Inoculation with the pH Lowering Plant Growth Promoting Bacterium Bacillus sp. ZV6 Enhances Ni Phytoextraction by Salix alba from a Ni-Polluted Soil Receiving Effluents from Ni Electroplating Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of the Bacterial Strain ZV6
2.2. Analysis of Ni-Polluted Soil
2.3. LM and AM Sources
2.4. Pot-Scale Experiment
2.5. Termination of Pot Experiment
2.6. Analysis
2.6.1. Status of Ni in Plant Portions and Soil
2.6.2. Assessment of Chlorophyll-a, Chlorophyll-b and Relative Water Content (RWTC)
2.6.3. Soil Enzymes
3. Results
3.1. Characterization of Rhizospheric Acidifying Bacterial Strain Bacillus sp. ZV6
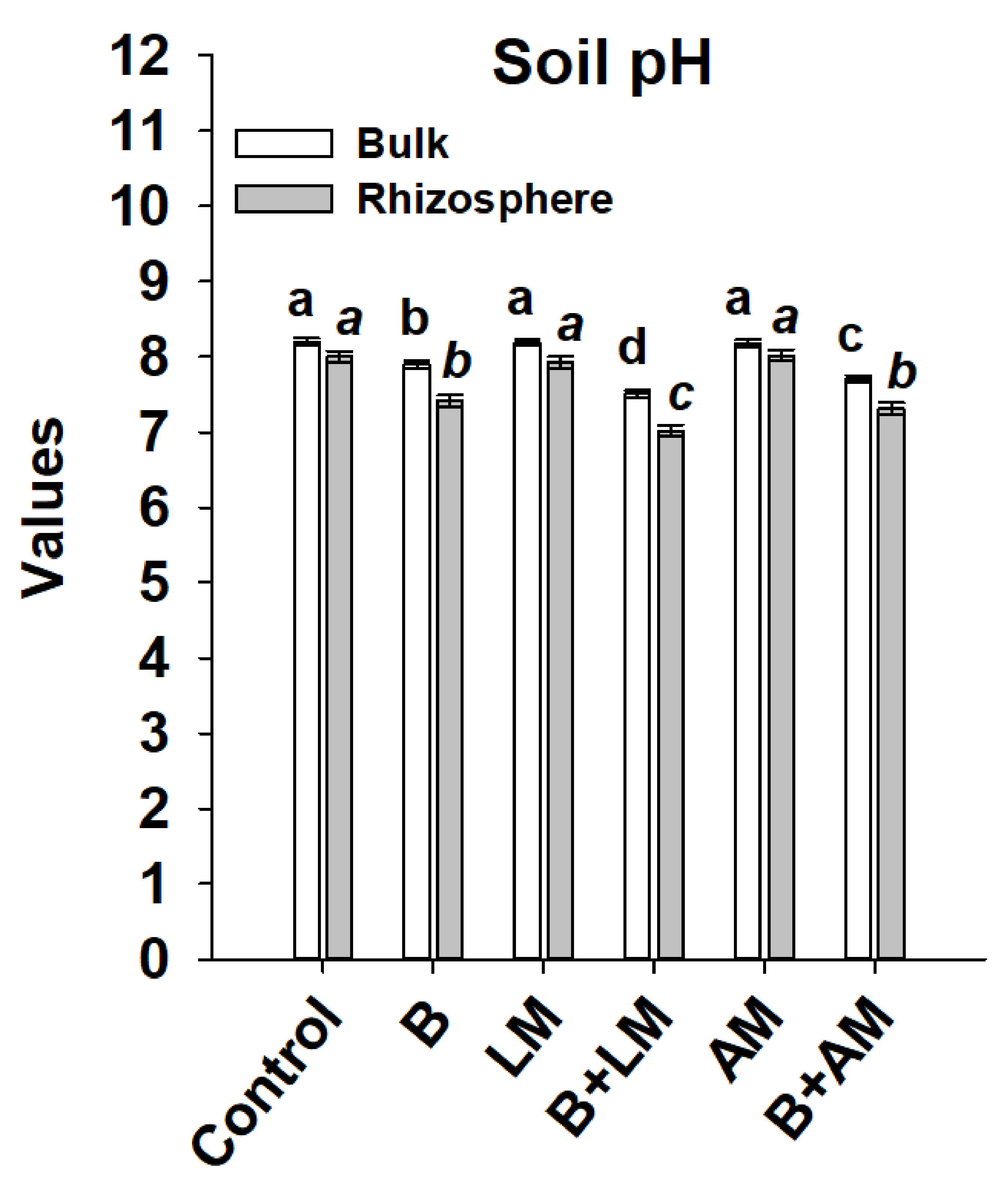
3.2. Changes in pH of Rhizosphere and Bulk Soils
3.3. Growth, Biomass, Chlorophyll and Relative Water Contents
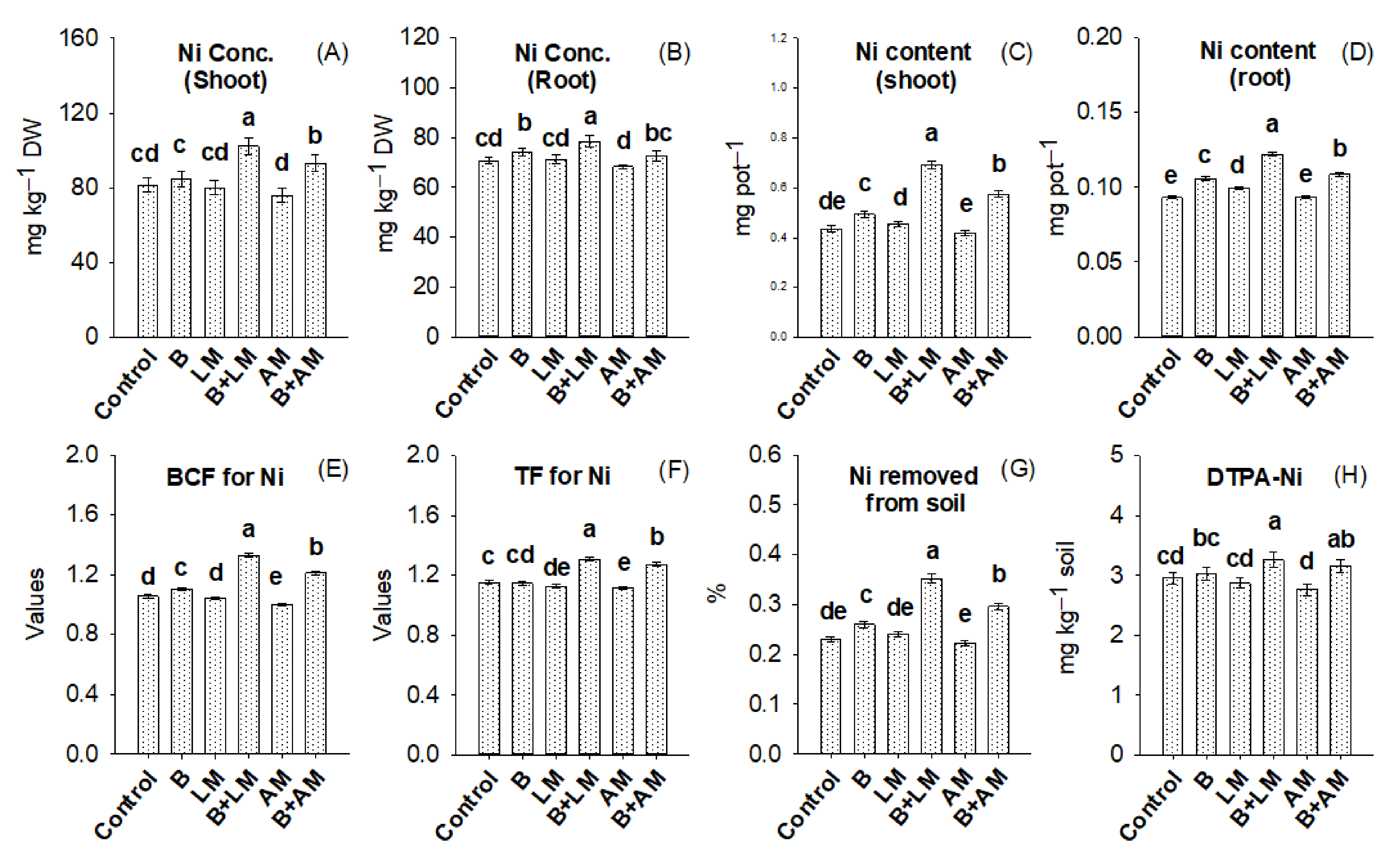
3.4. Ni Distribution in Salix, BCF and TF Values, and Ni Removed from the Soil
3.5. Soil Enzymatic Activities, Bacterial Count, Microbial Biomass, and Organic C
4. Discussion
4.1. Changes in pH of Broth Media and the Salix Bulk and Rhizospheric Soil Portions
4.2. Growth, Biomass, Chl-a, Chl-b, and RWTC
4.3. Ni Distribution in Salix Plant, Values of BCF and TF, and Ni Removed from the Soil
4.4. Soil Enzymatic Activities, Bacterial Count, Microbial Biomass, and Organic C
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasool, B.; Ramzani, P.M.A.; Zubair, M.; Khan, M.A.; Lewińska, K.; Turan, V.; Iqbal, M. Impacts of oxalic acid-activated phosphate rock and root-induced changes on Pb bioavailability in the rhizosphere and its distribution in mung bean plant. Environ. Pollut. 2021, 280, 116903. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, A.K.; Iqbal, M.; Jabbar, A.; Hussain, S.; Ibrahim, M. Assessment of nickel bioavailability through chemical extractants and red clover (Trifolium pratense L.) in an amended soil: Related changes in various parameters of red clover. Ecotoxicol. Environ. Saf. 2018, 149, 116–127. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Jiang, J.Y.; Wang, S.; Guo, Y.P.; Ding, H. Assessment of water-soluble thiourea-formaldehyde (WTF) resin for stabilization/solidification (S/S) of heavy metal contaminated soils. J. Hazard. Mater. 2018, 346, 167–173. [Google Scholar] [CrossRef]
- Pardo, T.; Rodríguez-Garrido, B.; Saad, R.F.; Soto-Vázquez, J.L.; Loureiro-Viñas, M.; Prieto-Fernández, Á.; Echevarria, G.; Benizri, E.; Kidd, P.S. Assessing the agromining potential of Mediterranean nickel-hyperaccumulating plant species at field-scale in ultramafic soils under humid-temperate climate. Sci. Total Environ. 2018, 630, 275–286. [Google Scholar] [CrossRef]
