Abstract
Oil well acidizing, although a stimulation process, induces the corrosion of metallic equipment and well tubing. There is, at present, a high demand for effective and less toxic high-temperature corrosion inhibitors for the acidizing process due to the failing of the existing inhibitors at high temperatures occasioned by increases in the well depths. In this study, aspartame (ASP), a commercially available natural compound, is examined as a corrosion inhibitor for T95 carbon steel in 15 wt.% HCl solution at 60, 70, 80, and 90 °C using the weight loss, electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP), scanning electron microscope (SEM), energy dispersive spectroscopy (EDX), and optical profilometry (OP) techniques. It was found that ASP possesses a corrosion inhibiting effect at the studied conditions. Inhibition efficiency increased with increases in temperature. With 2000 ppm ASP, inhibition efficiency of 86% was achieved from the weight loss method at 90 °C after 4 h of immersion. Results from the electrochemical techniques are in good agreement with the weight loss results. PDP results reveal that ASP acted as a mixed-type corrosion inhibitor under the investigated conditions. The inhibition ability of ASP is due to adsorption on the steel surface and has been confirmed by the SEM, OP, and EDX results. ASP is a promising active compound for the formulation of acidizing corrosion inhibitors.
1. Introduction
Well stimulation is one aspect of oil and gas production development in the petroleum industry. The acidizing approach in well stimulation to activate the oil well reservoirs is one of numerous operational activities. It involves the injection of strong acids into the reservoir to dissolve soluble components or create wormhole channels to increase permeability and eliminate formation damage [1]. The acidizing process dissolves acid-soluble components inside underground rock formations and aids in the removal of material that has clogged the formation at the wellbore face, allowing more oil or gas to flow out of the production wells or to allow oil-displacing fluids to flow into injection wells [2,3]. The main goal of acidizing is to boost oil well fluid production by improving the drainage efficiency of the reservoir rock surrounding the wellbore. Strong acids, such as HCl, HF, HF mixes, and mild acids, such as acetic and formic acids, are frequently employed [4]. HCl is the most used acid in this technique since the reaction products are water-soluble. Typically, 15–28% HCl is used to finish the process [5]. The corrosion of structural materials exposed to acid occurs throughout these procedures. Mixing tanks, pipelines, casings, tubing, and storage facilities are examples of such materials, typically made of API-grade low-carbon steel because it is inexpensive and readily available [6]. Steel grade refers to the steel’s yield strength. H-40, J-55, K-55, N-80, L-80, C-90, T-95, P-110, and Q-125 are the nine steel grade classifications. The letters denote the steel grade, which has nothing to do with the attributes, and the number denotes the minimum yield strength. The higher the number, the greater the yield strength. Furthermore, the API standard divides the nine categories into four groups, with H, J, K, N in the first, L, C, T in the second, P in the third, and Q in the fourth. The steel grade in the first group has low strength. Grades L, C, and T denote limited-yield casing and tubing with anti-sulfur and anti-corrosive properties. L-80 is a restricted-yield tubing grade that comes in three varieties. L80-1, L80-9CR, and L80-13Cr are the three different types of L80. C-90 is a brand-new API grade that comes in two varieties: C90-1 and C90-2. In sour conditions, only C90-1 is recommended. This grade must be specially ordered, and it has been gradually phased out in favor of T-95. T-95 has high strength and is resistant to sulfide stress corrosion. However, it is not weight loss resistant. It also comes in two varieties: T95-1 and T95-2. The T95-2 variety is used in this work, and for convenience’s sake, T95 shall be used henceforth.
Corrosion inhibitor technology is a practical, dependable, and cost-effective corrosion mitigation strategy [7,8,9]. Effective corrosion inhibitors must significantly reduce the rate of metallic corrosion during acidizing treatments. Corrosion inhibitors protect metal surfaces by adsorbing onto the surface through their active functionalities. The use of some natural compounds as corrosion inhibitors is motivated by the known dangers of most synthetic corrosion inhibitors. The traditional acidizing corrosion inhibitor, propargyl alcohol, according to the GHS classification, has a fatal dose (LD50) of 20 mg/kg, an acute oral toxicity classification of category 3, and a chronic aquatic toxicity rating of category 2 [10,11,12]; hence, it is short of the PARCOM requirement for environmentally benign chemicals [13,14]. From the standpoint of environmental compatibility, the area of corrosion inhibitors is undergoing dramatic changes as environmental organizations in various countries have imposed rigorous standards and restrictions on the use and release of corrosion inhibitors. As a result, developing novel corrosion inhibitors with little or no adverse effects has been deemed more vital. Green corrosion inhibitors are from a variety of natural sources. They have many polar atoms and electron-rich linkages, making them rich in organic molecules [1].
In addition to the clarion call for green chemicals, the recent depletion of shallower oil wells is pushing offshore oil and gas drilling activities to move further offshore and deeper underwater so as to find sources of production in low-risk areas. Meanwhile, this poses a serious challenge to the industry because of the higher temperature and pressure as the well gets deeper. The implication is that most of the effective inhibitors for the acidizing process are losing their potency. Most often, the inhibition efficiency of organic inhibitors [7], including plant extracts [15,16,17], decreases with increases in temperature. There is, therefore, a high demand for corrosion inhibitors that can perform effectively at high temperatures.
This study aims to create a low-cost, effective, and environmentally acceptable corrosion inhibitor for use in high-temperature acidizing processes. Aspartame, (N-(l-α-Aspartyl)-l-phenylalanine) was tested as a corrosion inhibitor for API T95 carbon steel in a 15 wt.% HCl solution. Aspartic acid and phenylalanine (amino acids) are two components of aspartame. It has an LD50 oral value of >10,000 mg/kg [18]. It is also very cheap and cost-effective. Figure 1 shows the chemical structure of aspartame. The examination included a variety of methods, including weight loss, electrochemical methods (electrochemical impedance spectroscopy and potentiodynamic polarization), and surface characterization (by scanning electron microscope (SEM) and optical profilometer (OP)).
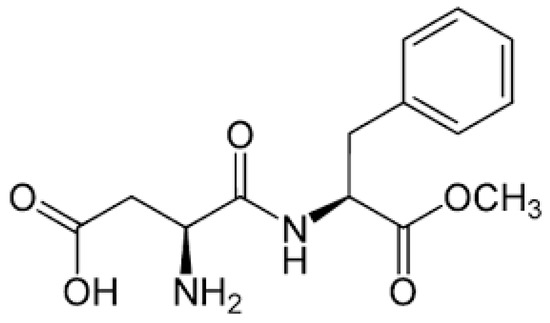
Figure 1.
