Freshwater Aquaculture Development in EU and Latin-America: Insight on Production Trends and Resource Endowments
Abstract
:1. Introduction
2. Data Sources for the Analysis
3. Aquaculture Production Trends in the Two Regions
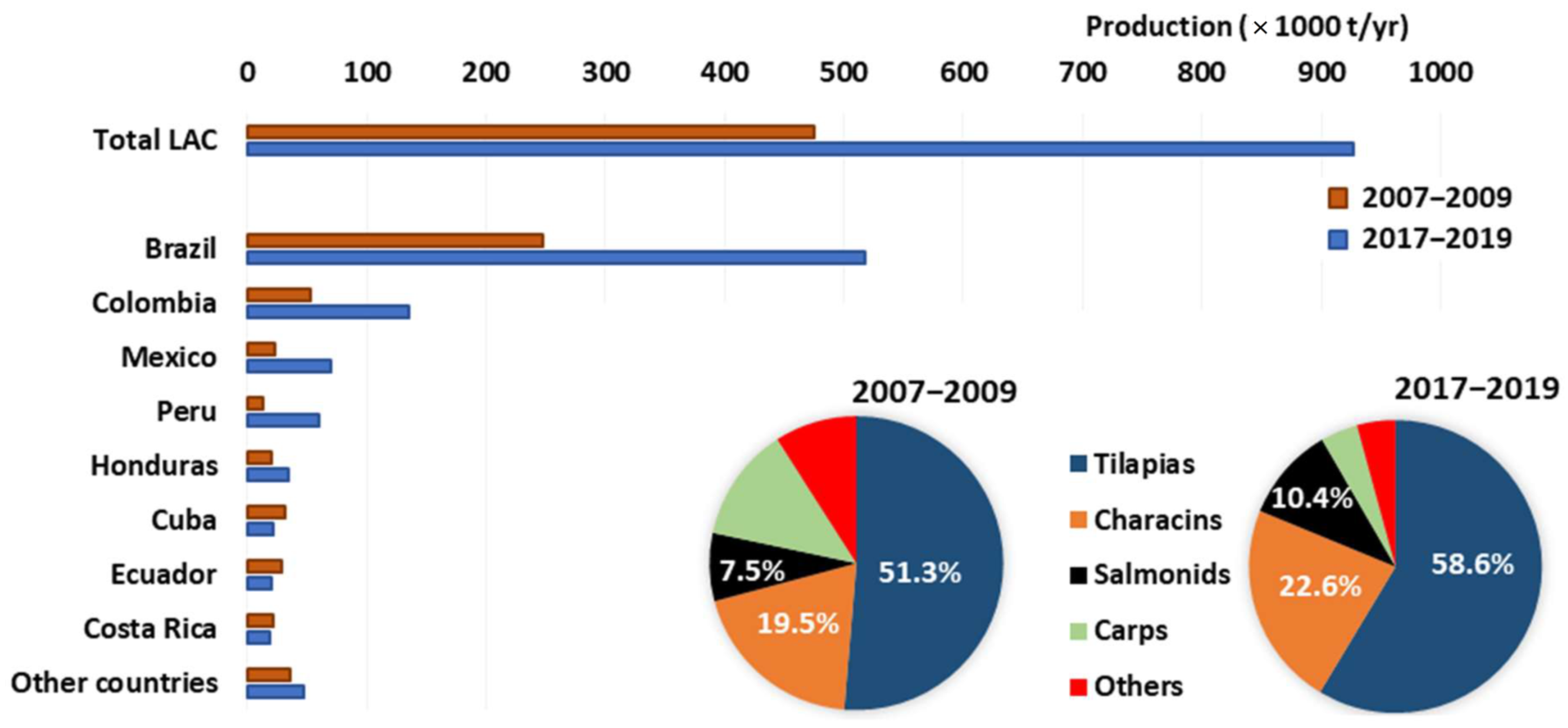
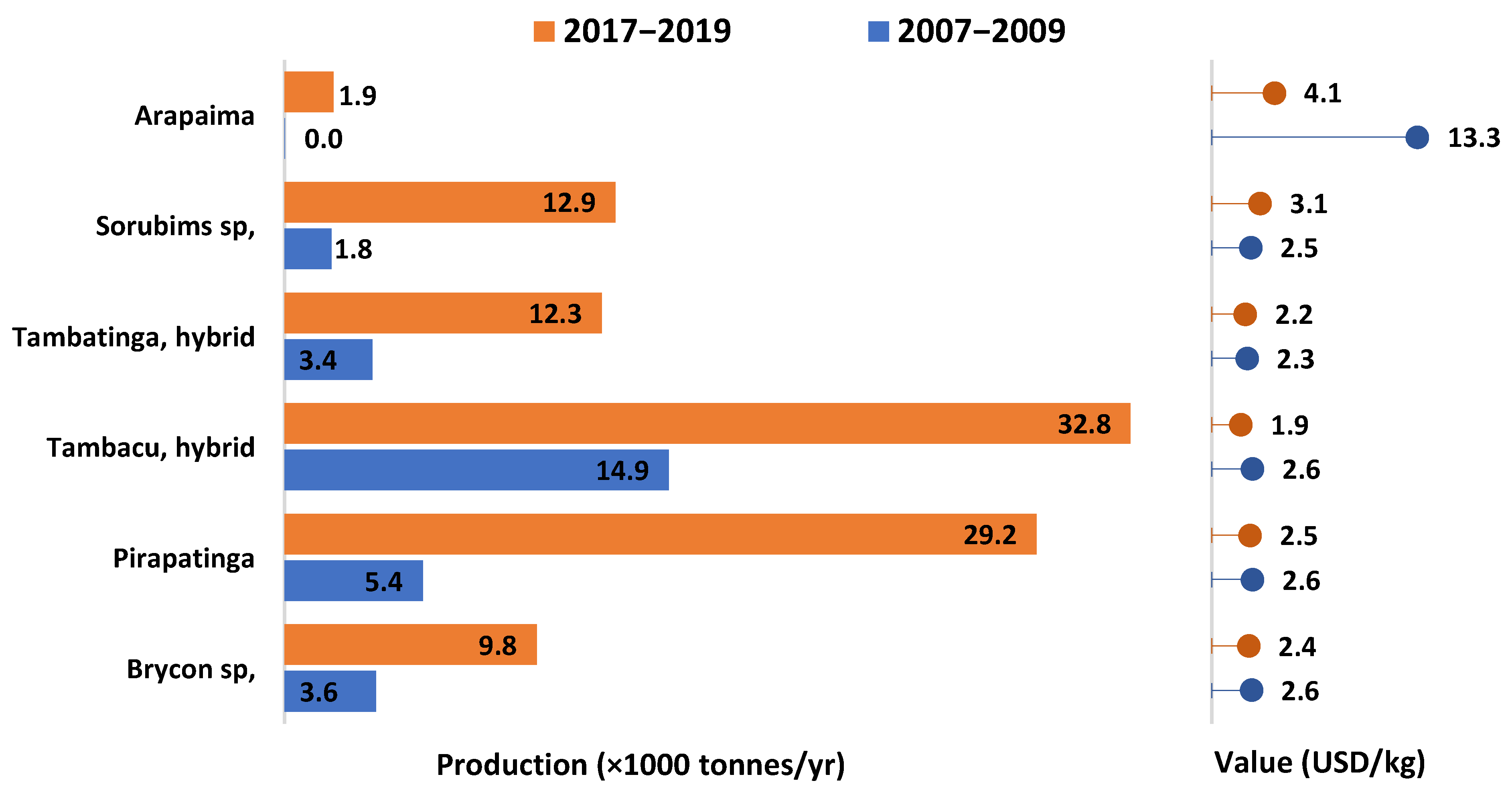
3.1. Production in LAC
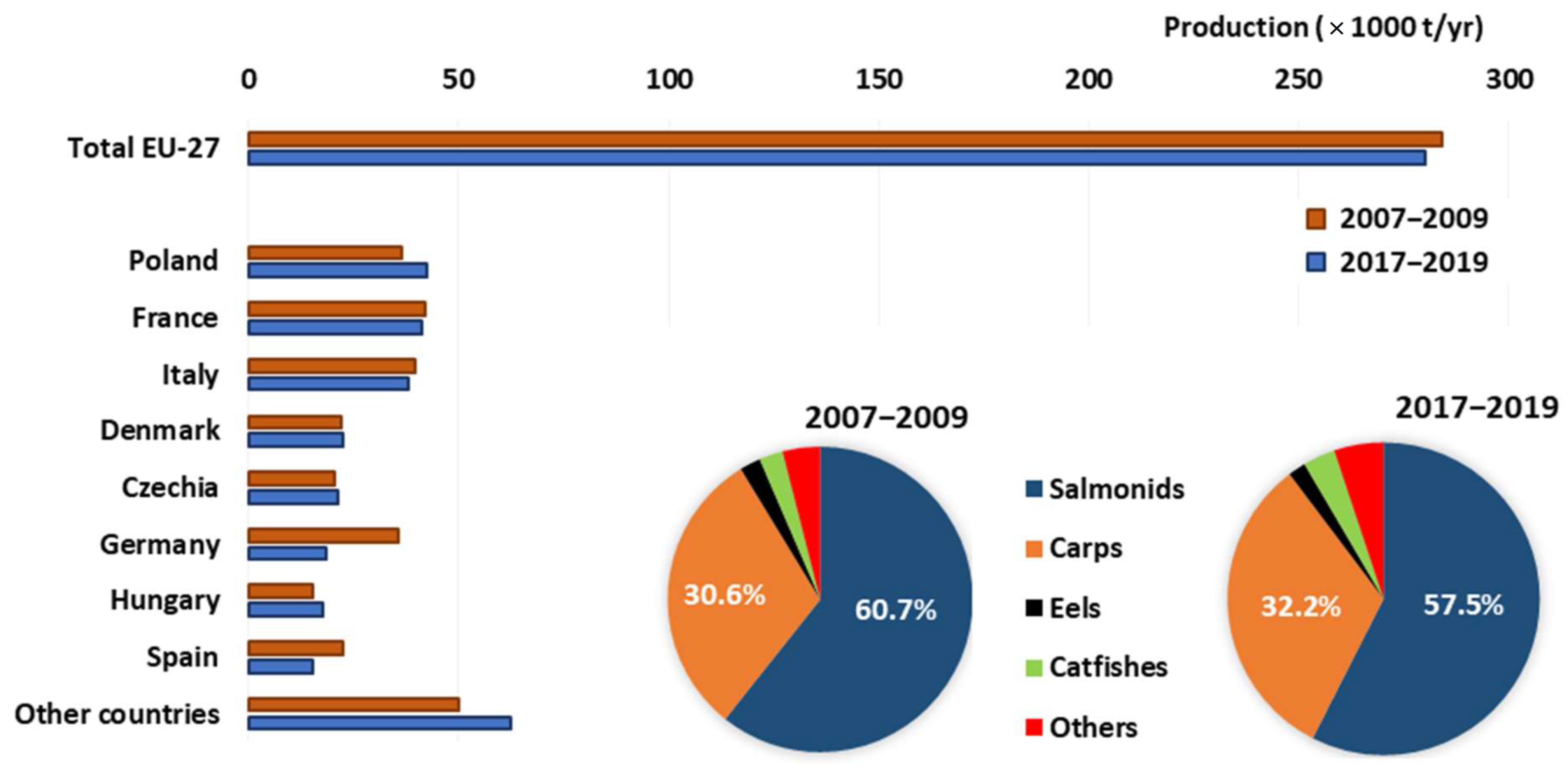
3.2. Production in EU
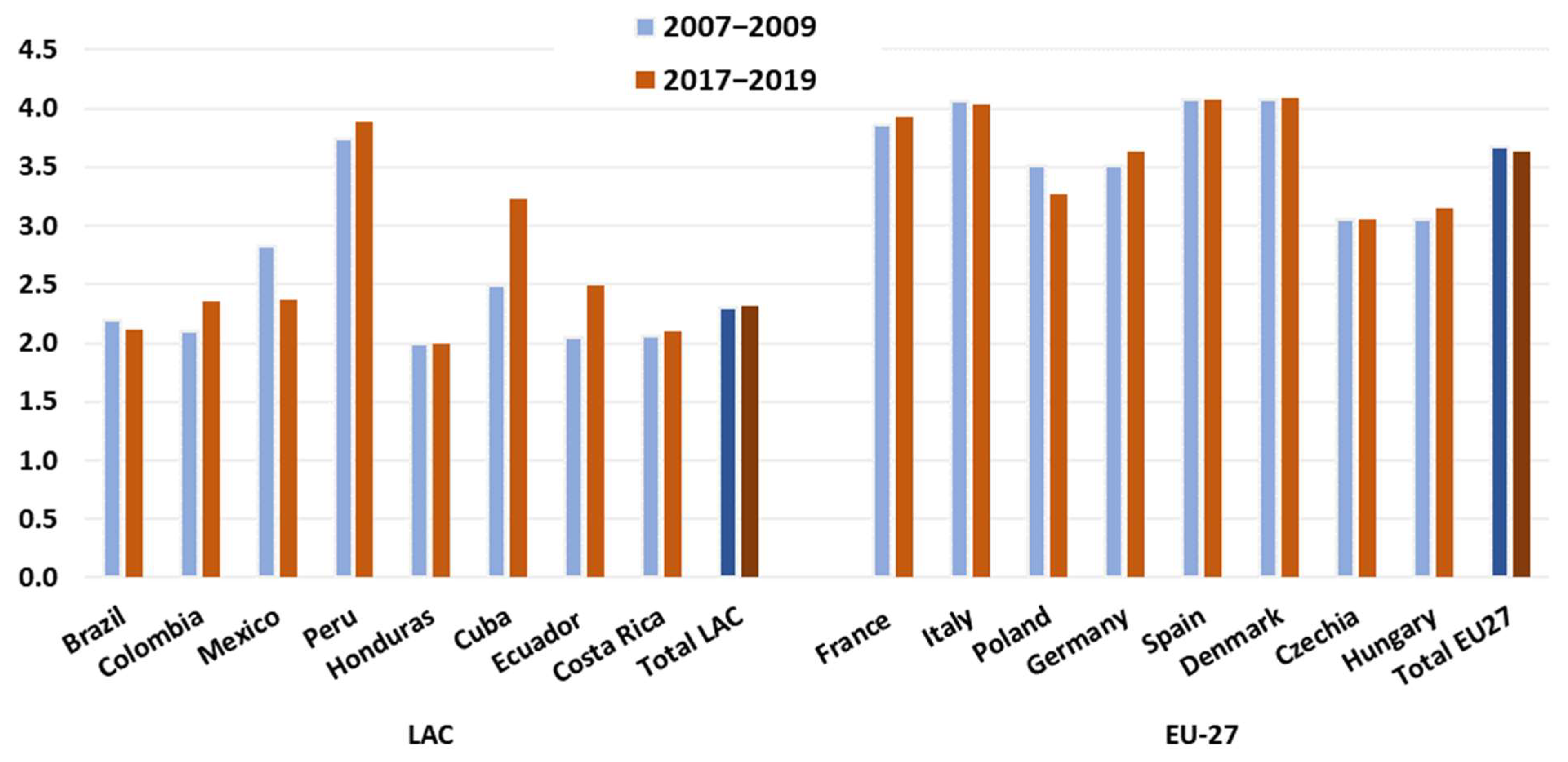
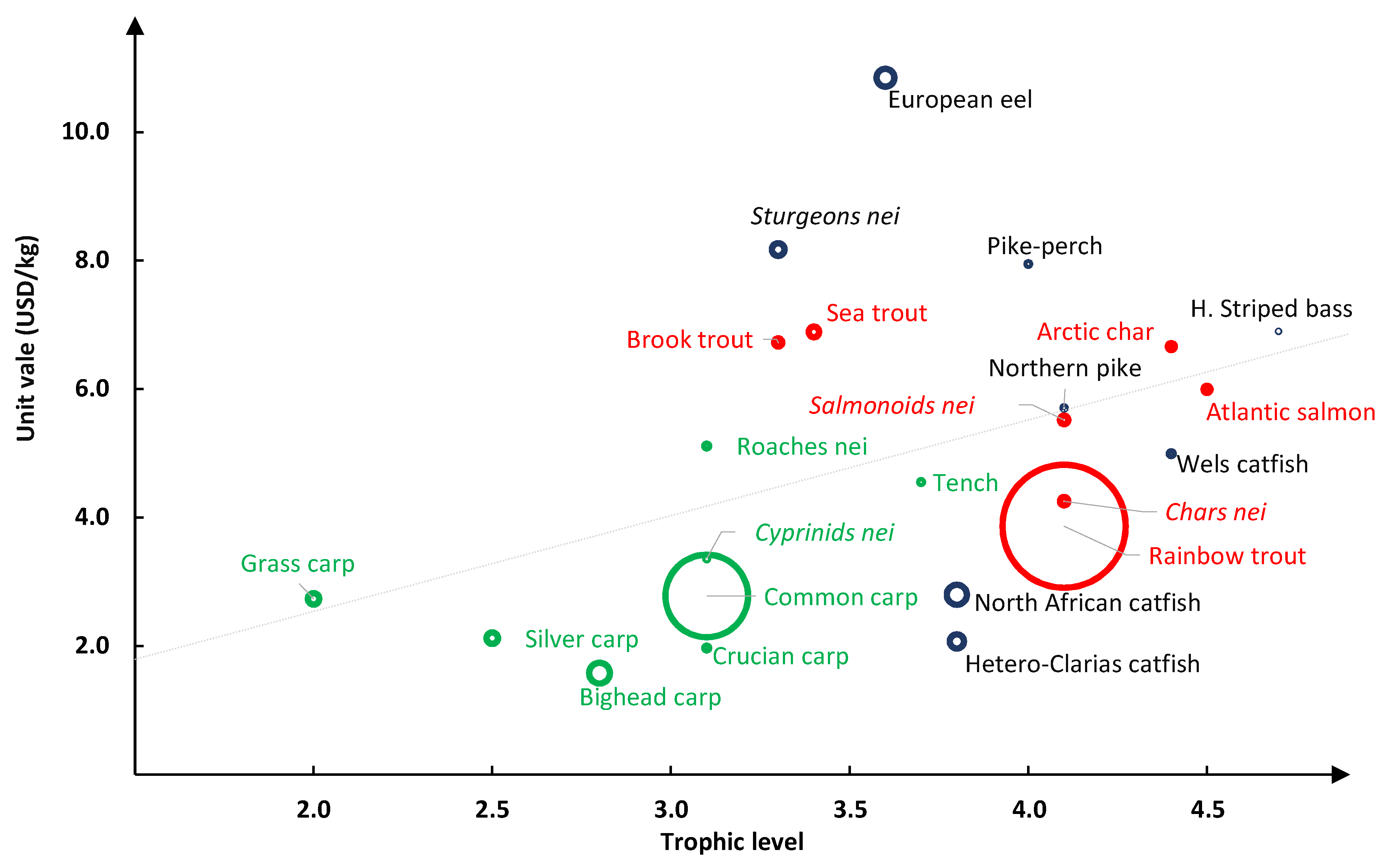
3.3. Trophic Level and Unit Value of Species Produced
3.4. Diversification and Emerging Species
4. Water Use and Resources in LAC and EU Aquaculture
4.1. Water Resource Intensity of LAC and EU Aquaculture Production
