Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Pretreatment
2.2. Microcosm Design
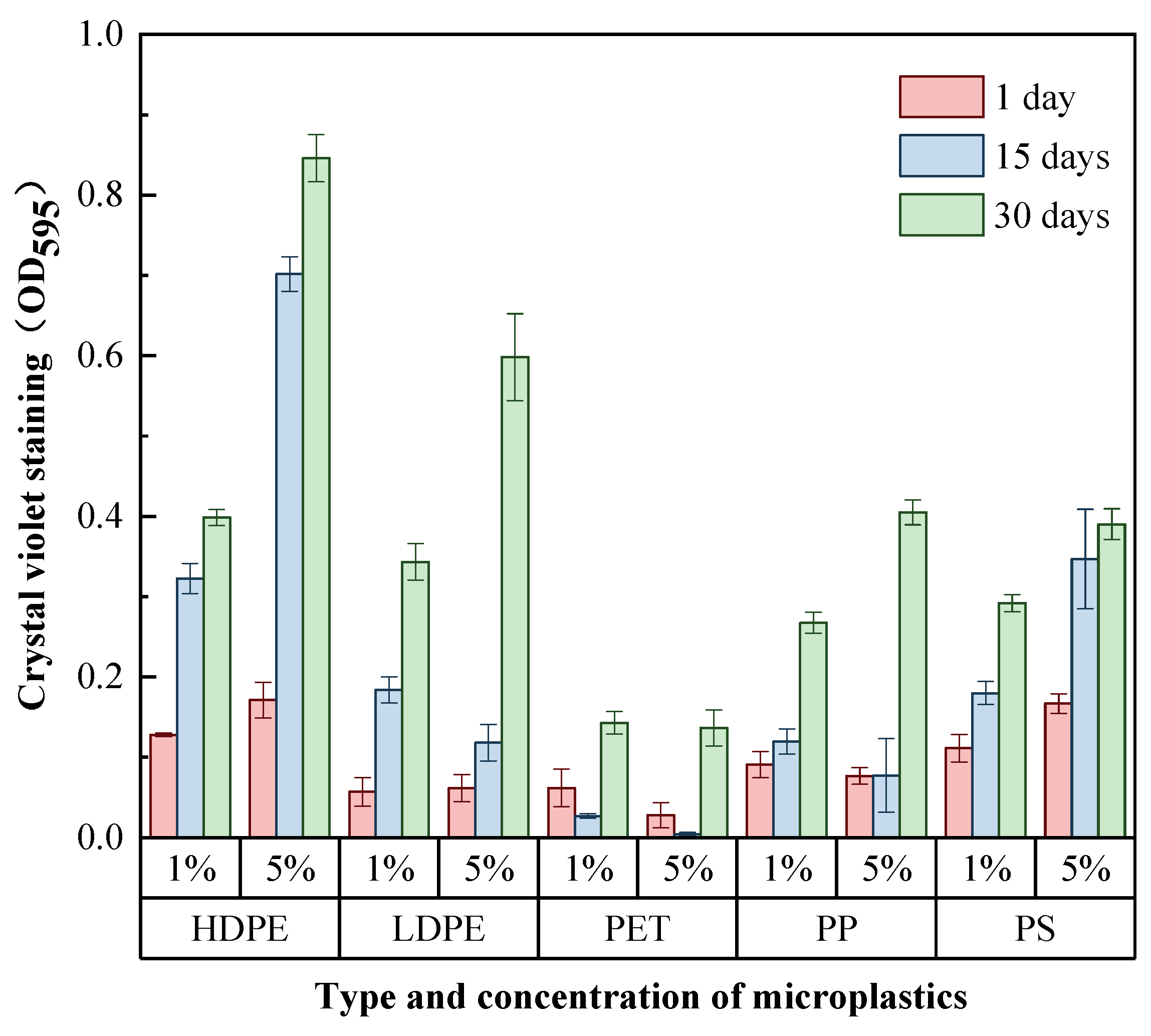
2.3. Biofilm Quantification by Use of Crystal Violet Assay
2.4. Determination of EPS in Biofilms on MP Surfaces
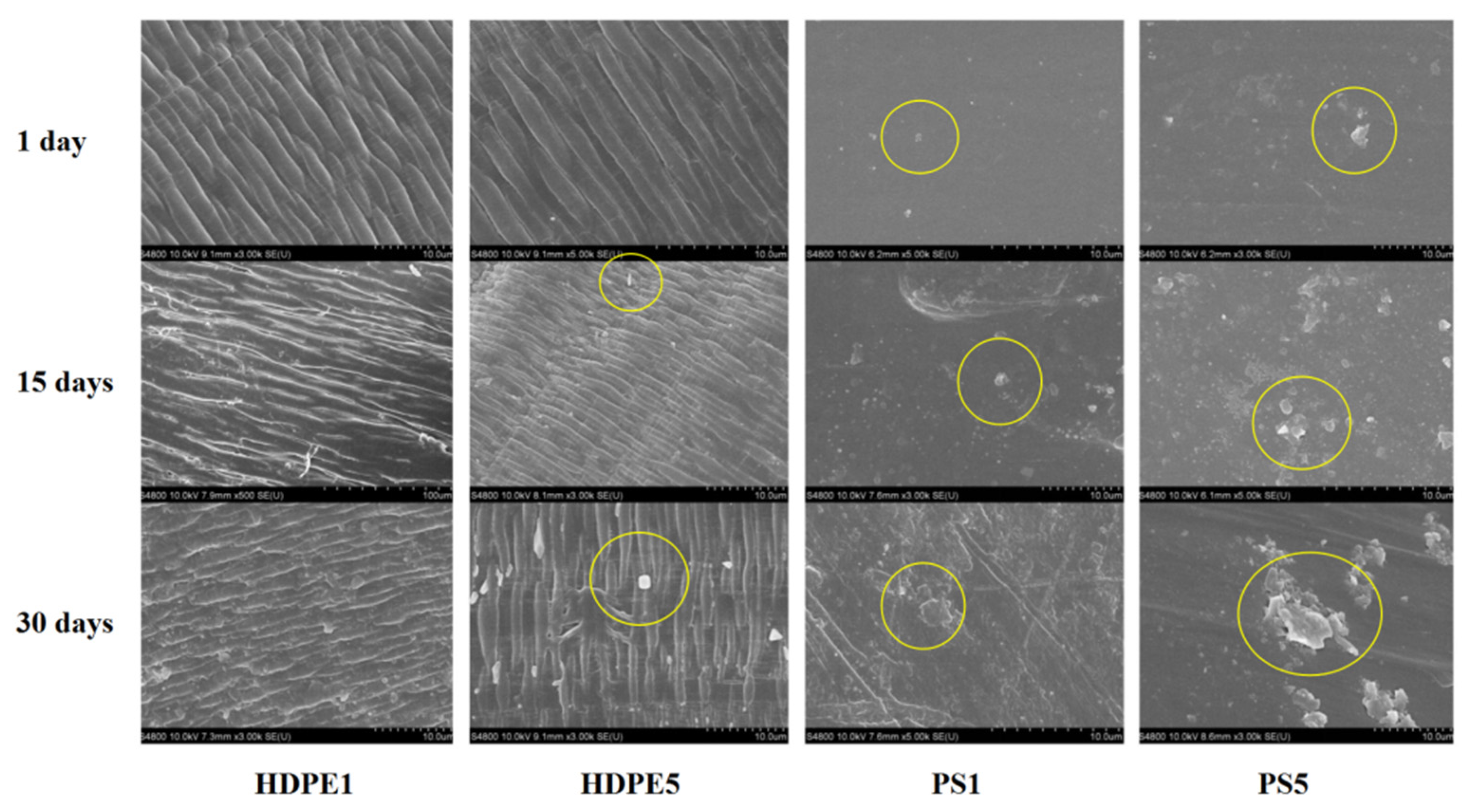
2.5. Characteristics of Microstructure
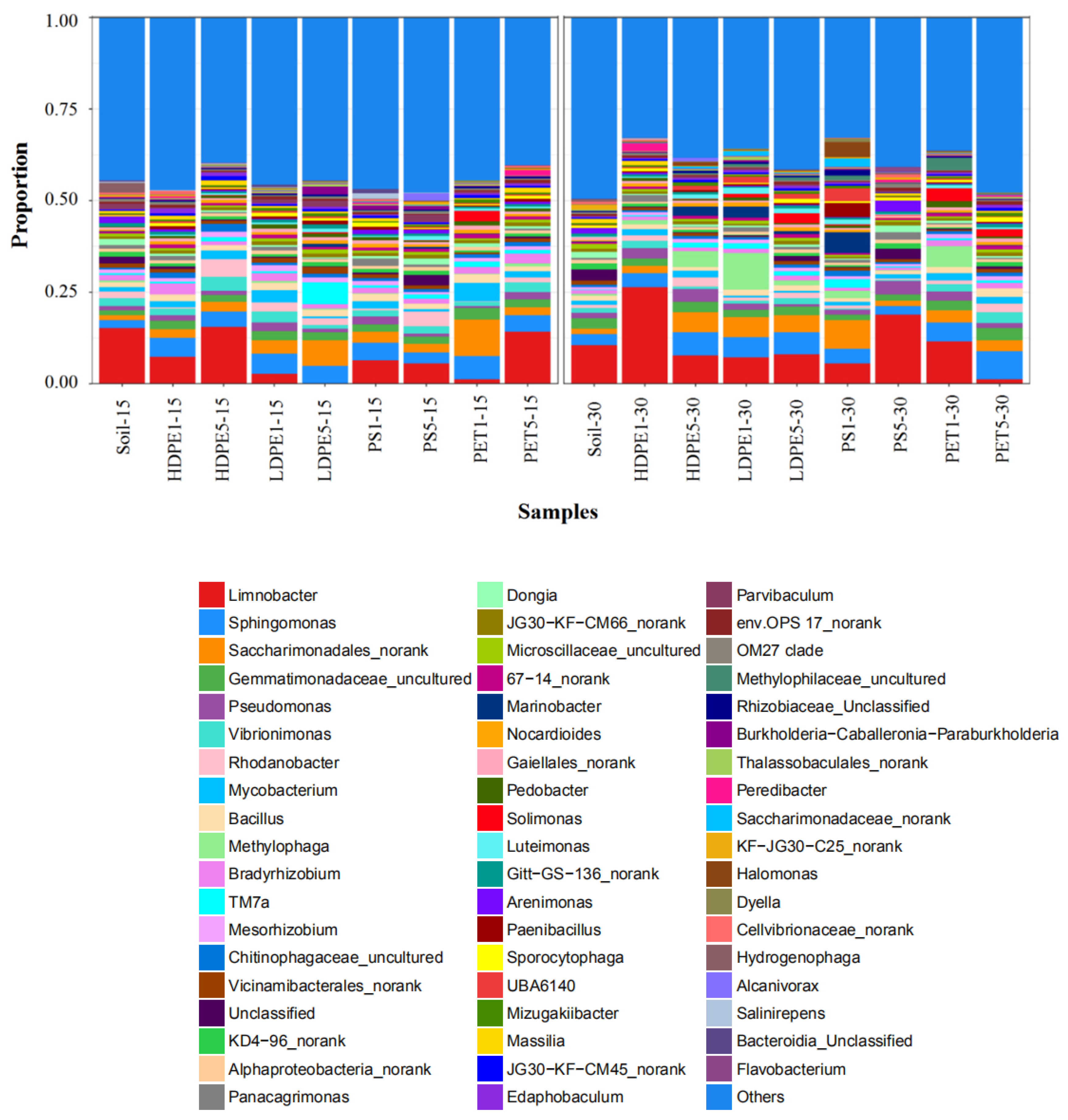
2.6. 16S rRNA Genes Sequencing
2.7. Data Statistics and Analysis
3. Results and Discussion
3.1. MP Surface Characteristics
3.2. Differences in the Total Amount of Biofilms Enriched on the Surface of MPs
3.3. Differences of EPS in Biofilms on MP Surfaces
3.4. Structural Diversity and Richness of Microorganisms Attached to MP Surfaces in Greenhouse Farmland
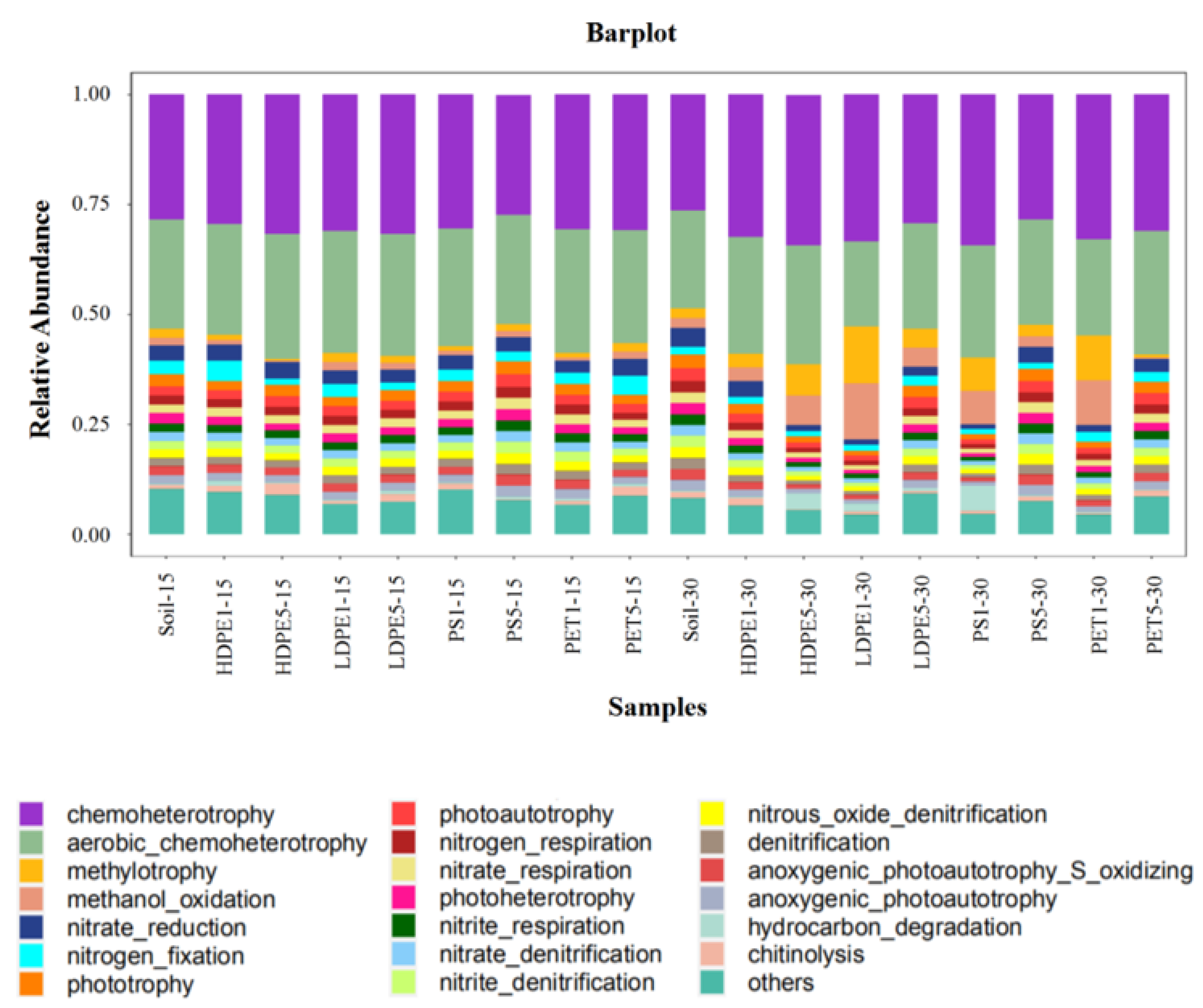
3.5. Prediction of the Potential Functional Diversity of Microbes by MPs in the Planting Area of Greenhouse Farmland
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xue, Y.H.; Cao, S.L.; Jia, T.; Jin, T.; Ju, X.H.; Xi, B.; Zhang, J.X.; Xu, Z.Y. Mechanized recycling and reuse technology to promote cleaner production of plastic film. Vegetable 2017, 8, 8–11. [Google Scholar]
- Rural Social and Economic Investigation Department of National Bureau of Statistics. 2020 China Rural Statistical Yearbook; China Statistics Press: Beijing, China, 2020; p. 42. [Google Scholar]
- Yan, C.R.; Liu, E.K.; Shu, F.; Liu, Q.; Liu, S. Characteristics and prevention and control technology of plastic film mulching and residual pollution in my country. J. Agricult. Res. Environ. 2014, 31, 95–102. [Google Scholar]
- Chen, H. Problems and countermeasures of recycling and utilization of waste agricultural film in Jiangsu. Sci. Pop. 2019, 11, 198. [Google Scholar]
