Bank Group: Washington, DC, USA, 2013; Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/16491/795480WP0Sourc0CF0Projects00PUBLIC0.pdf?sequence=1 (accessed on 10 March 2022).
- Allen, S.E. Chemical Analysis. Methods Plant Ecol. 1986, 285–344. Available online: https://cir.nii.ac.jp/crid/1571980076144721792 (accessed on 10 March 2022).
- Anderson, J.M.; Ingram, J.S.I. A handbook of methods. CAB Int. Wallingford 1993, 221, 62–65. [Google Scholar]
- Subedi, B.P.; Pandey, S.S.; Pandey, A.; Rana, E.B.; Bhattarai, S.; Banskota, T.R.; Charmakar, S.; Tamrakar, R. Forest Carbon Stock Measurement: Guidelines for Measuring Carbon Stocks in Community-Managed Forests; ANSAB, FECOFUN, ICIMOD: Kathmandu, Nepal, 2010. [Google Scholar]
- Zomer, R.J.; Neufeldt, H.; Xu, J.; Ahrends, A.; Bossio, D.; Trabucco, A.; Van Noordwijk, M.; Wang, M. Global Tree Cover and Biomass Carbon on Agricultural Land: The contribution of agroforestry to global and national carbon budgets. Sci. Rep. 2016, 6, 29987. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, L.K.; Sharma, K.C. Assessment of land use change and its effect on soil carbon stock using multitemporal satellite data in semiarid region of Rajasthan, India. Ecol. Process. 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Vesa, L.; Malimbwi, R.E.; Tomppo, E.; Zahabu, E.; Maliondo, S.; Chamuya, N.; Nsokko, E.; Otieno, J.; Dalsgaard, S. A National Forestry Resources Monitoring and Assessment of Tanzania (NAFORMA). 2010. Available online: http://suaire.sua.ac.tz/bitstream/handle/123456789/1287/Malimbwi17.pdf?sequence=1&isAllowed=y (accessed on 15 May 2022).
- Díaz-Zorita, M. Soil organic carbon recovery by the Walkley-Black method in a typic hapludoll. J. Commun. Soil Sci. Anal. Plant 1999, 30, 739–745. [Google Scholar] [CrossRef]
- Staff, S.S. Soil Survey laboratory methods manual. In Soil Survey Laboratory Investigations Report; No. 42; Version 5.0; 2014; Volume 182, pp. 1–8. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/ref/?cid=nrcs142p2_054247 (accessed on 10 March 2022). [CrossRef]
- Roderick, G.L. A History of Particle-Size Limits; 1962. Available online: http://publications.iowa.gov/17268/1/IADOT_hr99_History_Particle_Limits.pdf (accessed on 13 April 2022).
- Hoskins, B.; Ross, D. Soil sample preparation and extraction. Recomm. Soil Test. Proced. Northeast. U. S. Northeast. Reg. Publ. 2009, 493, 3–10. [Google Scholar]
- Wairiu, M.; Lal, R. Soil organic carbon in relation to cultivation and topsoil removal on sloping lands of Kolombangara, Solomon Islands. Soil Tillage Res. 2003, 70, 19–27. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 1149–1178. [Google Scholar]
- Bashour, I.I.; Sayegh, A.H. Methods of Analysis for Soils of Arid and Semi-Arid Regions; FAO: Rome, Italy, 2007; ISBN 9251056617. [Google Scholar]
- Buba, T. Impact of different types of land use on pattern of herbaceous plant community in the Nigerian Northern Guinea Savanna. J. Agric. Ecol. Res. Int. 2015, 4, 151–165. [Google Scholar] [CrossRef]
- Rolo, V.; Olivier, P.I.; Pfeifer, M.; van Aarde, R.J. Functional diversity mediates contrasting direct and indirect effects of fragmentation on below-and above-ground carbon stocks of coastal dune forests. For. Ecol. Manag. 2018, 407, 174–183. [Google Scholar] [CrossRef]
