Abstract
Several passive and active treatment approaches are available for dealing with Acid Mine Drainage (AMD). Despite a range of newly emergent techniques for the treatment of AMD, pH control using low-cost neutralizing reagents has been the most common and economical technique for the treatment of AMD. Thereby, owing to their widespread availability, ease of use, and cost effectiveness, active treatment techniques utilizing calcium-based reagents (particularly limestone) are considered the prime choice for treating AMD. Limestone is a well-known option worldwide for AMD neutralization thanks to its easy availability, low cost, and excellent efficiency. Generally, acidity is reduced by the presence of CaCO3 and alkalinity (i.e., HCO−3) is increased. pH can be increased from 2.5 to 7.5 by using limestone as a treating agent, resulting in the precipitation of heavy metals, which can then be removed by precipitation and sorption. Wargal limestone, a well-known limestone from the Salt Range, Indus basin, Pakistan, has high potential for neutralization and treatment of PTEs present in mine water or AMD. After selecting a suitable neutralization material at pilot scale, two different filters were designed using selected Wargal limestone: Filter 1 A (Oxic-based, Vertical bed-type Wargal Limestone Filter) and Filter 1 B (Anoxic-based Vertical bed-type Wargal Limestone Filter with Compost). The pH of the AMD under study was elevated from 2.5 to 7.65 and 7.60, respectively, in uncoated and coated media of limestone. Although the neutralization potential decreased over time, as an overall phenomenon the Ca concentration and net alkalinity (280–360 mg/L) were increased with the removal of metals such as Fe, Cu, Pb and Mn. The respective removal efficiency of these metals was 98%, 99%, 99% and 60%, with a threshold residence time of 5 h. in all columns of the developed filters.
1. Introduction
The mining sector plays a key role in the development of any country, however, its social and environments impacts cannot be neglected; a balance is required in both operations and environmental rehabilitation plans. Mine waste, which can be in the form of solids, such as tailings, liquid solutes, and gaseous products, represent a major health-related challenge during mining activities. Mine-related AMD consists of water solute from pyrite oxidation caused by chemical and biological reactions. These liquids have low pH (2.5–3) and can dissolve heavy metals at concentrations as high as 250 to 800 g/L [1,2]. Acid Mine Drainage (AMD) is produced by oxidation of sulfide minerals, and may contain high concentration of heavy metals and sulphuric acid. During last few decades, passive treatments for treating AMD have entered practice [3,4]. These systems do not rely on permanent energy sources, and can improve the quality of water via natural processes such as microbial metabolic, gravitational, photosynthesic and other geochemical processes [5]. Several geochemical-based processes include oxic and anoxic limestone drains, which create a reducing and alkalinity producing system (RAPS) [6,7,8,9]. This article introduces the characteristics of coal mine drainage in the sub-Himalayan region of Pakistan, the specific difficulties of coal mine drainage management and treatment, different solutions that have been applied, and their results. In addition, this article discusses the necessary requirements in terms of technology and management experience that must be present in order to effectively address coal mine drainage while contributing to the protection of the ecological environment and improving the living conditions of miners and the local community in sub-Himalayan Pakistan. Geochemically, the formation of AMD is a complex process that is influenced by the functions of microbiological control, the depositional environment, the acid/base balance of the tailings, and the inferred lithology, mineralogy, and hydrological conditions of the area. Heavy metals, being non-degradable and persistent, are proven potential activators of environmental fragility. Although quantitative analysis of mining’s impacts may have conflicting results due to the complexity of many interacting factors, high levels of metals have always been hazards to the environment and human health. Contamination of freshwater resources caused by anthropogenic factors is a major issue in many areas, and is associated with mining activities. Mine water, irrespective of its neutral or alkaline nature, has the propensity to dissolve metals, and the resulting metal contaminants, such as Copper, Cadmium, Iron and Sulphur, have potentially harmful impacts that are not limited to chemical, physical, and biological spoliation of soil and water bodies; their adverse consequences on human health are undeniable [10]. Higher concentrations of Iron (Fe) and Manganese (Mn) in water bodies associated with mining sites have adverse effects on indigenous public health, including lethargy, hepatic necrosis, tachycardia, shock, hypotension, metabolic acidosis DNA damage, and in severe cases, even death. This elevation in geochemical parameters can be a menace to local habitats during active mining operations. Many case studies reported in India, Japan, U.K, USA have shown that even after closure, coal mines may continue to result in the deterioration of the environment [11,12,13,14].
A wide variety of treatment system have been developed over the decades in different mine settings and environments. AMD can be treated using limestone by raising the pH with associated chemical reactions such as oxidation and precipitate formation [3,15,16,17]. Engineered limestone beds, a well-known option in passive treatment, are low-maintenance and a cheap choice for low-flow AMD discharges [18,19,20]. In this study, we aim to develop a geochemical filter established with locally identified limestone material from a well-known region of Asia, the Salt Range of Pakistan, for the improvement of ecological health in the coal basin areas of the Salt Range. This study demonstrates a novel potential use of limestone’s dissolution in a controlled laboratory setup in order to identify changes in effluent quality in coal mine areas of the Salt Range. These laboratory and pilot scale rate experiments were performed on a time span of three months using uncoated and coated limestone in order to develop a conceptual design for a treatment system while determining the retention time required for effective performance. The novelty of the geochemical filter developed here lies in the achievement of a very low retention time of five hr, which is far less than other similar geochemical systems developed around the world. The approach adopted here is in keeping with circular economy principles, as it contributes to the reuse of raw materials and sustainable water treatment efforts.
2. Materials and Methods
2.1. Study Area
The study area is located in well-known geological terrain comprising a system of hills. It lies between 71°30′–73°30′ east and between latitude 32°23′–33°00′ north, and is marked by its location between the Thal Desert and Potowar Plateau in the west and northeast, respectively [21]. This range starts from the Potowar region and ends at the north side of the River Jhelum. The River Jhelum is one of the major tributaries of the Indus; it flows through this region, and is considered the central hydrological unit of the study area. Climatically, the area is characterized by low rainfall of about 50 cm, and the months of July, August, and September are the more rain-prone periods. Subtropical dry and evergreen shrubs are the main vegetation types [22,23]. The location map of the Potowar region and Salt Range is presented in Figure 1.
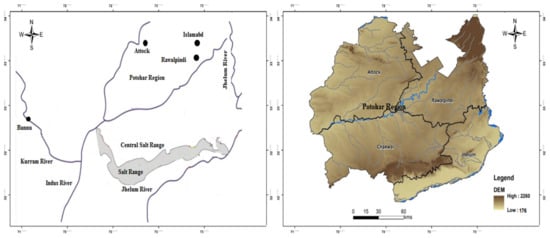
Figure 1.
Location map of the Potowar region and Salt Range.
In the first phase, understanding of AMD formation was realized and understanding of the local environment and local mine industry was established. After that, a review of available passive technologies was made and a suitable methodology was adopted after performing lab and pilot scale studies. A detailed review of the methodology is presented in Figure 2.
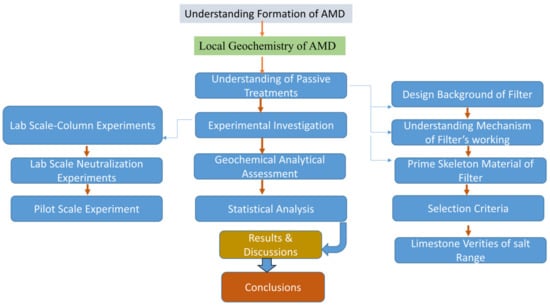
Figure 2.
Overview of the detailed methodology adopted in this study.
2.2. Formation of AMD and Local Geochemistry
Formation of acid mine drainage is linked with oxidation processes in mine waste, which causes a decrease in pH, elevated sulphate contents, and metals finding their way into mine drainage. The core ingredients of any mine drainage are water, reactive sulfide, and molecular oxygen. Pyrite present in different concentrations in waste of coal mine is the main source of AMD, typically by oxidation of sulfidic minerals such as iron pyrite [24]. Other influencing factors can include low pH, bacteria, temperature, and the presence of other oxidants such as manganese and iron [25].