4.2. Role of Water Resources in Aquaculture Development
5. Emission of Aquaculture Production
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| LAC Production (t/year) | EU 27 Production (t/year) | |||||
|---|---|---|---|---|---|---|
| ASFIS Species | 2007–2009 | 2017–2019 | ASFIS Species | 2007–2009 | 2017–2019 | |
| 1 | Nile tilapia (Oreochromis niloticus) | 200,785 | 416,322 | Rainbow trout (Oncorhynchus mykiss) | 167,173 | 151,721 |
| 2 | Tilapias nei (Oreochromis spp.) | 40,740 | 125,177 | Common carp (Cyprinus carpio) | 70,448 | 73,355 |
| 3 | Cachama (Colossoma macropomum) | 49,361 | 107,513 | Bighead carp (Hypophthalmichthys nobilis) | 3617 | 6255 |
| 4 | Rainbow trout (Oncorhynchus mykiss) | 31,021 | 95,987 | North African catfish (Clarias gariepinus) | 5296 | 5966 |
| 5 | Pirapatinga (Piaractus brachypomus) | 5381 | 29,174 | Freshwater fishes nei (Actinopterygii) | 5587 | 5620 |
| 6 | Tambacu, hybrid (P. mesopotamicus x C. macropomum) | 14,935 | 32,844 | European eel (Anguilla Anguilla) | 6280 | 5139 |
| 7 | Cyprinids nei (Cyprinidae) | 21,490 | 18,348 | Hetero-Clarias catfish, hybrid (H.longifilis x C.gariepinus) | 1822 | 3372 |
| 8 | Pacu (Piaractus mesopotamicus) | 15,921 | 16,283 | Sturgeons nei (Acipenseridae) | 1345 | 3008 |
| 9 | Silver carp (Hypophthalmichthys molitrix) | 19,189 | 13,447 | Silver carp (Hypophthalmichthys molitrix) | 4917 | 3086 |
