Abstract
In this study, flower-like MoS2 nanomaterials were synthesized by hydrothermal method with excess thiourea. The adsorption performance of MoS2 adsorbent for methylene blue (MB) in azo dye wastewater was studied. The morphology, crystal phase, and microstructure of nano MoS2 samples were characterized by X-ray diffraction, scanning electron microscopy, energy dispersive X-ray spectroscopy, and Fourier transform infrared spectroscopy. The effects of adsorption isotherm, kinetics, different hydrothermal time, and pH on the adsorption experiment were studied. The results showed that the MoS2 adsorbent with a hydrothermal time of 1 h had good adsorption properties for MB. The adsorption data accord with the Langmuir isotherm model, and the maximum adsorption capacity of MoS2 adsorbent is 200 mg/g, and the adsorption kinetics agrees well with the pseudo two-level model. The removal rate of MB is not significantly affected by the pH values. The large pH range can still maintain the removal rate above 93.47%, and the regeneration and recovery properties of MoS2 were also explored. Finally, the adsorption mechanism of MoS2 on MB is discussed.
1. Introduction
The textile industry needs to discharge 80~180 tons of wastewater for each ton of textiles processed. Therefore, the amount of dye wastewater is large, and it has the characteristics of complex composition, deep color, large changes of pH value, and high chemical oxygen demand (COD). It is one of the most difficult types of industrial wastewater to treat. There are carcinogens in untreated dye wastewater, threatening the environment and human health [1,2]. Azo dyes are the most widely used synthetic dyes in the printing and dyeing process. They are characterized by connecting aryl groups at both ends of azo groups, which can decompose more than 20 carcinogenic aromatic amines, cause lesions, and induce cancer by changing the structure of human DNA [3]. Methylene blue (MB) is an azo dye with a heterocyclic aromatic cation structure, which is often used in the textile industry. Because its complex aromatic structure is difficult to be degraded by traditional biological treatment processes [4,5], it is necessary to find an effective treatment method.
At present, the common methods for treating dye wastewater include coagulation [6], ozone oxidation [7], photocatalytic oxidation [8], Fenton oxidation [9], magnetic separation [10], adsorption [11,12], etc. Among them, adsorption method makes the dye molecules in the solution phase transfer to the surface of adsorbent, which is favored because of its high efficiency, low cost, and easy operation. Commonly used adsorbents include activated carbon, lignin, zeolite, metal organic framework, biomass residue, nano materials [13,14,15,16], etc. In recent years, with the rapid development of nanotechnology, the application of nano materials in the field of environment is also common. Similarly, the adsorption of dye wastewater by nano materials has also attracted the attention of researchers. Liu et al. [17] synthesized three-dimensional graphene oxide sponge as an adsorbent to explore the adsorption and removal of methyl violet by three-dimensional graphene oxide sponge. The adsorption efficiency of methyl violet by this process in 2 min is 98.8%. CoFe2O4/Go prepared by Anjali Gupta [18] et al. has a good adsorption effect on methylene blue and methyl violet under the assistance of ultrasound. The adsorption capacity of single molecular layer is 157 mg g−1 and 122 mg g−1. Synthesized by Allen [19] et al., MFe2O4@GO has a maximum adsorption capacity of (M = Cu, Co, or Ni) for methylene blue of 25.81, 50.15, and 76.34 mg g−1.
In this paper, MoS2 nano adsorption materials were synthesized by hydrothermal method. MB widely existing in industrial wastewater was used as the representative of organic azo pollutants. The adsorption isotherm and adsorption kinetics were studied. The materials were characterized by scanning electron microscope (SEM), X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), and Fourier transform infrared spectroscopy (FTIR). In addition, the effect of pH value on the experiment was also studied. According to the experimental results, the adsorption properties of the materials were discussed.
2. Materials and Methods
2.1. Reagents and Instruments
The reagents used in the experiment mainly include ammonium molybdate tetrahydrate ((NH4)6MoO24 4H2O) purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and thiourea (H2NCSNH2) purchased from Tianjin Bodi Chemical Co., Ltd. (Tianjin, China), which was directly used without further purification. Deionized water was prepared by FST-TOP-A24 super-pure-water mechanism, Shanghai FuShiTe Instrument Equipment Co., Ltd. (Shanghai, China) [20].
