Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria
Abstract
:1. Introduction
2. Materials and Methods
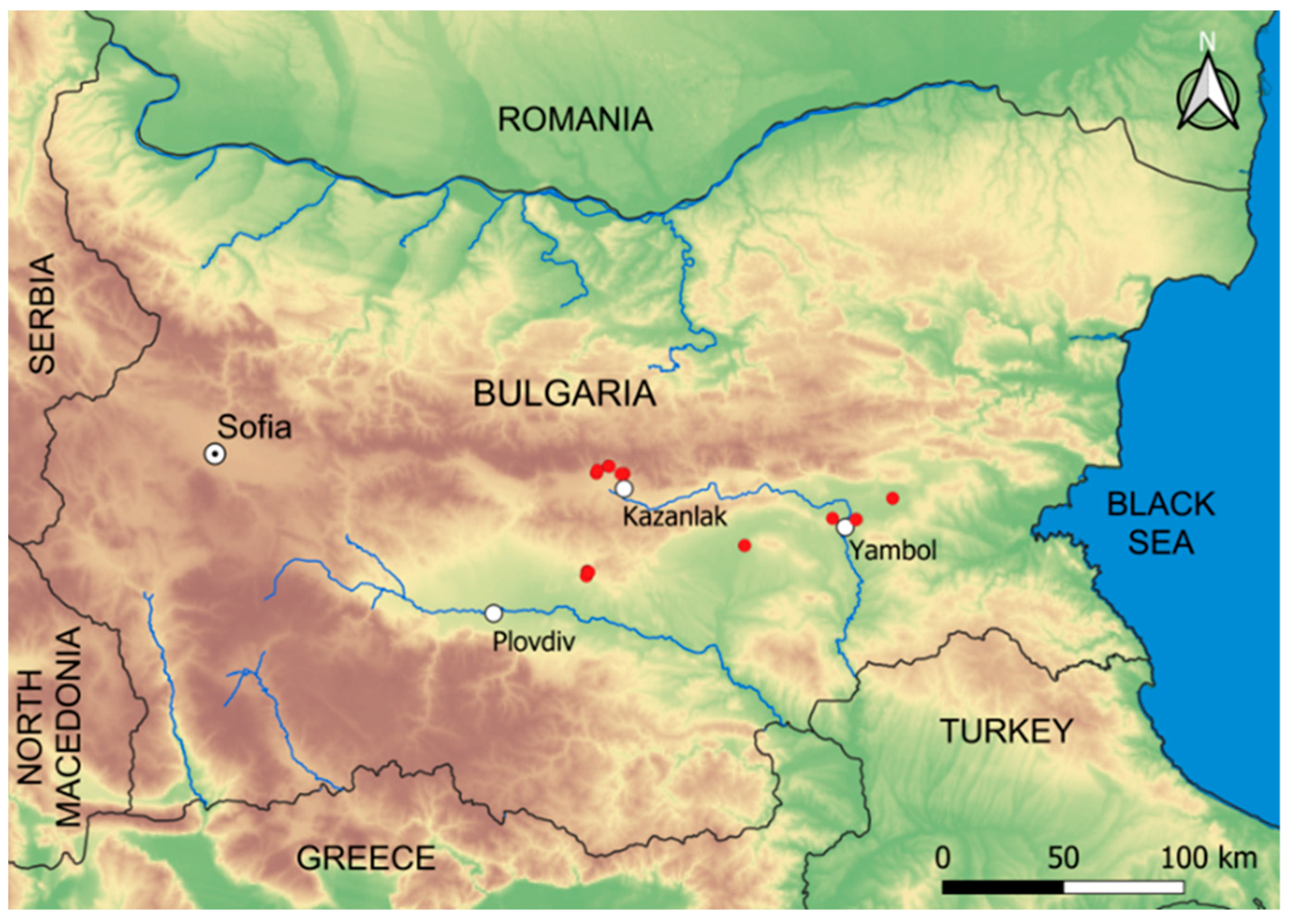
2.1. Study Objects and Area
2.2. Data Sampling and Processing
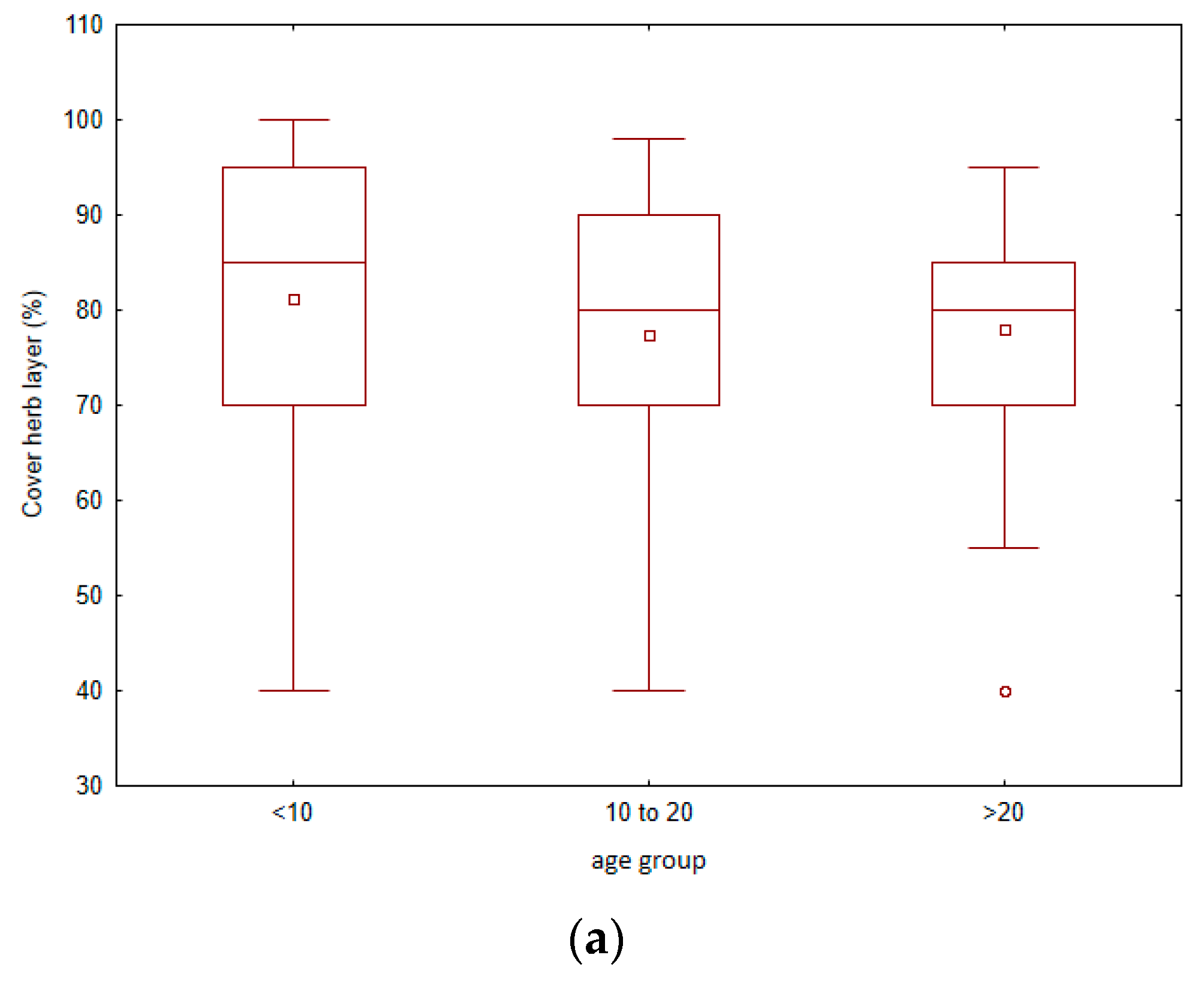
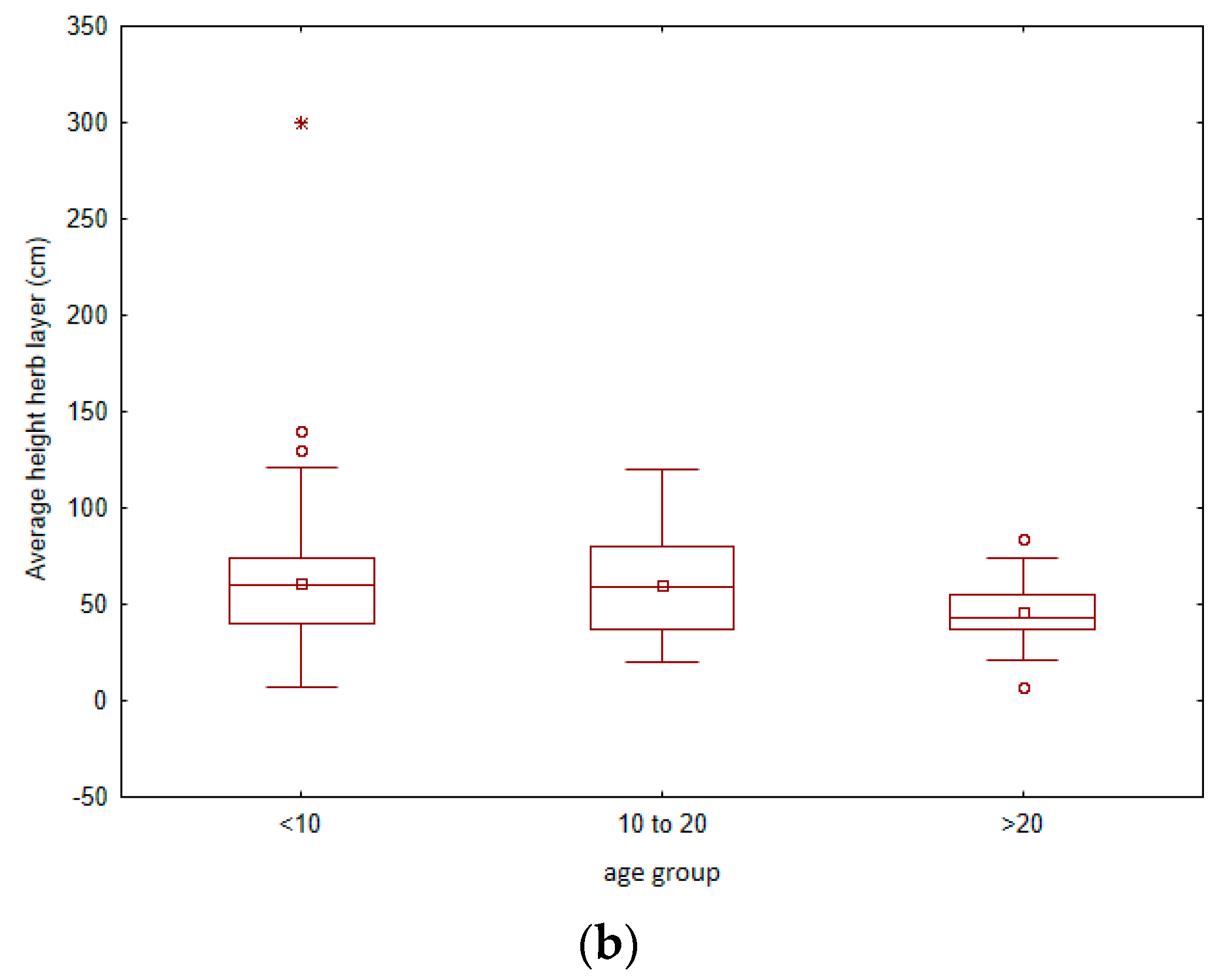
