The Possible Impact of COVID-19 on Respiratory Muscles Structure and Functions: A Literature Review
Abstract
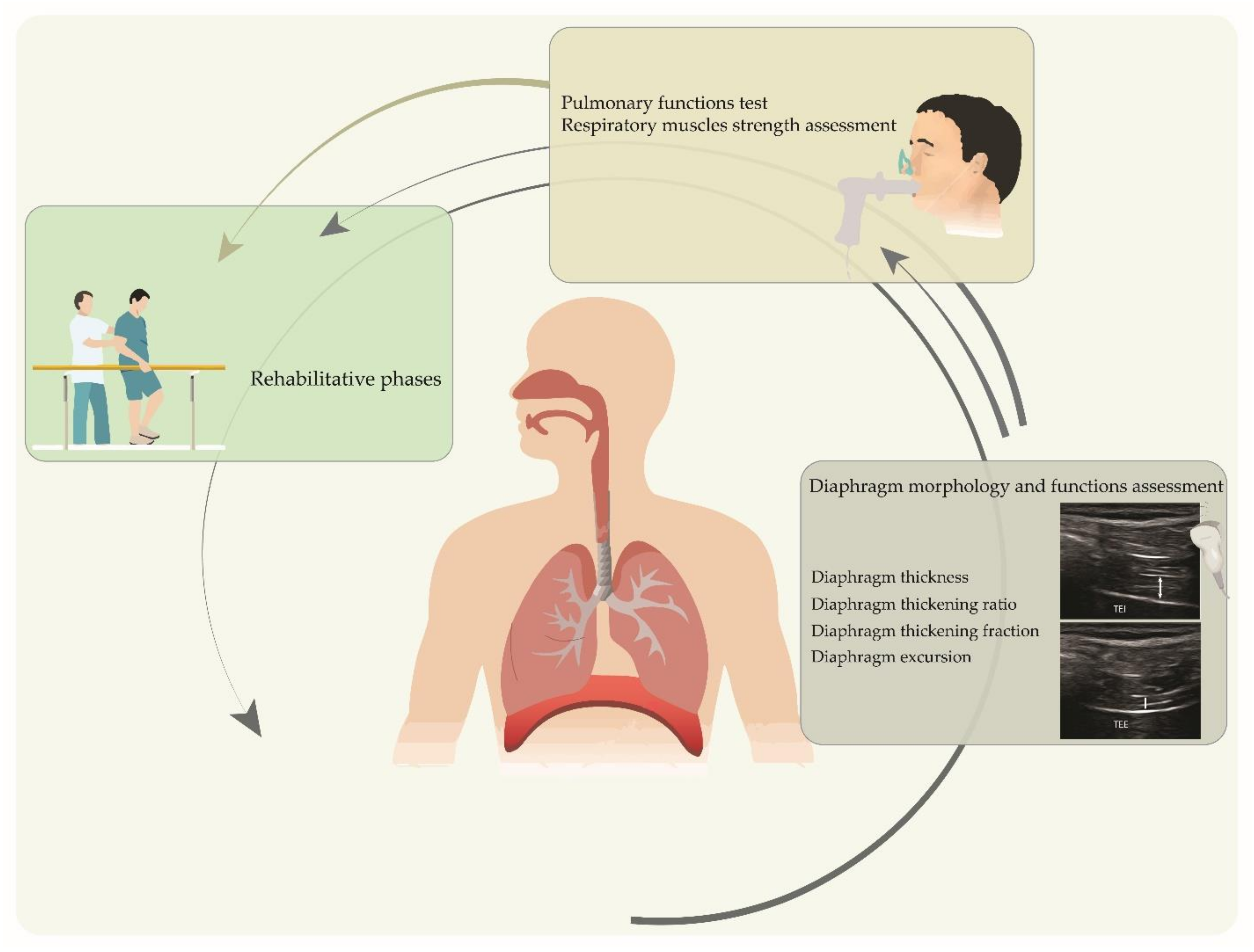
1. Introduction
2. Ultrasound Imaging of the Respiratory Muscles in COVID-19 Patients
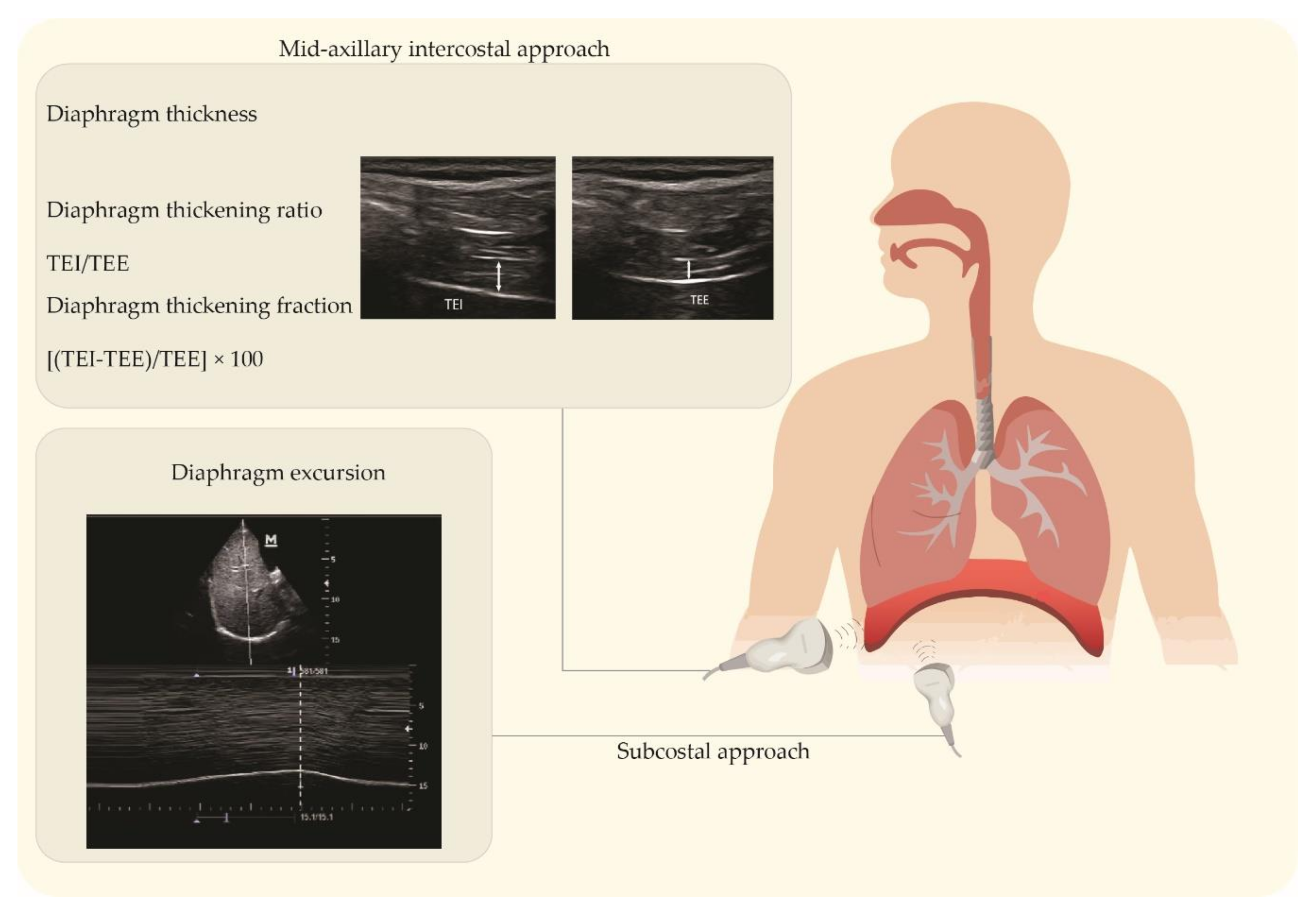
2.1. Ultrasound Imaging Techniques
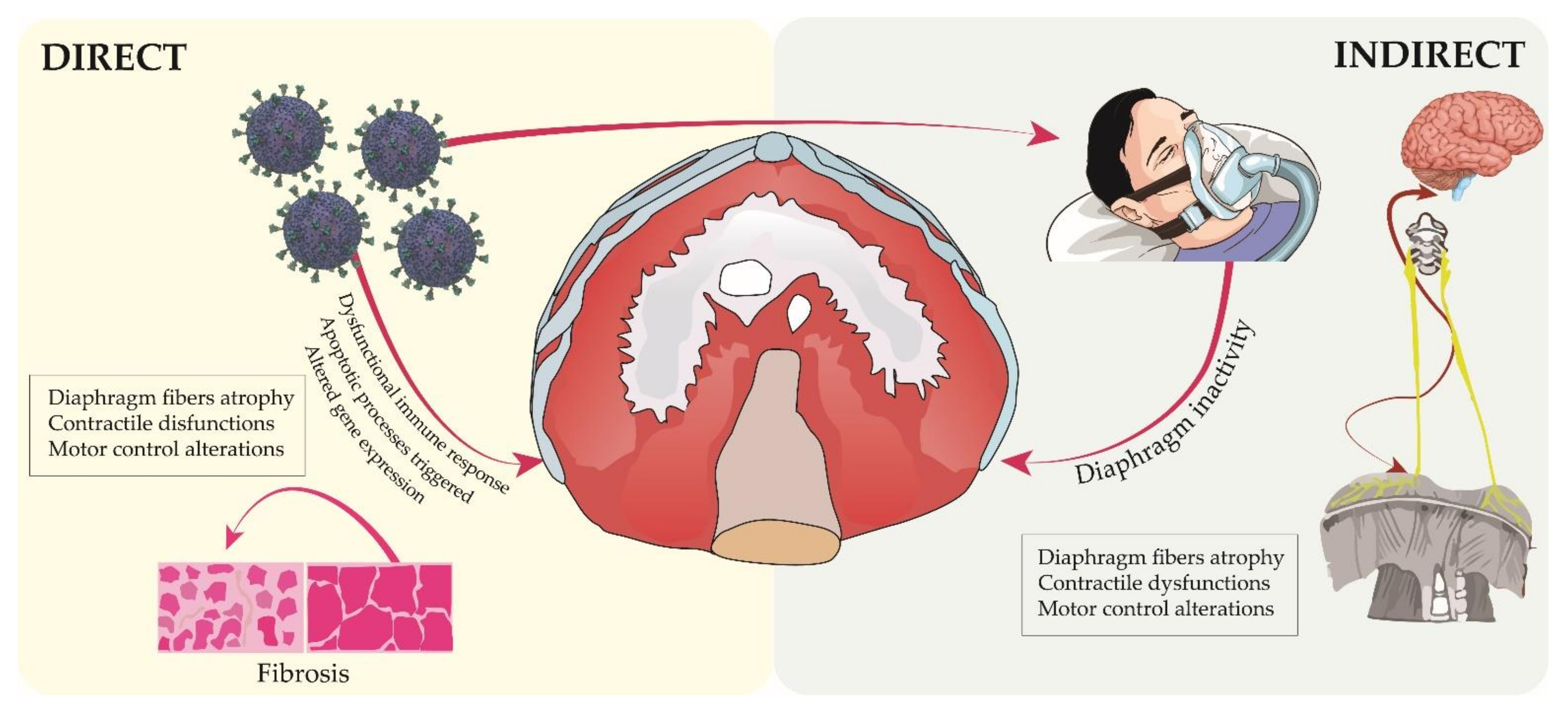
2.2. COVID-19 Context
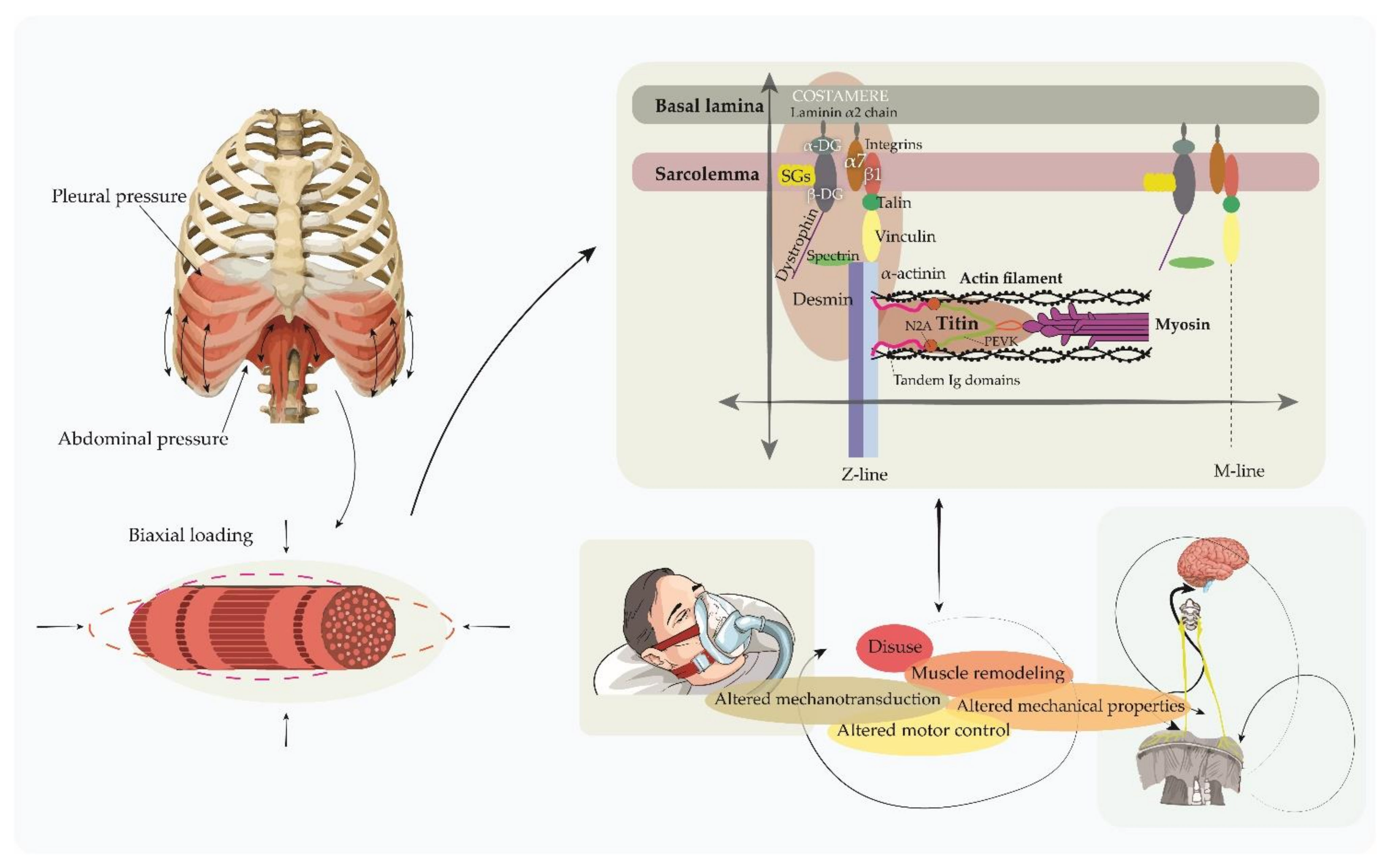
3. Mechanical Properties of the Respiratory Muscles and Its Potential Significance in COVID-19 Patients
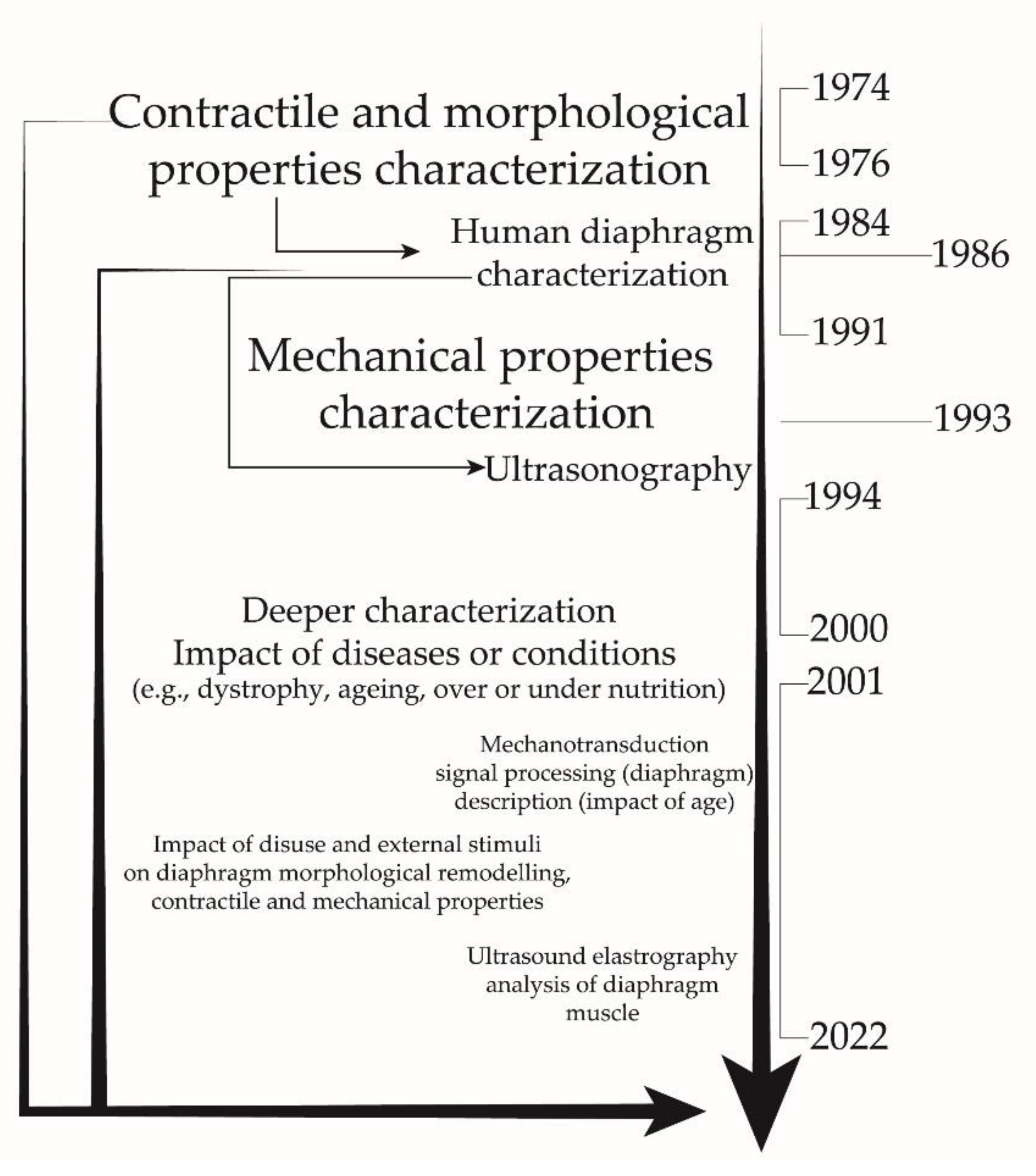
3.1. Historical Studies
3.2. Recent Research
4. Pulmonary Functions and Respiratory Muscles Strength Assessment in COVID-19 Patients
5. Rehabilitation Strategies Targeting Respiratory Muscles in COVID-19 Patients
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The First Documented Coronavirus Pandemic in History. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.; Rajakumar, T.; Malathi, M.; Manikandan, N.; Nagaraj, J.; Santhakumar, A.; Elangovan, A.; Malik, Y.S. Epidemiology and Pathobiology of SARS-CoV-2 (COVID-19) in Comparison with SARS, MERS: An Updated Overview of Current Knowledge and Future Perspectives. Clin. Epidemiol. Glob. Health 2021, 10, 100694. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, S.B.; Jonkman, A.H.; Kugler, M.C.; Munger, J.S.; Kaufman, D.A. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arter. Thromb. Vasc. Biol. 2020, 40, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Dua, A.; Gupta, S.; Injeti, E. A Research Update: Significance of Cytokine Storm and Diaphragm in COVID-19. Curr. Res. Pharm. Drug Discov. 2021, 2, 100031. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory Function in Patients Post-Infection by COVID-19: A Systematic Review and Meta-Analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Wen, Y.; Zhang, X.; Addinsall, A.B.; Cacciani, N.; Larsson, L. Multi-Omics Reveals Age-Related Differences in the Diaphragm Response to Mechanical Ventilation: A Pilot Study. Skelet. Muscle 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Bogaards, S.J.P.; Conijn, S.; Onderwater, Y.; Espinosa, P.; Bink, D.I.; van den Berg, M.; van de Locht, M.; Bugiani, M.; van der Hoeven, H.; et al. COVID-19 Is Associated with Distinct Myopathic Features in the Diaphragm of Critically Ill Patients. BMJ Open Respir. Res. 2021, 8, e001052. [Google Scholar] [CrossRef]
- Shi, Z.; de Vries, H.J.; Vlaar, A.P.J.; van der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C. Diaphragm Pathology in Critically Ill Patients With COVID-19 and Postmortem Findings From 3 Medical Centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621.e6. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Drake, D.H.; De Bonis, M.; Covella, M.; Agricola, E.; Zangrillo, A.; Zimmerman, K.G.; Cobey, F.C. Echocardiography in Pandemic: Front-Line Perspective, Expanding Role of Ultrasound, and Ethics of Resource Allocation. J. Am. Soc. Echocardiogr. 2020, 33, 683–689. [Google Scholar] [CrossRef]
- Van Steveninck, A.L.; Imming, L.M. Diaphragm Dysfunction Prior to Intubation in a Patient with Covid-19 Pneumonia; Assessment by Point of Care Ultrasound and Potential Implications for Patient Monitoring. Respir. Med. Case Rep. 2020, 31, 101284. [Google Scholar] [CrossRef]