The instruments required for the experiment are: DHG-9023A air drying box, Shanghai YiHeng Scientific Instrument Co., Ltd. (Shanghai, China); PHB-3 digital pH meter, Shanghai San-Xin Instrument Factory (Shanghai, China); FA2204N electronic balance, Shanghai Jinghai instrument Co., Ltd. (Shanghai, China); TU-1950 dual-beam ultraviolet-visible spectrophotometer, Beijing Puxi General Instrument Co., Ltd. (China). Making use of CuKα(λKα1 = 1.5418 Å) as a radiation source in the range of 10°–90°) in order to obtain an XRD profile of adsorbent in D/MaxIIIAX-ray diffrtometer (Almero, Netherlands), SEM image of the adsorbent was obtained with an FEI Quanta 200 FEG field emission scanning electron microscopy. The EDS spectrum and elemental mapping were performed on a Zeiss Auriga microscope ( Oberkochen, Germany) equipped with an Oxford Inca X-Max 50 detector. The FT-IR spectra of the adsorbents before and after adsorption were recorded with a NEXUS-870 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in the range of 4000–400 cm−1 [20].
2.2. Preparation of Adsorbent
MoS2 nano materials were prepared by one-step hydrothermal method using the hydrothermal process of excess thiourea [21]. We dissolved 0.353 g ammonium molybdate tetrahydrate and 1.830 g thiourea (molar S/Mo = 12.0) in 15 mL distilled water. After stirring for 30 min, we transferred the solution to a stainless steel autoclave lined with polytetrafluoroethylene. Next, we set the heating temperature to 240 °C. In order to consider the influence of different reaction time on the experiment, we set the heating time to 1 h, 3 h, and 6 h, respectively. After natural cooling to room temperature, it was washed by centrifugation for several times. Finally, it was dried in a vacuum drying oven at 50 °C for 12 h, and then, the solid was collected. Solid substances produced with heating times of 1.3 and 6 h are named, respectively, after MoS2-1, MoS2-2, and MoS2-3.
2.3. Batch Adsorption Experiments
In this paper, alkaline azo dye MB, which is difficult to biodegrade, was selected to simulate printing and dyeing wastewater. We set up the adsorption experiments of three groups of different samples, which weighed 20 mg for each of the three adsorbents MoS2-1, MoS2-2, and MoS2-3, respectively. That is, 20 mg adsorbent was added to 20 mg/L and 200 mL MB solution. The whole experimental process was put into a dark shaking box for adsorption treatment. The temperature was 25 °C at room temperature, and the rotating speed was 150 rpm/min. After the adsorption equilibrium, we took a certain amount of supernatant, measured its absorbance at 664 nm with UV–Vis, and analyzed it.
3. Results
3.1. Sample Characterization
SEM contains information about the surface topology and composition of the sample. The image of the synthetic sample shows the particle size and high pore structure. The SEM can obtain the morphology of MoS2 nanoparticles, and shown in Figure 1a–c are the SEM spectra of MoS2-1, MoS2-2, and MoS2-3, respectively. The three flower-like samples of MoS2 have morphologically similar structures (samples are shown as microsphere-like flowers under scanning electron microscopy), indicating that the reaction time has little effect on the morphology of molybdenum disulfide samples. The tiny gaps are closely connected with each other, and the surface of the material is loose and porous, indicating that the material has a large specific surface area, which is conducive to adsorption. Under the same proportion, the diameters of MoS2-1, MoS2-2, and MoS2-3 are also slightly different. As can be seen from Figure 1a–c, the diameter of MoS2-1 is about 0.8 μm. MoS2-2 and MoS2-3 are approximately 2 μm in diameter. Figure 1d shows the electron microscope spectrum of MoS2-1 after adsorbing MB for one hour. From the image, there is no obvious change on the surface of MoS2-1 after adsorbing MB for one hour, which is due to the tight gap, loose and porous nano flower MoS2, and large adsorption capacity.

Figure 1.
(a–c) SEM spectra of MoS2 generated at different preparation times (1 h, 3 h, and 6 h), and (d) SEM spectra of samples adsorbed for 1 h.
Figure 2 shows the XRD patterns of MoS2-1, MoS2-2, and MoS2-3. It can be seen from the figure that the positions of the main XRD diffraction peaks of the three samples obtained at different heating times change little; the main diffraction peak is 2θ = 14.37°, 33.50°, 35.87°, 39.53°, and 58.33°; the main diffraction peaks of the obtained products are consistent with the diffraction peaks of hexagonal phase molybdenum disulfide (MoS2, jcpdsno. 37-1492), corresponding to (002), (100), (102), (103), and (110) crystal planes, respectively. Apparently, the high characteristic peak strength of MoS2 is sufficient to prove that the sample has good crystal crystallinity. The figure shows essentially the same of the positions of the main diffraction peaks in the XRD spectra of the three samples. The results display that the crystallization time had no significant effect on the crystallization process of molybdenum disulfide. Figure 3 shows the EDS spectrum of molybdenum disulfide sample. Table 1 shows the element content of MoS2-1. It can be seen from the table that the sample contains o, s, and Mo, and the atomic percentage between S and Mo is close to 2:1, indicating that the prepared sample is MoS2. the result is consistent with the XRD characterization results.