Salt Range coal contains sulphur with a mean content of 5.50% to 11.03% and a fixed carbon plus ash content of 13.20% to 32.80%, whereas the moisture content ranges from 3.145 to 4.25% and the heating value lies in the range of 1114–1425 BTU/lb [26]. Based on previous research after [27], I-geo values, tailings sediment, and mine water have been found to be the main sources of toxicity, threatening the good ecological status of the Salt Range and the entire Indus Basin of Pakistan. Iron and copper are found as anthropic loads, with Mn, Cd, Hg, and Se as emerging toxic elements. The overall geochemistry of collected AMD is shown in Figure 3.
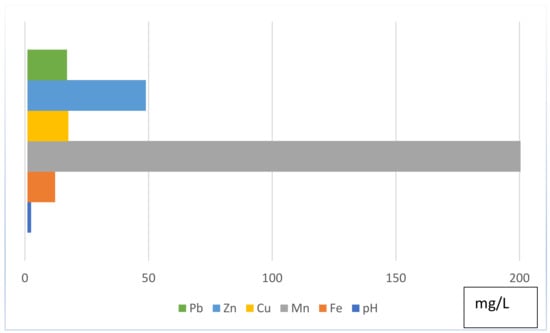
Figure 3.
Geochemical overview of collected AMD.
2.3. Understanding of Passive Treatments and Design Background
The basic principle of passive treatment systems is to retain mine water long enough to reduce the concentration of the contaminants to reasonably safe standards. The chemical and biological phenomena depend on the metals, pH, and oxidation–reduction potential. Performance of passive systems depends on special conditions which need to be created in order to enhance the productivity. Hence, a solid understanding of the mine water and the relevant chemical and biological reactions involved in the process is necessarily required to design an effective passive treatment system [28]. Several passive treatment approaches are available for dealing with Acid Mine Drainage (AMD), several of which have been explored in the literature [29].
The basic theme of FILTER is based on the reuse of wastewater for cropping after filtration through constructed of a soil/rock column to subsurface drainage followed by collection in a sump tank. Large volumes of wastewater can be treated in a small footprint area, and great environmental benefits can be achieved [30]. A schematic diagram of the FILTER technique is illustrated in Figure 4.
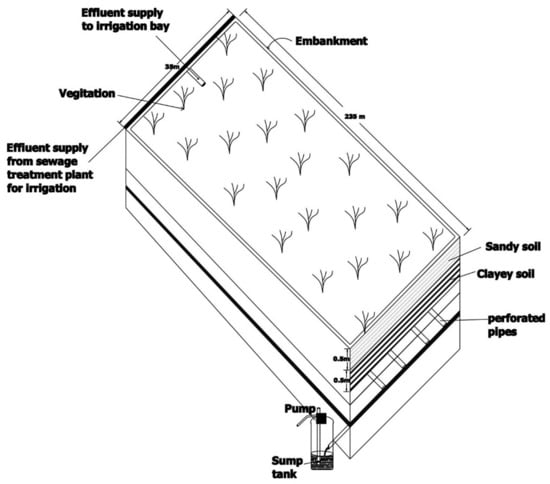
Figure 4.
Schematic Diagram of FILTER Plots after [30,31].
2.3.1. Prime Material of Filter
Limestone is a widely known option for AMD neutralization due to its easy availability, cost, and excellent efficiency [32,33]. Generally, acidity is consumed in the presence of CaCO3 and alkalinity, i.e., HCO−3, is produced [34]. pH can be increased from 2.5 to 7.5 by using limestone as a treating agent, which results in precipitation of heavy metals which can then be removed by precipitation and sorption [35]. Being inexpensive, limestone is considered the best fit for low acidic loads and post-closure treatment of many mine operations. These options may be oxic or anoxic drains or leach beds [36], diversion wells [37], successive alkalinity production systems, or vertical flow beds [38,39].
In the case of oxic mechanisms, AMD flows through trenches filled with limestone, which cause exclusion of O2 and inhibit Fe(OH)3 precipitation on the limestone. In case of anoxic mechanisms, pretreatment may be provided in an initial portion supplemented with suitable organic material or it may infiltrate directly into stratified beds of limestone [40].
2.3.2. Selection Criteria of Limestone
Microcrystalline limestone containing at least 50% CaCO3 is recommended, along with a low content of Dolomite and especially any siliceous matter, which may hamper the expected reactivity compared to Calcite [36]. In [32], the authors recommend of selecting limestone with a diameter in the range of 3–10 cm, however, other scientists suggest a diameter in the range of 2.1 cm to 2.8 cm and a maximum length of 0.5 cm to 1.3 cm [40]. Fine pieces with a diameter of 0.05 cm or less were removed, as they may cause clogging due to precipitation process. Limestone pieces with a diameter larger than 1.3 cm were removed as well, as their smaller surface area to volume ratio leads to poor reactivity [32]. Likewise, in pieces of very small diameter, a lower surface area is available. Rate measurement of infiltration can be measured with help of standard soil testing methods.
About fifty random samples were selected, and measurements were taken by observing the respective length and mass of individual particles using the methodology of Pearson and McDonnell, 1977 [35]; see Equation (1).
In Equation (1), m shows the mass (g) of limestone and D (cm) is the nominal diameter, which is equal to the diameter of a sphere of the same volume as the particles, as shown in Equation (2):
The pycnometer method from [41] was used to calculate particle density. In Equation (3), S is the shape factor of a particle:
S = 1.15 − 0.25 E
In Equation (3), E = l/d, where “l” is length and “d” is the nominal diameter of particles. Finally, the total surface area of particles can be found using Equation (4), as follows:
Residence time is the key factor when designing limestone drains for treatment of any mine water. In [6], the authors studied the construction characteristics, chemistry, and residence time required for efficient treatment of mine water. Residence time is the key factor affecting performance due to kinetic control of precipitation, sorption reactions, and dissolution, which together control the dissolved ion concentration and pH. Different filter drains may have varying porosities depending on their shape, size, and the mixed sizes and compaction properties of their media.
Based on a version of Darcy’s Formula [42], flow by media can be calculated by Equation (5) as follows:
where Q is the rate of flow, A is the cross-sectional area, and n is the porosity. If we substitute v = L/tR and rearrange it, inflow can be determined for Q at any distance by Equation (6), as follows:
where tR is proportional to the down-flow distance and the numerator of Equation (6). Residence time can now be calculated using a different version of Darcy’s Formula, modified by [6] as Equation (7):
where Ms represents limestone mass and is the bulk density of limestone.
V = Q/(An)
tR = (L.A.n)/Q
Absorption capacity measurements of the used material were made following Equation (8) [43]:
where q, V, Ci, Cf, and M are absorption capacity, volume of sample, initial concentration, final concentration, and adsorbent amount, respectively [44].
q = V(Ci − Cf)/M
The infiltration rate of the selected limestone aggregate (k) was determined = using Darcy’s Formula as follows:
where, k is the infiltration rate (cm/s), H is the hydraulic head (cm) and z is the thickness of the filter media.
Q = k(∆H)/z
2.4. Limestones Varieties of Salt Range Area
In order to find suitable neutralization material, sampling was carried out in the study area to select an appropriate variety of limestone. The Salt Range is full of stratigraphical succession and different geological formations, including many types of sandstone, shales and limestones. Several pure and famous limestones varieties can be found here, including Wargal Limestone, Lockhart Limestone, Chorgali Formation, Sakesar Limestone, Nammal Limestone, and a dolomite variety known as Jutana Dolomite. A summative geochemistry based on conventional petrography, XRD, and EDS techniques is presented in Figure 5.
