The Valorisation of Selected Quarry and Mine Waste for Sustainable Cement Production within the Concept of Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical and Mineralogical Characterization of the SRM
2.2.2. Radiological Characterization of SRM
2.2.3. Characterization of the Cements
3. Results and Discussion
3.1. Characterization of the Secondary Raw Materials
3.1.1. Mineralogical Composition
3.1.2. Major Oxides
3.1.3. Trace Elements
3.1.4. Rare Earth Elements
3.1.5. Radiological Characterization
Dose Calculations
3.2. Characterization of Cement
3.2.1. Phase Composition
3.2.2. Hydration Kinetics
3.2.3. Compressive Strength
4. Conclusions
- –
- Following the concept of a circular economy, it is possible to use the investigated quarry and mine waste for the synthesis of belite-sulfoaluminate cement.
- –
- Different experimental raw mixtures containing either 27.96 wt. % clay residue from a limestone quarry, 43.22 wt. % calcite residue from a limestone quarry, or 10.43 wt. % Pb-Zn mine waste were suitable for the synthesis of BSCA clinkers with targeted phase composition.
- –
- Radiological characterization (I-index and dose assessment) of the quarry and mine wastes are mainly (except D) comparable to the average ranges worldwide. Generally, it can be concluded that all three wastes have a low impact from a radiological point of view, especially given that they are only used in a certain proportion of the final product.
- –
- The hydraulic reactivity of cements, as evidenced by isothermal calorimetry, showed that C1 and C3 are slightly more reactive, with a shorter induction period in comparison to C2. The reactivity of cements is primarily connected to the highly reactive calcium sulfoaluminate phase (resulting in the formation of ettringite) and ferrite on one hand and the slowly reactive gehlenite on the other. In addition to the reactivity of the cement, the particle size distribution of the cements has also an impact (with D50 being lower in C1 and C3).
- –
- The slightly higher reactivity of the C1 and C3 cements result in a slightly higher compressive strength in comparison to C2, which is connected to the chemical composition of the waste used.
- –
- The present paper demonstrates the potential application of quarry (clay and calcite residues) and mine (Pb-Zn) wastes as raw materials in the production of BCSA cements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.iea.org/reports/global-status-report-for-buildings-and-construction-2019 (accessed on 15 February 2022).
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef] [Green Version]
- Garside, M. Global Cement Production 1995–2020, Statista 2022. Available online: https://www.statista.com/statistics/1087115/global-cement-production-volume (accessed on 30 March 2022).
- Favier, A.; De Wolf, C.; Scrivener, K.; Habert, G. A Sustainable Future for the European Cement and Technology Assessment for Full Decarbonisation of The Industry by 2050, EPFL. Available online: https://europeanclimate.org/wp-content/uploads/2018/10/AB_SP_Decarbonisation_report.pdf (accessed on 30 March 2022).
- Scrivener, K.L.; Vanderley, M.; John, V.M.; Ellis, M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Žibret, G.; Teran, K.; Žibret, L.; Šter, K.; Dolenec, S. Building of the Al-containing Secondary Raw Materials Registry for the Production of Low CO2 Mineral Binders in South-Eastern European Region. Sustainability 2021, 13, 1535. [Google Scholar] [CrossRef]
- Roadmap to a Resource Efficient Europe COM(2011) 571. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52011DC0571 (accessed on 15 February 2022).