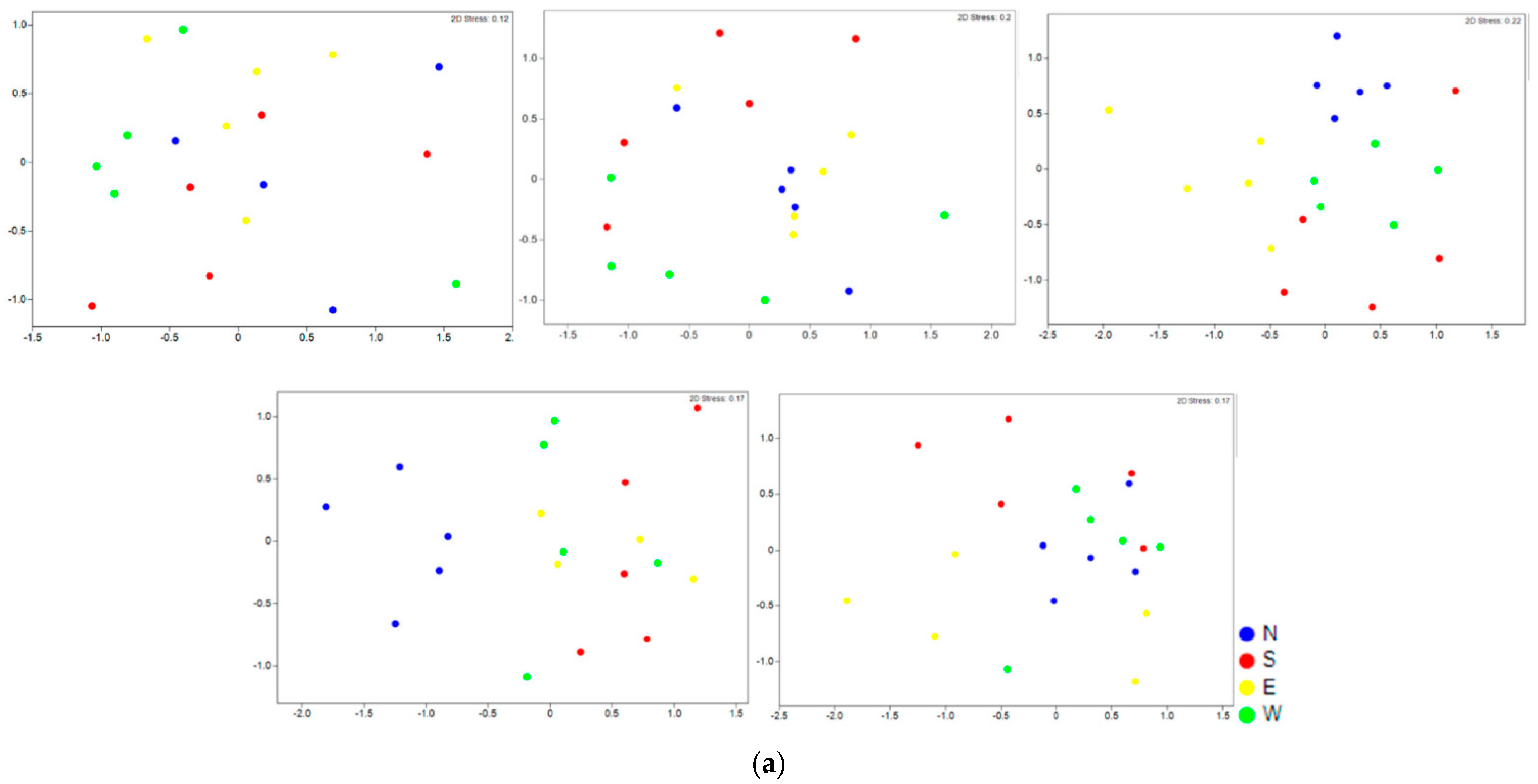
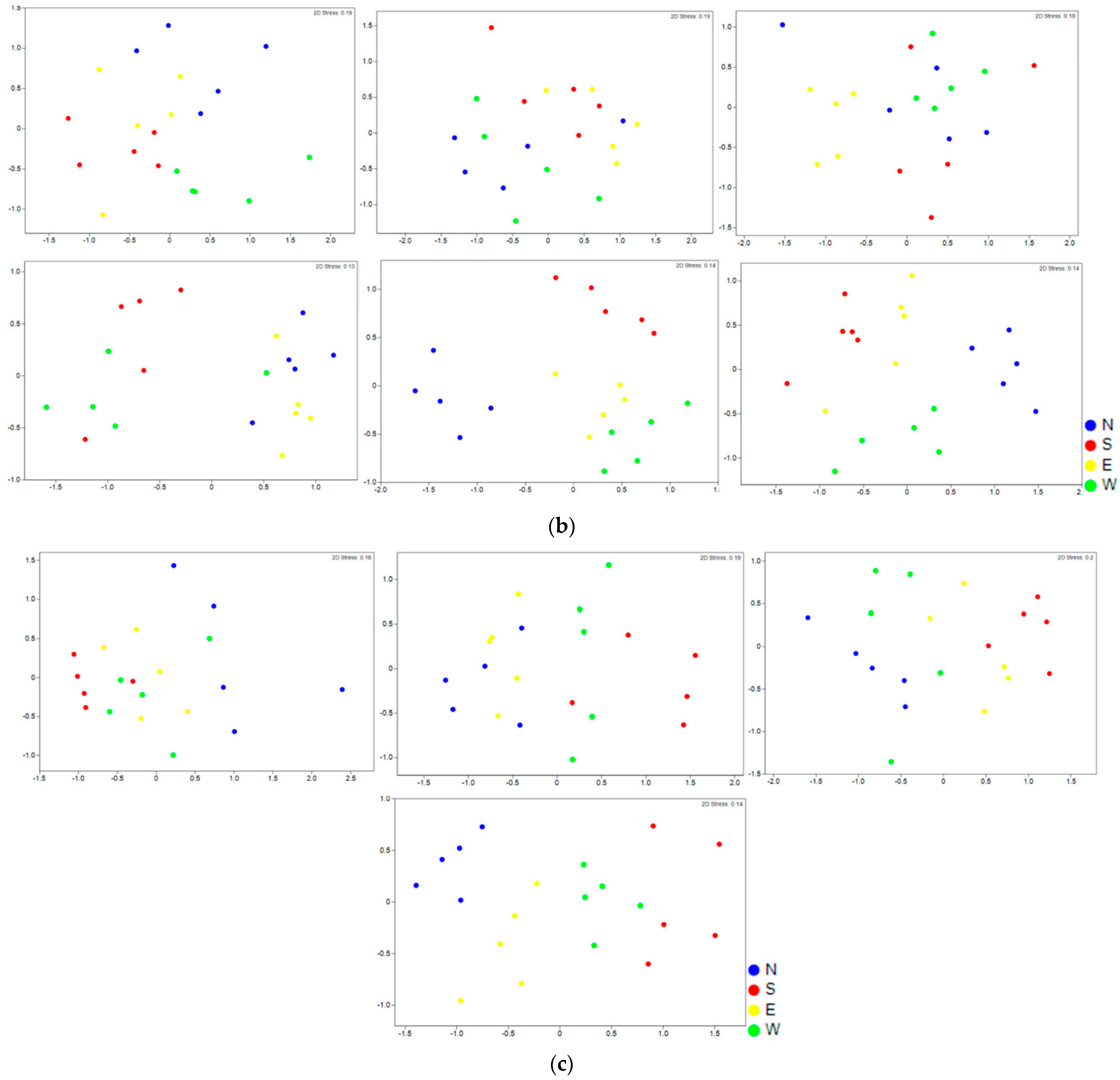
3. Results
4. Discussion
4.1. Vegetation Changes
4.2. Rate of the Recovery Success
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindborg, R.; Bengtsson, J.; Berg, Å.; Cousins, S.A.; Eriksson, O.; Gustafsson, T.; Hasund, K.P.; Lenoir, L.; Pihlgrn, A.; Sjödin, E.; et al. A landscape perspective on conservation of semi-natural grasslands. Agric. Ecosyst. Environ. 2008, 125, 213–222. [Google Scholar] [CrossRef]
- Schaich, H.; Bieling, C.; Plieninger, T. Linking ecosystem services with cultural landscape research. GAIA–Ecol. Perspect. Sci. Soc. 2010, 19, 269–277. [Google Scholar] [CrossRef]
- Council of Europe. European Landscape Convention, 2000 Signed at Florence on 20 October 2000. CETS No. 176. Available online: https://rm.coe.int/1680080621 (accessed on 15 January 2022).