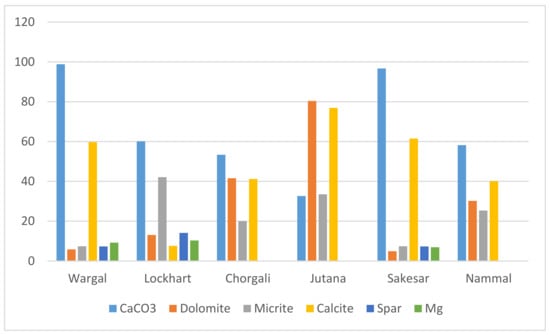
Figure 5.
Elemental concentration of different minerals in Salt Range limestone varieties, after [45,46,47,48,49,50,51].
3. Experimental Investigation
3.1. Lab-Scale Column Experiments
Based on available materials in the study area, it was decided to create a barrier based on selected lithology. For this purpose, heaps of available limestone material selected based on different local stratigraphy were transported to the laboratory to create filter stacks. Because a vertical flow system was selected, each layer was tested by replacing different type of limestone in the study area. For this purpose, different stratification design options were prepared and tested, and conclusive efficiency levels were determined for different types of limestone collected from Salt Range areas. In [52,53], the authors worked on different limestone beds to treat AMD in an efficient manner, especially for the removal of Mn and other heavy metals.
Neutral plastic containers (see Figure 6) were used for holding the columns of said limestones. Ten inches thickness was provided for treating AMD. The containers were filled with the selected type of limestone material and AMD was provided with a minimum retention time of 15 h. Then, all leachates were drained out for further analytical testing.

Figure 6.
Stacked gravels: column of different types of limestone from the Salt Rane.
3.2. Lab-Based Neutralization Potential Experiments
After finalizing the design and laboratory setup, neutralization rate experiments were conducted with different types of limestones selected and picked from Salt Range areas; see Figure 7. Different parameters such as retention time, effects of armouring by Fe(OH)3 coating, and encrustation in limestone surfaces were studied following the methodology from [5,54]. The rate of neutralization within experiments was expected to increase, as per [54,55]. Net acidity can be determined by understanding the contribution protons (H+; pH) and metals such as Fe, Cu, Cd, and Mn and the negative contribution from alkalinity as net acidity. After conducting lab-scale experiments, Wargal Limestone ranked better in neutralization potential and treatment of PTEs present in both mine water and AMD.
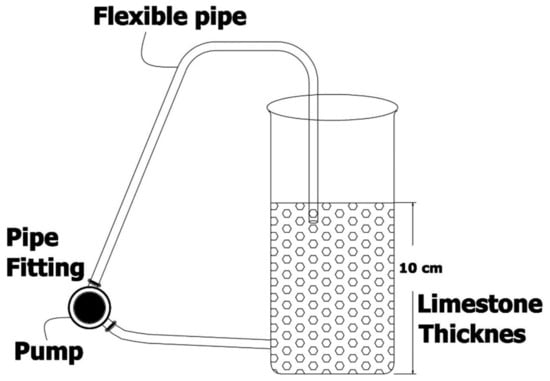
Figure 7.
Schematic projection for acrylic container packed with selected media of limestone from the Salt Range. The valve gear at the bottom introduces a constant flow of influent PTEs with the help of pump, whereas effluent PTEs are collected by the exit at end. Idea and picture after [56].
3.3. Pilot Scale Experiments
After selecting suitable materials and lithology, pilot scale experiments were performed using a polyethylene acrylic barrel (60 cm diameter) with a surface area of 0.25 m2 and height of 100 cm [57]. These barrels were filled with successive layers of the selected limestone. Treated mine water was collected at the bottom by placing perforated pipe/tile drains at bottom of the barrel and along the barrel’s diameter, as shown in Figure 6. Circulation was maintained with the help of a peristaltic pump with a pumping rate of 10–20 L/h in order to avoid any clogging and maintain simultaneous flow in the body of filter. Treated water was sampled at every half-hour interval during the first 7 h, then again after 24 h, then daily day, and finally after durations of two and three weeks. Two types of filters were designed using selected Wargal limestone, as described below.
3.3.1. Filter 1 A: Oxic-Based Vertical Bed-Type Wargal Limestone Filter
This filter was constructed by arranging selected aggregate of Wargal limestone with a bed thickness of 1.5 ft in a container made of acrylic material supported by a valve system and pump circulation arrangement. Mine water was recirculated and collected at different time intervals, as shown in Figure 8a.

Figure 8.
Designed Filters: (a) Oxic Type and (b) Anoxic Type.
3.3.2. Filter 1 B: Anoxic-Based Vertical Bed-Type Wargal Limestone Filter with Compost
This filter was constructed by adding compost material with 1 ft thickness over the selected aggregate of Wargal limestone with a bed thickness of 1.5 ft in a container made of acrylic material supported by a valve system and pump circulation arrangement. Mine water was recirculated and collected at different time intervals, as shown in Figure 8b.
4. Geochemical Analytical Assessment
The geochemistry of the AMD was already known, thus it was passed through the designed filters for treatment. The temperature and pH of the filtered leachate was determined on a daily basis during a three days span of one cycle in a single experiment. The pH and temperature after standard calibration were used to measure the parameters of concern. Drained effluent water was collected in plastic carboys and the volume was measured using graduated cylinders. The pH and temperature values were measured using a pH meter (Metrohm 704) after treatment with buffer solution (pH 4–6.8). PTE concentrations were determined using atomic absorption by observing standard practices, whereas ion chromatography was used for calculation of Cl and SO4. PHREEQC interactive V.2.6 was used to calculate aqueous species plus saturation of mineral indices (SI = log (activity product/Keq) [58]
Based on [59], asymptotic steady state increases in Ca or alkalinity container tests were observed; moreover, other researchers such as [60,61] have explained the asymptotic steady state using the kinetic relationship in Equation (10), as follows:
where C represents the Ca or alkalinity concentration, t is the time, CS represents the maximum steady state intensity, and K represents the rate along n, which is the order of the reaction.
dC/dt = k. (Cs − C)n
Dissolution of CaCO3 is a complicated process, whereas “n” is an integer with a value of 1 (first order model) or 2 (second order model). Thus, integration of Equation (10), being first order, yields the following Equation (11):
ln (Cs − Ct)/(Cs − Co) = K′.tr
Likewise, for the second order model, Equation (11) yields
(Cs − Ct) − 1/(Cs − Co) − 1 = K″.tr
In Equations (11) and (12), Co represents the initial concentration, Ct is the concentration at any time, and K′ and K″ are the first and second order constants, respectively. Thus, by using these parameters, the continuous duration for the retention time can be calculated. Finally, by rearranging and using the antilogarithm to calculate Ct, the following Equations (13) and (14) are suggested by rearranging.
For the first order model:
Ct = Cs − [(Cs − Co).exp{k′.tr}]
For the second order model:
Ct = Cs + {1/[k″.tr − 1/(Cs − Co)]}
Similarly, in order to use rate data for the executed tests and then for the applicable field-scale model with different types of limestone media, sizes, and types of fluids, the rate constant, i.e., k′, k″, can be found by dividing the ratio of the surface areas (A) of the selected media by the average volume of solution (V) in Equation (15), as follows:
K′ = k′/(A/V); k″ = k″/(A/V)
For geochemical understanding, PHREEQC software was used; PHREEQC operates on the basis of solubility to find solid phases in the context of thermodynamics by following Equation (16), known as the Activity and Mass Action Formula.
SI = Log IAP/KS
In Equation (16), SI represents the Saturation Index, which is the basis of dissolution and precipitation by water inclusion [61]. The Saturation Index (SI) can have a positive or negative value, which shows the condition of water as super-saturated, undersaturated, or saturated, respectively. In the case of undersaturation (SI values < −1), water has dissolving potential, whereas in the case of super-saturation (SI values > +1), precipitation takes place.