Chemical structure of Aspartame [19].
2. Materials and Methods
2.1. Materials, Sample Preparation, and Chemicals
The steel material used for this research is a pipe material, i.e., carbon steel material; more precisely, it is a T95 steel of chemical composition in wt.% (C:0.45, P:0.030, Mo:0.85, Si:0.45, S:0.010, Cr:1.50, Mn:1.20, Ni:0.99, and the balance Fe). For the weight loss experiments, the metal was cut into samples with surface areas of 12 cm2. Samples for electrochemical tests had surface areas of 1 cm2 and were soldered to a copper wire and abraded with Emery paper from 60 to 800 grits. The samples were thoroughly cleaned with distilled water, degreased with acetone, and dried at room temperature in a desiccator before being mounted using VersoCit-2 epoxy resin (Struers’ product). The corrodent, 15 wt.% hydrochloric acid, was prepared by diluting 37% concentrated HCl with distilled water. Aspartame (ASP) was purchased from Sigma-Aldrich, CAS No: 22839-47-0. The stock solution of the ASP inhibitor (100,000 ppm) was prepared by dissolving appropriate amount in 20 mL ethanol/water 1:1 ratio mixture. The required concentrations (500–2000 ppm) were obtained by diluting the stock solution with 15 wt.% HCl solution.
2.2. Weight Loss Measurements
Before weight loss testing, the samples were mechanically abraded with Emery paper from 60 to 800 grits. The samples were thoroughly cleaned with distilled water, degreased with acetone, and dried at room temperature in a desiccator. The initial weight of the samples was noted and recorded. The pre-weighed samples in triplicate were submerged in a 15 wt.% HCl solution without and with various inhibitor concentrations (500 ppm, 1000 ppm, 1500 ppm, and 2000 ppm) at several temperatures (60 °C, 70 °C, 80 °C, and 90 °C) for 4 h. After the immersion process, the samples were removed, cooled to room temperature, dipped in a pickling solution (zinc dust + NaOH solution) for 1 min, washed with distilled water, then degreased with acetone, dried, and re-weighed. The corrosion rate (v) and the inhibitor efficiency () of ASP were calculated using Equations (1) and (2) [20], respectively. The surface coverage, θ = .
where is the weight loss after immersion, is the density of the carbon steel (g cm3), T is the immersion time, A is surface area of the coupons, and 87,600 is the conversion factor.
2.3. Electrochemical Measurements
The electrochemical experiments (electrochemical impedance spectroscopy (EIS)) and potentiodynamic polarization (PDP)) were performed using a Gamry Potentiostat/Galvanostat/ZRA Reference 600+ workstation at different temperatures (60 °C, 70 °C, 80 °C, and 90 °C). The capacity of the reaction cell was 200 mL and the three–electrode system consisted of Ag/AgCl (sat. 4.2 M KCl) as reference electrode, a platinum rod as the counter electrode, and the T95 carbon steel as working electrode. Before the EIS measurements, the working electrode was allowed to freely corrode at open circuit potential (OCP) for 1800 s in order to establish a steady-state condition required for EIS measurement. Thereafter, the electrochemical impedance spectroscopy measurement was performed under potentiostatic mode by applying an alternating–current signal at the frequency range of 100,000 Hz to 0.01 Hz utilizing an amplitude of 10 mV. After the EIS experiments, the T95 carbon steel electrode was scanned within the potential range of −250 to +250 mV with respect to the open circuit potential, at the rate of 1 mV/s. The analysis of the EIS and the PDP data was achieved via the Echem Analyst software. The inhibition efficiency was calculated for EIS using Equation (3) and for PDP using Equation (4) [21]:
2.4. Surface Characterization
The surfaces of the corroded T95 carbon steel surfaces at 60 °C and 90 °C after 4 h of immersion were observed in a JEOL JSM-6610 LV scanning electron microscope (SEM) that is coupled with an energy dispersive X-ray spectrophotometer (EDX) for elemental composition determination. The samples for the surface characterization experiments were prepared by immersing the pre-cleaned coupons for 4 h in 15 wt.% HCl solution without and with 2000 ppm ASP at 60 °C and 90 °C. Afterward, the samples were rinsed with distilled water, dried in warm air, and submitted for SEM, EDX, and 3D optical surface examinations. The 3D optical observation was performed using a 3D optical profilometer (Contour GT-K, Bruker Nano GmBH, Berlin, Germany).
3. Results
3.1. Weight-Loss Studies on the Performance of Aspartame as Inhibitor
Gravimetric measurements were undertaken to study the corrosion inhibition performance of ASP against T95 corrosion in 15 wt.% HCl solution at different temperatures, and the calculated corrosion parameters are listed in Table 1. It is shown in the table that the metal coupons, whether in uninhibited or inhibited acid solutions, lost weight. The known anodic oxidation and cathodic reduction corrosion reactions of carbon steel in acidic media proceed as and [22]. These reactions, in addition to the high solubility of the corrosion products arising from the reactions, especially in the uninhibited acid solution, give rise to the metal samples losing weight [22]. Relative to the blank, the weight loss (WL) and corrosion rate (v) at all studied temperatures decreased in the inhibited systems. This indicates that ASP in the acid solution functioned as an inhibitor by protecting the metal surface from the corrosive attack. ASP, being an organic compound with O and N heteroatoms in its molecule (Figure 1), has the tendency to be adsorbed on the T95 surface and to obstruct active corrosion sites on the metal surface [23]. The influence of ASP on WL and v is seen to be concentration dependent. For instance, WL and v values decrease with increasing ASP concentration. This could suggest that, in the acid solutions containing higher concentrations of ASP, more molecules of ASP were adsorbed on the metal surface and larger active sites were covered. The variations in the surface coverage (θ) and inhibition efficiency (IEWL) according to ASP concentration seem to support this assertion. The observed increases in the values of θ and IEWL with increasing ASP concentrations imply a decrease in the electroactive area on the metal surface [24], meaning that more corrosion reaction sites were blocked upon the increase in the ASP concentration [24].

Table 1.
Weigh loss (WL), corrosion rate (v), surface coverage (θ), and inhibition efficiency (IE) for T95 carbon steel corrosion in 15 wt.% HCl solution in the absence and presence of different concentrations of aspartame (ASP) at different temperatures from weight-loss measurements.
