Root Distribution and Root Cohesion of Two Herbaceous Plants in the Loess Plateau of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Root Morphological Investigation
2.3. Root Tensile Tests
2.4. Root Cohesion Evaluation
2.5. Data Analysis
3. Results
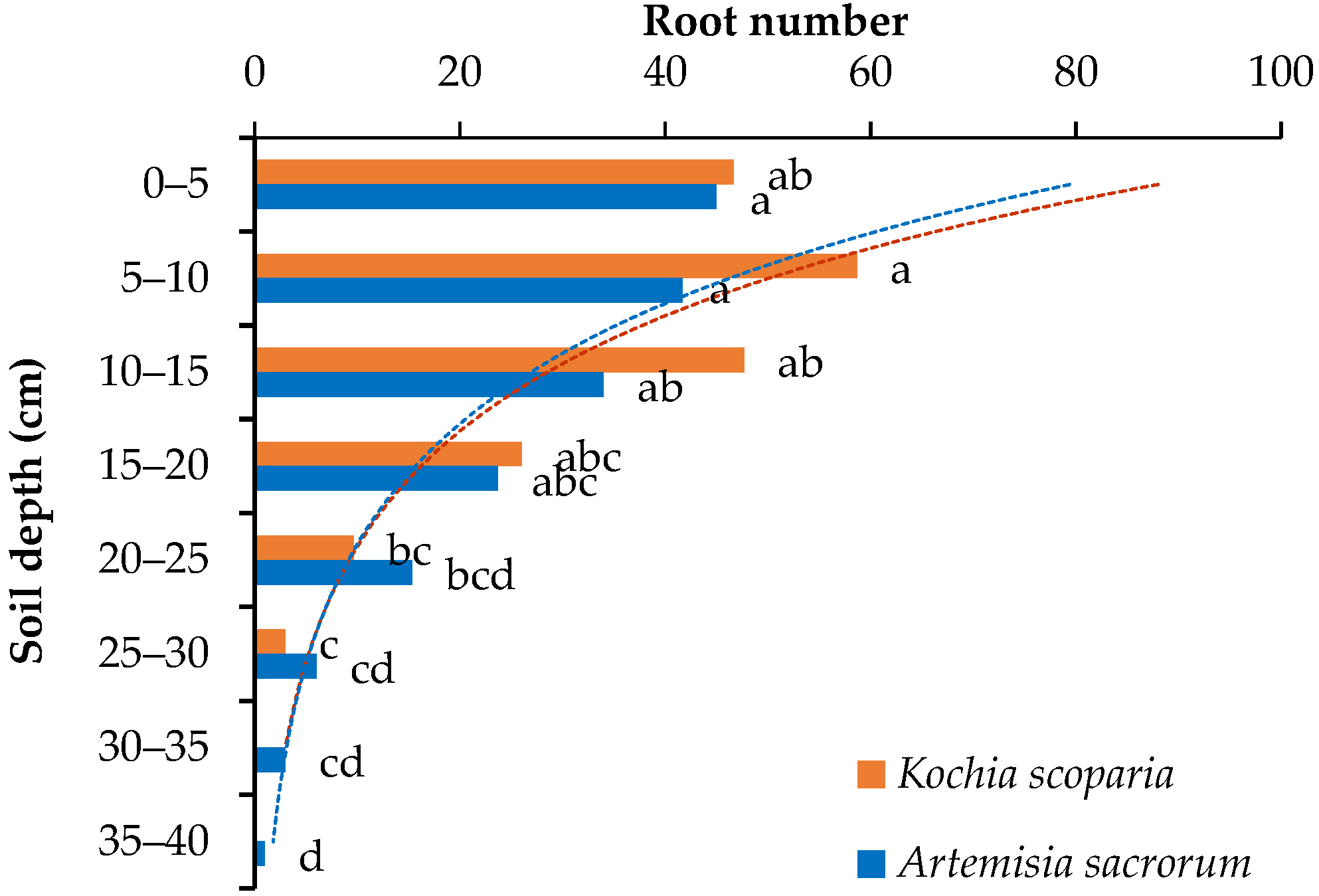
3.1. Root Number
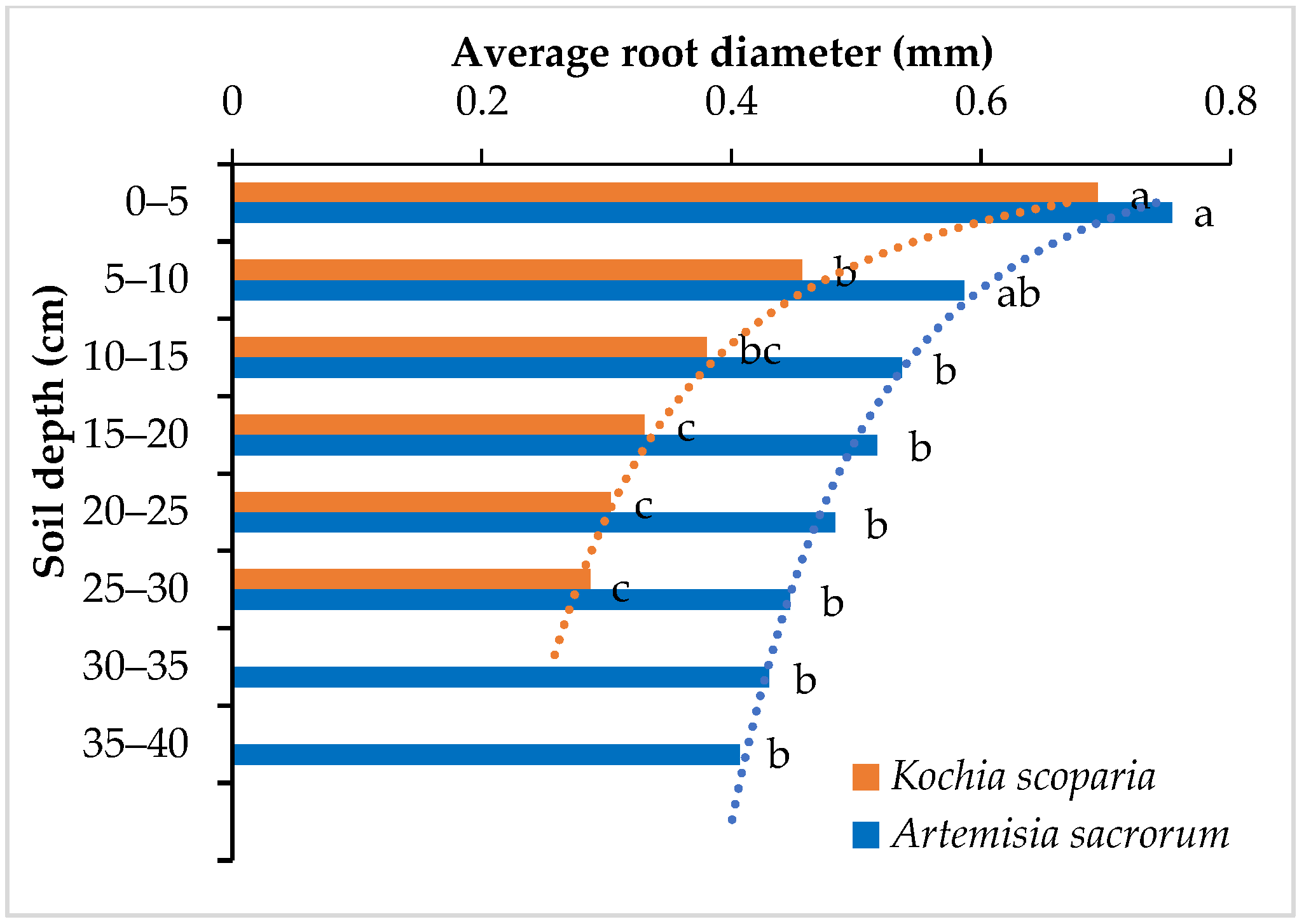