- Thapa, V.B.; Waldmann, D.; Simon, C. Gravel wash mud, a quarry waste material as supplementary cementitious T material (SCM). Cem. Conr. Res. 2019, 124, 105833. [Google Scholar] [CrossRef] [Green Version]
- Dolenec, S.; Malovrh Rebec, K.; Lesek, A.; Ster, K.; Zibret, L.; Zibret, G.; Teran, K.; Pucko, E.; Merta, I.; Poletanovic, B.; et al. Manual for Use of Al-Containing Residues in Low-Carbon Mineral Binders; Slovenian National Building and Civil Engineering Institute: Ljubljana, Slovenia, 2020; p. 124. ISBN 978-961-94071-9-6. [Google Scholar]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Efect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Da Costa, E.B.; Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Gobbo, L.A.; Kirchheim, A.P. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr. Build. Mater. 2016, 122, 373–383. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, Á.G.; Cuberos, A.J.M.; Álvarez-Pinazo, G.; Cuesta, A.; Aranda, M.A.G. In situ powder diffraction study of belite sulfoaluminate clinkering. J. Synchrotron Radiat. 2011, 18, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.P.; Zhang, L. High-Performance Cement Matrices Based on Calcium Sulfoaluminate–Belite Compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Mrak, M.; Winnefeld, F.; Lothenbach, B.; Dolenec, S. The influence of calcium sulfate content on the hydration of belite-calcium sulfoaluminate cements with different clinker phase compositions. Mater. Struct. 2021, 54, 212. [Google Scholar] [CrossRef]
- Zibret, L.; Ipavec, A.; Dolenec, S. Microstructural characteristics of belite–sulfoaluminate cement clinkers with bottom ash. Constr. Build. Mater. 2022, 321, 126289. [Google Scholar] [CrossRef]
- Žibret, L.; Šter, K.; Borštnar, M.; Loncnar, M.; Dolenec, S. The Incorporation of Steel Slag into Belite-Sulfoaluminate Cement Clinkers. Appl. Sci. 2021, 11, 1840. [Google Scholar] [CrossRef]
- Kramar, S.; Žibret, L.; Fidanchevska, E.; Jovanov, V.; Angjusheva, B.; Ducman, V. Use of fly ash and phosphogypsum for the synthesis of belite-sulfoaluminate clinker. Mater. Constr. 2019, 69, 1–12. [Google Scholar] [CrossRef]
- Gou, M.; Zhou, L.; Then, N.W.Y. Utilization of tailings in cement and concrete: A review. Sci. Eng. Compos. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Li, R.; He, W.; Zhang, J.; Wang, Y.; Zhang, Y.; Nie, D. Preparation of belite–sulphoaluminate cement using phosphate rock acid-insoluble residue. Constr. Build. Mater. 2022, 323, 126573. [Google Scholar] [CrossRef]
- Nouairi, J.; Hajjaji, W.; Costa, C.S.; Senff, L.; Patinha, C.; Ferreira da Silva, E.; Labrincha, J.A.; Rocha, F.; Medhioub, M. Study of Zn-Pb ore tailings and their potential in cement technology. J. Afr. Earth Sci. 2018, 139, 165–172. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Lu, Z.; Ng, S.; Niu, Y.; Jiang, J.; Xu, Y.; Lai, Z.; Liu, H. The Role of Brownmillerite in Preparation of High-Belite Sulfoaluminate Cement Clinker. Appl. Sci. 2022, 12, 4980. [Google Scholar] [CrossRef]
- RIS-ALiCE Project, RIS-ALiCE: Al-Rich Industrial Residues for Mineral Binders in ESEE Region. Available online: https://ris-alice.zag.si (accessed on 15 February 2022).
- Majling, J.; Strigác, J.; RoY, D.M. Generalized Bogue computations to forecast the mineralogical composition of sulfoaluminate cements based on fly ashes. Adv. Cem. Res. 1999, 11, 27–34. [Google Scholar] [CrossRef]
- EN 196-2; Method of Testing Cement—Part 2: Chemical Analysis of Cement. British Standards Institution: London, UK, 2013. Available online: https://www.en-standard.eu/bs-en-196-2-2013-method-of-testing-cement-chemical-analysis-of-cement/ (accessed on 30 June 2013).
- MSZ 525-17; Chemical Analysis of Cement. Part 17: Determination of Total Chrome Content as Cr2O3. Hungarian Standards Institution: Budapest, Hungary, 2013. Available online: http://www.mszt.hu/web/guest/webaruhaz;jsessionid=F7A616D8B7651350184650DE1E6EC186?p_p_id=msztwebshop_WAR_MsztWAportlet&p_p_lifecycle=1&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_msztwebshop_WAR_MsztWAportlet_ref=157252&_msztwebshop_WAR_MsztWAportlet_javax.portlet.action=search (accessed on 1 December 2013).