It is also shown in Table 1 and Figure 2 that WL, v, θ, and IEWL increase with rises in the system temperature. The increase of WL and v with rises in temperature is as expected considering the fact that the rate of a chemical reaction is accelerated at higher temperatures [25]. Nevertheless, the increase in WL and v is at a greater magnitude in the 15 wt.% HCl solution without ASP than in the solution containing ASP, indicating the inhibiting effect of ASP. The increases in θ and IEWL with rises in temperature are characteristics of an organic inhibitor that is adsorbed via a chemisorption mechanism [26,27]. Chemisorption involves an exchange of electrons between specific surface sites and inhibitor molecules or the transfer of electrons from the inhibitor molecule to the vacant orbital of the absorbent, resulting in the formation of chemical bond(s). Chemisorption bonds are stronger and more stable at high temperatures. Abdulazeez et al. [28] reported that a newly synthesized polyurea-based material (PUCorr-1) inhibited the corrosion of mild steel in a simulated acidic medium saturated with CO2 and H2S gases through the chemisorption mechanism. Other authors also reported corrosion inhibition via the chemisorption mechanism [29,30,31]. In the report, the inhibition efficiency of PUCorr-1 increased from 98.3% at 25 °C to 99.9% at 50 °C.
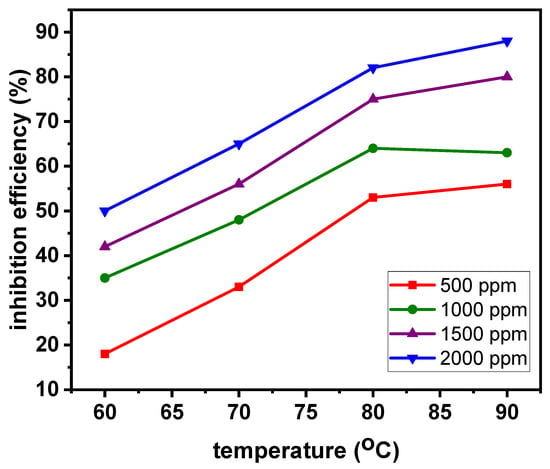
Figure 2.
Variations in inhibition efficiency of ASP with temperature from weight-loss measurements.
It is worth pointing out the significant reduction of v by the 2000 ppm ASP from 23.935 × 10−3 ± 0.048 g cm−2 h−1 to 3.415 × 10−3 ± 0.011 g cm−2 h−1 and the IEWL of 86% at 90 °C, which could imply stronger adsorption bonds and a dense and thick adsorption layer at this temperature. The solubility of ASP no doubt plays a remarkable role in the observed improved inhibition performance at higher temperatures. ASP solubility increased with increases in temperature [32]. It therefore means that, as the temperature of the solution was increased, more ASP dissolved and was available for adsorption and in extension inhibition.
3.2. Electrochemical Studies
3.2.1. OCP and EIS
It is a major requirement that system stability be achieved before electrochemical impedance spectroscopy (EIS) measurements are taken [33]. To satisfy this requirement, the T95 carbon steel working electrode was allowed to freely corrode in the 15 wt.% HCl solution under open circuit potential (OCP) for 1800 s. The variation in the OCP by immersion duration for the T95 steel immersed in 15% HCl solution without and with ASP at 60, 70, 80, and 90 °C is shown in Figure 3. It is obvious that, at least a pseudo-steady-state condition was achieved in all cases; hence, the EIS results presented herein can be relied upon. It should be mentioned that a completely steady-state condition is practically impossible since corrosion is dynamic in nature [34]. Worthy of noting also, as shown in Figure 3, is the mixed-type behavior of the ASP. As can be seen in the graphs, at all studied temperatures, the extent of the displacement of the OCP by ASP relative to the blank is less than 85 mV vs. Ag/AgCl is needed for ASP classification as an anodic- or cathodic-type inhibitor [35,36]. ASP thus behaves as a mixed-type corrosion inhibitor.
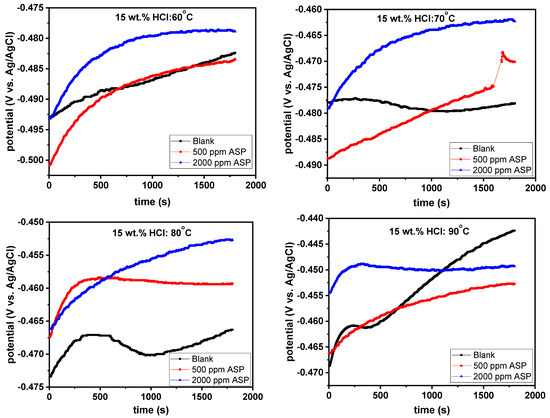
Figure 3.
Variations in open circuit potential (OCP) by time for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of aspartame at different temperatures.
At the steady OCP, the impedance characteristics of the T95 steel electrode in 15 wt.% HCl solution at the studied temperatures were collected. Figure 4 shows the Nyquist electrochemical plots for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of ASP at different temperatures. The corresponding Bode representation is shown in Figure 5. The Nyquist diagrams, except that of the blank at 90 °C, exhibit an imperfect, single capacitive loop at the high-to-medium frequencies and an inductive loop at the low frequencies. The semi-circle at the high-to-medium frequencies implies that the corrosion of the T95 steel in the examined environment is governed by a charge transfer process [37,38]. The inductive loop is caused by the relaxation of the corrosion products or the adsorbed ASP molecules [39]. The imperfectness of the semi-circle is due to the micro-roughness and heterogeneity of the steel electrode [40,40]. At 90 °C, the Nyquist graph, in addition to the charge-controlled capacitive loop and the inductive loop, exhibits a poorly resolved semi-circle at the medium frequencies, and in the Bode representation (Figure 5), at the lower frequencies, log /Z/ seems to be independent of log f. These behaviors are typical of Warburg impedance and are common with a corrosion system with diffusion contribution [41,42]. In such a system, reactants are transported from the bulk solution to the metal/solution interface or soluble products are transported from the metal/solution interface to the bulk solution [41,42]. Compared to the blank, it can be seen that, at all the studied temperatures, the size of the capacitive loop of the inhibited graphs is larger, with the 2000 ppm ASP-inhibited graphs being the largest. This is reflective of the inhibitive effect of ASP at 2000 ppm exhibiting a higher protective effect than the ASP at 500 ppm. The effect of concentration on the inhibitive performance of ASP is also seen in the Bode representation (Figure 5). In Figure 5, the impedance modulus and the phase angle tilt toward nobler values with increasing concentrations. The implication is that the rate of the charge transfer process was slowed down in the ASP-inhibited systems due to the adsorption of the ASP molecules onto the T95 surface and the covering of the active sites [43,44]. The anti-corrosion performance of ASP at higher concentrations is superior to that at lower concentrations.