- Kalaba, F.K.; Quinn, C.H.; Dougill, A.J.; Vinya, R. Floristic composition, species diversity and carbon storage in charcoal and agriculture fallows and management implications in Miombo woodlands of Zambia. For. Ecol. Manag. 2013, 304, 99–109. [Google Scholar] [CrossRef]
- Jarzebski, M.P.; Gasparatos, A. Land use change, carbon stocks and tree species diversity in green spaces of a secondary city in Myanmar, Pyin Oo Lwin. PLoS ONE 2019, 14, e0225331. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, C.; Zheng, Z.; Hu, L.; Feng, K.; Chang, J.; Xie, P. Plant community characteristics and their responses to environmental factors in the water level fluctuation zone of the three gorges reservoir in China. Environ. Sci. Pollut. Res. 2013, 20, 7080–7091. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, R.; Pukkala, T.; Kangas, A.; Packalen, P. Effects of errors in basal area and mean diameter on the optimality of forest management prescriptions. Ann. For. Sci. 2021, 78, 18. [Google Scholar] [CrossRef]
- Malimbwi, R.E.; Eid, T.; Chamshama, S. Allometric Volume and Biomass Models in Tanzania; ResearchGate: Berlin, Germany, 2016. [Google Scholar]
- Mugasha, W.A.; Mwakalukwa, E.E.; Luoga, E.; Malimbwi, R.E.; Zahabu, E.; Silayo, D.S.; Sola, G.; Crete, P.; Henry, M.; Kashindye, A. Allometric models for estimating tree volume and aboveground biomass in lowland forests of Tanzania. Int. J. For. Res. 2016, 2016, 8076271. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Global Wood Density Database. 2009. Dryland Identifier. Available online: http://hdl.handle.net/10255/drydad.235 (accessed on 5 May 2022).
- Rimal, B.; Sharma, R.; Kunwar, R.; Keshtkar, H.; Stork, N.E.; Rijal, S.; Rahman, S.A.; Baral, H. Effects of land use and land cover change on ecosystem services in the Koshi River Basin, Eastern Nepal. Ecosyst. Serv. 2019, 38, 100963. [Google Scholar] [CrossRef]
- Fearnside, P.M. Global Warming and Tropical Land-Use Change: Greenhouse Gas Emissions from Biomass Burning, Decomposition and Soils in Forest Conversion, Shifting Cultivation and Secondary Vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Gross, K.; Cardinale, B.J. Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am. Nat. 2007, 170, 207–220. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Hillebrand, H.; Harpole, W.S.; Gross, K.; Ptacnik, R. Separating the influence of resource ‘availability’ from resource ‘imbalance’ on productivity–diversity relationships. Ecol. Lett. 2009, 12, 475–487. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 5 May 2013).
- Mannan, A.; Liu, J.; Zhongke, F.; Khan, T.U.; Saeed, S.; Mukete, B.; ChaoYong, S.; Yongxiang, F.; Ahmad, A.; Amir, M. Application of land-use/land cover changes in monitoring and projecting forest biomass carbon loss in Pakistan. Glob. Ecol. Conserv. 2019, 17, e00535. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environ. Res. Lett. 2007, 2, 45023. [Google Scholar] [CrossRef]
- Jallat, H.; Khokhar, M.F.; Kudus, K.A.; Nazre, M.; Tahir, U.; Khan, W.R. Monitoring Carbon Stock and Land-Use Change in 5000-Year-Old Juniper Forest Stand of Ziarat, Balochistan, through a Synergistic Approach. Forests 2021, 12, 51. [Google Scholar] [CrossRef]
- Zhao, M.; He, Z.; Du, J.; Chen, L.; Lin, P.; Fang, S. Assessing the effects of ecological engineering on carbon storage by linking the CA-Markov and InVEST models. Ecol. Indic. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agriculture Organization: Rome, Italy, 2008; ISBN 9789251059814. [Google Scholar]