| 10 | Tambatinga, hybrid (C. macropomum x P. brachypomus) | 3422 | 11,894 | Grass carp (Ctenopharyngodon idellus) | 1696 | 2479 |
| 11 | Sorubims nei (Pseudoplatystoma spp.) | 0 | 12,404 | Sea trout (Salmo trutta) | 2860 | 2720 |
| 12 | Common carp (Cyprinus carpio) | 19,275 | 6145 | Salmonoids nei (Salmonidae) | 263 | 1628 |
| 13 | North African catfish (Clarias gariepinus) | 3838 | 6042 | Chars nei (Salvelinus spp) | 492 | 1913 |
| 14 | Brycon amazonicus | 0 | 4346 | Brook trout (Salvelinus fontinalis) | 693 | 1596 |
| 15 | Brycon spp | 0 | 4772 | Atlantic salmon (Salmo salar) | 26 | 1100 |
| 16 | Freshwater fishes nei (Actinopterygii) | 15,826 | 3472 | Arctic char (Salvelinus alpinus) | 404 | 1549 |
| 17 | Dorada (Brycon moorei) | 0 | 1346 | Crucian carp (Carassius carassius) | 33 | 337 |
| 18 | Streaked prochilod (Prochilodus lineatus) | 0 | 3167 | Roaches nei (Rutilus spp) | 13 | 710 |
| 19 | Leporinus spp | 0 | 3739 | Wels(=Som) catfish (Silurus glanis) | 1369 | 1051 |
| 20 | Magdalena River prochil (Prochilodus magdalenae) | 0 | 1634 | Silver, bighead carps nei (Hypophthalmichthys spp) | 0 | 771 |
| 21 | Arapaima (Arapaima gigas) | 8 | 1898 | Tench (Tinca tinca) | 1299 | 985 |
| 22 | Blue tilapia (Oreochromis aureus) | 2583 | 1862 | Pike-perch (Sander lucioperca) | 327 | 780 |
| 23 | Brycon melanopterus | 0 | 516 | Northern pike (Esox Lucius) | 286 | 585 |
| 24 | Catfishes nei (Ictalurus spp.) | 0 | 1347 | Cyprinids nei (Cyprinidae) | 1245 | 591 |
| 25 | Trahira (Hoplias malabaricus) | 186 | 746 | Striped bass, hybrid (Morone chrysops x M.saxatilis) | 197 | 344 |

References
- FAO. FishStatJ—Software for Fishery and Aquaculture Statistical Time Series; FAO Fisheries Division: Rome, Italy, 2021. [Google Scholar]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, Present and Future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- EUMOFA. European Market Observatory for Fisheries and Aquaculture Products (EUMOFA); The EU Fish Market: Luxembourg, 2021; Available online: https://www.eumofa.eu/documents/20178/477018/EN_The+EU+fish+market_2021.pdf/27a6d912-a758-6065-c973-c1146ac93d30?t=1636964632989 (accessed on 22 January 2022).