- Nigussie, A.; Kuyper, T.W.; Neergaard, A.D. Agricultural waste utilisation strategies and demand for urban waste compost: Evidence from smallholder farmers in Ethiopia. Waste Manag. 2015, 44, 82–93. [Google Scholar]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Luo, Y.M.; Zhou, Q.; Zhang, H.B.; Pan, X.L.; Tu, C.; Li, L.Z. Pay attention to research on microplastic pollution in soil to prevent ecological and food chain risks. Bull. Chin. Acad. Sci. 2018, 33, 1021–1030. [Google Scholar]
- Machado, A.A.d.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V.J.C. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Sci. Total Environ. 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Lehmann, A.; Fitschen, K.; Rillig, M.C. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Syst. 2019, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bacheher, J.B.; Faltin, E.; Becker, R.; Goerlich, A.S.; Rillig, M.C.J. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Technology, Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y.J. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; Machado, A.; Yang, G.J. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2019, 707, 135634. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Zhang, Y.J. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration. Geoderma 2019, 337, 444–452. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Mejia, P.A.; Esperanza, H.L.; Nicolas, B.; Henny, G.; Paolina, G.; Violette, G.J. Macro- and micro-plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M.; Letters, T. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. 2017, 4, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Zhu, J.J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2019, 707, 135601. [Google Scholar] [CrossRef]