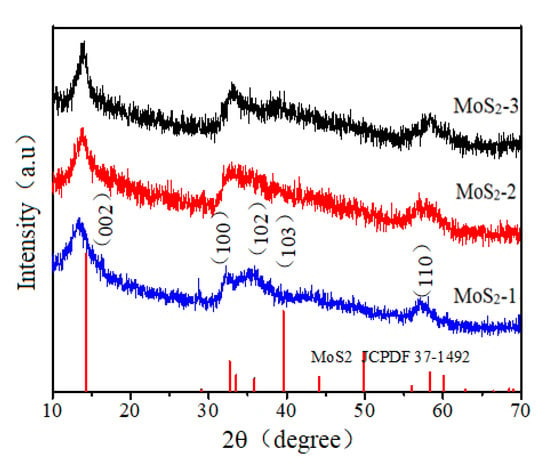
Figure 2.
XRD pattern of MoS2-1, MoS2-2, and MoS2-3.
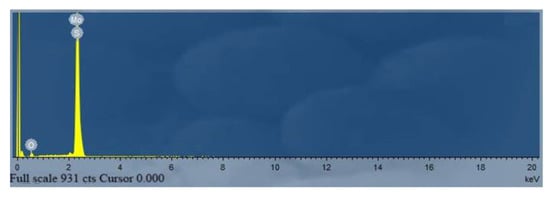
Figure 3.
EDS spectrum of MoS2 adsorbent.

Table 1.
Element content of MoS2-1 sample.
3.2. Adsorptive Property
We added 20 mg of MoS2-1, MoS2-2, and MoS2-3 to 20 mg/L and 200 mL of MB solution. After adsorption equilibrium, we took a certain amount of supernatant, measured its absorbance with UV–Vis, and analyzed it. Figure 4 shows the effect of different heating times on the adsorption performance of MoS2 adsorbent. The experimental results show that MoS2-1 has the maximum adsorption capacity, indicating that MoS2 adsorbent with reaction time of 1 h has high adsorption capacity for MB dye. By comparison of MB before and after MoS2-1 adsorption, shown in Figure 5, it is clear that MoS2 is a good adsorbent for MB.
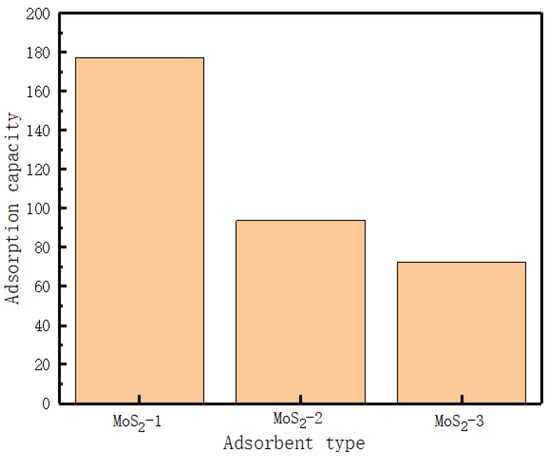
Figure 4.
Adsorption performance test of MoS2 nanoparticle adsorbent for MB.

Figure 5.
Comparison of MB solution before and after adsorption.
3.3. Adsorption Isotherm Models
Adsorption isotherm is used to describe the interaction process between adsorbent and adsorbate and the equilibrium performance of adsorbent [22]. Langmuir and Freundlich adsorption isotherm equations are commonly used. Langmuir isotherm equation assumes that the adsorption layer is single-molecule uniform adsorption, and each molecule has adsorption activation energy and isoenthalpy. Different from Langmuir isotherm equation, Freundlich adsorption isotherm equation can be applied to multilayer adsorption, which is a reversible, non-ideal, and uneven adsorption process [23]. In order to study the adsorption performance of MoS2-1, Langmuir and Freundlich models and linear equations were used. The equations corresponding to Langmuir and Freundlich are as follows (1) and (2):
where Ce (mg/L) is the concentration after equilibrium adsorption; Qe (mg/g) is the adsorption capacity after equilibrium, and Qm (mg/g) is the maximum adsorption capacity calculated according to Langmuir equation; Kl (L/mg) is the adsorption constant of Langmuir equation; KF (mg/g), n is the adsorption constant of Freundlich equation.