- Saad, R.F.; Kobaissi, A.; Machinet, G.; Villemin, G.; Echevarria, G.; Benizri, E. Crop rotation associating a legume and the nickel hyperaccumulator Alyssum murale improves the structure and biofunctioning of an ultramafic soil. Ecol. Res. 2018, 33, 799–810. [Google Scholar] [CrossRef]
- Houzelot, V.; Laubie, B.; Pontvianne, S.; Simonnot, M.O. Effect of up-scaling on the quality of ashes obtained from hyperaccumulator biomass to recover Ni by agromining. Chem. Eng. Res. Des. 2017, 120, 26–33. [Google Scholar] [CrossRef]
- Nkrumah, P.N.; Baker, A.J.; Chaney, R.L.; Erskine, P.D.; Echevarria, G.; Morel, J.L.; van der Ent, A. Current status and challenges in developing nickel phytomining: An agronomic perspective. Plant Soil 2016, 406, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Van der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Atma, W.; Larouci, M.; Meddah, B.; Benabdeli, K.; Sonnet, P. Evaluation of the phytoremediation potential of Arundo donax L. for nickel-contaminated soil. Int. J. Phytoremediat. 2017, 19, 377–386. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and nickel co-treatment alters metal uptake and stress parameters of Salix purpurea × viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. Phytoremediation 2016, 3, 29–64. [Google Scholar]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.; Cui, J.; Wang, Q. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytoremediat. 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Iqbal, M.; Puschenreiter, M.; Oburger, E.; Santner, J.; Wenzel, W.W. Sulfur-aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ. Pollut. 2012, 170, 222–231. [Google Scholar] [CrossRef]
- Wood, J.L.; Liu, W.; Tang, C.; Franks, A.E. Microorganisms in heavy metal bioremediation: Strategies for applying microbial-community engineering to remediate soils. AIMS Bioeng. 2016, 3, 211–229. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; De La Pena, T.C.; Pueyo, J.J.; Talebi, M. Isolation and characterization of Pb-solubilizing bacteria and their effects on Pb uptake by Brassica juncea: Implications for microbe-assisted phytoremediation. J. Microbiol. Biotechnol. 2018, 28, 1156–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ullah, S.; Ahmad, I.; Rauf, A.; Nadeem, S.M.; Khan, M.Y.; Bulgariu, L. Nickel phytoextraction through bacterial inoculation in Raphanus sativus. Chemosphere 2018, 190, 234–242. [Google Scholar] [CrossRef]