- Riggio, J.; Jacobson, A.P.; Hijmans, R.J.; Caro, T. How effective are the protected areas of East Africa? Glob. Ecol. Conserv. 2019, 17, e00573. [Google Scholar] [CrossRef]
- Twisa, S.; Buchroithner, M.F. Land-use and land-cover (LULC) change detection in Wami River Basin, Tanzania. Land 2019, 8, 136. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Morales-Hidalgo, D.; Oswalt, S.N.; Somanathan, E. Status and trends in global primary forest, protected areas, and areas designated for conservation of biodiversity from the Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 68–77. [Google Scholar] [CrossRef]
- Naikoo, M.W.; Islam, A.R.M.T.; Mallick, J.; Rahman, A. Land use/land cover change and its impact on surface urban heat island and urban thermal comfort in a metropolitan city. Urban Clim. 2022, 41, 101052. [Google Scholar] [CrossRef]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Estes, A.B.; Kuemmerle, T.; Kushnir, H.; Radeloff, V.C.; Shugart, H.H. Land-cover change and human population trends in the greater Serengeti ecosystem from 1984–2003. Biol. Conserv. 2012, 147, 255–263. [Google Scholar] [CrossRef]
- Gayo, L. Contribution of Wildlife Management Area on Wildlife Conservation and Livelihood: A Case of Wami-Mbiki “Wildlife Management Area”. Ph.D. Thesis, The University of Dodoma, Avenue, Tanzania, 2013. [Google Scholar]
- Kibet, S. Plant communities, species diversity, richness, and regeneration of a traditionally managed coastal forest, Kenya. For. Ecol. Manag. 2011, 261, 949–957. [Google Scholar] [CrossRef]
- Salehe, J. The Forests and Woodlands of the Coastal East Africa Region; World Wide Fund for Nature (WWF): Vaud, Switzerland, 2011. [Google Scholar]
- Yuan, Y.; Zeng, G.; Liang, J.; Li, X.; Li, Z.; Zhang, C.; Huang, L.; Lai, X.; Lu, L.; Wu, H.; et al. Effects of landscape structure, habitat and human disturbance on birds: A case study in East Dongting Lake wetland. Ecol. Eng. 2014, 67, 67–75. [Google Scholar] [CrossRef]
- Betemariyam, M.; Kefalew, T.; Tolera, M. Comparison of Woody Species Diversity and Carbon Stock along Natural Forest to Farmland Conversion Gradient in the Gura-Ferda District of Southwestern Ethiopia. J. Sustain. For. 2022, 1–17. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Du Toit, B.; Glèlè Kakaï, R.; Seifert, T. Aboveground Biomass and Carbon in a South African Mistbelt Forest and the Relationships with Tree Species Diversity and Forest Structures. Forests 2016, 7, 79. [Google Scholar] [CrossRef]
- Mangwale, K.; Shackleton, C.M.; Sigwela, A. Changes in forest cover and carbon stocks of the coastal scarp forests of the Wild Coast, South Africa. South. For. J. For. Sci. 2017, 79, 305–315. [Google Scholar] [CrossRef]
- Chisholm, R.A.; Muller-Landau, H.C.; Abdul Rahman, K.; Bebber, D.P.; Bin, Y.; Bohlman, S.A.; Bourg, N.A.; Brinks, J.; Bunyavejchewin, S.; Butt, N. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol. 2013, 101, 1214–1224. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef]
- Fang, J.; Bai, Y.; Wu, J. Towards a better understanding of landscape patterns and ecosystem processes of the Mongolian Plateau. Landsc. Ecol. 2015, 30, 1573–1578. [Google Scholar] [CrossRef]
- Daye, D.D.; Healey, J.R. Impacts of land-use change on sacred forests at the landscape scale. Glob. Ecol. Conserv. 2015, 3, 349–358. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Gómez-Aparicio, L.; Paquette, A.; Messier, C.; Kattge, J.; Zavala, M.A. Diversity increases carbon storage and tree productivity in Spanish forests. Glob. Ecol. Biogeogr. 2014, 23, 311–322. [Google Scholar] [CrossRef]