Figure 6a shows the fitting of Langmuir and Freundlich models to experimental data. Figure 5b,c are the linear fitting curves of Langmuir model and Freundlich model for MB adsorption by MoS2 adsorbent, respectively. The calculation results of the two models are shown in Table 2. The fitting results show that according to the correlation coefficients fitted by the two models, = 0.994 > = 0.975, the adsorption of MB by MoS2 conforms to the Langmuir isotherm model, the adsorption type is uniform monolayer adsorption, and the maximum adsorption capacity of MoS2 adsorbent is 200 mg/g. When the initial MB concentration was 50 and 5 mg/L, the adsorption capacity of MB solution reached 175.48 and 47.43 mg/g, respectively. It can be seen that the prepared MoS2 adsorbent has good adsorption performance for MB. Table 3 shows the comparison of the adsorption capacity of some adsorbents.
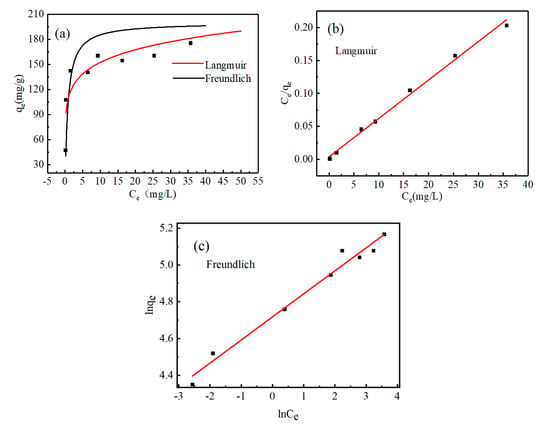
Figure 6.
(a) The isotherm of adsorbed MB; (b) and (c) are the linear fitting curves of Langmuir model and Freundlich model for the adsorption of MB solution by MoS2 adsorbent, respectively.

Table 2.
The isotherm model parameters of MB solution adsorbed by MoS2 adsorbent.

Table 3.
Comparison of adsorption capacity of different adsorbents.
3.4. Kinetic Study
The study of adsorption kinetics can determine the adsorption rate, efficiency, and influencing factors and analyze the adsorption mechanism. The pseudo first-order kinetic equation and pseudo second-order kinetic equation are usually used to fit the adsorption experimental data [20,33]. The pseudo first-order dynamic equations and pseudo second-order dynamic equations are as follows (3) and (4):
where qe and qt are MB adsorption capacity at equilibrium and at any time, respectively (mg/g); T (min) is the adsorption time. k1 (min−1) is the rate constant of pseudo first-order reaction. k2 (g/(mg·min)) is the rate constant of quasi second-order reaction.
The corresponding parameters of pseudo first-order dynamics and pseudo second-order dynamics models are calculated from the curves of ln(qe–qt) and t and (t/qt) and t, respectively. It can be seen from Figure 6a that the adsorption rate of MoS2 adsorbent increases rapidly within 0~3 min because the adsorption site is not occupied; in the stage of 3~25 min, the adsorption rate of MoS2 adsorbent gradually slows down; after 25 min, the MoS2 adsorbent gradually reaches equilibrium. Therefore, when the initial concentration of MB is 5 mg/L, the volume is 200 mL, and MoS2 is 20 mg, the adsorption equilibrium time of MoS2 is 25 min. Figure 7b shows the spectrum of UV–Vis measuring the absorbance of MB adsorbed by MoS2 adsorbent with time. It can be seen from Table 4 that the linear correlation coefficient of pseudo second-order kinetics R2 = 0.995 is greater than that of pseudo first-order kinetics R2 = 0.974, indicating that the adsorption process follows the pseudo second-order kinetic model, and the adsorption process is mainly electrostatic adsorption. The maximum adsorption capacity qe calculated by fitting the pseudo second-order kinetic model is 47.619 mg/g, which is close to the experimental value of qe = 43.385 mg/g measured when the initial MB concentration is 5 mg/L.
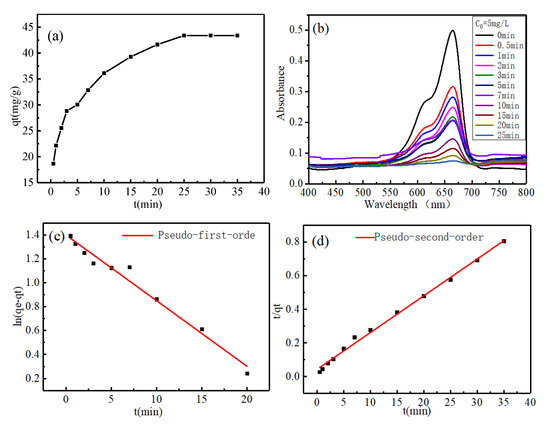
Figure 7.