- Zhang, C.J.; Tu, C.; Zhou, Q.; Li, L.Z.; Li, Y.; Fu, C.C.; Pan, X.L.; Luo, Y.M. Weathering characteristics of low-density polyethylene film microplastics in the coastal environment of the Yellow River estuary. Acta Pedol. Sin. 2021, 58, 456–463. [Google Scholar]
- Luo, Y.Y.; Zhang, Y.Y.; Xu, Y.B.; Guo, X.T.; Zhu, L.Y. Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties. Sci. Total Environ. 2020, 726, 138389. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y.J. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Grob, C.; Howat, A.M.; Burns, O.J.; Pratscher, J.; Jehmlich, N.; von Bergen, M.; Richnow, H.H.; Chen, Y.; Murrell, J.C. Methylamine as a nitrogen source for microorganisms from a coastal marine environment. Environ. Microbiol. 2017, 19, 2246–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennecke, D.; Duarte, B.; Paiva, F.; Ca Ador, I.; Ca Nning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Cox, S.B.; Peyron, M.; Bailes, G.; Kinloch, K.; Karich, K.; Miles, C.; Inglis, D.A.; Brodhagen, M. Native soil fungi associated with compostable plastics in three contrasting agricultural settings. Appl. Microbiol. Biotechnol. 2014, 98, 6467–6485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X.J.E.P. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2020, 93, 24–32. [Google Scholar] [CrossRef]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Sun, R.; He, L.; Li, T.; Dai, Z.; Sun, S.; Ren, L.; Li, C. Impact of the surrounding environment on antibiotic resistance genes carried by microplastics in mangroves. Sci. Total Environ. 2022, 837, 155771. [Google Scholar] [CrossRef]
- Tatsuya, M.; Akio, M.; Norimasa, I.; Tokifumi, M.; Shin-Ichiro, N.; Yuan, C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar]