Developed chemical encrustations on limestone surfaces were gently shredded by rinsing with deionized water, then dried at 1800 C. This material was dissolved in 50% solution of HCl-HNO3 (1:1) for determination of counted PTEs. Moreover, these treated hydrous oxides were qualitatively examined and analysed by EDS and X-ray diffractometry (XRD).
To understand expected armouring on the limestone surface, limestone particles were removed, air-dried for two weeks, and weighed. Coatings on limestone particles were cleaned using pincers and were studied under a Leica microscope to study the thickness and texture of the coatings. Powdery material on the surface of particles was removed and material was set in a Seimens D5000 XRD machine for further examination. At the same time, powder material was studied with help of a Cambridge S 150 SEM, which was run at 20 kv after facilitation with an lectron dispersive X-ray spectrometer, observing standard procedures.
5. Results
A logarithmic relation was seen, indicating a rapid increase in alkalinity generation in first few hours with slow dissolution of limestone with the passage of time in the context of calcite saturation; the same relationship was observed by [53]. In [8], the authors noted a similar relationship between net alkalinity and acidity generation, which they credited to higher dissolution of calcite under prevailing acidic environments.
Our laboratory experiments demonstrated the changes in AMD chemistry in the context of neutralization when brought into contact with the limestone of the designed filters. Because influents were recirculated with the installed pump, these tests may mimic kinetics such as the length of buried limestone‘s drainage system, mostly adopted in many parts of world [53]. The pH, alkalinity, and Ca of treated water were calculated and are summarized in Table 1 and Table 2. All of these parameters were assessed for both uncoated (fresh) limestone and coated limestone to determine the longevity of designed filters. For these two conditions (coated and uncoated), alkalinity, pH, and Ca concentrations increased during the first few days; thereafter, they approached equilibrium or steady state conditions within approximately two weeks of continuous contact. An asymptotic trend indicates the declining efficiency of limestone dissolution with the passage of time, especially as the effluent approaches equilibrium.

Table 1.
Geochemical parameters with uncoated media.

Table 2.
Geochemical parameters with coated media.
Initially, the influent water had a pH of 2.5 and a net acidity of 1 mg/L CaCO3, however, after a duration of one hour in contact with fresh limestone the pH increased to 4.5, and after 3 h It reached up to 5.9, with an alkalinity of 23 mg/L (see Table 1). Whereas for uncoated media a pH of 6.1 and alkalinity in the range of 38 mg/L was seen after 5 h, for coated media the pH of 2.5 increased to 3.2 in the first hour, which was elevated to 5.2 after 3 h and finally to 6.2 after 5 h, with an alkalinity of 62 mg/L (see Table 2). The concentrations of alkalinity and Ca content slightly increased with Fe(OH)3 coated limestone compared to uncoated limestone media, however, the tests converged and were roughly comparable after one day (24 h).
5.1. Filter Treatment Efficiency
As AMD was passed through the developed filter media, dissolution of calcite caused pH to be elevated with higher concentrations of Ca and HCO alkalinity. The major process responsible for bicarbonate production was the reaction of calcite with carbonic acid, a very simple reaction which, per Equations (17) and (18), can be shown as below:
CaCO3 + H+ = Ca2+ + HCO−
CaCO3 + H2CO3 = Ca2+ + 2HCO−
The results of the neutralization experiment conducted with different varieties of limestone from the Salt Range reveal the filtration ability of each limestone material, as presented in Figure 9.
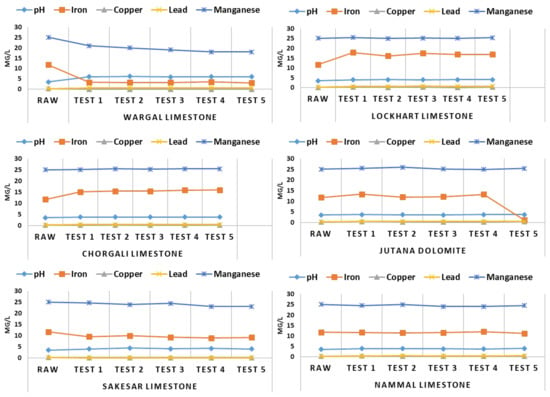
Figure 9.
Neutralization efficiency of different Salt Range limestones.
The fifth option was chosen as another famous limestone, known as Sakesar Limestone. It performed well, just after Wargal limestone, elevating pH to 4.09 and removing PTEs at an admirable level, as shown in Figure 9, making Sakesar limestone the second-best option as a neutralization material. Thee last option, Nammal limestone, was tested for its neutralization potential of AMD. Although the pH changed from 3.57 to 4.0, this limestone could not improve the quality of mine water, and further testing was declined due to the inefficiency of neutralization.
After conducting lab-scale experiments, Wargal Limestone was chosen for its neutralization potential and ability to treat PTEs present in mine water and AMD. After selecting a suitable neutralization material, pilot-scale experiments were conducted with the enhanced geochemical procedure and this limestone was tested under different scenarios to judge its efficiency, as shown in Figure 10.
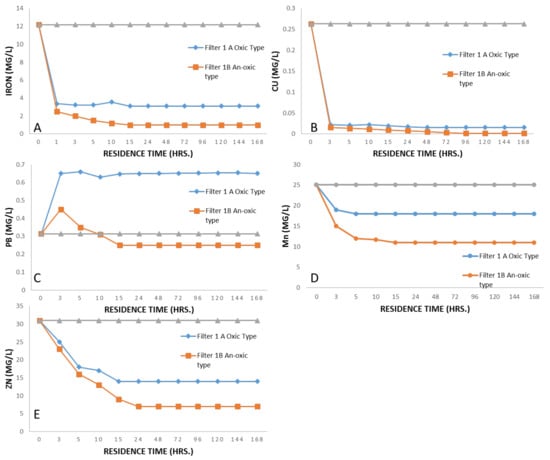
Figure 10.
Removal of PTEs by each system with different intervals of residence time: (A) Iron; (B) Copper; (C) Lead; (D) Manganese; (E) Zinc.
5.2. Filter Autopsies
To determine the intensity of limestone armouring after flushing of precipitate, three collective samples from the top, middle, and bottom were inspected and correlated with a Munsell chart. The top layers of the oxic bed were yellowish red (5YR 5/6), whereas the middle and bottom portions were a yellowish brown colour (10yR 6/6). An average 6% coating with a reddish yellow colour (7.5yR 5/6) was seen at the top portion of the oxic bed, whereas the middle and bottom portions had a 5% coating with a yellowish brown colour (10yR 6/8); see Figure 11.
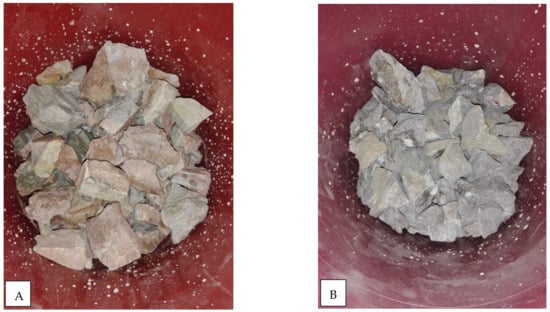
Figure 11.
Autopsies of filters (Oxic and Anoxic-types) showing development of armouring on limestone media and their shades have been matched with Munsel’s chart.
Likewise, the anoxic bed was a light medium grey colour (N4), whereas the bottom portion was a brownish yellow colour (19yR 6/6). Moreover, a 10% coating was observed having a black colour (N1), and a 2% red coating (10R 4/6) was observed as well. The bottom layers had a 2% coating with a red colour (10R 4/6), with other particle having a brownish yellow (10 yR 6/6) colour; see Figure 11A,B.