- IAEA. Measurement of Radionuclides in Food and the Environment; Technical Report Series No. 295; IAEA: Vienna, Austria, 1989. [Google Scholar]
- CMI. Radioactive Standard Solutions, ER X, Cert. No. 1035-SE-40844-17 Czech Metrology Institute Prague. 2017. Available online: https://www.cmi.cz/Certificates%20of%20accreditation?language=en (accessed on 1 January 2017).
- Vidmar, T. EFFTRAN—A Monte Carlo efficiency transfer code for gamma-ray spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. A 2005, 550, 603–608. [Google Scholar] [CrossRef]
- Nenadović, S.; Fereone, C.; Nenadović, M.; Cioffi, R.; Mirković, M.; Vukanac, I.; Kljajević, L.J. Chemical, physical and radiological evaluation of raw materials and geopolymers for building application. J. Radioanal. Nucl. Chem. 2020, 325, 435–445. [Google Scholar] [CrossRef]
- Snellings, R.; Chwast, J.; Cizer, O.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: Hydration stoppage methods for phase assemblage studies of blended cements—Results of a round robin test. Mater. Struct. 2018, 51, 111. [Google Scholar] [CrossRef]
- Snellings, R. X-ray powder diffraction applied to cement. In A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 126–195. [Google Scholar] [CrossRef]
- Cuesta, A.; Alvarez-Pinazo, G.; Sanfelix, S.G.; Pearl, I.; Aranda, M.A.G.; De la Tore, A.G. Hydration mechanisms of two polymorphs of synthetic ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y. Effect of MgO on the composition and properties of alite-sulphoaluminate cement. Cem. Concr. Res. 2005, 35, 1685–1687. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. CEN: Brussels, Belgium, 2011.
- Gosar, M.; Šajn, R.; Bavec, Š.; Gaberšek, M.; Pezdir, V.; Miler, M. Geochemical background and threshold for 47 chemical elements in Slovenian topsoil. Geologija 2019, 62, 7–59. [Google Scholar] [CrossRef]