- Goren, M.; Li, J. The Coomassie Brilliant Blue method underestimates drug-induced tubular proteinuria. Clin. Chem. 1986, 32, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, H.; Fu, C. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Sangale, M.K.; Shahnawar, M.; Ade, A.B. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci. Rep. 2019, 9, 5390. [Google Scholar] [CrossRef] [Green Version]
- Andrade, L.L.; Leite, D.C.A.; Ferreira, E.M.; Ferreira, L.Q.; Paula, G.R.; Maguire, M.J.; Hubert, C.R.J.; Peixoto, R.S.; Domingues, R.M.C.P.; Rosado, A.S. Microbial diversity and anaerobic hydrocarbon degradation potential in an oil-contaminated mangrove sediment. BMC Microbiol. 2012, 12, 186. [Google Scholar] [CrossRef] [Green Version]
- Gomes, N.C.M.; Flocco, C.G.; Costa, R.; Junca, H.; Vilchez, R.; Pieper, D.H.; Kroegerrecklenfort, E.; Paranhos, R.; Mendonca-Hagler, L.C.S.; Smalla, K. Mangrove microniches determine the structural and functional diversity of enriched petroleum hydrocarbon-degrading consortia. FEMS Microbiol. Ecol. 2010, 74, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qian, Z.J.; Hong, P.; Sun, S.; Li, C. Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere 2020, 252, 126565. [Google Scholar] [CrossRef]
- Tu, C.; Chen, T.; Zhou, Q.; Liu, Y.; Wei, J.; Waniek, J.J.; Luo, Y. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ. 2020, 734, 139237. [Google Scholar] [CrossRef]
- Nauendorf, A.; Krause, S.; Bigalke, N.K.; Gorb, E.V.; Gorb, S.N.; Haeckel, M.; Wahl, M.; Treude, T. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar. Pollut. Bull. 2016, 103, 168–178. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.r.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal dynamics of bacterial and fungal colonization on plasticdebris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Fletcher; Applied, L.J.; Microbiology, E. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microb. 