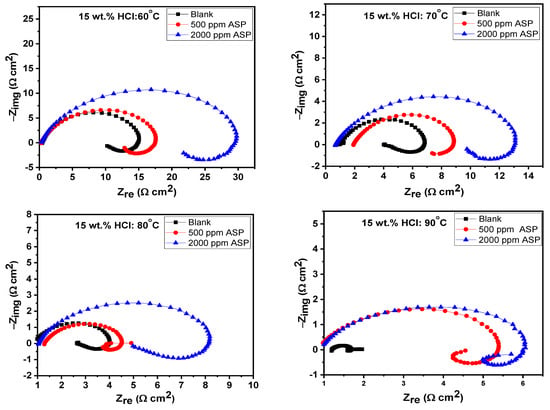
Figure 4.
Nyquist electrochemical plots for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of aspartame at different temperatures.
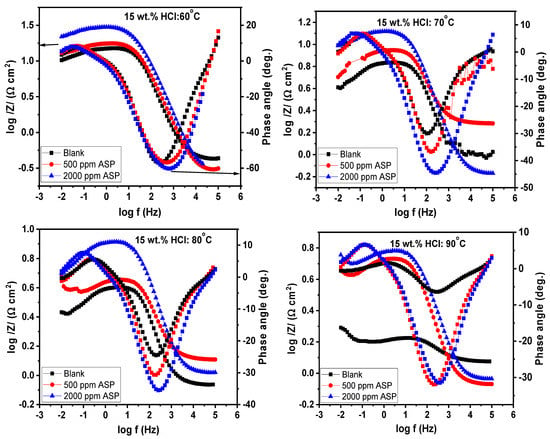
Figure 5.
Bode electrochemical plots for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of aspartame at different temperatures.
The impedance data were fitted using the equivalent circuits (ECs) shown in Figure 6. The various phenomena observed in Figure 4 and Figure 5 were taken into consideration in the selection of the ECs. The EC in Figure 6a was used for the analysis of all the EIS data except the data for the blank at 90 °C. The EC consists of the following elements: Rs (solution resistance), C (corrosion/adsorbed products capacitance), Rf (film resistance), Rct (charge transfer resistance), and L (inductor). Because of the diffusion phenomenon observed in Figure 4 and Figure 5 at 90 °C, the EC in Figure 6b was used for the analysis of the EIS data for the blank at 90 °C. It has the element, W (Warburg impedance), in addition to the other elements in Figure 6a. The suitability of the selected ECs is reflected in the low fitting error and the low chi square values listed in Table 2. All the values obtained from the fitting are given in Table 2. The inhibition efficiency (IEEIS) values, also given in the table, were calculated using Equation (3). Rp is the total resistance, that is Rct + Rf. It is clear from the table that Rp of the T95 steel increased in the ASP-inhibited systems relative to the uninhibited, indicating that the presence of the ASP in the HCl solution boosted the corrosion resistance property of the metal. The best effect is observed for the 2000 ppm–inhibited systems. At 60 °C, the presence of 2000 ppm ASP in the acid solution caused a 47% increase in Rp, from 13.52 ± 0.29 Ω cm2 to 25.31 ± 0.98 Ω cm2, meaning that the metal surface was more protected by 47%. It is also seen that the corrosion inhibition performance of ASP improves with increase in temperature and is in excellent agreement with the results from the weight-loss experiments (Table 1). For 2000 ppm ASP, the IEEIS increased from 47% at 60 °C to 51% at 70 °C. With further increase in the system temperature to 80 °C and 90 °C, IEEIS increased to 62% and 85%, respectively. These results indicate that ASP is a promising corrosion inhibitor for high-temperature acidizing applications. ASP could be employed as an active compound for inhibitor formulation intended for acidizing applications. It is worth mentioning that the value of n obtained for the blank at 90 °C is 0.51, which signifies Warburg impedance [41,42] and thus confirms the presence of diffusion at 90 °C [41,42].
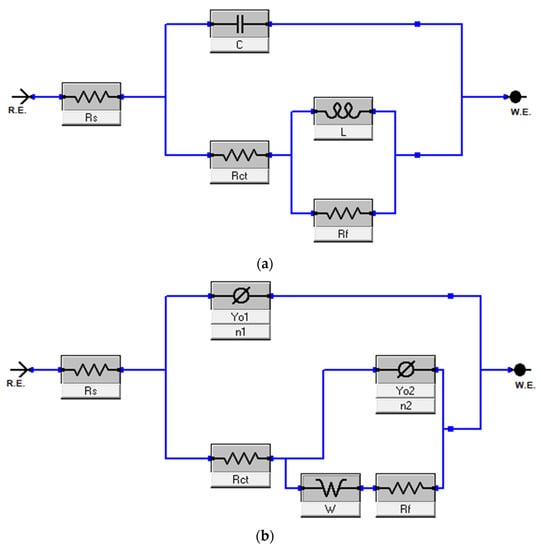
Figure 6.
Equivalent circuit used in the analysis of (a) all the electrochemical impedance data except the data for blank at 90 °C, in which (b) was used.

Table 2.
EIS parameters derived for T95 carbon steel during corrosion in 15 wt.% HCl at different temperatures without and with 500 ppm and 2000 ppm aspartame.
3.2.2. PDP
The potentiodynamic polarization technique was used to investigate the cathodic and anodic corrosion kinetics of T95 steel at 60–90 °C in 15% HCl in the presence and absence of ASP. The polarization curves plotted for the studied systems are presented in Figure 7. In the comparison of the polarization curves for the blank to those of the ASP-inhibited treatments, a shift in both the anodic and cathodic curves towards the lower current zone is observed. Additionally, the displacement of the corrosion potential (Ecorr) by the inhibitor is insignificant. These observations indicate that ASP acted as an inhibitor for T95 steel corrosion in the studied acid solution at all temperatures and that the inhibition was due to the ability of ASP to interrupt both the anodic oxidation of T95 steel and the cathodic reduction of adsorbed hydrogen to hydrogen gas [34,45]. In other words, ASP acted as a mixed-type corrosion inhibitor [34,45], which is in agreement with the OCP results (Figure 3).