- Adolf Helmy, M.; Magdy Milad, L.; Hasanin, A.; Mostafa, M. The Novel Use of Diaphragmatic Excursion on Hospital Admission to Predict the Need for Ventilatory Support in Patients with Coronavirus Disease 2019. Anaesth. Crit. Care Pain Med. 2021, 40, 100976. [Google Scholar] [CrossRef]
- Cammarota, G.; Rossi, E.; Vitali, L.; Simonte, R.; Sannipoli, T.; Anniciello, F.; Vetrugno, L.; Bignami, E.; Becattini, C.; Tesoro, S.; et al. Effect of Awake Prone Position on Diaphragmatic Thickening Fraction in Patients Assisted by Noninvasive Ventilation for Hypoxemic Acute Respiratory Failure Related to Novel Coronavirus Disease. Crit. Care 2021, 25, 305. [Google Scholar] [CrossRef]
- Corradi, F.; Isirdi, A.; Malacarne, P.; Santori, G.; Barbieri, G.; Romei, C.; Bove, T.; Vetrugno, L.; Falcone, M.; Bertini, P.; et al. Low Diaphragm Muscle Mass Predicts Adverse Outcome in Patients Hospitalized for COVID-19 Pneumonia: An Exploratory Pilot Study. Minerva Anestesiol. 2021, 87, 432–438. [Google Scholar] [CrossRef]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Boon, A.J.; Wolfe, L.F.; Franz, C.K. Diaphragm Dysfunction in Severe COVID-19 as Determined by Neuromuscular Ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef]
- Fayssoil, A.; Beaune, S.; Davido, B.; Mansencal, N. Diaphragm Ultrasound to Stratify COVID-19 Patients in the Emergency Department? J. Clin. Ultrasound 2021, 50, 106–107. [Google Scholar] [CrossRef]
- Guarracino, F.; Vetrugno, L.; Forfori, F.; Corradi, F.; Orso, D.; Bertini, P.; Ortalda, A.; Federici, N.; Copetti, R.; Bove, T. Lung, Heart, Vascular, and Diaphragm Ultrasound Examination of COVID-19 Patients: A Comprehensive Approach. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1866–1874. [Google Scholar] [CrossRef]
- Lassola, S.; Miori, S.; Sanna, A.; Cucino, A.; Magnoni, S.; Umbrello, M. Central Venous Pressure Swing Outperforms Diaphragm Ultrasound as a Measure of Inspiratory Effort during Pressure Support Ventilation in COVID-19 Patients. J. Clin. Monit. Comput. 2021, 36, 461–471. [Google Scholar] [CrossRef]
- Maheshwarappa, H.M.; Mishra, S.; Kulkarni, A.V.; Gunaseelan, V.; Kanchi, M. Use of Handheld Ultrasound Device with Artificial Intelligence for Evaluation of Cardiorespiratory System in COVID-19. Indian J. Crit. Care Med. 2021, 25, 524–527. [Google Scholar] [CrossRef]
- Nucci, R.A.B.; Dolhnikoff, M.; do Nascimento Saldiva, P.H.; Jacob-Filho, W. Ultrasound-Guided Minimally Invasive Autopsy of Respiratory Muscles as a Safe and Cost-Effective Technique in COVID-19 Pandemic Era. Acta Cytol. 2021, 65, 276–278. [Google Scholar] [CrossRef]
- Pivetta, E.; Cara, I.; Paglietta, G.; Scategni, V.; Labarile, G.; Tizzani, M.; Porrino, G.; Locatelli, S.; Calzolari, G.; Morello, F.; et al. Diaphragmatic Point-of-Care Ultrasound in COVID-19 Patients in the Emergency Department-A Proof-of-Concept Study. J. Clin. Med. 2021, 10, 5291. [Google Scholar] [CrossRef]
- Sánchez Sánchez, C.D.M.; Molina-Peña, M.E.; Rodriguez-Triviño, C.Y. Comprehensive Assessment of Respiratory Function, a Step towards Early Weaning from the Ventilator. Adv. Respir. Med. 2021, 89, 299–310. [Google Scholar] [CrossRef]