3.2. Root Diameter
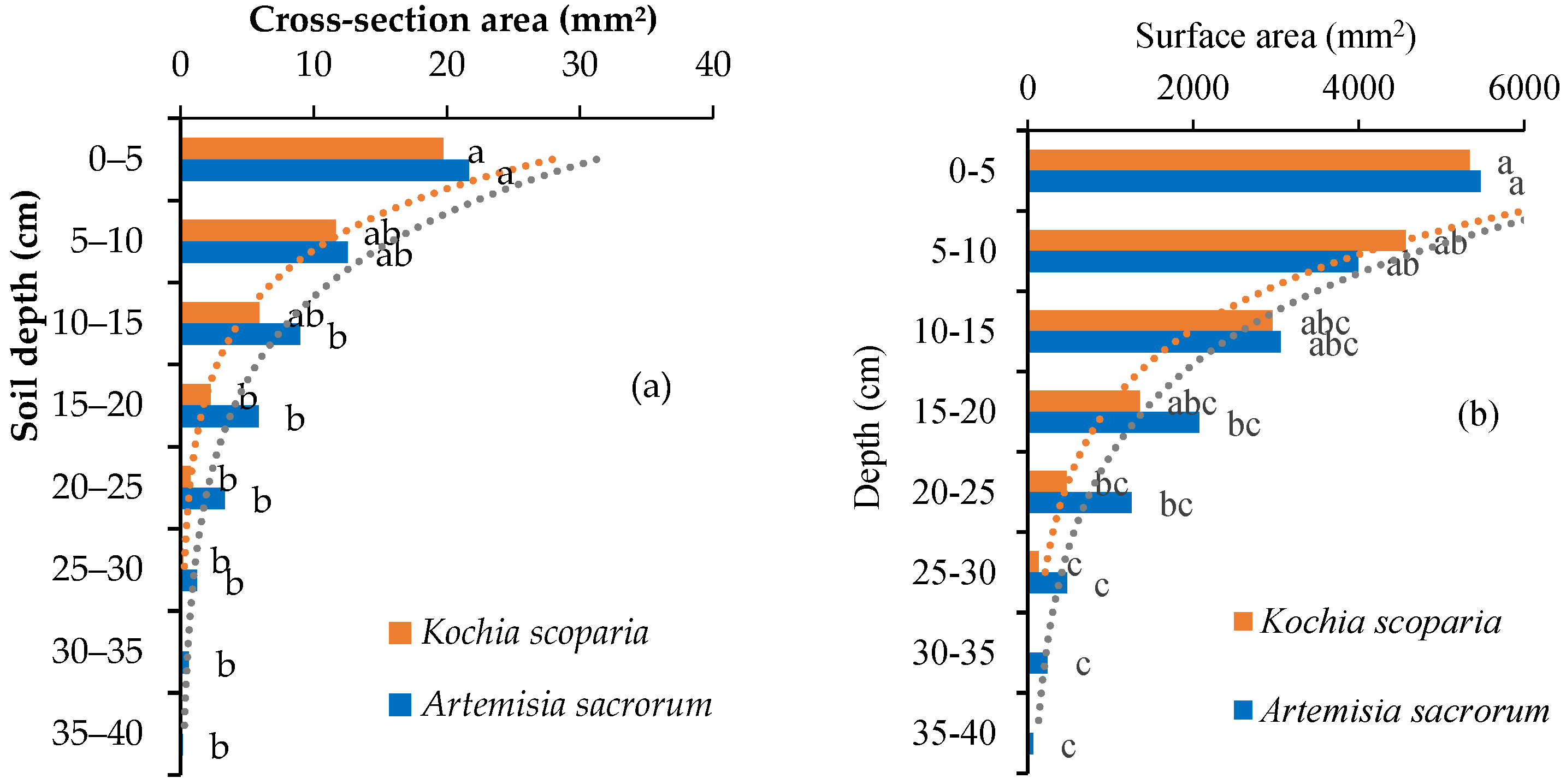
3.3. Other Root Morphological Indexes
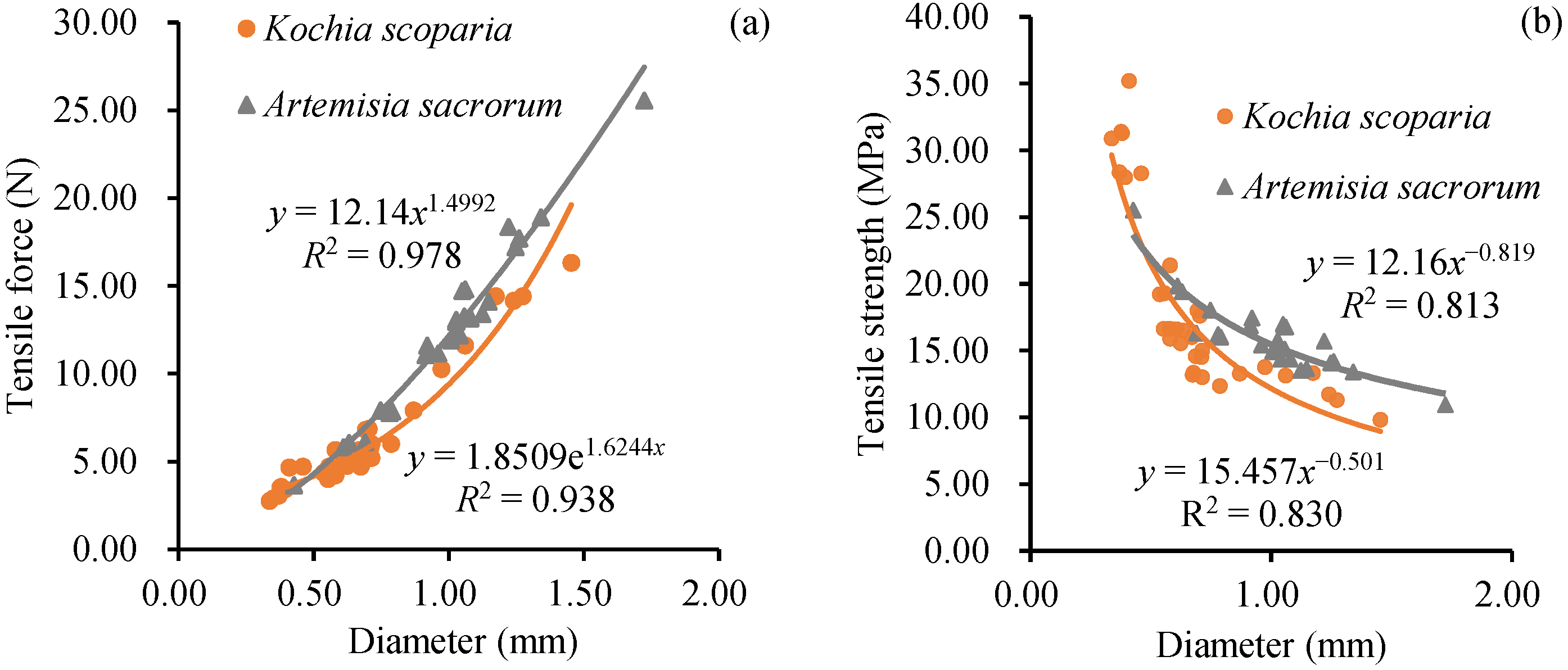
3.4. Root Tensile Properties
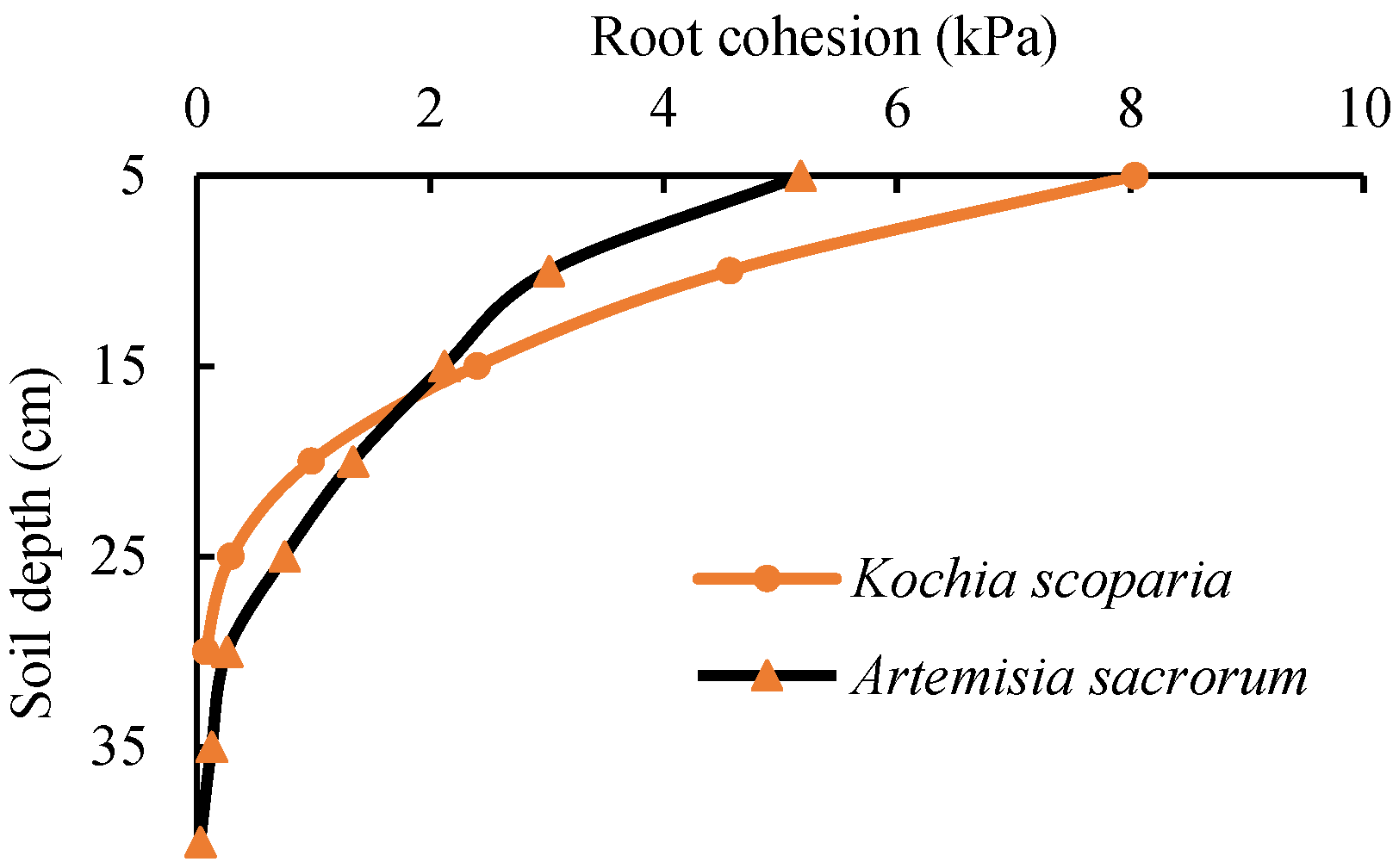
3.5. Root Cohesion
4. Discussion
4.1. Root Morphology and Soil Depth
4.2. Root Tensile Properties and Root Diameter
4.3. Root Cohesion and Soil Conservation
5. Conclusions
- (1)
- The root morphological indexes of the two plants decreased with the root depth in the power functions (root diameter) or exponential functions (root number, root cross-sectional area, root surface area, root volume and root area ratio). These morphological distribution indexes can comprehensively reflect the root distribution in different soil depth. It can be inferred that Kochia scoparia and Artemisia sacrorum are suitable for strengthening and enhancing the stability of the shallow soil of the slope.
- (2)
- The average tensile force of the roots of the two herbaceous plants was Artemisia sacrorum (12.4 N) > Kochia scoparia (6.48 N), and the average tensile strength was Kochia scoparia (18.34 MPa) > Artemisia sacrorum (15.84 MPa). The tensile force of Kochia scoparia and Artemisia sacrorum increased with the increase in the root diameter by the power function or exponential function, and the tensile strength was negatively correlated with root diameter by the power function.