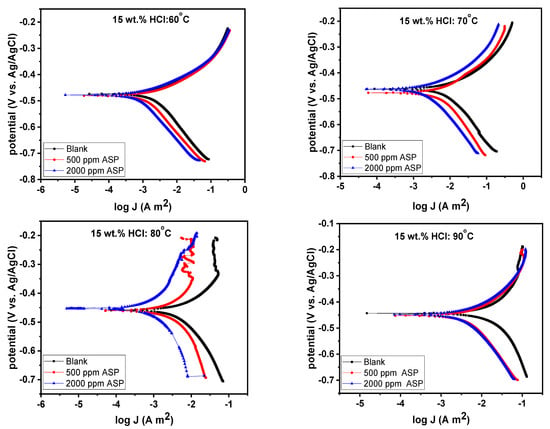
Figure 7.
Potentiodynamic polarization graphs for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of aspartame at different temperatures.
The anodic and cathodic branches show Tafel-like regions; that is, the linear portions signify majorly activation-controlled corrosion kinetics [36,46]. These linear portions were extrapolated to obtain the polarization parameters, namely corrosion potential (Ecorr), corrosion current density (icorr), anodic Tafel slope (βa), and cathodic Tafel slope (βc), the values for which are given in Table 3. The corrosion rate (v) values were calculated using Equation (5) [47] and the inhibition efficiency (IEPDP) values were calculated using Equation (4). From Table 3, the presence of ASP in the 15 wt.% HCl solution is seen to significantly diminish the corrosion current, particularly at 90 °C. For instance, at 90 °C, in the presence of 2000 ppm ASP, the icorr value decreased from 201.00 mA cm−2 to 25.97 mA cm−2. The low current density in the inhibited systems resulted in a low corrosion rate. The presence of 2000 ppm ASP reduced the v from 2326.686 mm/yr to 300.617 mm/yr, and this correspond to inhibition efficiency of 87%. Again, according to this technique, ASP is a highly promising corrosion inhibitor for high-temperature acidizing processes. A further inspection of Table 3 reveals that the changes in the Ecorr, βa, and βc values of the inhibited systems and those of the uninhibited system are minimal. For instance, 15 mV vs. Ag/AgCl. This confirms the mixed-type behavior [34,45] of ASP earlier proposed.
where 3.27 is a proportionality constant, e and are the equivalent weight and density of steel, respectively, and icorr is in mA cm−2.

Table 3.
Potentiodynamic polarization parameters derived for T95 carbon steel corrosion in 15 wt.% HCl solution without and with selected concentrations of aspartame at different temperatures.
3.3. Mechanism of Inhibition by Aspartame
The acceptable corrosion inhibition mechanism by organic inhibitors is the adsorption mechanism, whereby an organic molecule substitutes an adsorbed water molecule on a metal surface to form a protective film that decreases the rate at which corrosive species attack the surface [48,49]. It has already been established that the surface of carbon steel is positively charged in a highly concentrated HCl medium [36,50,51]. Additionally, chloride ions (Cl−) are believed to be preferentially adsorbed on the positively charged steel surface [36,50,51]. Accordingly, the anodic oxidation reactions are as shown in Equations (6)–(10) [52] and the overall anodic oxidation of the T95 carbon steel in 15 wt.% HCl solution is illustrated in Figure 8a.
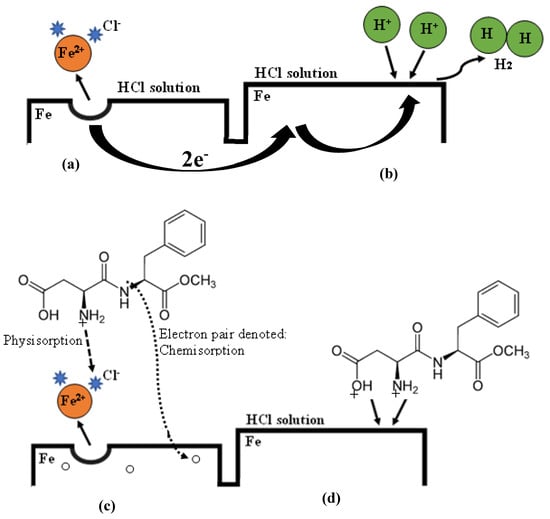
Figure 8.
An illustration of the corrosion of T95 carbon steel in 15 wt.% HCl solution and inhibition by aspartame. (a) Anodic reaction: , (b) Cathodic reaction: , (c) Adsorption of Aspartame on the anodic region: , and (d) Adsorption of Aspartame on the cathodic region: .
The corresponding cathodic reduction reactions are given in Equations (11)–(13) [52] and illustrated in Figure 8b.
In the HCl solution containing ASP, our potentiodynamic polarization results (Figure 5 and Table 3) reveal that both the anodic oxidation and the cathodic reduction reactions were interrupted. Obviously, in the 15 wt.% HCl solution, ASP is protonated through the functional group (see Figure 1) such that it exists in cationic form (). The is electrostatically attracted to the charged T95 steel surface [36,50,51], and thus, the anodic oxidation reaction in Equation (8) is interrupted, as shown in Equation (14) and illustrated in Figure 8c.
Moreover, our results (Table 1, Table 2 and Table 3) disclose that ASP molecules were chemically adsorbed on the steel surface; that is, chemical bonds were formed between ASP and the steel surface. ASP, as can be seen in Figure 1, still has other O, N, and even the benzene ring, which are rich in electron pairs. Therefore, it is possible that, upon attraction to the steel surface via coulombic electrostatic attraction, electron pairs were donated to the low energy 3d-orbital of Fe. Such behavior was previously reported for N-(2-(2-tridecyl-4,5-dihydro-1H-imidazol-1-yl)ethyl)tetradecanamide in 15 wt.% HCl solution [36].
At the cathodic region, and can undergo competitive adsorption [52] such that the adsorbed ASP inhibits the cathodic reduction reactions according to Equations (15) and (16) as well as the illustration in Figure 8d.