- Durand, A.; Piutti, S.; Rue, M.; Morel, J.L.; Echevarria, G.; Benizri, E. Improving nickel phytoextraction by co-cropping hyperaccumulator plants inoculated by plant growth promoting rhizobacteria. Plant Soil 2016, 399, 179–192. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Ghaderian, S.M.; Rodríguez-Garrido, B.; Prieto-Fernández, Á.; Kidd, P.S. Plant species-specificity and effects of bioinoculants and fertilization on plant performance for nickel phytomining. Plant Soil 2018, 425, 265–285. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, S.; Su, C. Impact of biochar on the bioremediation and phytoremediation of heavy metal (loid) s in soil. Adv. Bioremediat. Phytoremediat. 2018, 149, 183. [Google Scholar]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Dubey, L.; Dubey, M.; Jain, P. Role of green manuring in organic farming. Plant Arch. 2015, 15, 23–26. [Google Scholar]
- Tamele, R.A.; Ueno, H.; Toma, Y.; Morita, N. Nitrogen recoveries and nitrogen use efficiencies of organic fertilizers with different C/N ratios in maize cultivation with low-fertile soil by 15N method. Agriculture 2020, 10, 272. [Google Scholar] [CrossRef]
- Adediran, J.A.; Taiwo, L.B.; Sobulo, R.A. Effect of organic wastes and method of composting on compost maturity, nutrient composition of compost and yields of two vegetable crops. J. Sustain. Agric. 2003, 22, 95–109. [Google Scholar] [CrossRef]
- Wagh, S.P.; Gangurde, S.V. Effect of cow-dung slurry and Trichoderma spp. on quality and decomposition of teak and bamboo leaf compost. Res. J. Agric. For. Sci. 2015, 2320, 6063. [Google Scholar]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Lu, Y.; Yao, H.; Shan, D.; Jiang, Y.; Zhang, S.; Yang, J. Heavy metal residues in soil and accumulation in maize at long-term wastewater irrigation area in Tongliao, China. J. Chem. 2015, 2015, 628280. [Google Scholar] [CrossRef] [Green Version]
- Tauqeer, H.M.; Hussain, S.; Abbas, F.; Iqbal, M. The potential of an energy crop “Conocarpus erectus” for lead phytoextraction and phytostabilization of chromium, nickel, and cadmium: An excellent option for the management of multi-metal contaminated soils. Ecotoxicol. Environ. Saf. 2019, 173, 273–284. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Hafez, M.; Abo El-Ezz, S.F.; Popov, A.I.; Rashad, M. Organic amendments combined with plant growth-promoting rhizobacteria (Azospirillum brasilense) as an eco-friendly by-product to remediate and enhance the fertility of saline sodic-soils in Egypt. Commun. Soil Sci. Plant Anal. 2021, 52, 1416–1433. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S.; Méndez, A.; Gascó, G. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J. Soils Sediments 2014, 14, 483–494. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 2014, 3, 53–66. [Google Scholar] [CrossRef]
- Kisiel, A.; Kępczyńska, E. Medicago truncatula Gaertn. as a model for understanding the mechanism of growth promotion by bacteria from rhizosphere and nodules of alfalfa. Planta 2016, 243, 1169–1189. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Abou-Shanab, R.I.; Delorme, T.A.; Angle, J.S.; Chaney, R.L.; Ghanem, K.; Moawad, H.; Ghozlan, H.A. Phenotypic characterization of microbes in the rhizosphere of Alyssum murale. Int. J. Phytoremediat. 2003, 5, 367–379. [Google Scholar] [CrossRef]
- Adele, N.C.; Ngwenya, B.T.; Heal, K.V.; Mosselmans, J.F.W. Role of plant growth promoting bacteria in driving speciation gradients across soil-rhizosphere-plant interfaces in zinc-contaminated soils. Environ. Pollut. 2021, 279, 116909. [Google Scholar] [CrossRef]
- Zeng, T.; Khaliq, M.A.; Li, H.; Jayasuriya, P.; Guo, J.; Li, Y.; Wang, G. Assessment of Cd availability in rice cultivation (Oryza sativa): Effects of amendments and the spatiotemporal chemical changes in the rhizosphere and bulk soil. Ecotoxicol. Environ. Saf. 2020, 196, 110490. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Magdziak, Z.; Mleczek, M.; Rutkowski, P.; Goliński, P. Diversity of low-molecular weight organic acids synthesized by Salix growing in soils characterized by different Cu, Pb and Zn concentrations. Acta Physiol. Plant 2017, 39, 137. [Google Scholar] [CrossRef]
- Reddy, G.B.; Babu, A.J.; Supriya, R.A.; Swetha, C.S.; Sravanthi, M.; Rao, T.M. Estimation of Nickel from the Poultry Organs Surrounding Tirupati, Andhra Pradesh, India. Int. J. Sci. Environ. Technol. 2017, 6, 1934–1939. [Google Scholar]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Ugbe, J.O. Green manures and NPK fertilizer effects on soil properties, growth, yield, mineral and vitamin C composition of okra (Abelmoschus esculentus (L.) Moench). J. Saudi Soc. Agric. Sci. 2019, 18, 218–223. [Google Scholar] [CrossRef]