(a) The adsorption capacity of MoS2 to MB solution in different time periods; (b) UV absorption spectrum of MB solution concentration change; (c) and (d) linear fitting diagrams of pseudo first-order and second-order kinetic equations, respectively.

Table 4.
The pseudo first- and second-order kinetic parameters of MB adsorbed by MoS2.
3.5. Effect of pH on MB Adsorption
The pH value of the solution is an important factor affecting the adsorption effect [34]. In this paper, the pH range of the solution is adjusted to 3–12 with a certain concentration of concentrated sulfuric acid and sodium hydroxide. It can be seen from Figure 8 that different pH values have little effect on the adsorption effect. The adsorption rate of MoS2 on MB can be maintained at more than 93.47%, indicating that the adsorbent has a wide pH adsorption range. In the local pH range (3–5), the removal rate of MB solution by MoS2 adsorbent decreased slightly. This is because the surface of MoS2 adsorbent has anionic charge, and MB belongs to cationic dye. Therefore, in the strong acid environment, the strong acid may change the charge distribution on the sample surface and affect its adsorption effect [35].
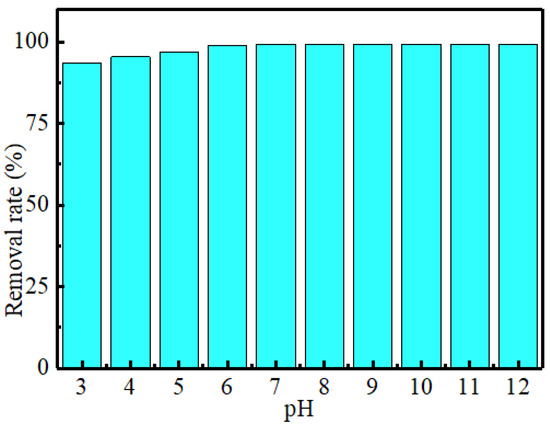
Figure 8.
Effect of pH on adsorption of MB solution by MoS2 adsorbent.
3.6. Effect of Recycling of MoS2-1 on Adsorption
The regeneration and recycling performance of adsorbent is also an important index to evaluate the function of adsorbent [35]. Therefore, it is necessary to study the regeneration and cycle performance of MoS2. In alkaline solution, OH- easily competes with the active adsorption site on the surface of MoS2 to desorb cationic dyes [36]. In this paper, 0.05 mol/L NaOH solution was used as the elution solution to treat the adsorption material [37]. In Figure 9, the removal efficiency of MB by MoS2 for the first time is 99.5%, and the removal efficiency of MB by MoS2 after five cycles is still 89.34%, which indicates that MoS2 adsorbent has good regeneration and recycling performance.
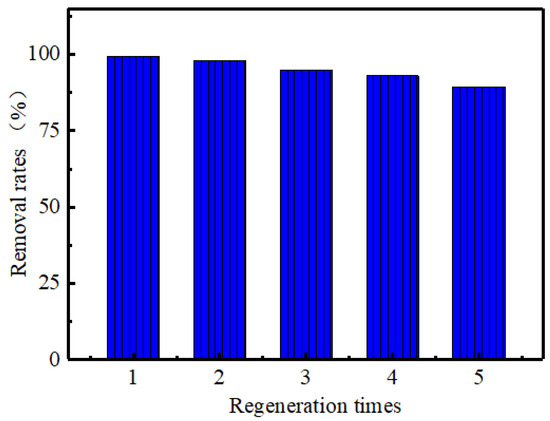
Figure 9.
Effect of recycling on MB adsorption.
3.7. Adsorption Mechanism
The MoS2 adsorbent before and after adsorption was analyzed by FT-IR spectrum. As shown in Figure 10, the strong peak around 590 cm−1 is the characteristic tensile vibration peak of Mo-S [38,39]. The C-H vibration in the plane is the main reason for the occurrence of new characteristic peaks at 792 cm−1. The peak at 901 cm−1 is caused by C-O vibration. Partial oxidation of MoS2 after contact with air causes an absorption peak at 1009 cm−1 [40]. The displacement of the absorption peak at 1125 cm−1 is caused by the vibrational stretching of the C-O. The peak of 1380 cm−1 is S=O peak, which is also the representative peak of MoS2 [41]. The peak at 1600 cm−1 is caused by O-H tensile vibration and bending vibration caused by partial oxidation of MoS2 after contact with adsorbed water [42,43]. In addition, it can be clearly seen that the peak at 3410 cm−1 after adsorption is enhanced, which indicates that more O-H groups appear on the surface of MoS2 [44].