3.4. Surface Analysis
3.4.1. SEM and EDX
The surface morphology of the abraded T95 steel, the steel specimens immersed in 15 wt.% HCl solution without ASP at 60 °C and 90 °C, and the steel specimens immersed in 15 wt.% HCl solution containing 2000 ppm of ASP at 60 °C and 90 °C after 4 h of immersion are shown in Figure 9. The elemental composition of the surfaces at 90 °C, as obtained by energy dispersive X-ray spectrophotometry (EDX), are given in Figure 10. As can be seen in Figure 9a, the steel surface after mechanical abrasion was smooth, and Fe (85.9%), Cr (8.6%), C (3.6%), Mo (1.1%), Si (0.4%), Mn (0.3%), and Ni (0.1%) were detected (Figure 10a), which agrees with the composition of the T95 steel listed in Section 2.1. The steel surface was significantly impacted by corrosion in the absence of the inhibitor, resulting in a rough surface with heaps of porous corrosion products (Figure 9b,c). The EDX results in Figure 10b disclose that the corrosion product is enriched with Cl (20.5%), O (11.0%) and C (10.5%). This indicates that the corrosion products on the steel surface are mixtures of carbonates, chlorides, and oxides, in conformity with previous reports [36]. Compared to the surfaces in Figure 9b,c, the ASP-inhibited surfaces in Figure 9d,e exhibit smoother morphology and the surface deposits are more compact. In the EDX spectrum in Figure 10c, there is a noticeable decrease in the percentage of Cl (16.3%) and O (8.3%), meaning that the formation of the corrosion products was abated. The detection of N (1.0%) and the increase in C (12.7%) content provide experimental evidence of the adsorption of ASP on the steel surface. The adsorbed ASP film was thus responsible for the inhibition of the steel corrosion, in agreement with the results from other applied techniques.
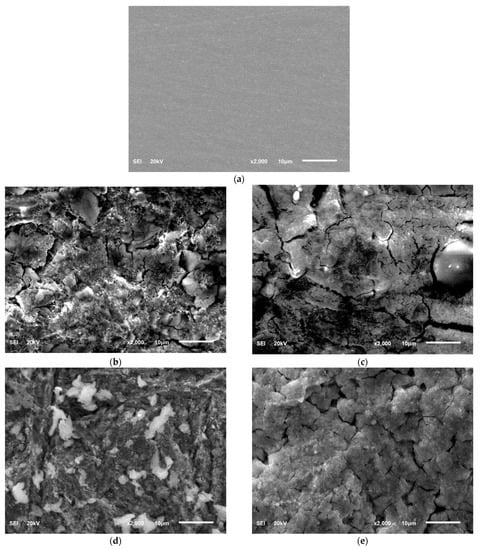
Figure 9.
SEM micrographs of (a) the abraded T95 steel, the steel specimens immersed in 15 wt.% HCl solution without the inhibitor at (b) 60 °C and (c) 90 °C, and the steel specimens immersed in 15 wt.% HCl solution containing 2000 ppm of aspartame at (d) 60 °C and (e) 90 °C after 4 h of immersion.
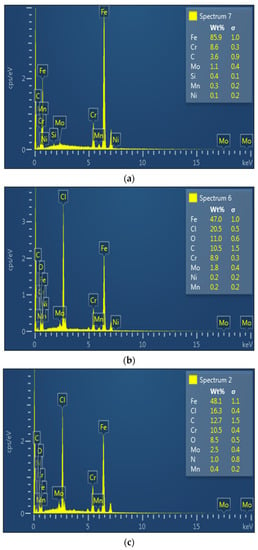
Figure 10.
EDX spectra of (a) the abraded T95 steel, (b) the steel specimens immersed in 15 wt.% HCl solution without the inhibitor, and (c) the steel specimens immersed in 15 wt.% HCl solution containing 2000 ppm of inhibitor at 90 °C after 4 h of immersion.
3.4.2. Optical Profilometer
The surface of the corroded T95 steel was further studied using an optical profilometer. Our interest in this study was on the roughness characteristics of the corroded surface in comparison with the roughness properties of the abraded surface. Figure 11 shows the surface profilometer images taken for the T95 steel specimen after mechanical abrasion and after exposure to a 15 wt.% HCl solutions devoid of and containing 2000 ppm ASP for 4 h at 60 and 90 °C. The values of the roughness parameters derived for the surfaces are presented in Table 4. The parameters include Ra, which is the average value of profile deviation from the mean line; Rt, the total height which is used to describe the largest peak to valley height; and the mean width of the primary profile element (RRMS) [36,43]. The smooth surface in Figure 11a has Ra, Rt, and RRMS values of 0.154 µm, 2.119 µm, and 0.186 µm, respectively. It is very visible that the metal surface was severely damaged by corrosion at the studied temperatures. The surface became very rough due to corrosion (Figure 11b,c). The Ra, Rt, and RRMS values increased to 2.269 µm, 39.708 µm, and 2.933 µm, respectively, at 60 °C and to 4.350 µm, 93.215 µm, and 5.679 µm, respectively, at 90 °C. However, the steel surface was protected in the acid solution containing ASP, such that the surface roughness greatly decreased (Figure 11d,e). As can be seen in Table 4, the Ra, Rt, and RRMS values are reduced by 41%, 73%, and 40%, respectively, at 60 °C and by 86%, 66%, and 89%, respectively, at 90 °C. This shows the excellent corrosion-protective ability of ASP. It can again be seen that ASP is very effective at 90 °C.
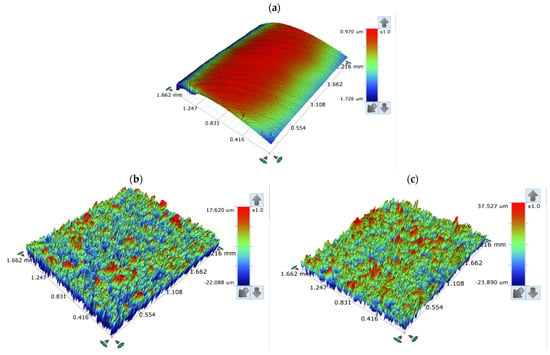
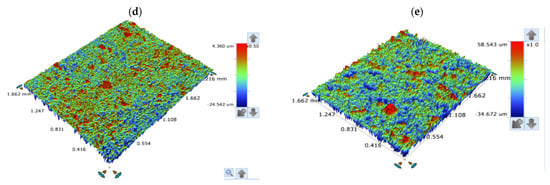
Figure 11.
Surface profilometer images of (a) the abraded T95 steel, the steel specimens immersed in 15 wt.% HCl solution without the inhibitor at (b) 60 °C and (c) 90 °C, and the steel specimens immersed in 15 wt.% HCl solution containing 2000 ppm of aspartame at (d) 60 °C and (e) 90 °C after 4 h of immersion.

Table 4.
Surface parameters derived from profilometer surface analysis of the corroded T95 steel immersed in 15% HCl without and with additives at 90 °C after 4 h immersion.