- Keppert, M.; Scheinherrová, L.; Jerman, M.; Doušová, B.; Kobera, L.; Brus, J.; Černý, R. Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent. Materials 2019, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Shimosaka, K.; Inoue, T.; Tanaka, H.; Kishimoto, Y. Influence of Minor Elements in Clinker on the Properties of Cement: A New Approach for Application to Commercial Cement Manufacturing. Trans. Mater. Res. Soc. Jpn. 2007, 32, 647–652. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Zhang, L.; Yang, K.; Guan, X.; Zhao, R. Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. Reuse of base-metal tailings as aggregates for rendering mortars: Assessment of immobilization performances and environmental behavior, Constr. Build. Mater. 2015, 96, 296–306. [Google Scholar] [CrossRef]
- EU Directive 2003/53/EC on Chromium in Cement. Directive 2003/53/EC of the European Parliament and of the Council of 18 June 2003 Amending for the 26th Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Nonylphenol, Nonylphenol Ethoxylate and Cement). Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003L0053:en:NOT (accessed on 30 March 2022).
- Rudnick, R.L.; Gao, S. The Composition of the Continental Crust. In The Crust; Holland, H.D., Turekian, K.K., Eds.; Treatise on Geochemistry; Elsevier-Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar] [CrossRef]
- UN Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR 2008 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 2010; ISBN 978-92-1-142274-0. [Google Scholar]