In-depth autopsies were performed using XRD and SEM (EDS) to investigate the presence of different deposited precipitates of minerals in the body of the developed filter. Figure 11 shows the autopsies before treatment; the XRD data confirm the presence of sulfide minerals such as pyrite and pyrrhotite. SEM analysis (Figure 12, A—spectrum 1, B—Spectrum 2 and C—spectrum 3) further confirms the presence of sulphur (up to 15.53%) along with associated other minerals such as Fe (3.67%), Mg (2.43) and Ca (0.16%). Post-treatment autopsies are provided in Figure 12, revealing the precipitation of iron oxyhydroxide (Fe 9.21%), represented by mackinawite. The surface material shows iron in secondary solid phases. Several others elements, such as Ca, Si, Na, Mg, and Mn, can be seen in the SEM results as shown in Figure 13, A—spectrum 1, B—spectrum 2, C—spectrum 3). The presence of gypsum, Mn, and calcite can be observed in Figure 13 (A—spectrum 1 and C—spectrum 3, respectively).
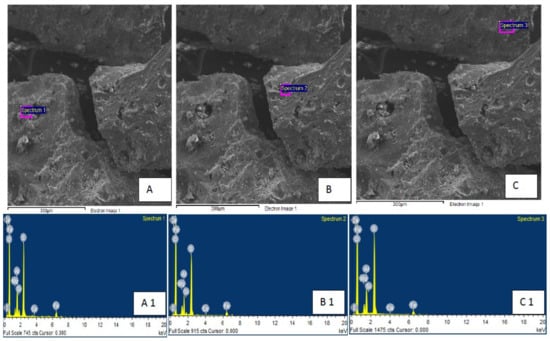
Figure 12.
SEM and XRD of filter body after pilot scale experiments ((A–C) show SEM whereas (A1–C1) show XRD results).
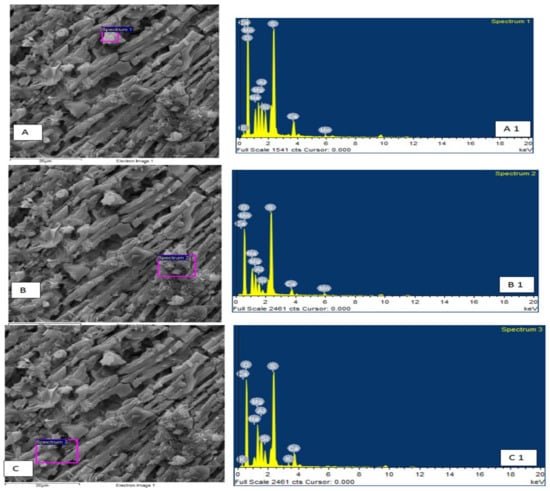
Figure 13.
SEM and XRD of filter body after pilot scale experiments ((A–C) show SEM whereas (A1–C1) show XRD results).
5.3. Metal Removal Mechanisms and Geochemical Stability
As a removal mechanism, Fe and other PTEs are removed by formation of hydroxide, oxyhydroxide, and hydroxy-sulfate precipitates as the pH rises in both systems of treatments [62,63,64,65,66]. Likewise, adsorption mechanisms are responsible for the removal of Mn and Zn [67,68]. During the system autopsies, a yellowish, red colour was seen at the entrance of the influent whereas a light colour was observed further down in the system, reflecting the chemical reactions at the inlets and precipitation of PTEs at the outlet of the designed systems [20].
Moreover, in the anoxic filter type, the prevailing reducing environments led ferric iron to reduce to ferrous iron and sulfate to hydrogen via the production of bicarbonate alkalinity [8,32]. Under reducing conditions, mackinawite (FeS) then leads to reaction with sulfide, then formation of greigite (Fe3S4), and finally to formation of Pyrite [68]. Likewise, Zn removal is facilitated by the formation of Zn sulfide or the similar sphalerite. The light colour of the limestone aggregate in the upper portions suggest that most of the precipitate was held by compost layer; moreover, when the black precipitate from the upper layer was exposed to air, the colour faded over a time span of 8 h, which shows the presence of Fe monosulfide (mackinawite). On the other hand, the light colour in the lower layer shows the presence of an amount of Cu with the increase in pH [20]. Furthermore, reduction may be supported by adsorption on hydroxide surfaces or by precipitation of Fe hydroxide in stable reducing conditions [69].
Chemical stability is determined by examination of the influent and effluent over different stages of time, and even up to months afterwards; here, it was examined during the first 15 and 24 h, then at the 1, 7, 14, and 30 day mark of the experiments. Subsequently, experiments were conducted over a time duration of three months, and during this period, the filters were exposed to varied temperature and climatic conditions. Effluent sampling at different intervals (presented in Table 1 and Table 2 and in Figure 11) showed the stability of the filters over time and under different conditions.
6. Discussion
The relatively small diameter of the selected media prevents passivation, as particles dissolve before a crust of precipitates is able to form. Although this causes fast reactions, it may lead to clogging of the whole system. Similarly, in the case of larger grain sizes, as per our observations, a majority of the fluid starts to follow certain preferential pathways and the purpose of the proposed reactions is not achieved, resulting in failure of the suggested scheme.
Successful acidity neutralization of AMD was carried out based on laboratory column experiments using selected filter media. The pH of the AMD under study was elevated from 2.5 to 7.65 and 7.60 in uncoated and coated limestone media, respectively. Although, neutralization potential shows a decrease over time, as an overall phenomenon the Ca concentration and net alkalinity (280–360 mg/L) were increased, and metals such as Fe, Cu, Pb, and Mn were removed with a cleaning efficiency of 88%, 92%, 88%, and 30% respectively. In continued experiments, a threshold residence time of 5 h was identified as having neutral pH in all columns of our developed filters using selected limestone from the Salt Range area of Pakistan.
The results of the neutralization experiment conducted with different varieties of limestone from the Salt Range reveal the filtration ability of each limestone material, as presented in Figure 9. Figure 9 shows the acid neutralization results and removal of certain elements based on five trials conducted over a time span of 5 h. Wargal limestone, a well-known limestone from the Salt Range, proved an excellent option for treatment of AMD originating from different coal mines. The pH of raw mine water was raised from 3.57 to 7.0, leading to precipitation of dissolved PTEs over the minimum retention time of 5 h. The second-best option was Lockhart Limestone; its neutralization potential was tested by same experimental procedure, and although it improved the pH of raw mine water, it could not remove the PTEs as effectively. The third-best option was Chorgali limestone; in experiments conducted under same protocol, Chorgali limestone did not perform as expected, elevating the pH of raw mine water while not removing the expected amount of PTEs found in the AMD. The fourth selected material was Jutana Dolomite, which is a famous reserve of dolomite in the Salt Range area. Likewise, it could not remove the expected load of PTEs, and only provided a slight elevation in the pH of the mine water. In addition, the important aspect of cost is shown below in Table 3.

Table 3.
Cost of different materials used for production of alkalinity.
6.1. Performance Comparison of Oxic and Anoxic Filters
The goals set for pilot scale testing were to increase pH and to remove dissolved metals from the mine water. An additional goal was to compare the performance efficiencies over the time for the oxic and anoxic systems with selected limestone media from the Salt range. Different problems, such as armouring, plugging, and formation of hydroxide precipitants, can cause reductions in performance efficiency, ultimately leading to un-dissolution of limestone [63,64].
After completion of our pilot scale experiments, system autopsies were carried out to determine the amount of metals retained in the systems, the extent of limestone armouring caused by Fe hydroxide, and colour changes, which were compared using Munsell colour charts. The systems were furnished with flushing tabs in order to remove accumulated precipitates, and each system was flushed once a month during the three month time span of the experiments.
6.1.1. Oxic Limestone Bed
Under a controlled flow rate ranging from 0.0020–0.025 L/s with an average time of residence of 5 to 20 h, pH was increased from 6.6 to 8 and approached neutral at a residence time of more than 10 h (see Figure 14A–C). Fe, Mn, and Zn were depleted over a time frame of 5 h and treatment performance was obvious at end of this experiment. The removal efficiencies were rated as 99% for Fe and Cu (at 20 h), 50% for Mn (at 20 h), and 75% for Zn (at 20 h); see Figure 10A–C.