4. Summary and Conclusions
The corrosion inhibition ability of aspartame (ASP) for T95 carbon steel in 15 wt.% HCl at 60–90 °C was studied using the weight-loss, electrochemical impedance spectroscopy, potentiodynamic polarization, scanning electron microscope, energy dispersive X-ray, and optical profilometric techniques. It was found, from all the applied techniques, that ASP is a promising corrosion inhibitor for acidizing processes. The inhibition efficiency of ASP increased with increases in temperature, reaching 86% with 2000 ppm ASP at 90 °C. ASP acts as a mixed-type corrosion inhibitor, interfering in both the anodic and cathodic reactions. The optical profilometry results show that the Ra, Rt, and RRMS values were reduced by 41%, 73%, and 40%, respectively, at 60 °C and by 86%, 66%, and 89%, respectively, at 90 °C in the presence of ASP. This shows the excellent corrosion-protective ability of ASP. All the results are in good agreement and the EDX results provide evidence of ASP adsorption on the steel surface. ASP is recommended as an active compound for inhibitor formulations intended for acidizing applications at high temperatures.
Author Contributions
Conceptualization, M.M.S. and I.E.U.; methodology, M.M.S., I.E.U. and S.A.U.; validation, M.M.S. and S.A.U.; formal analysis, M.M.S., R.T.L., I.E.U. and S.A.U.; investigation, M.M.S. and I.E.U.; resources, M.M.S., R.T.L., I.E.U. and S.A.U.; data curation, M.M.S., S.A.U., and I.E.U., writing—original draft preparation M.M.S. and I.E.U.; writing—review and editing, M.M.S., R.T.L., I.E.U. and S.A.U.; visualization, M.M.S.; supervision, M.M.S. and S.A.U.; project administration, M.M.S.; funding acquisition M.M.S. and I.E.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided upon request.
Acknowledgments
The authors are grateful to the Interdisciplinary Research Center for Advanced Materials at the King Fahd University of Petroleum and Minerals, Dhahran 31261, Saudi Arabia, for allowing some of the center’s facilities to be used in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Thompson, J.W.; Robinson, J.R. Understanding gas production mechanism and effectiveness of well stimulation in the Haynesville Shale through reservoir simulation. Soc. Pet. Eng. Can. Unconv. Resour. Int. Pet. Conf. 2010, 1, 352–366. [Google Scholar]
- Crowe, C.; Masmonteil, J.; Thomas, R. Trends in matrix acidizing. Oilf. Rev. 1992, 4, 24–40. [Google Scholar]
- Abdel Ghany, N.A.; Shehata, M.F.; Saleh, R.M.; El Hosary, A.A. Novel corrosion inhibitors for acidizing oil wells. Mater. Corros. 2017, 68, 355–360. [Google Scholar] [CrossRef]
- Huizinga, S.; Liek, W.E. Corrosion behavior of 13% chromium steel in acid stimulations|Corrosion|OnePetro. Corrosion 1994, 50, NACE-94070555. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Lateef, H.M. Synergistic effect of polyethylene glycols and rare earth Ce4+ on the corrosion inhibition of carbon steel in sulfuric acid solution: Electrochemical, computational, and surface morphology studies. Res. Chem. Intermed. 2015, 42, 3219–3240. [Google Scholar] [CrossRef]
- Singh, A.; Quraishi, M.A. Acidizing Corrosion Inhibitors: A Review. J. Mater. Environ. Sci. 2015, 6, 224–235. [Google Scholar]
- Frenler, F.B.; Growcock, W.W.; Lopp, V.R. Mechanisms of Corrosion Inhibitors Used in Acidizing Wells; SPE Production Engineering: Richardson, TX, USA, 1988; pp. 584–590. [Google Scholar]
- Zhang, J.; Sun, X.; Ren, Y.; Du, M. The synergistic effect between imidazoline-based dissymmetric bis-quaternary ammonium salts and thiourea against CO2 corrosion at high temperature. J. Surfactants Deterg. 2015, 18, 981–987. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Solomon, M.M.; Umoren, S.A.; Ali, S.A. Comparative studies of the corrosion inhibition efficacy of a dicationic monomer and its polymer against API X60 steel corrosion in simulated acidizing fluid under static and hydrodynamic conditions. ACS Omega 2020, 5, 27057–27071. [Google Scholar] [CrossRef]
- Nations, U. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 6th ed.; UNECE: Geneva, Switzerland, 2015; ISBN 9789211170870. [Google Scholar]
- Alhaffar, M.T.; Umoren, S.A.; Obot, I.B.; Ali, S.A.; Solomon, M.M. Studies of the anticorrosion property of a newly synthesized green isoxazolidine for API 5L X60 steel in acid environment. J. Mater. Res. Technol. 2019, 8, 4399–4416. [Google Scholar] [CrossRef]
- Mohammed, A.R.I.; Solomon, M.M.; Haruna, K.; Umoren, S.A.; Saleh, T.A. Evaluation of the corrosion inhibition efficacy of Cola acuminata extract for low carbon steel in simulated acid pickling environment. Environ. Sci. Pollut. Res. 2020, 27, 34270–34288. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Shang, Z.; Zhu, J. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2021, 15, 5078–5094. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Cao, X.; Fu, A.; Guo, L.; Marzouki, R.; Li, W. Insight into the anti-corrosion performance of two food flavors as eco-friendly and ultra-high performance inhibitors for copper in sulfuric acid medium. J. Colloid Interface Sci. 2022, 609, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Lan, W.; Zhang, S.; Deng, H.; Qiang, Y.; Fu, A.; Ran, Y.; Xiong, J.; Marzouki, R.; Li, W. Passiflora edulia Sims leaves extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128892. [Google Scholar] [CrossRef]
- Supelco. Safety Data Sheet—Aspartame.
- Aspartame—American Chemical Society. Available online: https://www.acs.org/content/acs/en/molecule-of-the-week/archive/a/aspartame.html (accessed on 20 May 2022).