1979, 37, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natasa, M.D.; James, W.; Radu, C.M.; Paul, R.S. Impact of nano-topography on bacterial attachment. Biotechnol. J. 2008, 3, 536–544. [Google Scholar]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J.J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Smart, J. Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology 1986, 67, 1328–1340. [Google Scholar]
- Hou, X.; Liu, S.; Zhang, Z.J. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Wen, Y.; Ren, Y.; Hao, G.; Zhang, Y.J. Role and significance of extracellular polymeric substances from granular sludge for simultaneous removal of organic matter and ammonia nitrogen. Bioresour. Technol. 2015, 179, 460–466. [Google Scholar] [CrossRef]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, A.; Mehert, A.; Prescott, A.; Kiley, T.B.; Stanley-Wall, N.R.J. YuaB functions synergistically with the exopolysaccharide and TasA amyloid fibers to allow biofilm formation by Bacillus subtilis. J. Bacteriol 2011, 193, 4821–4831. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Mayer, C. Cohesiveness in biofilm matrix. Community Struct. Co-Oper. Biofilms 2000, 59, 87. [Google Scholar]
- QIan, Y.X.; Li, Z.J.; Yang, B.; Zhang, X.W. Study on the Mechanism of Removal of Pollutants from Wastewater by Extracellular Polymers. Indust. Water Treat. 2016, 7, 16–21. [Google Scholar]
- Li, S.; Peng, Y.Z. The role of extracellular polymers in biological wastewater treatment. J. Nat. Sci. Heilongjiang Univ. 2016, 33, 515–520. [Google Scholar]
- Liang, Y.; Jiang, Y.; Wang, F.; Wen, C.; Deng, Y.; Xue, K.; Qin, Y.; Yang, Y.; Wu, L.; Zhou, J.J. Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J. 2015, 9, 2561–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; He, Z.L.; Xiong, J.B. Elevated carbon dioxide accelerates the spatial turnover of soil microbial communities. Global. Chang. Biol. 2015, 22, 957–964. [Google Scholar] [CrossRef]