- Satıcı, C.; Aydın, Ş.; Tuna, L.; Köybaşı, G.; Koşar, F. Electromyographic and Sonographic Assessment of Diaphragm Dysfunction in Patients Who Recovered from the COVID-19 Pneumonia. Tuberk. Toraks 2021, 69, 425–428. [Google Scholar] [CrossRef]
- Umbrello, M.; Guglielmetti, L.; Formenti, P.; Antonucci, E.; Cereghini, S.; Filardo, C.; Montanari, G.; Muttini, S. Qualitative and Quantitative Muscle Ultrasound Changes in Patients with COVID-19-Related ARDS. Nutrition 2021, 91–92, 111449. [Google Scholar] [CrossRef] [PubMed]
- Formenti, P.; Umbrello, M.; Castagna, V.; Cenci, S.; Bichi, F.; Pozzi, T.; Bonifazi, M.; Coppola, S.; Chiumello, D. Respiratory and Peripheral Muscular Ultrasound Characteristics in ICU COVID 19 ARDS Patients. J. Crit Care 2022, 67, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.; Franz, C.K.; Bharat, A.; Walter, J.M.; Wolfe, L.F.; Koralnik, I.J.; Deshmukh, S. Diaphragm and Phrenic Nerve Ultrasound in COVID-19 Patients and Beyond: Imaging Technique, Findings, and Clinical Applications. J. Ultrasound Med. 2022, 41, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Somhorst, P.; Gommers, D.; Endeman, H. Advanced Respiratory Monitoring in Mechanically Ventilated Patients with Coronavirus Disease 2019-Associated Acute Respiratory Distress Syndrome. Curr. Opin. Crit. Care 2022, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tuinman, P.R.; Jonkman, A.H.; Dres, M.; Shi, Z.-H.; Goligher, E.C.; Goffi, A.; de Korte, C.; Demoule, A.; Heunks, L. Respiratory Muscle Ultrasonography: Methodology, Basic and Advanced Principles and Clinical Applications in ICU and ED Patients-a Narrative Review. Intensive Care Med. 2020, 46, 594–605. [Google Scholar] [CrossRef]
- Stock, M.S.; Thompson, B.J. Echo Intensity as an Indicator of Skeletal Muscle Quality: Applications, Methodology, and Future Directions. Eur. J. Appl. Physiol. 2021, 121, 369–380. [Google Scholar] [CrossRef]
- Jonkman, A.H.; de Korte, C.L. Shear Wave Elastography of the Diaphragm: Good Vibrations? Am. J. Respir. Crit Care Med. 2021, 204, 748–750. [Google Scholar] [CrossRef]
- Buras, E.D.; Converso-Baran, K.; Davis, C.S.; Akama, T.; Hikage, F.; Michele, D.E.; Brooks, S.V.; Chun, T.-H. Fibro-Adipogenic Remodeling of the Diaphragm in Obesity-Associated Respiratory Dysfunction. Diabetes 2019, 68, 45–56. [Google Scholar] [CrossRef]
- Blazevich, A.J. Adaptations in the Passive Mechanical Properties of Skeletal Muscle to Altered Patterns of Use. J. Appl. Physiol. 2019, 126, 1483–1491. [Google Scholar] [CrossRef]
- Sood, A. Altered Resting and Exercise Respiratory Physiology in Obesity. Clin. Chest Med. 2009, 30, 445–454. [Google Scholar] [CrossRef]
- Sieck, G.C.; Ferreira, L.F.; Reid, M.B.; Mantilla, C.B. Mechanical Properties of Respiratory Muscles. Compr. Physiol. 2013, 3, 1553–1567. [Google Scholar] [CrossRef]
- Herbert, R. The Passive Mechanical Properties of Muscle and Their Adaptations to Altered Patterns of Use. Aust. J. Physiother. 1988, 34, 141–149. [Google Scholar] [CrossRef]
- Herbert, R.D.; Gandevia, S.C. The Passive Mechanical Properties of Muscle. J. Appl. Physiol. 2019, 126, 1442–1444. [Google Scholar] [CrossRef]