- Agnoletti, M. Rural landscape, nature conservation and culture: Some notes on research trends and management approaches from a (southern) European perspective. Landsc. Urban. Plan. 2014, 126, 66–73. [Google Scholar] [CrossRef]
- Deák, B.; Tóthmérész, B.; Valkó, O.; Sudnik-Wójcikowska, B.; Moysiyenko, I.; Bragina, T.; Apostolova, I.; Dembicz, I.; Bykov, N.; Török, P. Cultural monuments and nature conservation: A review of the role of kurgans in the conservation and restoration of steppe vegetation. Biodivers. Conserv. 2016, 25, 2473–2490. [Google Scholar] [CrossRef]
- Rassamakin, Y. The main directions of the development of early pastoral societies of Northern Pontic Zone: 4500-2450 BC (Pre-yamnaya cultures and Yamnaya culture). Balt.-Potnic-Stud. 1994, 2, 29–90. [Google Scholar]
- Parvin, M. The Kazanlak Tomb, 1st ed.; Faber Publishing: Veliko Tarnovo, Bulgaria, 2015; 47p. [Google Scholar]
- Sadykov, T.; Caspari, G.; Blochin, J. Kurgan Tunnug 1—New data on the earliest horizon of scythian material culture. J. Field Archaeol. 2020, 45, 556–570. [Google Scholar] [CrossRef]
- Kitov, G. The Thracian tumuli. Thracia 1993, 10, 39–80. [Google Scholar]
- Agre, D. The Tumulus of Golyamata Mogila Near the Villages of Malomirovo and Zlatinitsa, 1st ed.; Avalon Publishing: Sofia, Bulgaria, 2011; 240p. [Google Scholar]
- Chichikova, M.; Stoyanova, D.; Stoyanov, T. The Caryatids Royal Tomb Near the Village of Sveshtari. 30 Years of Discovery, 1st ed.; Historical Museum of Isperih: Isperih, Bulgaria, 2012; 128p. [Google Scholar]
- Dimitrova, D. Shushmanets Tumular Temple near Shipka (Central Bulgaria). In The Thracians and Their Neighbors in the Bronze and Iron Ages. Necropolises, Cult Places, Religion, Mythology, Proceedings of the 12th International Congress of Thracology, Târgoviște, Romania, 10–14 September 2013; Sîrbu, V., Ștefănescu, R., Eds.; Muzeul Brăilei Editura Istros: Brașov, Romania, 2013; Volume II, pp. 133–152. [Google Scholar]
- Dimitrova, D. The Tomb of King Seuthes III in Golyama Kosmatka Tumulus, 1st ed.; Aros Publishing: Sofia, Bulgaria, 2015; 378p. [Google Scholar]
- Caneva, G. A botanical approach to the planning of archaeological, parks in Italy. Conserv. Manag. Archaeol. Sites 1999, 3, 127–134. [Google Scholar] [CrossRef]
- Caneva, G.; Benelli, F.; Bartoli, F.; Cicinelli, E. Safeguarding natural and cultural heritage on Etruscan tombs (La Banditaccia, Cerveteri, Italy). Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 891–907. [Google Scholar] [CrossRef]
- Cicinelli, E.; Salerno, G.; Caneva, G. An assessment methodology to combine the preservation of biodiversity and cultural heritage: The San Vincenzo al Volturno historical site (Molise, Italy). Biodivers. Conserv. 2018, 27, 1073–1093. [Google Scholar] [CrossRef]
- Deák, B. Nature and Culture: The Role of Ancient Burial Mounds in the Conservation of Eurasian Steppe Vegetation; Centre for Ecological Research: Tihany, Hungary, 2020; 172p. [Google Scholar]
- Sudnik-Wójcikowska, B.; Moysiyenko, I.I.; Zachwatowicz, M.; Jabłońska, E. The value and need for protection of kurgan flora in the anthropogenic landscape of steppe zone in Ukraine. Plant Biosyst. 2011, 145, 638–653. [Google Scholar] [CrossRef]
- Moysiyenko, I.; Zachwatowicz, M.; Sudnik-Wójcikowska, B.; Jabłonska, E. Kurgans help to protect endangered steppe species in the Pontic grass steppe zone, Ukraine. Wulfenia 2014, 21, 83–94. [Google Scholar]
- Deák, B.; Valkó, O.; Nagy, D.D.; Török, P.; Torma, A.; Lórinczi, G.; Kelemen, A.; Nagy, A.; Bede, Á.; Mizser, S.; et al. Habitat islands outside nature reserves—Threatened biodiversity hotspots of grassland specialist plant and arthropod species. Biol. Conserv. 2020, 241, 108254. [Google Scholar] [CrossRef]