- Breister, A.M.; Imam, M.A.; Zhou, Z.; Ahsan, M.A.; Noveron, J.C.; Anantharaman, K.; Prabhakar, P.J. Soil microbiomes mediate degradation of vinyl ester-based polymer composites. Commun. Mater. 2020, 1, 101. [Google Scholar] [CrossRef]
- Zhelezova, A.D.; Zverev, A.O.; Zueva, A.I.; Leonov, V.D.; Tiunov, A.V.J. Prokaryotic community formation on polyethylene films incubated for six months in a tropical soil. Environ. Pollut. 2021, 269, 116126. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef]
- Weihong, Z.; Zheng, L.U.; Jianmeng, C.; Xiao, C.; Kedan, S. Conditions and intermediate metabolites of MTBE degradation by beta-Proteobacteria. Environ. Sci. 2006, 27, 2536–2541. [Google Scholar]
- Leen, B.; Dirk, S.; Pierre, W.; Hauke, H.; Rupert, D.W.; Hubert, V.; Ludo, D. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microb. 2000, 66, 1834–1843. [Google Scholar]
- Ko-ichi, O.; Yuji, T.; Tomoaki, N.; Yoshinobu, M. Isolation and characterization of a novel bacterium, Sphingomonas bisphenolicum strain AO1, that degrades bisphenol A. Biodegradation 2007, 18, 247–255. [Google Scholar]
- Martikainen, P.J.; Reittu, A.; Arnold, M.; Von, W.A.; Suihko, M.L. Bacterial degradation of styrene in waste gases using a peat filter. Appl. Microbiol. Biot. 1997, 48, 738–744. [Google Scholar]
- Li, J.; Gu, J.D.; Yao, J.H. Biodegradation, Degradation of dimethyl terephthalate by Pasteurella multocida Sa and Sphingomonas paucimobilis Sy isolated from mangrove sediment. Int. Biodeter. Biodegr. 2005, 56, 158–165. [Google Scholar] [CrossRef]