- Lieber, R.L.; Fridén, J. Muscle Contracture and Passive Mechanics in Cerebral Palsy. J. Appl. Physiol. 2019, 126, 1492–1501. [Google Scholar] [CrossRef]
- Purslow, P.P. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The Effect of Intramuscular Fat on Skeletal Muscle Mechanics: Implications for the Elderly and Obese. J. R Soc. Interface 2015, 12, 20150365. [Google Scholar] [CrossRef]
- Proske, U.; Rack, P.M. Short-Range Stiffness of Slow Fibers and Twitch Fibers in Reptilian Muscle. Am. J. Physiol. 1976, 231, 449–453. [Google Scholar] [CrossRef]
- Walmsley, B.; Proske, U. Comparison of Stiffness of Soleus and Medial Gastrocnemius Muscles in Cats. J. Neurophysiol. 1981, 46, 250–259. [Google Scholar] [CrossRef]
- Torrejais, M.M.; Soares, J.C.; Matheus, S.M.M.; Francia-Farje, L.A.D.; Mello, J.M.; Vicente, E.J.D. Morphologic Alterations Resulting from Denervation of the Diaphragm in Rats. Int. J. Morphol. 2012, 30, 1150–1157. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, G.C.; Rocha, N.N.; de A Maia, L.; Melo, I.; Simões, A.C.; Antunes, M.A.; Bloise, F.F.; Woyames, J.; da Silva, W.S.; Capelozzi, V.L.; et al. Impact of Experimental Obesity on Diaphragm Structure, Function, and Bioenergetics. J. Appl. Physiol. 2020, 129, 1062–1074. [Google Scholar] [CrossRef]
- Freundt, J.K.; Linke, W.A. Titin as a Force-Generating Muscle Protein under Regulatory Control. J. Appl. Physiol. 2019, 126, 1474–1482. [Google Scholar] [CrossRef]
- Hidalgo, C.; Saripalli, C.; Granzier, H.L. Effect of Exercise Training on Post-Translational and Post-Transcriptional Regulation of Titin Stiffness in Striated Muscle of Wild Type and IG KO Mice. Arch. Biochem. Biophys. 2014, 552–553, 100–107. [Google Scholar] [CrossRef]
- Crul-Sluijter, E.J.; Crul, J.F.; Lukassen, A.M.; Wijdeveld, A. In Vivo Phrenic Nerve Diaphragm Preparation in the Cat Its Possibilities for Studying Mechanical Properties and Sensitivity to Curariform Drugs. Arch. Int. Pharm. 1974, 207, 69–76. [Google Scholar]
- Vachon, B.R.; Kunov, H.; Zingg, W. Mechanical Properties of Diaphragm Muscle in Dogs. Med. Biol. Eng. 1975, 13, 252–260. [Google Scholar] [CrossRef]
- Silla, L.M.; Stephens, N.L. Mechanical Properties of Diaphragm of Three-Toed Sloth. Comp. Biochem. Physiol. A Comp. Physiol. 1976, 55, 393–397. [Google Scholar] [CrossRef]
- Pengelly, L.D. Mechanical Properties of the Diaphragm and Their Application to a Mathematical Model. Am. Rev. Respir. Dis. 1979, 119, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.A.; Gosselin, L.E.; Zhan, W.Z.; Schlenker, E.H.; Sieck, G.C. Histochemical and Mechanical Properties of Diaphragm Muscle in Morbidly Obese Zucker Rats. J. Appl. Physiol. 1994, 77, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Ameredes, B.T.; Rogers, R.M.; Donahoe, M.P.; Rosas, J.F.; Daood, M.J.; Watchko, J.F. Diaphragm Myosin Heavy Chain Composition Shifts in Aging Chronically-Undernourished Fischer 344 Male Rats. Aging 1998, 10, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, N.; Rafael, J.A.; Beckers-Bleukx, G.; Kahn, D.; Deconinck, A.E.; Davies, K.E.; Gillis, J.M. Consequences of the Combined Deficiency in Dystrophin and Utrophin on the Mechanical Properties and Myosin Composition of Some Limb and Respiratory Muscles of the Mouse. Neuromuscul. Disord. 1998, 8, 362–370. [Google Scholar] [CrossRef]