- NACE; ASTM. ASTMG31-12a Standard Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2010; Volume 100. [Google Scholar]
- Singh, A.; Caihong, Y.; Yaocheng, Y.; Soni, N.; Wu, Y.; Lin, Y. Analyses of new electrochemical techniques to study the behavior of some corrosion mitigating polymers on N80 tubing steel. ACS Omega 2019, 4, 3420–3431. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.M.; Umoren, S.A.; Udosoro, I.I.; Udoh, A.P. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Tripathi, D.; Abai, E.J. Effect of akyl chain length, flow, and temperature on the corrosion inhibition of carbon steel in a simulated acidizing environment by an imidazoline-based inhibitor. J. Pet. Sci. Eng. 2020, 187, 106801. [Google Scholar] [CrossRef]
- Key, J.A. Factors that Affect the Rate of Reactions. In Introductory Chemistry; Prentice Hall: Hoboken, NJ, USA, 2007. [Google Scholar]
- Umoren, S.A.; Obot, I.B.; Ebenso, E.E.; Okafor, P.C.; Ogbobe, O.; Oguzie, E.E. Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros. Methods Mater. 2006, 53, 277–282. [Google Scholar] [CrossRef]
- Khadraoui, A.; Khelifa, A. Ethanolic extract of Ruta chalepensis as an eco-friendly inhibitor of acid corrosion of steel. Res. Chem. Intermed. 2013, 39, 3937–3948. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Al-Hamouz, O.C.S.; Khaled, M.; Al-Saadi, A.A. Inhibition of mild steel corrosion in CO2 and H2S-saturated acidic media by a new polyurea-based material. Mater. Corros. 2020, 71, 646–662. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Jing, C.; Wang, Z.; Gong, Y.; Huang, H.; Ma, Y.; Xie, H.; Li, H.; Zhang, S.; Gao, F. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2018, 138, 353–371. [Google Scholar] [CrossRef]
- Azghandi, M.V.; Davoodi, A.; Farzi, G.A.; Kosari, A. Corrosion inhibitive evaluation of an environmentally friendly water-base acrylic terpolymer on mild steel in hydrochloric acid media. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 5493–5504. [Google Scholar] [CrossRef] [Green Version]
- Leng, Y.; Qi, H. Solubility of aspartame in water, methanol, ethanol and different binary mixtures in the temperature range of (278.15 to 333.15) K. J. Chem. Eng. Data 2014, 59, 1549–1555. [Google Scholar] [CrossRef]
- Gamry.com Basics of EIS: Electrochemical Research-Impedance. Available online: https://www.gamry.com/application-notes/EIS/basics-of-electrochemical-impedance-spectroscopy (accessed on 8 May 2022).
- Öztürk, S.; Gerengi, H.; Solomon, M.M.; Gece, G.; Yıldırım, A.; Yıldız, M. A newly synthesized ionic liquid as an effective corrosion inhibitor for carbon steel in HCl medium: A combined experimental and computational studies. Mater. Today Commun. 2021, 29, 102905. [Google Scholar] [CrossRef]
- Srikanth, A.P.; Sunitha, T.G.; Raman, V.; Nanjundan, S.; Rajendran, N. Synthesis, characterization and corrosion protection properties of poly(N-(acryloyloxymethyl) benzotriazole-co-glycidyl methacrylate) coatings on mild steel. Mater. Chem. Phys. 2007, 103, 241–247. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid Interface Sci. 2019, 551, 47–60. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Corrales-Luna, M.; Le Manh, T.; Romero-Romo, M.; Palomar-Pardavé, M.; Arce-Estrada, E.M. 1-Ethyl 3-methylimidazolium thiocyanate ionic liquid as corrosion inhibitor of API 5L X52 steel in H 2 SO 4 and HCl media. Corros. Sci. 2019, 153, 85–99. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Romero-Romo, M.; Herrera-Hernández, H.; Abreu-Quijano, M.A.; Likhanova, N.V.; Uruchurtu, J.; Juárez-García, J.M. Influence of the alkyl chain length of 2 amino 5 alkyl 1, 3, 4 thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions. Corros. Sci. 2012, 54, 231–243. [Google Scholar] [CrossRef]
- Sedik, A.; Lerari, D.; Salci, A.; Athmani, S.; Bachari, K.; Gecibesler, H.; Solmaz, R. Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies. J. Taiwan Inst. Chem. Eng. 2020, 107, 189–200. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Zhou, G.D. Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2,5-thiadiazoly)-disulfide in chloride media. Appl. Surf. Sci. 2004, 225, 287–293. [Google Scholar] [CrossRef]
- Gerengi, H.; Mielniczek, M.; Gece, G.; Solomon, M.M. Experimental and Quantum Chemical Evaluation of 8-Hydroxyquinoline as a Corrosion Inhibitor for Copper in 0.1 M HCl. Ind. Eng. Chem. Res. 2016, 55, 9614–9624. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl cellulose/silver nanoparticles composite: Synthesis, characterization and application as a benign corrosion inhibitor for St37 steel in 15% H2SO4 medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef]
- de Araújo Macedo, R.G.M.; do Nascimento Marques, N.; Tonholo, J.; de Carvalho Balaban, R. Water-soluble carboxymethylchitosan used as corrosion inhibitor for carbon steel in saline medium. Carbohydr. Polym. 2019, 205, 371–376. [Google Scholar] [CrossRef]
- Ghelichkhah, Z.; Dehkharghani, F.K.; Sharifi-Asl, S.; Obot, I.B.; Macdonald, D.D.; Farhadi, K.; Avestan, M.S.; Petrossians, A. The inhibition of type 304LSS general corrosion in hydrochloric acid by the New Fuchsin compound. Corros. Sci. 2021, 178, 109072. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Figà, V.; Privitera, A.; Bruno, M.; Napolitano, A.; Piacente, S. Inhibition of Cor-Ten steel corrosion by “green” extracts of Brassica campestris. Corros. Sci. 2018, 136, 91–105. [Google Scholar] [CrossRef]
- Sangeetha, Y.; Meenakshi, S.; Sundaram, C.S. Interactions at the mild steel acid solution interface in the presence of O-fumaryl-chitosan: Electrochemical and surface studies. Carbohydr. Polym. 2016, 136, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mathew, Z.P.; Rajan, K.; Augustine, C.; Joseph, B.; John, S. Corrosion inhibition of mild steel using poly (2-ethyl -2-oxazoline) in 0.1 M HCl solution. Heliyon 2020, 6, e05560. [Google Scholar] [CrossRef] [PubMed]
- Subramania, A.; Sathiya Priya, A.R.; Muralidharan, V.S. Development of novel acidizing inhibitors for carbon steel corrosion in 15% boiling hydrochloric acid. Corrosion 2008, 64, 687. [Google Scholar]
- Haruna, K.; Obot, I.B.; Ankah, N.K.; Sorour, A.A.; Saleh, T.A. Gelatin: A green corrosion inhibitor for carbon steel in oil well acidizing environment. J. Mol. Liq. 2018, 264, 515–525. [Google Scholar] [CrossRef]
- Kowsari, E.; Payami, M.; Amini, R.; Ramezanzadeh, B.; Javanbakht, M. Task-specific ionic liquid as a new green inhibitor of mild steel corrosion. Appl. Surf. Sci. 2014, 289, 478–486. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).