- Czieborowski, M.; Hübenthal, A.; Poehlein, A.; Vogt, I.; Philipp, B.J.M. Genetic and physiological analysis of biofilm formation on different plastic surfaces by Sphingomonas sp. Strain S2M10 reveals an essential function of sphingan biosynthesis. Microbiol. Res. 2020, 166, 918–935. [Google Scholar] [CrossRef] [PubMed]
- Chistoserdov, A.Y.; Boyd, J.; Mathews, F.S.; Lidstrom, M.E.J.B. The genetic organization of the mau gene cluster of the facultative autotroph Paracoccus denitrificans. Environ. Microbiol. 1992, 184, 1181–1189. [Google Scholar] [CrossRef]
- Starr, E.P.; Shi, S.; Blazewicz, S.J.; Probst, A.J.; Herman, D.J.; Firestone, M.K.; Banfield, J.F. Stable isotope informed genome-resolved metagenomics reveals that Saccharibacteria utilize microbially-processed plant-derived carbon. Microbiome 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Hu, B.X.; Ren, H.; Zhang, J. Composition and functional diversity of microbial community across a mangrove-inhabited mudflat as revealed by 16S rDNA gene sequences. Sci. Total Environ. 2018, 633, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, X.; Wang, X.; Cheng, T.; Fu, K.; Qin, Z.; Feng, K. Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil. Sustainability 2022, 14, 7024. https://doi.org/10.3390/su14127024
Chen Y, Wang X, Wang X, Cheng T, Fu K, Qin Z, Feng K. Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil. Sustainability. 2022; 14(12):7024. https://doi.org/10.3390/su14127024
Chicago/Turabian StyleChen, Yue, Xiaobing Wang, Xiaoli Wang, Tong Cheng, Kuankuan Fu, Zhentian Qin, and Ke Feng. 2022. "Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil" Sustainability 14, no. 12: 7024. https://doi.org/10.3390/su14127024
APA StyleChen, Y., Wang, X., Wang, X., Cheng, T., Fu, K., Qin, Z., & Feng, K. (2022). Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil. Sustainability, 14(12), 7024. https://doi.org/10.3390/su14127024