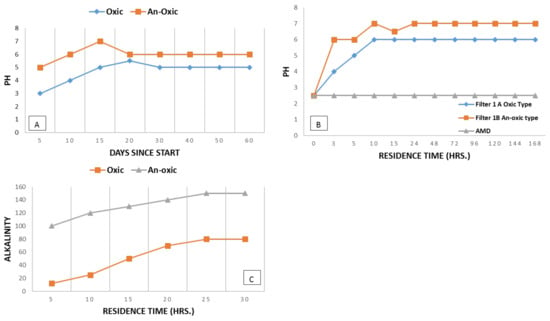
Figure 14.
Graph showing ranges of residence time for AMD during pilot scale experiments and changes in pH (A,B) and alkalinity (C) for oxic and anoxic filter types.
6.1.2. Anoxic Compost Bed
The anoxic vertical flow system with compost at the top was provided with a constant flow rate of 0.00090–0.25 L/s with a residence time of 5 to 15 h. pH was increased from 7.1 to 7.9 over the residence time of 5 to 15 h; see Figure 14A–C. Dissolved Oxygen was kept lower at one mg/L, whereas oxidation and reduction capability decreased to 282 mv with Fe in ferrous state, indicating the development of reducing conditions. The removal efficiencies were rated as 98% for Fe (at 20 h), 99% for Pb (at 20 h), and 60% for Zn (at 20 h). Acid removal of 10 to 55 g/m2 per day was observed with a residence time of 5 h; see Figure 10A–E.
6.2. Failures of AMD Treatment System
Although there are many success stories when using limestone media as a filter, failures are a part of the literature as well. Failure of treatment systems is associated with accumulated precipitates, such as rust-coloured coatings which fill voids and coat limestone. This phenomenon depends on pH, constituent concentrations, redox state, and various dissolved metals such as Fe+2, Fe+3, Ca, and Al. Many coatings have an orange colour, which is related to the presence of Oxyhydroxysulphate and Fe(OH)3. Initially deposited Fe+3 is in a weak bond with the media (limestone) and is easily recognized [55,69]. On the contrary, precipitates of gypsum and Al hydroxysulfate are more adherent, and may inhibit dissolution of limestone [40,70,71,72,73]. However, gypsum and Al hydroxysulfate are favoured for formation in diluted AMD and other leachates with low SO4 concentrations.
Due to this limitation, systems may require periodic flushing and graveling of filter media after several months of operation in order to avoid any sort of clogging and crust formation, which can hamper the void spaces of developed media. Other techniques, such as sweep precipitation, cementation, solvent extraction, and coagulation, can be used for the collection of secondary sludge [74].
As ours was a lab-scale study, further research field-scaled application needs to be understood and readdressed for long operation times, as stability can be governed by dissolution, physical disintegration, and the presence of colloidal matter and microorganisms. Nevertheless, under the studied circumstances, the developed filters can be utilized to deal with anthropogenic loads from coal mines [75,76].
7. Conclusions
Coal mine drainage has often been discharged into the natural hydrographical system (streams and canals) and then not been collected and discharged without any treatment; therefore, it often greatly affects the natural ecological system and makes the whole natural hydrographical system into a mine drainage system. This characteristic has been shown clearly in the rainy season, with its huge drainage volumes. Thus, proper management of mine tailing by concerned organizations should be mandatory, as they are a potential source of poisonous trace elements in the local ecosystem of the Salt Range portion of the Indus Basin. Regular monitoring of heavy metals in water quality is recommended around active and abandoned mining sites for the conservation and protection of water resources. The following conclusions have been drawn from this research:
- Previously, a wide range of techniques have been used for treating Acid and Mine Drainage (AMD). These techniques include pH control, chemical precipitation, chelation/complexation, ion exchange, membrane filtration, floatation, electrochemical treatments, coagulation/flocculation, and adsorption and biosorption techniques. However, controlling the pH is the most common practice for the treatment of AMD.
- Despite a range of newly emergent techniques for the treatment of AMD, pH control using low-cost neutralizing reagents has been the most common and economical technique for the treatment of AMD. Therefore, owing to its widespread availability, ease of use, and cost effectiveness, active treatment technique utilizing calcium-based reagents (particularly limestone) are considered the prime choice for treating AMD.
- All coal mines located in the Salt Range area contain notable loads of PTEs, with an average pH of 3.5 making for a typically acidic nature; thus, as a remedial measure, different low-cost options for passive treatments were cited and two out of many options were finally selected. These two selected treatment types, oxic and anoxic, were constructed and operated on the lab- and pilot-scale levels in order to judge the effectiveness of the designed systems.
- The proposed treatment process is based on chemical reactions between the selected limestone and the acidic mine water for the removal of PTEs in column- and pilot-scale experiments. In both experiments, the removal efficiencies were greater than 90% for treating 150 L of mine water, which is 200 times greater than the media used in the development of the filters. Moreover, XRF and SEM results confirmed the flocs developed by precipitation of heavy metals, which were confirmed by visual autopsies of the developed media as well.
- Both systems performed up to the expected levels, and PTEs were efficiently removed from mine water from the Salt Range area. The anoxic filter supported by compost material performed well compared to the oxic filter, although the efficiency of the oxic filter was satisfactory and it can be used for PTEs with periodic maintenance. Optimal removal in both systems were encouraging, with the following results: Fe 98% (anoxic), 99% (oxic); Cu 99% (anoxic and oxic); Mn 60% (anoxic), 30% (oxic); Zn 75% (anoxic), 30% (oxic).
- The most encouraging discovery is the attainment of a residence time of 5 h, which is low compared to most recommended residence times in the literature, typically around 15 h. This might be due to the small size of the limestone media and their higher reactive surface areas. The anoxic filter autopsies suggest insignificant Fe monosulfides and armouring, making it an attractive option for treatment in the Salt Range area.
- This paper has explained the necessary approach, lab- and pilot-scale experiments, and computational methods for evaluating the presence of heavy metals and their possible removal from the ecosystem of the Salt Range. This approach and results can be applied to other similar coal mining sites and effluents. On the basis of our results, moderately low acidic AMD with low levels of metal concentrations can be treated with selected types of limestone and formulated filters for removal and neutralization of effluent mine water.