- Tinsley, J.; Deconinck, N.; Fisher, R.; Kahn, D.; Phelps, S.; Gillis, J.M.; Davies, K. Expression of Full-Length Utrophin Prevents Muscular Dystrophy in Mdx Mice. Nat. Med. 1998, 4, 1441–1444. [Google Scholar] [CrossRef]
- Boriek, A.M.; Capetanaki, Y.; Hwang, W.; Officer, T.; Badshah, M.; Rodarte, J.; Tidball, J.G. Desmin Integrates the Three-Dimensional Mechanical Properties of Muscles. Am. J. Physiol. Cell. Physiol. 2001, 280, C46–C52. [Google Scholar] [CrossRef]
- Sahani, A.K.; Joseph, J.; Sivaprakasam, M. Evaluation of the Algorithm for Automatic Identification of the Common Carotid Artery in ARTSENS. Physiol Meas 2014, 35, 1299–1317. [Google Scholar] [CrossRef]
- Farkas, G.A.; Roussos, C. Acute Diaphragmatic Shortening: In Vitro Mechanics and Fatigue. Am. Rev. Respir. Dis. 1984, 130, 434–438. [Google Scholar] [CrossRef]
- Farkas, G.A. Mechanical Properties of Respiratory Muscles in Primates. Respir. Physiol. 1991, 86, 41–50. [Google Scholar] [CrossRef]
- Kelly, N.G.; McCarter, R.J.; Barnwell, G.M. Respiratory Muscle Stiffness Is Age- and Muscle-Specific. Aging 1993, 5, 229–238. [Google Scholar] [CrossRef]
- Strumpf, R.K.; Humphrey, J.D.; Yin, F.C. Biaxial Mechanical Properties of Passive and Tetanized Canine Diaphragm. Am. J. Physiol. 1993, 265, H469–H475. [Google Scholar] [CrossRef]
- Jannapureddy, S.R.; Patel, N.D.; Hwang, W.; Boriek, A.M. Genetic Models in Applied Physiology. Merosin Deficiency Leads to Alterations in Passive and Active Skeletal Muscle Mechanics. J. Appl. Physiol. 2003, 94, 2524–2533; discussion 2523. [Google Scholar] [CrossRef]
- Hwang, W.; Kelly, N.G.; Boriek, A.M. Passive Mechanics of Muscle Tendinous Junction of Canine Diaphragm. J. Appl. Physiol. 2005, 98, 1328–1333. [Google Scholar] [CrossRef]
- Song, C.; Alijani, A.; Frank, T.; Hanna, G.B.; Cuschieri, A. Mechanical Properties of the Human Abdominal Wall Measured in Vivo during Insufflation for Laparoscopic Surgery. Surg. Endosc. 2006, 20, 987–990. [Google Scholar] [CrossRef]
- Rowe, J.; Chen, Q.; Domire, Z.J.; McCullough, M.B.; Sieck, G.; Zhan, W.-Z.; An, K.-N. Effect of Collagen Digestion on the Passive Elastic Properties of Diaphragm Muscle in Rat. Med. Eng. Phys. 2010, 32, 90–94. [Google Scholar] [CrossRef]
- Smith, L.R.; Barton, E.R. Collagen Content Does Not Alter the Passive Mechanical Properties of Fibrotic Skeletal Muscle in Mdx Mice. Am. J. Physiol. Cell Physiol. 2014, 306, C889–C898. [Google Scholar] [CrossRef]
- Aarab, Y.; Flatres, A.; Garnier, F.; Capdevila, M.; Raynaud, F.; Lacampagne, A.; Chapeau, D.; Klouche, K.; Etienne, P.; Jaber, S.; et al. Shear Wave Elastography, a New Tool for Diaphragmatic Qualitative Assessment: A Translational Study. Am. J. Respir. Crit. Care Med. 2021, 204, 797–806. [Google Scholar] [CrossRef]
- Bellemare, F.; Bigland-Ritchie, B.; Woods, J.J. Contractile Properties of the Human Diaphragm in Vivo. J. Appl. Physiol. 1986, 61, 1153–1161. [Google Scholar] [CrossRef]
- Reid, M.B.; Feldman, H.A.; Miller, M.J. Isometric Contractile Properties of Diaphragm Strips from Alcoholic Rats. J. Appl. Physiol. 1987, 63, 1156–1164. [Google Scholar] [CrossRef]