- Kamran, M.A.; Eqani, S.A.M.A.S.; Bibi, S.; Xu, R.K.; Monis, M.F.H.; Katsoyiannis, A.; Bokhari, H.; Chaudhary, H.J. Bioaccumulation of nickel by E. sativa and role of plant growth promoting rhizobacteria (PGPRs) under nickel stress. Ecotoxicol. Environ. Saf. 2016, 126, 256–263. [Google Scholar] [CrossRef]
- Khatun, M.F.; Chowdhury, M.A.H.; Islam, M.S.; Haider, S.M.S.; Talukder, M.Z.A. Effects of different tree leaf litters on growth and yield of Okra in Madhupur Forest Soil. Int. J. Exp. Agric. 2010, 1, 5–11. [Google Scholar]
- Sharma, M.; Reynnells, R. Importance of soil amendments: Survival of bacterial pathogens in manure and compost used as organic fertilizers. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Ewees, M.S.; Abdel Hafeez, A.A.A. Response of maize grain yield to a partial substitution of N-mineral by applying organic manure, bio-inoculation and elemental sulphur as an alternative strategy to avoid the possible chemical pollution. Egypt. J. Soil Sci. 2010, 50, 141–166. [Google Scholar]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Baig, K.S.; Arshad, M.; Khalid, A.; Hussain, S.; Abbas, M.N.; Imran, M. Improving growth and yield of maize through bioinoculants carrying auxin production and phosphate solubilizing activity. Soil Environ. 2014, 33, 159–168. [Google Scholar]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef]
- Naeem, I.; Masood, N.; Turan, V.; Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2021, 208, 111723. [Google Scholar] [CrossRef] [PubMed]
- Sajad, M.A.; Khan, M.S.; Bahadur, S.; Shuaib, M.; Naeem, A.; Zaman, W.; Hazrat, A. Nickel phytoremediation potential of some plant species of the Lower Dir, Khyber Pakhtunkhwa, Pakistan. Limnol. Rev. 2020, 20, 13–22. [Google Scholar] [CrossRef]
- Tőzsér, D.; Harangi, S.; Baranyai, E.; Lakatos, G.; Fülöp, Z.; Tóthmérész, B.; Simon, E. Phytoextraction with Salix viminalis in a moderately to strongly contaminated area. Environ. Sci. Pollut. Res. 2018, 25, 3275–3290. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.D.; Wang, Y.Y.; Zhao, F.L.; Ding, Z.L.; Zhang, X.C.; Zhu, Z.Q.; Yang, X.E. Variation in copper and zinc tolerance and accumulation in 12 willow clones: Implications for phytoextraction. J. Zhejiang Univ. Sci. B 2014, 15, 788–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello-Conejo, M.I.; Becerra-Castro, C.; Prieto-Fernández, A.; Monterroso, C.; Saavedra-Ferro, A.; Mench, M.; Kidd, P.S. Rhizobacterial inoculants can improve nickel phytoextraction by the hyperaccumulator Alyssum pintodasilvae. Plant Soil 2014, 379, 35–50. [Google Scholar] [CrossRef]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Bai, X.; Liang, J.; Wei, Y.; Huang, S.; Li, Y.; Dong, L.; Liu, X.; Qu, J.; Yan, L. Inoculation of Pseudomonas sp. GHD-4 and mushroom residue carrier increased the soil enzyme activities and microbial community diversity in Pb-contaminated soils. J. Soils Sediments 2019, 19, 1064–1076. [Google Scholar] [CrossRef]