Author Contributions
A.J.K. and G.A. designed this research; A.J.K. performed data analysis and wrote the first draft of the manuscript; Y.G. provided important advice on the conceptual methodology and structuring of the manuscript; M.S. and K.U.R. performed extensive editing and revisions to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financially supported by the Chinese Academy of Sciences President’s International Fellowship Initiative (Grant No. 2021VCA0010) and the International Science and Technology Cooperation Program of China (2018YFE0100100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the Chinese Academy of Sciences President’s International Fellowship Initiative and the International Science & Technology Cooperation Program of China financially supported the current research. The authors would like to greatly acknowledge the support extended by PCRWR, the geotechnical department of NUST University & the Department of Chemistry, QAU for testing work. Special thanks of gratitude to the Director and Executive Director of the China-Pakistan Joint Research Centre (CPJRC) on Earth Sciences for their encouragement, and support during the entire period of research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine water hydrology. In Mine Water; Springer: Dordrecht, The Netherlands, 2002; pp. 127–270. [Google Scholar]
- Ziemkiewicz, P.F.; Skousen, J.G.; Simmons, J. Long-term performance of passive acid mine drainage treatment systems. Mine Water Environ. 2003, 22, 118–129. [Google Scholar] [CrossRef]
- Piramid Consortium. Engineering Guidelines for the Passive Remediation of Acidic and/or Metalliferous Mine Drainage and Similar Wastewaters; University of Newcastle: Newcastle Upon Tyne, UK, 2003. [Google Scholar]
- Hedin, R.S.; Watzlaf, G.R. The effects of anoxic limestone drains on mine water chemistry. J. Am. Soc. Min. Reclam. 1994, 6, 185–194. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N.; Ptacek, C.J.; Blowes, D.W. Negative pH and extremely acidic mine waters from Iron Mountain, California. Environ. Sci. Technol. 2000, 34, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Jage, C.R.; Zipper, C.E.; Noble, R. Factors Affecting Alkalinity Generation by Successive Alkalinity-Producing Systems: Regression Analysis. J. Environ. Qual. 2001, 30, 1015–1022. [Google Scholar] [CrossRef]
- Stantec Consulting Ltd. Review of Water Quality Issues in Neutral pH Drainage: Examples and Emerging Priorities for the Mining Industry in Canada; Report prepared for the MEND initiative; MEND Publications: Quebec, QC, Canada, 2004. [Google Scholar]
- Koul, B.; Poonia, A.K.; Singh, R.; Kajla, S. Strategies to cope with the emerging waste water contaminants through adsorption regimes. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–106. [Google Scholar]
- Younger, P.L. Hydrogeochemistry of minewaters flowing from abandoned coal workings in County Durham. Q. J. Eng. Geol. 1995, 28 (Suppl. S2), S101–S113. [Google Scholar] [CrossRef]
- Burrell, R.; Whitworth, K. The influence of minewater recovery on surface gas and water discharges in the Yorkshire Coalfield. In Proceedings of the 7th Congress of International Mine Water Association, Ustron, Poland, 11–15 September 2000; pp. 81–90. [Google Scholar]
- Leavitt, B.R.; Donovan, J.J.; Morris, A.J.; Werner, E. Modeling of mine flooding in the Pittsburgh Coal Basin, USA. In Proceedings of the 6th International Committee on Acid Rock Drainage, Cairns, Australia, 14–17 July 2003; pp. 1065–1071. [Google Scholar]
- Okamoto, N.; Shiokawa, S.; Kawano, S.; Yamaji, N.; Sakurai, H.; Kurihara, M. World’s first lifting test for seafloor massive sulphides in the Okinawa Trough in the EEZ of Japan. In Proceedings of the 29th International Ocean and Polar Engineering Conference, Honolulu, HI, USA, 16–21 June 2019. [Google Scholar]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Walton-Day, K. Geochemistry of Active and Passive Treatment Processes Used to Treat Mine Drainage; Mineralogical Association of Canada: Québec, QC, Canada, 2003. [Google Scholar]
- Lottermoser, B.G. Mining Environments: The Good, the Bad, and the Ugly. 2007. Available online: http://www.sudbury2007.ca/ (accessed on 22 April 2022).
- Skousen, J.; Rose, A.; Geidel, G.; Foreman, J.; Evans, R.; Hellier, W. Handbook of Technologies for Avoidance and Remediation of Acid Mine Drainage; National Mine Land Reclamation Center: Morgantown, WV, USA, 1998; p. 131. [Google Scholar]
- Cravotta, C.A. Size and performance of anoxic limestone drains to neutralize acidic mine drainage. J. Environ. Qual. 2003, 32, 1277–1289. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Sibrell, P.L.; Belkin, H.E. Characterization of limestone reacted with acid-mine drainage in a pulsed limestone bed treatment system at the Friendship Hill National Historical Site, Pennsylvania, USA. Appl. Geochem. 2003, 18, 1705–1721. [Google Scholar] [CrossRef]
- Yeats, R.S.; Khan, S.H.; Akhtar, M. Late quaternary deformation of the Salt Range of Pakistan. Geol. Soc. Am. Bull. 1984, 95, 958–966. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, A.; Jan, M.M. The medicinal plants of Salt Range. J. Biol. Sci. 2002, 2, 175–177. [Google Scholar] [CrossRef] [Green Version]
- Malkani, M.S.; Mahmood, Z. Mineral Resources of Pakistan: Provinces and basins wise. Geol. Surv. Pak. Mem. 2017, 25, 1–179. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Pitfalls of passive mine water treatment. Rev. Environ. Sci. Biotechnol. 2005, 1, 335–343. [Google Scholar] [CrossRef]
- Tripole, S.; Gonzalez, P.; Vallania, A.; Garbagnati, M.; Mallea, M. Evaluation of the impact of acid mine drainage on the chemistry and the macrobenthos in the Carolina stream (San Luis–Argentina). Environ. Monit. Assess. 2006, 114, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Gazder, U. Energy consumption trends in energy scarce and rich countries: Comparative study for Pakistan and Saudi Arabia. EDP Sci. 2017, 23, 07002. [Google Scholar] [CrossRef] [Green Version]
- Jabbar Khan, A.; Akhter, G.; Gabriel, H.F.; Shahid, M. Anthropogenic effects of coal mining on ecological resources of the Central Indus Basin, Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 1255. [Google Scholar] [CrossRef] [Green Version]
- Watzlaf, G.R.; Schroeder, K.T.; Kleinmann, R.L.; Kairies, C.L.; Nairn, R.W. The Passive Treatment of Coal Mine Drainage; United States Department of Energy National Energy Technology Laboratory Internal Publication: Norman, OK, USA, 2004; pp. 1–72.
- Aoyagi, T.; Hamai, T.; Hori, T.; Sato, Y.; Kobayashi, M.; Sato, Y.; Sakata, T. Hydraulic retention time and pH affect the performance and microbial communities of passive bioreactors for treatment of acid mine drainage. Amb. Express 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Biswas, T.K.; Song, Y.; Cheng, X.; Fu, M.; Li, D.; Blackwell, J. Planning, Installation and Operation of the FILTER Trial in Wuqing, China. 2000, CSIRO Land and Water Report 79/07, Report to ACIAR June 2007. Available online: https://publications.csiro.au/rpr/download?pid=procite:db4f5f5e-f637-4985-833d-85a35dd53a90&dsid=DS1 (accessed on 22 April 2022).
- Xianjun, C.; Zhanyi, G.; Jayawardane, N.; Blackwell, J.; Biswas, T. Filter technology: Integrated wastewater irrigation and treatment, a way of water scarcity alleviation, pollution elimination and health risk prevention. Water Resour. J. 2003, 78–86. Available online: https://publications.iwmi.org/pdf/H033504.pdf (accessed on 22 April 2022).
- Hedin, R.S.; Watzlaf, G.R.; Nairn, R.W. Passive Treatment of Acid Mine Drainage with Limestone. Am. Soc. Agron. Crop Sci. Soc. Am. Soil Sci. Soc. Am. 1994, 23, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Prasad, H.; Lohchab, R.K.; Singh, B.; Nain, A.; Kumari, M. Lime treatment of wastewater in a plywood industry to achieve the zero liquid discharge. J. Clean. Prod. 2019, 240, 118176. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Rodríguez-Navarro, C.; González-Muñoz, M.T.; Arias, J.M.; Cultrone, G.; Rodríguez-Gallego, M. Precipitation and growth morphology of calcium carbonate induced by Myxococcus xanthus: Implications for recognition of bacterial carbonates. J. Sediment. Res. 2004, 74, 868–876. [Google Scholar] [CrossRef]
- Pearson, F.H.; McDonnell, A.J. Characterization of coarse porous media. J. Environ. Eng. Div. 1997, 103, 615–624. [Google Scholar] [CrossRef]
- Turner, D.; McCoy, D. Anoxic alkaline drain treatment system, a low cost acid mine drainage treatment alternative. In Proceedings of the 1990 National Symposium on Mining, University of Kentucky, Lexington, KY, USA, 23–26 May 1990. [Google Scholar]
- Faulkner, B.B.; Skousen, J.G. Effects of land reclamation and passive treatment systems on improving water quality. Green Lands 1995, 25, 34–40. [Google Scholar]
- Kepler, D.A.; McCleary, E.C. Successive alkalinity-producing systems (SAPS) for the treatment of acidic mine drainage. In Proceedings of the International Land Reclamation and Mine Drainage Conference and the 3rd International Conference on the Abatement of Acidic Drainage; ASMR: Champaign, IL, USA, 1994; Volume 1, pp. 195–204. [Google Scholar]
- Pulles, W.; Rose, P.; Coetser, L.; Heath, R. Development of Integrated Passive Water Treatment Systems for the Treatment of Mine Waters. 2003. Available online: https://era.library.ualberta.ca/items/0a6e2028-f899-481f-baae-ecf198390de6 (accessed on 22 April 2022).