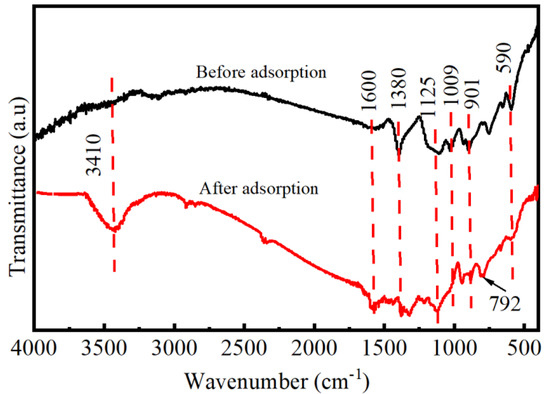
Figure 10.
FTIR spectra of MoS2-1 before and after adsorption.
4. Conclusions
The three-dimensional nano flower-shaped MoS2 adsorbent was synthesized by a simple, one-step hydrothermal method. When the hydrothermal time was 1 h, the prepared MoS2-1 adsorbent had good adsorption for cationic dye MB. According to the analysis of adsorption isotherm model, Langmuir isothermal adsorption model can better fit the adsorption data. The results show that the maximum adsorption capacity of MoS2 nano adsorbent is 200 mg/g, which belongs to uniform monolayer adsorption. The adsorption kinetic process of MoS2 on MB follows the pseudo second-order kinetic model, which shows that the adsorption process is mainly electrostatic adsorption. pH value has little effect on the removal rate of MB and can maintain the removal rate of more than 93.47%, indicating that the adsorbent can maintain high adsorption performance in a wide pH range. In addition, the adsorbent has good recoverability, regeneration, and recycling performance and has application potential. The treatment object of this paper is simulated printing and dyeing wastewater, and the research results are expected to provide a theoretical basis for the practical application of practical printing and dyeing wastewater treatment.
Author Contributions
Data curation, P.Z. and F.X.; Methodology, G.H. and L.B.; Validation, B.S.; Writing—original draft, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of National Key Research and Development Program (2019YFC0408505), the Science and Technology Major projects of Anhui Province (18030801106), the Natural Science Foundation of Anhui Province (No. 1908085QE241), the Natural Science Research Project of the Higher Education Institutions of Anhui Province (KJ2021A0616, KJ2020A0468), the National Natural Science Foundation of China (52103104, 61873003), the Key Research and Development Plan of Anhui Province (201904a07020070), the first batch of natural science projects supported by surplus funds in 2021 of Anhui Jianzhu University (JZ202129, JZ202134), and the Scientific Research Start-up Foundation for Introduction of Talent, Anhui Jianzhu University (2016QD113).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajoriya, S.; Bargole, S.; George, S. Treatment of textile dyeing industry effluent using hydrodynamic cavitation in combination with advanced oxidation reagents. J. Hazard. Mater. 2018, 344, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.M.; Shao, G.Y.; Zhang, W.J.; Chen, J.J.; Qu, Y.X.; Zhang, F.; Tian, S.; Zhou, Z.; Ren, Z. The degradation of printing and dyeing wastewater by manganese-based catalysts. Sci. Total Environ. 2022, 828, 15439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, Y.; Zan, F.; Khanal, S.K.; Hao, T. Biogenic sulfide for azo dye decolorization from textile dyeing wastewater. Chemosphere 2021, 283, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Q.; Liu, S.H.; Pei, Y.; Luo, X.G. Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. Int. J. Biol. Macromol. 2021, 166, 1419–1428. [Google Scholar] [CrossRef]
- Alvarenga, G.; Lima, J.P.; Goszczynski, A.; Rosa, C.H. Methylene blue adsorption by timbaúva (Enterolobium contortisiliquum)-erived materials. Environ. Sci. Pollut. Res. 2020, 27, 27893–27903. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Characterization and optimization of spectrophotometric colour removal from dye containing wastewater by Coagulation-Flocculation. Pol. J. Chem. Technol. 2018, 4, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Dai, Y.; Han, X.Y. Decolorization of azo dye C.I. Reactive Black 5 by ozonation in aqueous solution: Influencing factors, degradation products, reaction pathway and toxicity assessment. Water Sci. Technol. 2016, 73, 1500–1510. [Google Scholar] [CrossRef]