- Sharma, M.; Chakraborty, A.; Garg, J.K.; Joshi, P.K. Assessing forest fragmentation in north-western Himalaya: A case study from Ranikhet forest range, Uttarakhand, India. J. For. Res. 2017, 28, 319–327. [Google Scholar] [CrossRef]
- Tesfaye, M.A.; Bravo-Oviedo, A.; Bravo, F.; Ruiz-Peinado, R.J.A. Aboveground biomass equations for sustainable production of fuelwood in a native dry tropical afro-montane forest of Ethiopia. Ann. For. Sci. 2016, 73, 411–423. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Poorter, L.; Van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Masota, A.M.; Zahabu, E.; Malimbwi, R.E.; Bollandsås, O.M.; Eid, T.H. Volume models for single trees in tropical rainforests in Tanzania. J. Energy Nat. Resour. 2014, 3, 66–76. [Google Scholar] [CrossRef]
- Godoy, F.L.; Tabor, K.; Burgess, N.D.; Mbilinyi, B.P.; Kashaigili, J.J.; Steininger, M.K. Deforestation and CO2 emissions in coastal Tanzania from 1990 to 2007. Environ. Conserv. 2012, 39, 62–71. [Google Scholar] [CrossRef]
- Baccini, A.; Laporte, N.; Goetz, S.J.; Sun, M.; Dong, H. A first map of tropical Africa’s above-ground biomass derived from satellite imagery. Environ. Res. Lett. 2008, 3, 45011. [Google Scholar] [CrossRef]
- Capitani, C.; Van Soesbergen, A.; Mukama, K.; Malugu, I.; Mbilinyi, B.; Chamuya, N.; Kempen, B.; Malimbwi, R.; Mant, R.; Munishi, P.J.E.C. Scenarios of Land Use and Land Cover Change and Their Multiple Impacts on Natural Capital in Tanzania. Environ. Conserv. 2019, 46, 17–24. [Google Scholar] [CrossRef]
- De la Cruz-Amo, L.; Bañares-de-Dios, G.; Cala, V.; Granzow-de la Cerda, Í.; Espinosa, C.I.; Ledo, A.; Salinas, N.; Macía, M.J.; Cayuela, L. Trade-Offs Among Aboveground, Belowground, and Soil Organic Carbon Stocks Along Altitudinal Gradients in Andean Tropical Montane Forests. Front. Plant Sci. 2020, 11, 106. [Google Scholar] [CrossRef]
- Mauya, E.W.; Madundo, S. Aboveground Biomass and Carbon Stock of Usambara Tropical Rainforests In Tanzania. Tanzan. J. For. Nat. Conserv. 2021, 90, 63–82. [Google Scholar]
- Shirima, D.D.; Totland, Ø.; Munishi, P.K.T.; Moe, S.R. Relationships between tree species richness, evenness and aboveground carbon storage in montane forests and miombo woodlands of Tanzania. Basic Appl. Ecol. 2015, 16, 239–249. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Potvin, C. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 2011, 189, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Kacholi, D.S.; Whitbread, A.M.; Worbes, M. Diversity, abundance, and structure of tree communities in the Uluguru forests in the Morogoro region, Tanzania. J. For. Res. 2015, 26, 557–569. [Google Scholar] [CrossRef]
- Bhandari, S.; Twayana, R.; Shrestha, R.; Sharma, K. Future Land Use Land Cover Scenario Simulation Using Open-Source GIS for the City of Banepa and Dhulikhel Municipality, Nepal. Available online: https://www.foss4g-asia.org/2020/wp-content/uploads/Papers/Theme%203_%20Spatial%20Landuse%20Planning%20and%20Decision%20Support%20System%20(SPDSS)/%5BPaper%20ID-11%5D%20Future%20land%20use%20land%20cover%20scenario%20simulation%20using%20Open%20Source%20GIS%20for%20the%20City%20of%20Banepa%20and%20Dhulikhel%20Municipality,%20Nepal.pdf (accessed on 10 April 2022).