- Dembicz, I.; Moysiyenko, I.I.; Kozub, Ł.; Dengler, J.; Zakharova, M.; Sudnik-Wójcikowska, B. Steppe islands in a sea of fields: Where island biogeography meets the reality of a severely transformed landscape. J. Veg. Sci. 2021, 32, e12930. [Google Scholar] [CrossRef]
- Apostolova, I.; Palpurina, S.; Sopotlieva, D.; Terziyska, T.; Velev, N.; Vassilev, K.; Nekhrizov, G.; Tsvetkova, N. Ancient Burial Mounds–Stepping Stones for Semi-Natural Habitats in Agricultural Landscape. Ecol. Balk. 2020, 12, 43–52. [Google Scholar]
- Apostolova, I.; Sopotlieva, D.; Valcheva, M.; Ganeva, A.; Shivarov, V.; Velev, N.; Vassilev, K.; Terziyska, T.; Nekhrizov, G. First Survey of the Vascular and Cryptogam Flora on Bulgaria’s Ancient Mounds. Plants 2022, 11, 705. [Google Scholar] [CrossRef]
- Lindborg, R.; Plue, J.; Andersson, K.; Cousins, S.A.O. Function of small habitat elements for enhancing plant diversity in different agricultural landscapes. Biol. Conserv. 2014, 169, 206–213. [Google Scholar] [CrossRef]
- Bridgewater, P.; Rotherham, I.D. A critical perspective on the concept of biocultural diversity and its emerging role in nature and heritage conservation. People Nat. 2019, 1, 291–304. [Google Scholar] [CrossRef] [Green Version]
- European Parliament Resolution of 8 September 2015 towards an Integrated Approach to Cultural Heritage for Europe (2014/2149(INI). OJ C 316. 22 September 2017, pp. 88–98. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52015IP0293 (accessed on 15 February 2022).
- Pykälä, J. Effects of restoration with cattle grazing on plant species composition and richness of semi-natural grasslands. Biodivers. Conserv. 2003, 12, 2211–2226. [Google Scholar] [CrossRef]
- Mota, J.; Sola, A.; Jiménez-Sánchez, M.; Pérez-García, F.; Merlo, M. Gypsicolous flora, conservation and restoration of quarries in the southeast of the Iberian Peninsula. Biodivers. Conserv. 2004, 13, 1797–1808. [Google Scholar] [CrossRef]
- Rydgren, K.; Halvorsen, R.; Odland, A.; Skjerdal, G. Restoration of alpine spoil heaps: Successional rates predict vegetation recovery in 50 years. Ecol. Eng. 2011, 37, 294–301. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P. Using spontaneous succession for restoration of human-disturbed habitats: Experience from Central Europe. Ecol. Eng. 2001, 17, 55–62. [Google Scholar] [CrossRef]
- Török, P.; Vida, E.; Deák, B.; Lengyel, S.; Tóthmérész, B. Grassland restoration on former croplands in Europe: An assessment of applicability of techniques and costs. Biodivers. Conserv. 2011, 20, 2311–2332. [Google Scholar] [CrossRef]
- Prach, K.; Řehounková, K.; Lencová, K.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Študent, V.; Vaněček, Z.; Tichý, L.; Petřík, P.; et al. Vegetation succession in restoration of disturbed sites in Central Europe: The direction of succession and species richness across 19 seres. Appl. Veg. Sci. 2014, 17, 193–200. [Google Scholar] [CrossRef]
- Prach, K.; Šebelíková, L.; Řehounková, K.; del Moral, R. Possibilities and limitations of passive restoration of heavily disturbed sites. Landsc. Res. 2020, 45, 247–253. [Google Scholar] [CrossRef]
- Cicinelli, E.; Benelli, F.; Bartoli, F.; Traversetti, L.; Caneva, G. Trends of plant communities growing on the Etruscan tombs (Cerveteri, Italy) related to different management practices. Plant Biosyst. 2020, 154, 158–164. [Google Scholar] [CrossRef]
- Kanellou, E.; Papafotiou, M.; Economou, G.; Paraskevopoulou, A.; Kartsonas, E.; Ntoulas, N. Response of sown herbaceous forb mixtures suitable for aesthetic improvement and vegetation management at archaeological sites of the Mediterranean region. Ecol. Eng. 2021, 167, 106256. [Google Scholar] [CrossRef]