- Chen, P.; Liang, Y.M.; Xu, Y.F.; Zhao, Y.L.; Song, S.X. Synchronous photosensitized degradation of methyl orange and methylene blue in water by visible-light irradiation. J. Mol. Liq. 2021, 334, 116159. [Google Scholar] [CrossRef]
- Ertugay, N.; Acar, F.N. Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: Kinetic study. Arabian J. Chem. 2017, 10, 1158–1163. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.Q.; Xiao, X.; Ye, Z.W.; Guan, Z.J.; Sun, S.Y.; Ren, J.; Yan, P.F. Research on magnetic separation for complex nickel deep removal and magnetic seed recycling. Environ. Sci. Pollut. Res. 2017, 24, 9294–9304. [Google Scholar] [CrossRef] [Green Version]
- Dhananasekaran, S.; Palanivel, R.; Pappu, S. Adsorption of Methylene Blue, Bromophenol Blue, and Coomassie Brilliant Blue by α-chitin nanoparticles. J. Adv. Res. 2016, 7, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia, D.D.R.A.; Carissimi, E.; Dotto, G.L.; Sander, H.; Feris, L.A. Biosorption of rhodamine B dye from dyeing stones effluents using the green microalgae Chlorella pyrenoidosa. J. Clean. Prod. 2018, 198, 1302–1310. [Google Scholar]
- Kheddo, A.; Rhyman, L.; Elzagheid, M.I. Adsorption of synthetic dyed wastewater using activated carbon from rice husk. SN Appl. Sci. 2020, 2, 2170. [Google Scholar] [CrossRef]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpaa, M. Emerging adsorptive removal of azo dye by metal-organic frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Khan, T.A.; Islam, A.; Tabrez, U. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour. Technol. 2022, 354, 127168. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, Z.M.; Qamar, D.; Faisal, N.; Naveed, Z.M.; Munawar, I.; Faizan, N.M. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Water-Soluble Dye Removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- Gupta, A.; Cobas, H.V.; Gupta, N.K. Sono-adsorption of organic dyes onto CoFe2O4/Graphene oxide nanocomposite. Surf. Interfaces 2020, 20, 100563. [Google Scholar] [CrossRef]
- Shu, Y.J.; Zhang, W.B.; Cai, H.H.; Yang, Y.; Yu, X.; Gao, Q. Expanding the interlayers of molybdenum disulfide toward the highly sensitive sensing of hydrogen peroxide. Nanoscale 2019, 11, 6644–6653. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, J.; Ding, C.F.; Xu, C.F.; Cheng, Y.M.; Tang, Z.; Pan, F.K.; Liu, J.Y.; Zhu, S.G. Synthesis and characterization of Fe-Al-Ni ternary composite metal oxides as highly efficient adsorbent for fluoride removal from water. Desalination Water Treat. 2021, 229, 243–251. [Google Scholar] [CrossRef]
- Yuan, W.; Kuang, J.; Yu, M.; Huang, Z.; Zhu, L. Facile preparation of mos2@kaolin composite by one-step hydrothermal method for efficient removal of pb(ii). J. Hazard. Mater. 2020, 405, 124261. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.F.; Fan, X.R.; Qiang, W.; Yue, S. An adsorption isotherm model for adsorption performance of silver-loaded activated carbon. Therm. Sci. 2017, 21, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghouti, M.A.; Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Liu, H. A novel carbon aerogel prepared for adsorption of copper(II) ion in water. J. Porous Mater 2017, 24, 1575–1580. [Google Scholar] [CrossRef]
- Yu, W.B.; Xu, H.F.; Roden, E.E.; Wan, Q. Efficient adsorption of iodide from water by chrysotile bundles with wedge-shaped nanopores. Appl. Clay Sci. 2019, 183, 105331. [Google Scholar] [CrossRef]
- Dong, W.; Liang, K.; Qin, Y.; Ma, H.; Zhao, X.; Zhang, L.; Zhu, S.; Yu, Y.; Bian, D.; Yang, J. Hydrothermal Conversion of Red Mud into Magnetic Adsorbent for Effective Adsorption of Zn(II) in Water. Appl. Sci. 2019, 9, 1519. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.F.; Zhu, T.Y.; Liu, X.; Zhang, W.Q. Simultaneous oxidation and adsorption of As(III) from water by cerium modified chitosan ultrafine nanobiosorbent. J. Hazard. Mater. 2016, 308, 1–10. [Google Scholar] [CrossRef]