- Robbins, E.I.; Cravotta III, C.A.; Savela, C.E.; Nord, G.L., Jr. Hydrobiogeochemical interactions inanoxic’limestone drains for neutralization of acidic mine drainage. Fuel 1999, 78, 259–270. [Google Scholar] [CrossRef]
- McIntyre, D.S.; Stirk, G.B. A method for determination of apparent density of soil aggregates. Aust. J. Agric. Res. 1954, 5, 291–296. [Google Scholar] [CrossRef]
- Odong, J. Evaluation of empirical formulae for determination of hydraulic conductivity based on grain-size analysis. J. Am. Sci. 2007, 3, 54–60. [Google Scholar]
- Ahmad, K.; Bhatti, I.A.; Muneer, M.; Iqbal, M.; Iqbal, Z. Removal of heavy metals (Zn, Cr, Pb, Cd, Cu and Fe) in aqueous media by calcium carbonate as an adsorbent. Int. J. Chem. Biochem. Sci. 2012, 2, 48–53. [Google Scholar]
- Munir, M.J.; Kazmi, S.M.S.; Wu, Y.F. Efficiency of waste marble powder in controlling alkali–silica reaction of concrete: A sustainable approach. Constr. Build. Mater. 2017, 154, 590–599. [Google Scholar] [CrossRef]
- Afzal, S. Determination of Selenium Speciation of Saline Lakes from Soan-Sakesar Valley Salt Range Pakistan. Ph.D. Thesis, University of the Punjab, Lahore, Pakistan, 1999. [Google Scholar]
- Boustani, M. Depositional and Diagenetic Environments of the (Eocene) Sakesar Litiestote in the Salt Range Area. Ph.D. Thesis, Quad-e-Azam University, Islamabad, Pakistan, 2000; pp. 204–205. [Google Scholar]
- Khan, S.; Ahmad, S.; Hanif, M.; Jan, I.U.; Swati, M.A.F.; Khan, S.; Saboor, A. Lithofacies, paleoenvironments and sequence stratigraphic modelling of the Wargal Limestone: Implication for reservoir characterization in the Salt Range, northwest, Pakistan. J. Himal. Earth Sci. 2014, 47, 41. [Google Scholar]
- Bilal, A.; Khan, M.S. Petrography of paleogene carbonates in Kalamula and Khursheedabad area, Kahuta, Azad Kashmir. Earth Sci. Malays. 2017, 1, 36–41. [Google Scholar] [CrossRef]
- Naeem, M.; Khalid, P.; Sanaullah, M.; ud Din, Z. Physio-mechanical and aggregate properties of limestones from Pakistan. Acta Geod. Geophys. 2014, 49, 369–380. [Google Scholar] [CrossRef]
- Khan, M.W.; Alam, I.; Shah, M.Z. Microfacies and diagenetic fabric of the Lockhart Limestone, Kotal Pass section, northeast of Kohat, Pakistan. Int. J. Econ. Environ. Geol. 2019, 7, 65–72. [Google Scholar]
- Awais, M.; Hanif, M.; Ishaq, M.; Jan, I.U. An integrated approach to evaluate dolomite in the Eocene Chorgali Formation, Khair-e-Murat Range, Pakistan: Implications for reservoir geology. J. Himal. Earth Sci. 2020, 53, 12. [Google Scholar]
- Means, B.; Rose, A.W. Rate of manganese removal in limestone bed systems. In Proceedings of the National Meeting of the American Society of Mining and Reclamation, Breckenridge, CO, USA, 19–23 June 2005; pp. 702–716. [Google Scholar]
- Rose, A.W. Advances in passive treatment of coal mine drainage 1998–2009. In Proceedings of the 27th ASMR, Pittsburgh, PA, USA, 27 October 2010; pp. 847–887. [Google Scholar]
- Cravotta, C.A., III; Trahan, M.K. Limestone drains to increase pH and remove dissolved metals from acidic mine drainage. Appl. Geochem. 1999, 14, 581–606. [Google Scholar] [CrossRef]
- Cravotta, C.A., III; Watzlaf, G.R. Design and performance of limestone drains to increase pH and remove dissolved metals from acidic mine drainage. In Handbook of Groundwater Remediation Using Permeable Reactive Barriers—Applications to Radionuclides, Trace Metals, and Nutrients; Naftz, D.L., Morrison, S.J., Fuller, C.C., Davis, J.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 19–66. [Google Scholar]
- Lee, M.; Paik, I.S.; Kim, I.; Kang, H.; Lee, S. Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazard. Mater. 2007, 144, 208–214. [Google Scholar] [CrossRef]
- Prochaska, C.A.; Zouboulis, A.I. Removal of phosphates by pilot vertical-flow constructed wetlands using a mixture of sand and dolomite as substrate. Ecol. Eng. 2006, 26, 293–303. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (Version 2): A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Cravotta, C.A. Size and performance of anoxic limestone drains to neutralize acidic mine drainage. J. Environ. Qual. 2004, 33, 1164. [Google Scholar] [CrossRef]
- Langmuir, D. Solutions Manual: Aqueous Environmental Geochemistry; Prentice Hal: Upper Saddle River, NJ, USA, 1997; ISBN 0-02-367412-1. [Google Scholar]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophys. Res. Solid Earth 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126, ISBN 0-471-51184-6. [Google Scholar]
- Ziemkiewicz, P.F.; Skousen, J.G.; Brant, D.L.; Sterner, P.L.; Lovett, R.J. Acid Mine Drainage Treatment with Armored Limestone in Open Limestone Channels. Am. Soc. Agron. Crop Sci. Soc. Am. Soil Sci. Soc. Am. 1997, 26, 1017–1024. [Google Scholar] [CrossRef]
- Watzlaf, G.R.; Schroeder, K.T.; Kairies, C.L. Long-term performance of anoxic limestone drains. Mine Water Environ. 2000, 19, 98–110. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid mine drainage control and treatment. Reclam. Drastically Disturb. Lands 2000, 41, 131–168. [Google Scholar]
- Bostick, B.C.; Fendorf, S. Arsenite sorption on troilite (FeS) and pyrite (FeS2). Geochim. Cosmochim. Acta 2003, 67, 909–921. [Google Scholar] [CrossRef]
- Hunger, S.; Benning, L.G. Greigite: A true intermediate on the polysulfide pathway to pyrite. Geochem. Trans. 2007, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A. Elemental sulfur in drain sediments associated with acid sulfate soils. Appl. Geochem. 2006, 21, 1240–1247. [Google Scholar] [CrossRef]
- Santomartino, S.; Webb, J.A. Estimating the longevity of limestone drains in treating acid mine drainage containing high concentrations of iron. Appl. Geochem. 2007, 22, 2344–2361. [Google Scholar] [CrossRef]
- Huminicki, D.M.; Rimstidt, J.D. Neutralization of sulfuric acid solutions by calcite dissolution and the application to anoxic limestone drain design. Appl. Geochem. 2008, 23, 148–165. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Potvin, R.; Benzaazoua, M.; Zagury, G.J. Dissolution of calcitic marble and dolomitic rock in high iron concentrated acid mine drainage: Application to anoxic limestone drains. Environ. Earth Sci. 2012, 66, 2387–2401. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Equeenuddin, S.M.; Powell, M.A. Current approaches for mitigating acid mine drainage. Rev. Environ. Contam. Toxicol. 2013, 226, 1–32. [Google Scholar] [PubMed]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Shi, T.; Wang, S.; Huang, X.; Zhang, T.; Tang, Y.; Qiu, R. Silane-based coatings on the pyrite for remediation of acid mine drainage. Water Res. 2013, 47, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).