- (3)
- According to the Wu–Waldron model, the average root cohesion of the two herbaceous plants was Kochia scoparia (2.73 kPa) > Artemisia sacrorum (1.60 kPa). Considering the overestimation effect of the Wu–Waldron model, the average root cohesion was estimated to be 0.92 kPa–1.37 kPa for Kochia scoparia and 0.54 kPa–0.8 kPa for Artemisia sacrorum. The soil reinforcement effect of Kochia scoparia is obviously higher than that of Artemisia sacrorum, which can be considered the dominant herb species in the ecological slope protection project.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Zhu, Q.; Tong, X.; Wang, Y.; Chen, W.; Lu, J. Characteristic analysis of temporal and spatial variation of precipitation during recent 50 years in Loess Plateau. Agric. Res. Arid Areas 2016, 34, 206–212. [Google Scholar]
- Chen, Y.Z. Modern Erosion and Control on Loess Plateau; Science Press: Beijing, China, 1988. [Google Scholar]
- Liu, G.B.; Shangguan, Z.P.; Yao, W.Y.; Yang, Q.K.; Zhao, M.J.; Tang, X.H.; Guo, M.H.; Wang, G.L.; Wang, B. Ecological effect of ecological engineering in loess plateau. Bull. Chin. Acad. Sci. 2017, 32, 11–19. [Google Scholar]
- Odum, H.T. Ecological Tools and Their Use: Man and the Ecosystem. In Proceedings of the Lockwood Conference on the Suburban Forest and Ecology, New Haven, CT, USA, 26 March 1962; Volume 652, pp. 57–75. [Google Scholar]
- Adhikari, A.R.; Gautam, M.R.; Yu, Z.B.; Imada, S.; Acharya, K. Estimation of root cohesion for desert shrub species in the Lower Colorado riparian ecosystem and its potential for streambank stabilization. Ecol. Eng. 2013, 51, 33–44. [Google Scholar] [CrossRef]
- Gonzalez-Ollauri, A.; Mickovski, S.B. Plant-Best: A novel plant selection tool for slope protection. Ecol. Eng. 2017, 106, 154–173. [Google Scholar] [CrossRef]
- Gobinath, R.; Ganapathy, G.P.; Akinwumi, I.I. Stabilisation of natural slopes using natural plant root as reinforcing agent. Mater. Today Proc. 2021, 39, 493–499. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, X.W.; Xu, X.C. Ecological restoration and mechanical reinforcement effect of slope of tailings reservoir. Environ. Earth Sci. 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Wu, T.H.; McKinnell, W.P.I.; Swanston, D.N. Strength of tree roots and landslides on Prince of Wales Island, Alaska. Can. Geotech. J. 1979, 16, 19–33. [Google Scholar] [CrossRef]
- Ji, X.L. A Root Distribution-Based Study on Stability of Ecological Slope. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2013. [Google Scholar]
- Rossi, R.; Picuno, P.; Fagnano, M.; Amato, M. Soil reinforcement potential of cultivated cardoon (Cynara cardunculus L.): First data of root tensile strength and density. Catena 2022, 211, 106016. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J. Vertical root distribution and root cohesion of typical tree species on the Loess Plateau, China. J. Arid Land 2014, 6, 601–611. [Google Scholar] [CrossRef]
- Esmaiili, M.; Abdi, E.; Nieber, J.L.; Jafary, M.; Majnounian, B. How roots of Picea abies and Fraxinus excelsior plantations contribute to soil strength and slope stability: Evidence from a study case in the Hyrcanian Forest, Iran. Soil Res. 2021, 59, 287–298. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Zhong, R.H.; He, X.B.; Bao, Y.H.; Tang, Q.; Gao, J.Z.; Yan, D.D.; Wang, M.F.; Li, Y. Estimation of soil reinforcement by the roots of four post-dam prevailing grass species in the riparian zone of Three Gorges Reservoir, China. J. Mt. Sci. 2016, 13, 508–521. [Google Scholar] [CrossRef]
- Abe, K. An evaluation of tree-root effect on slope stability by tree-root strength. J. Jap. For. Soc. 1986, 68, 505–510. [Google Scholar]
- Watson, A.J.; Marden, M. Live root-wood tensile strengths of some common New Zealand indigenous and plantation tree species. N. Z. J. For. Sci. 2004, 34, 344–353. [Google Scholar]
- Tosi, M. Root tensile strength relationships and their slope stability implications of three shrub species in the Northern Apennines (Italy). Geomorphology 2007, 87, 268–283. [Google Scholar] [CrossRef]