- Caneva, G.; Langone, S.; Bartoli, F.; Cecchini, A.; Meneghini, C. Vegetation Cover and Tumuli’s Shape as Affecting Factors of Microclimate and Biodeterioration Risk for the Conservation of Etruscan Tombs (Tarquinia, Italy). Sustainability 2021, 13, 3393. [Google Scholar] [CrossRef]
- Valkó, O.; Tóth, K.; Kelemen, A.; Miglécz, T.; Radócz, S.; Sonkoly, J.; Tóthmérész, B.; Török, P.; Deák, B. Cultural heritage and biodiversity conservation–plant introduction and practical restoration on ancient burial mounds. Nat. Conserv. 2018, 24, 65–80. [Google Scholar] [CrossRef]
- Deák, B.; Valkó, O.; Tóth, C.A.; Botos, Á.; Novák, T.J. Legacies of past land use challenge grassland recovery—An example from dry grasslands on ancient burial mounds. Nat. Conserv. 2020, 39, 113–132. [Google Scholar] [CrossRef]
- Prach, K.; Hobbs, R.J. Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Clewell, B.; McDonald, T. Relevance of Natural Recovery to Ecological Restoration. Ecol. Restor. 2009, 27, 122–124. [Google Scholar] [CrossRef]
- Kitov, G. The Valley of the Thracian Rulers, 2nd ed.; Slavena Publishing: Varna, Bulgaria, 2005; 99p. [Google Scholar]
- Velev, S. Climatic regioning. In Geography of Bulgaria, 1st ed.; Kopralev, I., Ed.; Publishing House ForCom: Sofia, Bulgaria, 2002; pp. 155–156. [Google Scholar]
- Connor, S.E.; Ross, S.A.; Sobotkova, A.; Herries, A.I.; Mooney, S.D.; Longford, C.; Iliev, I. Environmental conditions in the SE Balkans since the Last Glacial Maximum and their influence on the spread of agriculture into Europe. Quat. Sci. Rev. 2013, 68, 200–215. [Google Scholar] [CrossRef]
- Tonkov, S.; Bozilova, E.; Marinova, E.; Jűngner, H. History of vegetation and landscape during the last 4000 years in the area of Straldzha mire (southeastern Bulgaria). Phytol. Balcan. 2008, 14, 158–191. [Google Scholar]
- Euro+Med, 2006–2021. Euro+Med–The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://www.europlusmed.org (accessed on 2 June 2022).
- Delipavlov, D.; Cheshmedzhiev, I.; Popova, M.; Terziyski, D.; Kovachev, I. Guide to Vascular Plants in Bulgaria; Academic Publishing of the Agricultural University: Plovdiv, Bulgaria, 2003; 587p. [Google Scholar]
- Stoyanov, K.; Raycheva, T.; Cheschmedzhiev, I. Key to the Native and Foreign Vascular Plants in Bulgaria; Academic Publishing of the Agricultural University: Plovdiv, Bulgaria, 2021; 678p. [Google Scholar]
- Hill, M.O.; Bell, N.; Bruggeman-Nannenga, M.A.; Brugués, M.; Cano, M.J.; Enroth, J.; Flatberg, K.I.; Frahm, J.-P.; Gallego, M.T.; Garilleti, R.; et al. An annotated checklist of the mosses of Europe and Macaronesia. J. Bryol. 2006, 28, 198–267. [Google Scholar] [CrossRef]
- Ganeva, A.; Düll, R. A contribution to the Bulgarian bryoflora. Checklist of Bulgarian bryophytes. In Contributions to the Bryoflora of former Yugoslavia and Bulgaria, 1 Auflage; Düll, R., Ganeva, A., Martincic, A., Pavletic, Z., Eds.; IDH-Verlag: Bad Münstereifel, Germany, 1999; pp. 111–199. [Google Scholar]
- Prach, K. Spontaneous succession in Central-European man-made habitats: What information can be used in restoration practice? Appl. Veg. Sci. 2003, 6, 125–129. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), ver. 13. 2016. Available online: http://www.dell.com (accessed on 15 February 2022).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- PRIMER-E Ltd. PRIMER (Plymouth Routines in Multivariate Ecological Research), Version 7. 2017. Available online: http://www.primer-e.com (accessed on 15 February 2022).