- Coirault, C.; Chemla, D.; Pourny, J.C.; Lambert, F.; Lecarpentier, Y. Instantaneous Force-Velocity-Length Relationship in Diaphragmatic Sarcomere. J. Appl. Physiol. 1997, 82, 404–412. [Google Scholar] [CrossRef][Green Version]
- Tallis, J.; James, R.S.; Little, A.G.; Cox, V.M.; Duncan, M.J.; Seebacher, F. Early Effects of Ageing on the Mechanical Performance of Isolated Locomotory (EDL) and Respiratory (Diaphragm) Skeletal Muscle Using the Work-Loop Technique. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R670–R684. [Google Scholar] [CrossRef] [PubMed]
- Müller-Felber, W.; Riepl, R.; Reimers, C.D.; Wagner, S.; Pongratz, D. Combined Ultrasonographic and Neurographic Examination: A New Technique to Evaluate Phrenic Nerve Function. Electromyogr. Clin. Neurophysiol. 1993, 33, 335–340. [Google Scholar] [PubMed]
- Flatres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S.; et al. Real-Time Shear Wave Ultrasound Elastography: A New Tool for the Evaluation of Diaphragm and Limb Muscle Stiffness in Critically Ill Patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Eddinger, T.J.; Moss, R.L. Mechanical Properties of Skinned Single Fibers of Identified Types from Rat Diaphragm. Am. J. Physiol. 1987, 253, C210–C218. [Google Scholar] [CrossRef]
- Dias, C.M.; Pássaro, C.P.; Cagido, V.R.; Einicker-Lamas, M.; Lowe, J.; Negri, E.M.; Capelozzi, V.L.; Zin, W.A.; Rocco, P.R.M. Effects of Undernutrition on Respiratory Mechanics and Lung Parenchyma Remodeling. J. Appl. Physiol. 2004, 97, 1888–1896. [Google Scholar] [CrossRef]
- Gayan-Ramirez, G.; Decramer, M. Clinical relevance of myosin isoforms in the diaphragm. Rev. Mal. Respir. 2000, 17, 574–584. [Google Scholar]
- Patel, N.D.; Jannapureddy, S.R.; Hwang, W.; Chaudhry, I.; Boriek, A.M. Altered Muscle Force and Stiffness of Skeletal Muscles in α-Sarcoglycan-Deficient Mice. Am. J. Physiol. -Cell Physiol. 2003, 284, C962–C968. [Google Scholar] [CrossRef][Green Version]
- Lopez, M.A.; Mayer, U.; Hwang, W.; Taylor, T.; Hashmi, M.A.; Jannapureddy, S.R.; Boriek, A.M. Force Transmission, Compliance, and Viscoelasticity Are Altered in the A7-Integrin-Null Mouse Diaphragm. Am. J. Physiol. -Cell Physiol. 2005, 288, C282–C289. [Google Scholar] [CrossRef][Green Version]
- Margulies, S.S.; Lei, G.T.; Farkas, G.A.; Rodarte, J.R. Finite-Element Analysis of Stress in the Canine Diaphragm. J. Appl. Physiol. 1994, 76, 2070–2075. [Google Scholar] [CrossRef]
- Pardo, P.S.; Lopez, M.A.; Mohamed, J.S.; Boriek, A.M. Anisotropic Mechanosensitive Pathways in the Diaphragm and Their Implications in Muscular Dystrophies. J. Muscle Res. Cell Motil. 2017, 38, 437–446. [Google Scholar] [CrossRef]
- Lopez, M.A.; Pardo, P.S.; Cox, G.A.; Boriek, A.M. Early Mechanical Dysfunction of the Diaphragm in the Muscular Dystrophy with Myositis (Ttnmdm) Model. Am. J. Physiol. Cell Physiol. 2008, 295, C1092–C1102. [Google Scholar] [CrossRef]
- Pardo, P.S.; Lopez, M.A.; Boriek, A.M. FOXO Transcription Factors Are Mechanosensitive and Their Regulation Is Altered with Aging in the Respiratory Pump. Am. J. Physiol. Cell Physiol. 2008, 294, C1056–C1066. [Google Scholar] [CrossRef]
- Klimathianaki, M.; Vaporidi, K.; Georgopoulos, D. Respiratory Muscle Dysfunction in COPD: From Muscles to Cell. Curr. Drug Targets 2011, 12, 478–488. [Google Scholar] [CrossRef]