- Su, M.H.; Li, H.; Liu, Z.Q.; Peng, H.R.; Huang, S.; Zhou, Y.; Liao, C.Z.; Song, G.; Chen, D.Y. Highly-efficient and easy separation of γ-Fe2O3 selectively adsorbs U(VI) in waters. Environ. Res. 2022, 210, 112917. [Google Scholar] [CrossRef]
- Hossini, H.; Soltani, R.D.C.; Safari, M.; Maleki, A.; Rezaee, R.; Ghanbari, R. The application of a natural chitosan/bone char composite in adsorbing textile dyes from water. Chem. Eng. Commun. 2017, 204, 1082–1093. [Google Scholar] [CrossRef]
- Nnaji, C.C.; Agim, A.E.; Mama, C.N.; Emenike, P.C.; Ogarekpe, N.M. Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernelchaff. Sci. Rep. 2021, 11, 7808. [Google Scholar] [CrossRef]
- Jian, N.G.; Dai, Y.Y.; Wang, Y.L.; Qi, F.F.; Li, S.J.; Wu, Y.J. Preparation of polydopamine nanofibers mat as a recyclable and efficient adsorbent for simultaneous adsorption of multiple tetracyclines in water. J. Clean. Prod. 2021, 320, 128875. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Liu, W.; Li, Z.Y.; He, K. Efficient removal of dyes from dyeing wastewater by powder activated charcoal/titanate nanotube nanocomposites: Adsorption and photoregeneration. Environ. Sci. Pollut. Res. 2019, 26, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Song, F.M.; Ge, H.G.; Shi, J.; Liu, Z.F.; Li, C.; Tang, B. RETRACTED ARTICLE: Adsorption kinetics and thermodynamics of Ni (II) by Pisha sandstone. J. Nanoparticle Res. 2020, 22, 179. [Google Scholar] [CrossRef]
- Sun, B.; Cheng, Y.M.; Xu, F.W.; Liu, F.; Zhang, J.; Tang, Z.; Wang, Y.; Liu, J.Y.; Zhu, S.G.; Cai, X.L. Study on the adsorption performance of Ni-Mo-S nanomaterials for Congo Red in azo wastewater. Desalination Water Treat. 2021, 234, 267–276. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.M.; Jia, F.F.; Song, S.X. Adsorption of heavy metals on molybdenum disulfide in water: A critical review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Han, S.C.; Liu, K.R.; Hu, L.F.; Teng, F.; Yu, P.P.; Zhu, Y.F. Superior Adsorption and Regenerable Dye Adsorbent Based on Flower-Like Molybdenum Disulfide Nanostructure. Sci. Rep. 2017, 7, 43599. [Google Scholar] [CrossRef]
- Lin, S.N.; Zhang, T.; Fu, D.X.; Zhou, X.Y. Utilization of magnesium resources in salt lake brine and catalytic degradation of dye wastewater by doping cobalt and nickel. Sep. Purif. Technol. 2021, 270, 118808. [Google Scholar] [CrossRef]
- Ma, W.; Row, K.H. Solid-Phase Extraction of Catechins from Green Tea with Deep Eutectic Solvent Immobilized Magnetic Molybdenum Disulfide Molecularly Imprinted Polymer. Molecules 2020, 25, 280. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, R.H.; Huo, Y.Z.; Ai, Y.J.; Gu, P.C.; Wang, X.X.; Li, Q.; Yu, S.J.; Chen, Y.T.; Yu, Z.M.; et al. Efficient elimination of Cr(VI) from aqueous solutions using sodium dodecyl sulfate intercalated molybdenum disulfide. Ecotoxicol. Environ. Saf. 2019, 175, 251–262. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.X.; Zhao, G.X.; Song, G.; Chen, D.Y.; Chen, H.X.; Xie, J.; Hayat, T.; Alsaedi, A.; Wang, X.K. Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chem. Eng. J. 2018, 334, 569–578. [Google Scholar] [CrossRef]
- Shahzad, A.; Jang, J.; Lim, S.R.; Lee, D.S. Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized mxene nanocomposite for mercury adsorption. Environ. Res. 2020, 182, 109005. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A 2013, 1, 959–965. [Google Scholar] [CrossRef]
- Massey, A.T.; Gusain, R.; Kumari, S.; Khatri, O.P. Hierarchical microspheres of MoS2 Nanosheets: Efficient and Regenerative Adsorbent for Removal of Water-Soluble Dyes. Ind. Eng. Chem. Res. 2016, 55, 7124–7131. [Google Scholar] [CrossRef]
- Tong, S.; Deng, H.; Wang, L.; Huang, T.; Liu, S.; Wang, J. Multi-functional nanohybrid of ultrathin molybdenum disulfide nanosheets decorated with cerium oxide nanoparticles for preferential uptake of lead (II) ions. Chem. Eng. J. 2018, 335, 22–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).