- Gbenu, S.T.; Oladejo, O.F.; Alayande, O.; Olukotun, S.F.; Fasasi, M.K.; Balogun, F.A. Assessment of radiological hazard of quarry products from southwest Nigeria. J. Radiat. Res. Appl. Sci. 2016, 9, 20–25. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Radiation Protection 112. Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; Directorate-General Environment, Nuclear Safety and Civil Protection: Luxembourg, 1999; ISBN 92-828-8376-0. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Garcıa-Mate, M.; De la Torre, A.G.; Leon-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Muller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSAcements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Morin, V.; Termkhajornkit, P.; Huet, B.; Pham, G. Impact of quantity of anhydrite, water to binder ratio, fineness on kinetics and phase assemblage of belite-ye’elimite-ferrite cement. Cem. Concr. Res. 2017, 99, 8–17. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 15. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781138747234. [Google Scholar]
- Rungchet, A.; Poon, C.S.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Chen, X.; Zhang, W.; Yang, D. Synthesis and calorimetric study of hydration behavior of sulfate-rich belite sulfoaluminate cements with different phase compositions. J. Therm. Anal. Calorim. 2018, 133, 1281–1289. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
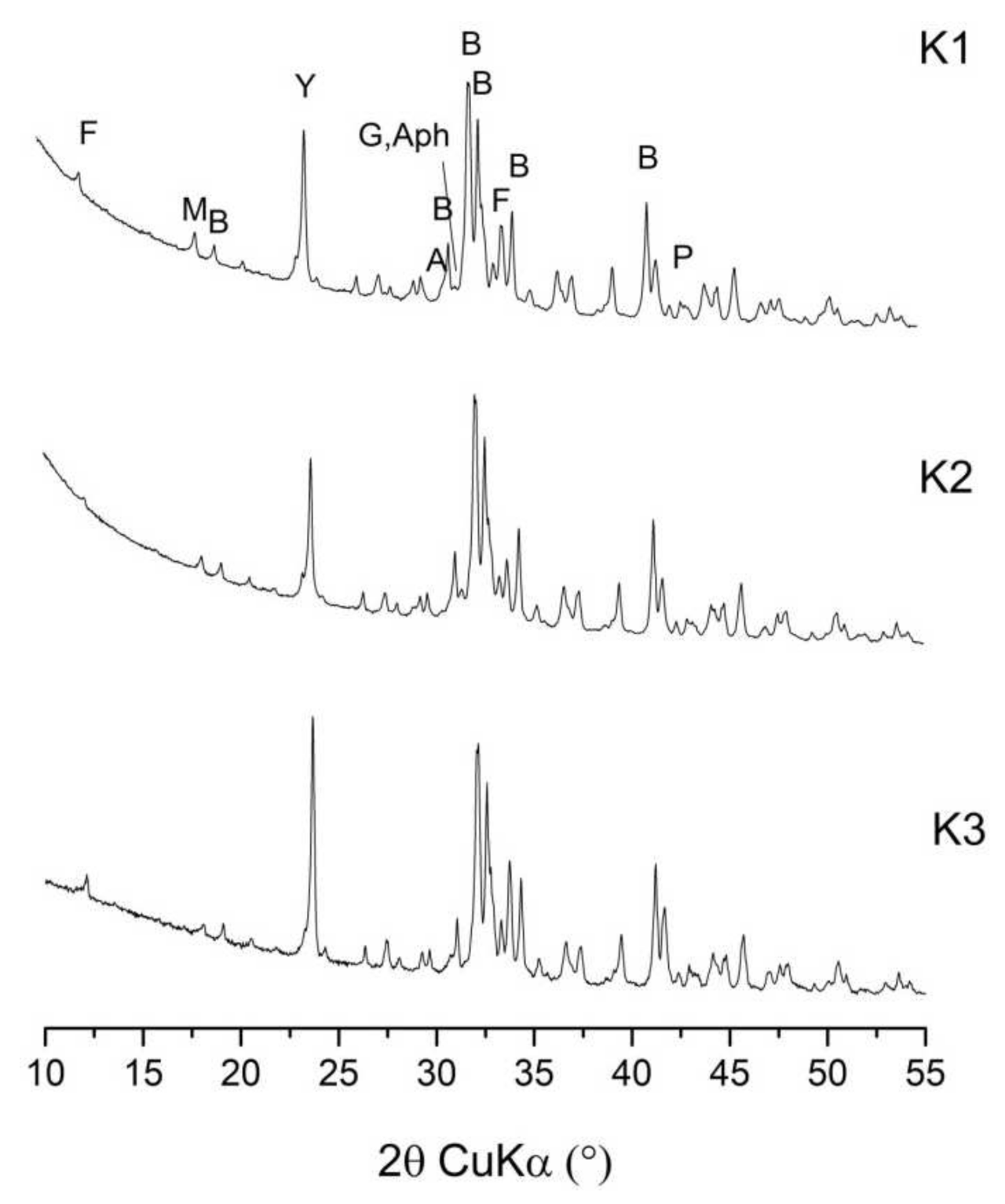

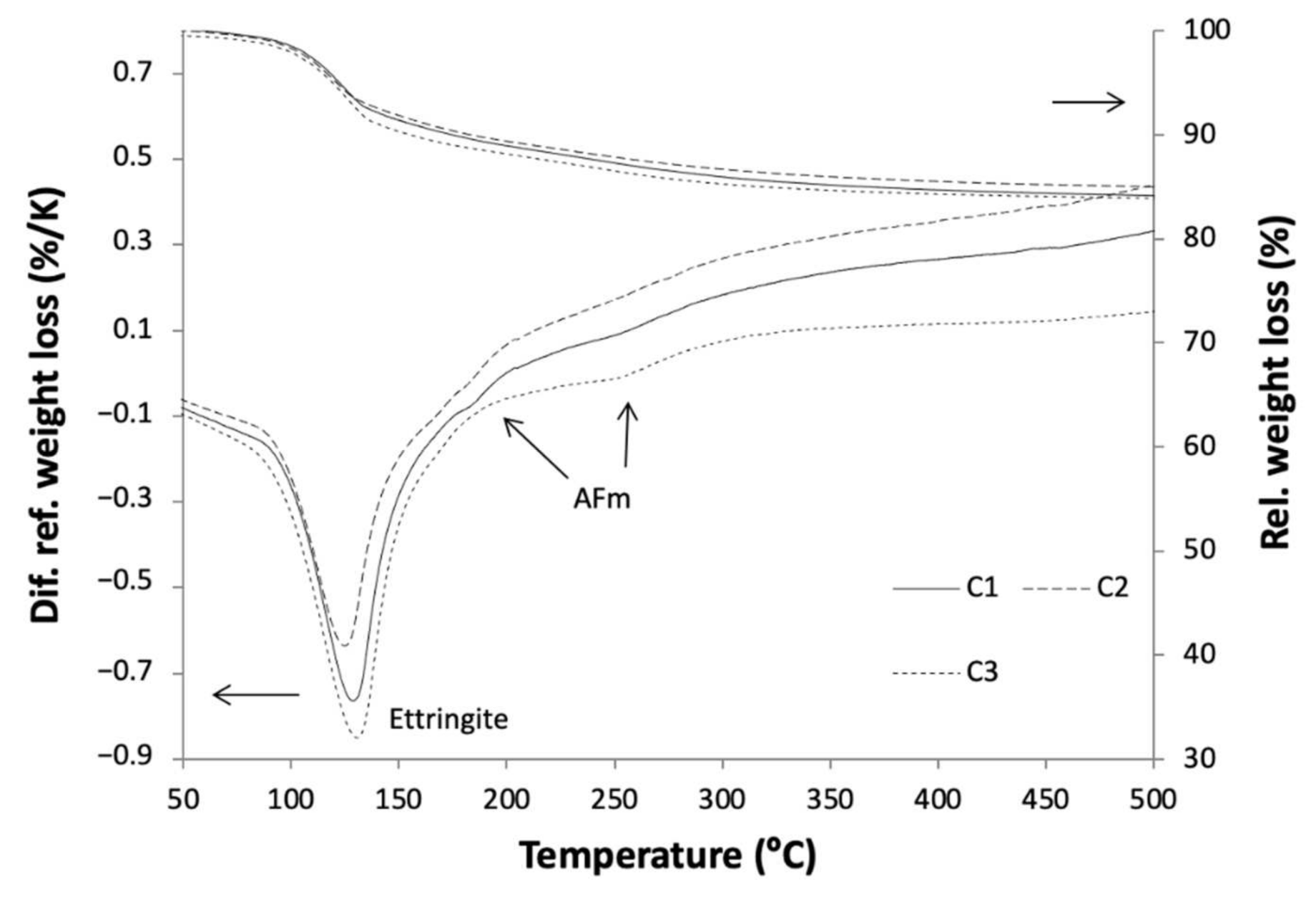
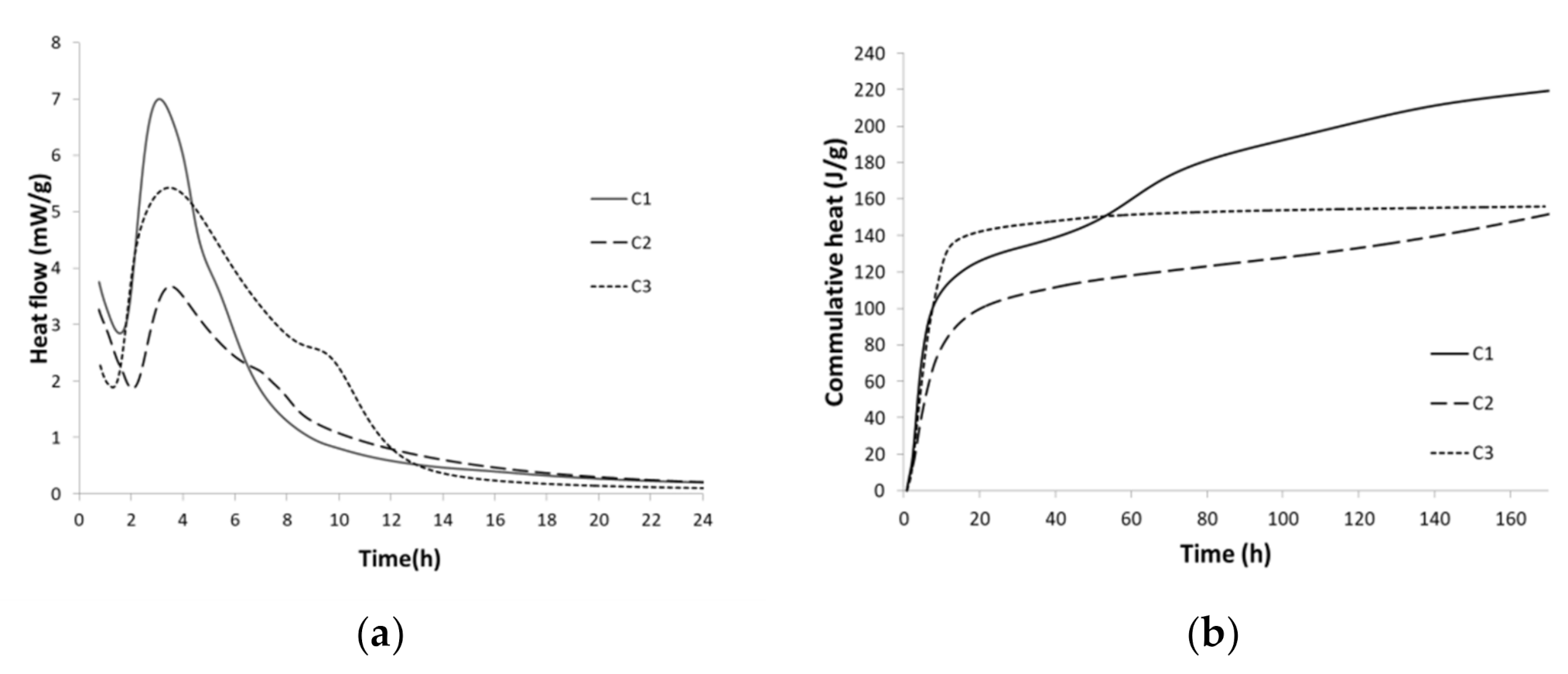
| Clinker | Limestone | Flysch | W1 | W2 | W3 | Gypsum | Bauxite | Mill Scale | Sum |
|---|---|---|---|---|---|---|---|---|---|
| K1 | 63.83 | 0.00 | 27.97 | 0.00 | 0.00 | 3.71 | 4.07 | 0.42 | 100.00 |
| K2 | 0.00 | 47.75 | 0.00 | 43.22. | 0.00 | 3.78 | 4.80 | 0.45 | 100.00 |
| K3 | 56.10 | 24.01 | 0.00 | 0.00 | 10.49 | 3.83 | 5.57 | 0.00 | 100.00 |