- Greising, S.M.; Medina-Martínez, J.S.; Vasdev, A.K.; Sieck, G.C.; Mantilla, C.B. Analysis of Muscle Fiber Clustering in the Diaphragm Muscle of Sarcopenic Mice. Muscle Nerve. 2015, 52, 76–82. [Google Scholar] [CrossRef]
- Sahani, R.; Wallace, C.H.; Jones, B.K.; Blemker, S.S. Diaphragm Muscle Fibrosis Involves Changes in Collagen Organization with Mechanical Implications in Duchenne Muscular Dystrophy. J. Appl. Physiol. 2022, 132, 653–672. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, Z.; Wu, L. One-Step Compensation Downward Continuation Method Free of Iteration in Wave Number Domain. Acta Geod. Geophys. 2020, 55, 163–181. [Google Scholar] [CrossRef]
- Frija-Masson, J.; Debray, M.-P.; Gilbert, M.; Lescure, F.-X.; Travert, F.; Borie, R.; Khalil, A.; Crestani, B.; d’Ortho, M.-P.; Bancal, C. Functional Characteristics of Patients with SARS-CoV-2 Pneumonia at 30 Days Post-Infection. Eur. Respir. J. 2020, 56, 2001754. [Google Scholar] [CrossRef]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal Pulmonary Function in COVID-19 Patients at Time of Hospital Discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, Y.; Song, W.; Li, Q.; Xie, H.; Xu, Q.; Jia, J.; Li, L.; Mao, H.; Zhou, X.; et al. Follow-up Study of the Pulmonary Function and Related Physiological Characteristics of COVID-19 Survivors Three Months after Recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Xiaohong, Y.; Tingyuan, L.; Zhicheng, H.; Yifang, P.; Huawen, L.; Shicang, Y.; Huaming, M.; Lihua, W.; Huarong, Z.; Wenjuan, F.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Chin. J. Pathol. 2020, 49, 411–417. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section CT and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia. Korean J. Radiol. 2020, 21, 746–755. [Google Scholar] [CrossRef]
- Çelik, Z.; Güzel, N.A.; Kafa, N.; Köktürk, N. Respiratory Muscle Strength in Volleyball Players Suffered from COVID-19. Ir. J. Med. Sci. 2021, 1971, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Macera, C.; Addy, C.; Sy, F.; Wieland, D.; Blair, S. Effects of Physical Activity on Exercise Tests and Respiratory Function. Br. J. Sports Med. 2003, 37, 521–528. [Google Scholar] [CrossRef]
- Myers, J. Exercise and Cardiovascular Health. Circulation 2003, 107, e2–e5. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold. Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Gjaka, M.; Feka, K.; Bianco, A.; Tishukaj, F.; Giustino, V.; Parroco, A.M.; Palma, A.; Battaglia, G. The Effect of COVID-19 Lockdown Measures on Physical Activity Levels and Sedentary Behaviour in a Relatively Young Population Living in Kosovo. J. Clin. Med. 2021, 10, 763. [Google Scholar] [CrossRef]
- Dimitrova, A.; Izov, N.; Maznev, I.; Vasileva, D.; Nikolova, M. Physiotherapy in Patients with Chronic Obstructive Pulmonary Disease. Open Access Maced. J. Med. Sci. 2017, 5, 720–723. [Google Scholar] [CrossRef]
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of Inspiratory Muscle Training in COPD Patients: A Systematic Review and Meta-Analysis. Clin. Respir. J. 2018, 12, 2178–2188. [Google Scholar] [CrossRef]