- Tang, X.; Liu, B.; Deng, Q.; Zhang, R.; Li, X.; Xu, H. Strengthening detoxication impacts of Coprinus comatus on nickel and fluoranthene co-contaminated soil by bacterial inoculation. J. Environ. Manag. 2018, 206, 633–641. [Google Scholar] [CrossRef]
- Antonious, G.F. Biochar and animal manure impact on soil, crop yield and quality. In Agricultural Waste and Residues; Aladjadjiyan, A., Ed.; IntechOpen: London, UK, 2018; pp. 45–67. [Google Scholar]
- Li, X.; Dong, S.; Yao, Y.; Shi, W.; Wu, M.; Xu, H. Inoculation of bacteria for the bioremediation of heavy metals contaminated soil by Agrocybe aegerita. RSC Adv. 2016, 6, 65816–65824. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Ramzani, P.M.A.; Zubair, M.; Rasool, B.; Khan, M.K.; Ahmed, A.; Ali Khan, S.; Turan, V.; Iqbal, M. Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Sci. Total Environ. 2020, 710, 136294. [Google Scholar] [CrossRef]
- Nosheen, A.; Yasmin, H.; Naz, R.; Bano, A.; Keyani, R.; Hussain, I. Pseudomonas putida improved soil enzyme activity and growth of kasumbha under low input of mineral fertilizers. Soil Sci. Plant Nutr. 2018, 64, 520–525. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef] [Green Version]
- Kucharski, J.; Boros, E.; Wyszkowska, J. Biochemical Activity of Nickel-Contaminated Soil. Pol. J. Environ. Stud. 2009, 18, 1039–1044. [Google Scholar]

| Properties | Units | Values |
|---|---|---|
| Sand | % | 16.0 |
| Silt | % | 41.0 |
| Clay | % | 43.0 |
| pH (H2O) | - | 8.20 |
| Organic matter (OM) | % | 1.10 |
| Calcium carbonate (CaCO3) | % | 3.10 |
| Bicarbonate (HCO3) | % | 0.04 |
| Electrical conductivity (EC) | dSm−1 | 1.90 |
| Cation exchange capacity (CEC) | cmolc kg−1 | 16.7 |
| Nitrogen (N) | mg kg−1 | 139.0 |
| Phosphorus (P) | mg kg−1 | 6.90 |
| Potassium (K) | mg kg−1 | 138.0 |
| DTPA-extractable Ni | mg kg−1 | 3.94 |
| Total Ni | mg kg−1 | 77.0 |
| Treatments | Abbreviations | Input Amounts of LM and AM (g kg−1 Soil) | Bacterial Inoculation |
|---|---|---|---|
| Control | Control | - | - |
| Bacteria | B | - | 10 mL bacterial suspension (OD600 = 1.0) |
| Leaf Manure | LM | 50 | - |