- Imada, S.; Yamanaka, N.; Tamai, S. Water table depth affects populus alba fine root growth and whole plant biomass. Funct. Ecol. 2008, 22, 1018–1026. [Google Scholar] [CrossRef]
- Cannon, W.A. Some relations between root characters, ground water and species distribution. Science 1913, 37, 420–423. [Google Scholar] [CrossRef][Green Version]
- Huang, J.K.; Wang, X.L.; Ji, J.N.; Chen, L.H.; Zhang, Z.W. Numerical simulation of root reinforcement for herbs in Loess Plateau based on asymptotic homogenization theory. Transac. Chin. Soc. Agr. Eng. 2020, 36, 168–176. [Google Scholar]
- Ji, J.N.; Kokutse, N.; Genet, M.; Fourcaud, T.; Zhang, Z.Q. Effect of spatial variation of tree root characteristics on slope stability. A case study on Black Locust (Robinia pseudoacacia) and Arborvitae (Platycladus orientalis) stands on the Loess Plateau, China. Catena 2012, 92, 139–154. [Google Scholar] [CrossRef]
- Lian, B.Q.; Peng, J.B.; Zhan, H.B.; Wang, X.G. Mechanical response of root-reinforced loess with various water contents. Soil Tillage Res. 2019, 193, 85–94. [Google Scholar] [CrossRef]
- Liu, Y.B.; Hu, X.S.; Yu, D.M.; Zhu, H.L.; Li, G.R. Influence of the roots of mixed-planting species on the shear strength of saline loess soil. J. Mt. Sci. 2021, 18, 806–818. [Google Scholar] [CrossRef]
- Su, X.M.; Zhou, Z.C.; Liu, J.; Wang, P.P.; Liu, J.Y.; Li, Q.J.; Zhao, F.W. The role of roots traits of climax community species to shear strength in the Loess Hilly Region, China. Soil Tillage Res. 2022, 221, 105417. [Google Scholar] [CrossRef]
- Comino, E.; Marengo, P. Root tensile strength of three shrub species: Rosa canina, Cotoneaster dammeri and Juniperus horizontalis. Catena 2010, 82, 227–235. [Google Scholar] [CrossRef]
- Hubble, T.C.T.; Airey, D.W.; Sealey, H.K.; Carli, E.V.D.; Clarke, S.L. A little cohesion goes a long way: Estimating appropriate values of additional root cohesion for evaluating slope stability in the Eastern Australian highlands. Ecol. Eng. 2013, 61, 621–632. [Google Scholar] [CrossRef]
- Liu, F.; Liu, J.; Yao, X.; Zhang, Y.; Yuan, S. Mechanical factors influencing soil-reinforcement by roots and identifying appropriate plant species for erosion control. Acta Ecol. Sin. 2015, 35, 6306–6315. [Google Scholar]
- Zhang, C.B.; Li, D.R.; Jiang, J.; Zhou, X.; Niu, X.Y.; Wei, Y.; Ma, J.J. Evaluating the potential slope plants using new method for soil reinforcement program. Catena 2019, 180, 346–354. [Google Scholar] [CrossRef]
- Schiechtl, H.M. FAO Watershed Management Field Manual. Vegetative and Soil Treatment Measures; FAO: Rome, Italy, 1985. [Google Scholar]
- Niu, X.; Li, H.; Yan, J. Characterization of general climate change patterns from 1951 to 2012 in Taiyuan, Shanxi Province. J. Shanxi Agric. Sci. 2013, 41, 1352–1357. [Google Scholar]
- Böhm, W. Methods of Studying Root Systems; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Su, X.M.; Zhou, Z.C.; Liu, J.E.; Cao, L.G.; Liu, J.Y.; Wang, P.P. Estimating slope stability by the root reinforcement mechanism of Artemisia sacrorum on the Loess Plateau of China. Ecol. Model. 2021, 444, 109473. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J.; Zhou, S. Effects of gauge length and strain rate on the tensile strength of tree roots. Trees-Struct. Funct. 2012, 26, 1577–1584. [Google Scholar] [CrossRef]
- Waldron, L.J. The shear resistance of root-permeated homogeneous and stratified soil. Soil Sci. Soc. Am. J. 1977, 41, 843–849. [Google Scholar] [CrossRef]
- Mattia, C.; Bischetti, G.B.; Gentile, F. Biotechnical characteristics of root systems of typical Mediterranean species. Plant Soil 2005, 278, 23–32. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, L.F.; Xiao, H.B.; Wang, H.L.; Yang, H. Distribution characteristics of vetiver’s roots in highway slope. J. Basic Sci. Eng. 2017, 25, 102–112. [Google Scholar]
- Burylo, M.; Hudek, C.; Rey, F. Soil reinforcement by the roots of six dominant species on eroded mountainous marly slopes (Southern Alps, France). Catena 2010, 84, 70–78. [Google Scholar] [CrossRef]