- EUMOFA. European Market Observatory for Fisheries and Aquaculture Products (EUMOFA); The EU Fish Market: Luxembourg, 2020. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; Volume 32. [Google Scholar] [CrossRef]
- FAO. Globefish Highlights April 2019 ISSUE, with Jan.–Dec. 2018 Statistics—A Quarterly Update on World Seafood Markets, 2nd ed.; FAO: Rome, Italy, 2019. [Google Scholar]
- World Bank. World Bank Database. Available online: https://databank.worldbank.org/home.aspx (accessed on 22 January 2022).
- Fishbase. Available online: https://www.fishbase.de/ (accessed on 14 January 2022).
- FAO. AQUASTAT Database, Global Information System on Water and Agriculture. Available online: https://www.fao.org/aquastat/en/databases/ (accessed on 11 August 2021).
- Boyd, C.E.; Li, L.; Brummett, R. Relationship of Freshwater Aquaculture Production to Renewable Freshwater Resources. J. Appl. Aquac. 2012, 24, 99–106. [Google Scholar] [CrossRef]
- World Bank. Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 24 April 2022).
- Longline Environment Ltd. META Database—Maritime and Environmental Thresholds for Aquaculture. 2022. Available online: https://longline.co.uk/meta/ (accessed on 10 May 2022).
- Hofherr, J.; Natale, F.; Fiore, G. Indicators for Sustainable Aquaculture in the European Union an Approach towards European Aquaculture Performance Indicators, 1st ed.; Publication office of the European Union: Luxembourg, 2012. [Google Scholar] [CrossRef]
- Gephart, J.A.; Troell, M.; Henriksson, P.J.G.; Beveridge, M.C.M.; Verdegem, M.; Metian, M.; Mateos, L.D.; Deutsch, L. The ‘Seafood Gap’ in the Food-Water Nexus Literature—Issues Surrounding Freshwater Use in Seafood Production Chains. Adv. Water Resour. 2017, 110, 505–514. [Google Scholar] [CrossRef]
- Peixe, B.R.; Anuario Peixe-BR da Piscicultura. Associacao Brasileira da Piscicultura. Available online: https://www.peixebr.com.br/anuario-peixebr-2018/ (accessed on 21 November 2021).
- Wurmann, C.; Soto, D.; Norambuena, R. Regional Review on Status and Trends in Aquaculture Development in Latin America and the Caribbean-2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Neori, A.; Nobre, A.M. Relationship between Trophic Level and Economics in Aquaculture. Aquac. Econ. Manag. 2012, 16, 40–67. [Google Scholar] [CrossRef]
- Perschbacher, P.W. Sustainability Needs and Challenges: Freshwater Systems. In Tilapia in Intensive Co-Culture; Perschbacher, P.W., Stickney, R.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 114–128. [Google Scholar]
- Lucas, S.; Soler, L.G.; Irz, X.; Gascuel, D.; Aubin, J.; Cloâtre, T. The Environmental Impact of the Consumption of Fishery and Aquaculture Products in France. J. Clean. Prod. 2021, 299, 126718. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M.; Turchini, G.M.; de Silva, S.S. Responsible Aquaculture and Trophic Level Implications to Global Fish Supply. Rev. Fish. Sci. 2010, 18, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, R.S.; Metian, M.; Froehlich, H.E.; Blanchard, J.L.; Sand Jacobsen, N.; McIntyre, P.B.; Nash, K.L.; Williams, D.R.; Bouwman, L.; Gephart, J.A.; et al. Time to Rethink Trophic Levels in Aquaculture Policy. Rev. Aquac. 2021, 13, 1583–1593. [Google Scholar] [CrossRef]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.D.L.; Moraes-Valenti, P. Indicators of Sustainability to Assess Aquaculture Systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving Sustainable Aquaculture: Historical and Current Perspectives and Future Needs and Challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Metian, M.; Troell, M.; Christensen, V.; Steenbeek, J.; Pouil, S. Mapping Diversity of Species in Global Aquaculture. Rev. Aquac. 2020, 12, 1090–1100. [Google Scholar] [CrossRef]