| Bacteria + Leaf Manure | B + LM | 50 | 10 mL bacterial suspension (OD600 = 1.0) |
| Animal Manure | AM | 50 | - |
| Bacteria + Animal Manure | B + AM | 50 | 10 mL bacterial suspension (OD600 = 1.0) |
| Treatments | Plant Height (A) | Shoot DRW (B) | Root DRW (C) | Chlorophyll-a (D) | Chlorophyll-b (E) | RWTC (F) |
|---|---|---|---|---|---|---|
| (cm) | (g pot−1) | (mg g−1 FRW) | (%) | |||
| Control | 81.1 ± 2.05 d | 5.36 ± 0.13 d | 1.32 ± 0.02 d | 1.25 ± 0.04 d | 0.90 ± 0.02 d | 72.1 ± 1.82 d |
| B | 91.0 ± 2.28 bc | 5.82 ± 0.14 bc | 1.43 ± 0.03 bc | 1.42 ± 0.04 bc | 1.12 ± 0.05 bc | 79.0 ± 1.99 abc |
| LM | 87.6 ± 2.22 bcd | 5.69 ± 0.14 cd | 1.40 ± 0.03 bcd | 1.39 ± 0.04 c | 1.05 ± 0.04 c | 77.3 ± 1.94 bcd |
| B + LM | 98.3 ± 2.48 a | 6.76 ± 0.17 a | 1.56 ± 0.03 a | 1.60 ± 0.03 a | 1.25 ± 0.04 a | 85.0 ± 2.14 a |
| AM | 84.4 ± 2.14 cd | 5.51 ± 0.14 cd | 1.37 ± 0.03 cd | 1.37 ± 0.05 cd | 0.94 ± 0.03 d | 75.0 ± 1.88 cd |
| B + AM | 93.0 ± 2.34 ab | 6.16 ± 0.15 b | 1.50 ± 0.04 ab | 1.52 ± 0.04 ab | 1.17 ± 0.03 ab | 81.5 ± 2.05 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virk, Z.A.; Al Farraj, D.A.; Iqbal, M.; Lewińska, K.; Hussain, S. Inoculation with the pH Lowering Plant Growth Promoting Bacterium Bacillus sp. ZV6 Enhances Ni Phytoextraction by Salix alba from a Ni-Polluted Soil Receiving Effluents from Ni Electroplating Industry. Sustainability 2022, 14, 6975. https://doi.org/10.3390/su14126975
Virk ZA, Al Farraj DA, Iqbal M, Lewińska K, Hussain S. Inoculation with the pH Lowering Plant Growth Promoting Bacterium Bacillus sp. ZV6 Enhances Ni Phytoextraction by Salix alba from a Ni-Polluted Soil Receiving Effluents from Ni Electroplating Industry. Sustainability. 2022; 14(12):6975. https://doi.org/10.3390/su14126975
Chicago/Turabian StyleVirk, Zaheer Abbas, Dunia A. Al Farraj, Muhammad Iqbal, Karolina Lewińska, and Sabir Hussain. 2022. "Inoculation with the pH Lowering Plant Growth Promoting Bacterium Bacillus sp. ZV6 Enhances Ni Phytoextraction by Salix alba from a Ni-Polluted Soil Receiving Effluents from Ni Electroplating Industry" Sustainability 14, no. 12: 6975. https://doi.org/10.3390/su14126975
APA StyleVirk, Z. A., Al Farraj, D. A., Iqbal, M., Lewińska, K., & Hussain, S. (2022). Inoculation with the pH Lowering Plant Growth Promoting Bacterium Bacillus sp. ZV6 Enhances Ni Phytoextraction by Salix alba from a Ni-Polluted Soil Receiving Effluents from Ni Electroplating Industry. Sustainability, 14(12), 6975. https://doi.org/10.3390/su14126975