- Parker, M.M.; Lear, D.H.V. Soil heterogeneity and root distribution of mature loblolly pine stands in piedmont soils. Soil Sci. Soc. Am. J. 1996, 60, 1920–1925. [Google Scholar] [CrossRef]
- Cheng, L.; Hao, Y.Z. Experimental study on the root characteristic parameters impact on soil strength. Sci. Tech. Eng. 2018, 2018, 271–276. [Google Scholar]
- Ye, C.; Guo, Z.L.; Li, Z.X.; Cai, C.F. The effect of Bahiagrass roots on soil erosion resistance of Aquults in subtropical China. Geomorphology 2017, 285, 82–93. [Google Scholar] [CrossRef]
- Abdi, E. Root tensile force and resistance of several tree and shrub species of Hyrcanian Forest, Iran. Croat. J. Forest Eng. 2018, 39, 255–270. [Google Scholar]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.F.; Beek, R.V. The Influence of Cellulose Content on Tensile Strength in Tree Roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Pollen, N.; Simon, A. Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour. Res. 2005, 41, 226–244. [Google Scholar] [CrossRef]
- Docker, B.B.; Hubble, T.C.T. Quantifying root-reinforcement of river bank soils by four Australian tree species. Geomorphology 2008, 100, 401–418. [Google Scholar] [CrossRef]
- Preti, F.; Giadrossich, F. Root reinforcement and slope bioengineering stabilization by Spanish Broom ( Spartium junceum L.). Hydrol. Earth Syst. Sci. 2009, 13, 1713–1726. [Google Scholar] [CrossRef]
- Schwarz, M.; Preti, F.; Giadrossich, F.; Lehmann, P.; Or, D. Quantifying the role of vegetation in slope stability: A case study in Tuscany (Italy). Ecol. Eng. 2009, 36, 285–291. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Wang, Q.; Wang, Y.; Zhang, H.; Wang, B.; Wang, J.; Li, Y. Change of soil fixation effects in the process of gradual damage. J. B. Forestry Univ. 2015, 37, 85–92. [Google Scholar]
- Chen, H. Effect of vegetation root system on stability and soil consolidation of loess slope. Water Resour. Power 2019, 37, 97–100. [Google Scholar]
- Yang, P.; Xia, J.; Zhan, C.S.; Mo, X.G.; Chen, X.J.; Hu, S.; Chen, J. Estimation of water consumption for ecosystems based on Vegetation Interfaces Processes Model: A case study of the Aksu River Basin, Northwest China. Sci. Total Environ. 2018, 613, 186–195. [Google Scholar] [CrossRef]
- Bueno, A.; Greenfield, L.; Pritsch, K.; Schmidt, S.; Simon, J. Responses to competition for nitrogen between subtropical native tree seedlings and exotic grasses are species-specific and mediated by soil N availability. Tree Physiol. 2019, 39, 404–416. [Google Scholar] [CrossRef]


| Location | Clay (g·100 g−1) | Silt (g·100 g−1) | Sand (g·100 g−1) | Texture Class |
|---|---|---|---|---|
| Western Taiyuan | 15.2 | 28.3 | 56.5 | Sandy loam |
| Plant Species | Fitting Formula | Coefficient of Determination R2 |
|---|---|---|
| Kochia scoparia | s = 70.668e−0.929h | 0.974 |
| S = 18,280e−0.744h | 0.927 | |
| V = 3523.6e−0.928h | 0.974 | |
| RAR = 0.0013e−0.916h | 0.972 | |
| Artemisia sacrorum | s = 61.696e−0.681h | 0.949 |
| S = 16,117e−0.612h | 0.933 | |
| V = 3100.1e−0.682h | 0.948 | |
| RAR = 0.0008e−0.695h | 0.956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, C.; Yao, S.; Jiang, J. Root Distribution and Root Cohesion of Two Herbaceous Plants in the Loess Plateau of China. Sustainability 2022, 14, 8053. https://doi.org/10.3390/su14138053
Yang Q, Zhang C, Yao S, Jiang J. Root Distribution and Root Cohesion of Two Herbaceous Plants in the Loess Plateau of China. Sustainability. 2022; 14(13):8053. https://doi.org/10.3390/su14138053
Chicago/Turabian StyleYang, Qihong, Chaobo Zhang, Shiming Yao, and Jing Jiang. 2022. "Root Distribution and Root Cohesion of Two Herbaceous Plants in the Loess Plateau of China" Sustainability 14, no. 13: 8053. https://doi.org/10.3390/su14138053
APA StyleYang, Q., Zhang, C., Yao, S., & Jiang, J. (2022). Root Distribution and Root Cohesion of Two Herbaceous Plants in the Loess Plateau of China. Sustainability, 14(13), 8053. https://doi.org/10.3390/su14138053