- Zádorová, T.; Penížek, V.; Vašát, R.; Žížala, D.; Chuman, T.; Vaněk, A. Colluvial soils as a soil organic carbon pool in different soil regions. Geoderma 2015, 253, 122–134. [Google Scholar] [CrossRef]
- Sarathchandra, C.; Alemu Abebe, Y.; Worthy, F.R.; Lakmali Wijerathne, I.; Ma, H.; Yingfeng, B.; Jiayu, G.; Chen, H.; Yan, Q.; Geng, Y. Impact of land use and land cover changes on carbon storage in rubber dominated tropical Xishuangbanna, South West China. Ecosyst. Health Sustain. 2021, 7, 1915183. [Google Scholar] [CrossRef]
- Assefa, D.; Rewald, B.; Sandén, H.; Rosinger, C.; Abiyu, A.; Yitaferu, B.; Godbold, D.L. Deforestation and land use strongly effect soil organic carbon and nitrogen stock in Northwest Ethiopia. Catena 2017, 153, 89–99. [Google Scholar] [CrossRef]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. For. Ecol. 2018, 361, 1108–1111. [Google Scholar] [CrossRef]
- Kashaigili, J.J.; Majaliwa, A.M. Implications of land use and land cover changes on hydrological regimes of the Malagarasi River, Tanzania. J. Agric. Sci. Appl. 2013, 2, 45–50. [Google Scholar] [CrossRef]
| LULC Classes | Diversity | Evenness | Richness |
|---|---|---|---|
| Bushland | 1.27 ± 0.16 a | 0.29 ± 0.04 a | 4.16 ± 0.63 a |
| Agriculture | 0.38 ± 0.38 a | 0.28 ± 0.14 a | 1.00 ± 0.00 b |
| Forest | 0.92 ± 0.18 a | 0.28 ± 0.04 a | 5.70 ± 0.79 c |
| Grassland | 0.16 ± 0.03 a | 0.25 ± 0.21 a | 2.54 ± 0.69 a |
| Land Use | AGC (tC/ha) | BGC (tC/ha) |
|---|---|---|
| Grassland | 2.51 ± 0.1 a | 0.6 ± 0.0 a |
| Bushland | 15.4 ± 5.6 a | 8.9 ± 2.0 a |
| Agriculture | 6.4 ± 5.8 a | 2.3 ± 1.7 b |
| Forest | 36.9 ± 6.3 b | 23.0 ± 3.6 c |
| DBH Class (cm) | AGC (t/ha) | BGC (t/ha) |
|---|---|---|
| 10–15 | 46.0 ± 15.2 a | 26.3 ± 6.3 a |
| >15 | 41.4 ± 11.3 a | 23.2 ± 5.1 a |
| 3–5 | 15.2 ± 2.6 a | 11.2 ± 1.9 a |
| 5–10 | 18.5 ± 4.6 a | 11.9 ± 2.8 a |
| Land Use | pH | % Clay | % Silt | % Sand |
|---|---|---|---|---|
| Bushland | 6.5 ± 0.1 a | 36.8 ± 2.3 a | 6.4 ± 0.6 a | 56.8 ± 2.5 a |
| Agriculture | 6.4 ± 0.1 b | 50.3 ± 5.8 a | 6.0 ± 1.0 a | 43.7 ± 6.1 a |
| Forest | 6.5 ± 0.2 c | 46.0 ± 2.9 a | 5.6 ± 0.6 a | 48.4 ± 3.3 a |
| Grassland | 7.4 ± 0.2 d | 36.2 ± 3.2 a | 5.4 ± 0.6 a | 58.3 ± 3.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntukey, L.T.; Munishi, L.K.; Treydte, A.C. Land Use Land/Cover Change Reduces Woody Plant Diversity and Carbon Stocks in a Lowland Coastal Forest Ecosystem, Tanzania. Sustainability 2022, 14, 8551. https://doi.org/10.3390/su14148551
Ntukey LT, Munishi LK, Treydte AC. Land Use Land/Cover Change Reduces Woody Plant Diversity and Carbon Stocks in a Lowland Coastal Forest Ecosystem, Tanzania. Sustainability. 2022; 14(14):8551. https://doi.org/10.3390/su14148551
Chicago/Turabian StyleNtukey, Lucas Theodori, Linus Kasian Munishi, and Anna Christina Treydte. 2022. "Land Use Land/Cover Change Reduces Woody Plant Diversity and Carbon Stocks in a Lowland Coastal Forest Ecosystem, Tanzania" Sustainability 14, no. 14: 8551. https://doi.org/10.3390/su14148551
APA StyleNtukey, L. T., Munishi, L. K., & Treydte, A. C. (2022). Land Use Land/Cover Change Reduces Woody Plant Diversity and Carbon Stocks in a Lowland Coastal Forest Ecosystem, Tanzania. Sustainability, 14(14), 8551. https://doi.org/10.3390/su14148551