- Bollmeier, S.G.; Hartmann, A.P. Management of Chronic Obstructive Pulmonary Disease: A Review Focusing on Exacerbations. Am. J. Health Syst. Pharm. 2020, 77, 259–268. [Google Scholar] [CrossRef]
- Yun, R.; Bai, Y.; Lu, Y.; Wu, X.; Lee, S.-D. How Breathing Exercises Influence on Respiratory Muscles and Quality of Life among Patients with COPD? A Systematic Review and Meta-Analysis. Can. Respir. J. 2021, 2021, 1904231. [Google Scholar] [CrossRef]
- Pancera, S.; Galeri, S.; Porta, R.; Pietta, I.; Bianchi, L.N.C.; Carrozza, M.C.; Villafañe, J.H. Feasibility and Efficacy of the Pulmonary Rehabilitation Program in a Rehabilitation Center: Case report of a young patient developing severe covid-19 acute respiratory distress syndrome. J. Cardiopulm. Rehabil. Prev. 2020, 40, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, C.; Gray, A. Pelvic Floor Considerations in COVID-19. J. Womens Health Phys. Ther. 2020, 44, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory Muscle Training for Recovered COVID-19 Patients after Weaning from Mechanical Ventilation. Medicine 2021, 100, e25339. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-L.; Yang, T. Pulmonary Rehabilitation for Patients with Coronavirus Disease 2019 (COVID-19). Chronic. Dis. Transl. Med. 2020, 6, 79–86. [Google Scholar] [CrossRef]
- Feng, F.; Tuchman, S.; Denninger, J.W.; Fricchione, G.L.; Yeung, A. Qigong for the Prevention, Treatment, and Rehabilitation of COVID-19 Infection in Older Adults. Am. J. Geriatr. Psychiatry 2020, 28, 812–819. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, J.; Shen, P.; Li, M.; You, H.; Liu, C.; Chen, L.; Wang, Z.; Zhou, C.; Feng, Z. Liuzijue Is a Promising Exercise Option for Rehabilitating Discharged COVID-19 Patients. Medicine 2021, 100, e24564. [Google Scholar] [CrossRef]
- Gao, P.; Tang, F.; Liu, W.; He, K.; Mo, Y. Effect of Liuzijue Qigong on Patients with Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e27344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesanelli, L.; Satkunskiene, D.; Bileviciute-Ljungar, I.; Kubilius, R.; Repečkaite, G.; Cesanelli, F.; Iovane, A.; Messina, G. The Possible Impact of COVID-19 on Respiratory Muscles Structure and Functions: A Literature Review. Sustainability 2022, 14, 7446. https://doi.org/10.3390/su14127446
Cesanelli L, Satkunskiene D, Bileviciute-Ljungar I, Kubilius R, Repečkaite G, Cesanelli F, Iovane A, Messina G. The Possible Impact of COVID-19 on Respiratory Muscles Structure and Functions: A Literature Review. Sustainability. 2022; 14(12):7446. https://doi.org/10.3390/su14127446
Chicago/Turabian StyleCesanelli, Leonardo, Danguole Satkunskiene, Indre Bileviciute-Ljungar, Raimondas Kubilius, Gintare Repečkaite, Federico Cesanelli, Angelo Iovane, and Giuseppe Messina. 2022. "The Possible Impact of COVID-19 on Respiratory Muscles Structure and Functions: A Literature Review" Sustainability 14, no. 12: 7446. https://doi.org/10.3390/su14127446
APA StyleCesanelli, L., Satkunskiene, D., Bileviciute-Ljungar, I., Kubilius, R., Repečkaite, G., Cesanelli, F., Iovane, A., & Messina, G. (2022). The Possible Impact of COVID-19 on Respiratory Muscles Structure and Functions: A Literature Review. Sustainability, 14(12), 7446. https://doi.org/10.3390/su14127446