| Phase Composition | K1 | K2 | K3 |
|---|---|---|---|
| Calcium sulfoaluminate | 17.3 | 15.8 | 22.6 |
| Belite | 69.1 | 71.4 | 64.8 |
| Mayenite | 1.7 | 1.4 | 0.4 |
| Ferrite | 8.9 | 4.9 | 9.0 |
| Gehlenite | 0.4 | 1.5 | 0.0 |
| Periclase | 1.1 | 1.1 | 1.2 |
| Perovskite | 0.0 | 1.4 | 1.2 |
| Arcanite | 1.0 | 1.1 | 0.2 |
| Aphthitalite | 0.5 | 1.4 | 0.6 |
| Sum | 100.0 | 100.0 | 100.0 |
| Cement | D10 | D50 | D90 |
|---|---|---|---|
| C1 | 1.46 | 8.60 | 44.03 |
| C2 | 1.38 | 14.48 | 57.07 |
| C3 | 1.34 | 7.31 | 44.89 |
| Sample | Amorphous | Crystalline | Sum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | C | I/M | K | F | Chl | D | P | H | S | |||
| W1 | 42.3 | 21.1 | 16.6 | 16.0 | 2.6 | - | 1.1 | 0.3 | - | - | - | 100.00 |
| W2 | 2.8 | 0.1 | 96.2 | - | - | - | - | 0.9 | - | - | - | 100.00 |
| W3 | 32.9 | 23.8 | 9.3 | 5.3 | - | 4.8 | 22.3 | 1.2 | 0.3 | 0.1 | 100.00 | |
| Sample | LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl− | Na2O | K2O | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 16.17 | 45.29 | 14.72 | 5.15 | 13.60 | 1.62 | 0.12 | 0.008 | 0.12 | 1.84 | 0.11 | 0.81 |
| W2 | 42.70 | 1.01 | 1.01 | 0.17 | 53.61 | 0.91 | 0.09 | 0.002 | 0.01 | 0.02 | <0.01 | 0.02 |
| W3 | 5.54 | 48.98 | 11.61 | 11.62 | 9.15 | 2.58 | 0.07 | 0.009 | 0.47 | 1.84 | 0.41 | 0.85 |
| Trace/Heavy Elements | W1 | W2 | W3 |
|---|---|---|---|
| Ag | 0.034 * | 0.035 * | b.q.l. |
| As | 21.2 | 4.1 | 34.7 |
| Ba | 249 | 11.0 | 513 |
| Be | 3.0 | <1 | 3.3 |
| Cd | 0.5 | 0.5 | 9.9 |
| Co | 18.3 | 0.6 | 12.9 |
| Cr | 88.0 | 8.0 | 118 |
| Cu | 24.6 | 4.4 | 101 |
| Ga | 18.4 | 0.9 | b.q.l |
| Hg | 0.169 | 0.026 | 0.025 |
| Li | 69.5 | 2.1 | n.d |
| Mn | 353 | 54 | 10,672 |
| Nb | 10.0 | 0.6 | b.q.l. |
| Ni | 63.2 | 2.8 | 77.6 |