- Holzapfel, C.; Schmidt, W.; Shmida, A. The role of seed bank and seed rain in the recolonization of disturbed sites along an aridity gradient. Phytocoenologia 1993, 23, 561–580. [Google Scholar] [CrossRef]
- Poschlod, P.; Bakker, J.; Bonn, S.; Fischer, S. Dispersal of plants in fragmented landscapes. In Species Survival in Fragmented Landscapes, 1st ed.; Settele, J., Margules, C., Poschlod, P., Henle, K., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 123–127. [Google Scholar]
- Valkó, O.; Rádai, Z.; Deák, B. Hay transfer is a nature-based and sustainable solution for restoring grassland biodiversity. J. Environ. Manag. 2022, 311, 114816. [Google Scholar] [CrossRef]
- Sopotlieva, D.; Velev, N.; Tsvetkova, N.; Vassilev, V.; Apostolova, I. Ecosystem condition assessment of semi-natural grasslands outside the Natura 2000 network in Bulgaria, using vegetation data. Tuexenia 2018, 38, 385–404. [Google Scholar]
- Michalska-Hejduk, D.; Wolski, G.J.; Harnisch, M.; Otte, A.; Bomanowska, A.; Donath, T.W. Restoration of floodplain meadows: Effects on the re-establishment of mosses. PLoS ONE 2017, 12, e0187944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmalholz, M.; Hylander, K. Succession of bryophyte assemblages following clear-cut logging in boreal spruce-dominated forests in south-central Sweden—Does retrogressive succession occur? Can. J. For. Res. 2009, 39, 1871–1880. [Google Scholar] [CrossRef]
- Jeschke, M. Cryptogams in calcareous grassland restoration: Perspectives for artificial vs. natural colonization. Tuexenia 2012, 32, 269–279. [Google Scholar]
- Walker, L.R.; Del Moral, R. Primary Succession and Ecosystem Rehabilitation, 1st ed.; Cambridge University Press: Cambridge, UK, 2003; 443p. [Google Scholar]
- Deák, B.; Kovács, B.; Rádai, Z.; Apostolova, I.; Kelemen, A.; Kiss, R.; Lukács, K.; Palpurina, S.; Sopotlieva, D.; Báthori, F.; et al. Linking environmental heterogeneity and plant diversity: The ecological role of small natural features in homogeneous landscapes. Sci. Total Environ. 2021, 763, 144199. [Google Scholar] [CrossRef]

| Height (m) | 2D Area (m2) | |||||
|---|---|---|---|---|---|---|
| Age Group | Min | Max | Average ± SD | Min | Max | Average ± SD |
| Mound excavated less than 10 years, n = 5 | 2.0 | 3.4 | 2.3 ± 0.6 | 78.5 | 736.4 | 337.5 ± 250.7 |
| Mound excavated between 10–20 years, n = 6 | 2.0 | 7.9 | 4.3 ± 2.3 | 78.5 | 1481.2 | 588.5 ± 5.9.1 |
| Mound excavated between 20–30 years, n = 4 | 2.2 | 3.5 | 3.2 ± 0.6 | 78.5 | 570.9 | 338.6 ± 188.9 |
| <10 Years | 10 to 20 Years | 20 to 30 Years | p | |
|---|---|---|---|---|
| Number of cases (plots) | 100 | 120 | 80 | |
| Parameter | ||||
| Total species richness | 14.3 ±6.3 a | 18.9 ± 7.5 b | 23.8 ± 4.4 c | ** |
| Species richness of short-lived plants | 9.9 ± 4.5 a | 10.45 ± 5.3 a | 13.7 ± 4.2 b | *** |
| Species richness of perennial plants | 4.3 ± 2.6 a | 8.2 ± 4.1 b | 9.8 ± 3.5 c | ** |
| Species richness of anthropophytes | 8.3 ± 2.6 | 8.5 ± 3.7 | 9.2 ± 2.6 | 0.125 |
| Species richness of target species | 5.9 ± 4.7 a | 10.1 ± 6.3 b | 14.3 ± 3.6 c | ** |
| Cover of short-lived species | 73.4 ± 20.4 a | 40.8 ± 34.9 b | 34.6 ± 18.5 b | ** |
| Cover of perennial species | 16.5 ± 15.0 a | 50.9 ± 27.4 b | 50.7 ± 28.4 b | ** |
| Cover of anthropophytes | 52.6 ±25.6 a | 43.4 ± 35.9 b | 22.1 ± 14.9 c | *** |
| Cover of target species | 37.3 ± 25.3 a | 48.3 ± 31.0 b | 63.2 ± 20.8 c | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolova, I.; Valcheva, M.; Sopotlieva, D.; Velev, N.; Ganeva, A.; Nekhrizov, G. Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria. Sustainability 2022, 14, 7318. https://doi.org/10.3390/su14127318
Apostolova I, Valcheva M, Sopotlieva D, Velev N, Ganeva A, Nekhrizov G. Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria. Sustainability. 2022; 14(12):7318. https://doi.org/10.3390/su14127318
Chicago/Turabian StyleApostolova, Iva, Magdalena Valcheva, Desislava Sopotlieva, Nikolay Velev, Anna Ganeva, and Georgi Nekhrizov. 2022. "Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria" Sustainability 14, no. 12: 7318. https://doi.org/10.3390/su14127318
APA StyleApostolova, I., Valcheva, M., Sopotlieva, D., Velev, N., Ganeva, A., & Nekhrizov, G. (2022). Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria. Sustainability, 14(12), 7318. https://doi.org/10.3390/su14127318