| Pb | 26.3 | 7.4 | 3217 |
| Sb | 1.5 | 0.2 | b.q.l. |
| Se | 0.5 | <0.3 | n.d |
| Sr | 74.0 | 116 | 274 |
| Ta | 0.7 | <0.1 | b.q.l. |
| Th | 10.7 | 0.9 | b.q.l. |
| TI | 0.9 | 0.1 | n.d |
| U | 2.7 | 0.2 | b.q.l |
| V | 121 | 3.0 | 103 |
| Zn | 98.6 | 13.5 | 2189 |
| Zr | 67.0 | 5.3 | 150 |
| Sample | Light REE | Heavy REE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce | Eu | Gd | La | Nd | Pr | Sm | Sc | Dy | Er | Ho | Lu | Tb | Tm | Yb | Y | |
| W1 | 64.4 | 0.9 | 4.6 | 30.9 | 26.8 | 6.8 | 5.3 | 14.0 | 3.7 | 1.6 | 0.7 | 0.2 | 0.6 | 0.3 | 2 | 19.1 |
| W2 | 5.0 | <0.1 | 0.5 | 2.9 | 2.4 | 0.5 | 0.4 | 0.6 | 0.5 | 0.1 | 0.1 | <0.1 | <0.1 | <0.1 | 0.3 | 2.4 |
| W3 | 50.6 | <5.0 | <15 | 27.4 | 22.8 | <15 | <7.5 | 9.5 | <7.5 | <7.5 | <7.5 | * | <7.5 | <7.5 | <2 | 18.7 |
| Activity Concentration, [Bq·kg−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | 210Pb | 226Ra | 232Th | 40K | 137Cs | 238U | 235U | 235U/238U |
| W1 | 51.4 ± 6.1 | 41.2 ± 2.7 | 41.8 ± 3.1 | 568.0 ± 3.1 | <0.04 | 56.6 ± 6.4 | 2.5 ± 0.4 | 0.044 |
| W2 | <1 | <10.0 | 2.3 ± 0.4 | 18.0 ± 1.9 | <0.03 | 3.1 ± 1.0 | <0.2 | <0.065 |
| W3 | 17.6 ± 3.3 | 34.0 ± 2.0 | 15.0 ± 2.0 | 345.0 ± 25.0 | <0.2 | 19.3 ± 4.7 | 1.3 ± 0.2 | 0.067 |
| Sample | Gamma index I | Raeq [Bqkg−1] | Hex [Bqkg−1] | Ḋ [nGyh−1] | EDR [mSvy−1] |
|---|---|---|---|---|---|
| W1 | 0.54 | 145 | 0.409 | 129 | 0.634 |
| W2 | 0.02 | 14.7 | 0.040 | 13.2 | 0.645 |
| W3 | 0.30 | 96.3 | 0.271 | 86.4 | 0.423 |
| Phase Composition | C1 | C2 | C3 |
|---|---|---|---|
| ∑ Calcium sulfoaluminate | 0 | 0 | 0 |
| Belite-beta | 35.6 | 33.3 | 25.7 |
| Belite-gamma | 4.1 | 4.4 | 5.8 |
| ∑ Belite | 39.7 | 37.7 | 31.5 |
| Mayenite | 0.0 | 0.0 | 0.0 |
| Ferrite | 2.0 | 0.0 | 1.6 |
| Gehlenite | 0.6 | 1.1 | 0.0 |
| Perovskite | 0.1 | 0.7 | 0.0 |
| Periclase | 0.3 | 0.4 | 0.0 |
| Ettringite | 13.0 | 11.6 | 19.5 |
| Monosulfate | 1.9 | 2 | 0.6 |
| Strätlingite | 0.9 | 2.2 | 0.1 |
| Amorphous | 41.5 | 44.3 | 46.7 |
| Sum | 100.0 | 100.0 | 100.0 |
| Sample | Compressive Strength (N/mm2) |
|---|---|
| C1 | 20.0 ± 0.9 |
| C2 | 18.5 ± 1.3 |
| C3 | 20.6 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidanchevski, E.; Šter, K.; Mrak, M.; Kljajević, L.; Žibret, G.; Teran, K.; Poletanovic, B.; Fidanchevska, M.; Dolenec, S.; Merta, I. The Valorisation of Selected Quarry and Mine Waste for Sustainable Cement Production within the Concept of Circular Economy. Sustainability 2022, 14, 6833. https://doi.org/10.3390/su14116833
Fidanchevski E, Šter K, Mrak M, Kljajević L, Žibret G, Teran K, Poletanovic B, Fidanchevska M, Dolenec S, Merta I. The Valorisation of Selected Quarry and Mine Waste for Sustainable Cement Production within the Concept of Circular Economy. Sustainability. 2022; 14(11):6833. https://doi.org/10.3390/su14116833
Chicago/Turabian StyleFidanchevski, Emilija, Katarina Šter, Maruša Mrak, Ljiljana Kljajević, Gorazd Žibret, Klemen Teran, Bojan Poletanovic, Monika Fidanchevska, Sabina Dolenec, and Ildiko Merta. 2022. "The Valorisation of Selected Quarry and Mine Waste for Sustainable Cement Production within the Concept of Circular Economy" Sustainability 14, no. 11: 6833. https://doi.org/10.3390/su14116833
APA StyleFidanchevski, E., Šter, K., Mrak, M., Kljajević, L., Žibret, G., Teran, K., Poletanovic, B., Fidanchevska, M., Dolenec, S., & Merta, I. (2022). The Valorisation of Selected Quarry and Mine Waste for Sustainable Cement Production within the Concept of Circular Economy. Sustainability, 14(11), 6833. https://doi.org/10.3390/su14116833