- Popp, J.; Váradi, L.; Békefi, E.; Péteri, A.; Gyalog, G.; Lakner, Z.; Oláh, J. Evolution of Integrated Open Aquaculture Systems in Hungary: Results from a Case Study. Sustainability 2018, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Piria, M.; Jelkić, D.; Gavrilović, A.; Horváth, Á.; Kovács, B.; Balogh, R.E.; Špelić, I.; Radočaj, T.; Vilizzi, L.; Ozimec, S.; et al. Finding of Hybrid African Catfish “Clariobranchus” in the River Danube. J. Vertebr. Biol. 2022, 71, 22008. [Google Scholar] [CrossRef]
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon Meat and Caviar Production: Global Update 2017. J. Appl. Ichthyol. 2019, 35, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Samuel-Fitwi, B.; Nagel, F.; Meyer, S.; Schroeder, J.P.; Schulz, C. Comparative Life Cycle Assessment (LCA) of Raising Rainbow Trout (Oncorhynchus Mykiss) in Different Production Systems. Aquac. Eng. 2013, 54, 85–92. [Google Scholar] [CrossRef]
- Vielma, J.; Kankainen, M.; Setälä, J. Current Status of Recirculation Aquaculture Systems (RAS) and Their Profitability and Competitiveness in the Baltic Sea Area; Natural Resources Institute Finland: Helsinki, Finland, 2021. [Google Scholar]
- Guzmán-Luna, P.; Gerbens-Leenes, P.W.; Vaca-Jiménez, S.D. The Water, Energy, and Land Footprint of Tilapia Aquaculture in Mexico, a Comparison of the Footprints of Fish and Meat. Resour. Conserv. Recycl. 2021, 165, 105224. [Google Scholar] [CrossRef]
- de Godoy, E.M.; David, F.S.; Fialho, N.S.; Proença, D.C.; Camargo, T.R.; Bueno, G.W. Environmental Sustainability of Nile Tilapia Production on Rural Family Farms in the Tropical Atlantic Forest Region. Aquaculture 2022, 547, 737481. [Google Scholar] [CrossRef]
- Nobile, A.B.; Cunico, A.M.; Vitule, J.R.S.; Queiroz, J.; Vidotto-Magnoni, A.P.; Garcia, D.A.Z.; Orsi, M.L.; Lima, F.P.; Acosta, A.A.; da Silva, R.J.; et al. Status and Recommendations for Sustainable Freshwater Aquaculture in Brazil. Rev. Aquac. 2020, 12, 1495–1517. [Google Scholar] [CrossRef]
- Fialho, N.S.; Valenti, W.C.; David, F.S.; Godoy, E.M.; Proenca, D.C.; Roubach, R.; Bueno, G.W. Environmental Sustainability of Nile Tilapia Net-Cage Culture in a Neotropical Region. Ecol. Indic. 2021, 129, 108008. [Google Scholar] [CrossRef]
- Saint-Paul, U. Native Fish Species Boosting Brazilian’s Aquaculture Development. Acta Fish. Aquat. Resour. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Verdegem, M.C.J.; Bosma, R.H. Water Withdrawal for Brackish and Inland Aquaculture, and Options to Produce More Fish in Ponds with Present Water Use. Water Policy 2009, 11, 52–68. [Google Scholar] [CrossRef]
- D’orbcastel, E.R.; Blancheton, J.P.; Belaud, A. Water Quality and Rainbow Trout Performance in a Danish Model Farm Recirculating System: Comparison with a Flow through System. Aquac. Eng. 2009, 40, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.J. Reducing Water Use for Animal Production through Aquaculture. Int. J. Water Resour. Dev. 2006, 22, 101–113. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; van der Werf, H.M.G.; Petit, J.; Morvan, Y.M. Characterisation of the Environmental Impact of a Turbot (Scophthalmus Maximus) Re-Circulating Production System Using Life Cycle Assessment. Aquaculture 2006, 261, 1259–1268. [Google Scholar] [CrossRef]
- Yacout, D.M.M.; Soliman, N.F.; Yacout, M.M. Comparative Life Cycle Assessment (LCA) of Tilapia in Two Production Systems: Semi-Intensive and Intensive. Int. J. Life Cycle Assess. 2016, 21, 806–819. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Boxman, S.E.; Main, K.L.; Zhang, Q.; Trotz, M.A.; Hicks, A. Life Cycle Assessment of Aquaculture Systems: Does Burden Shifting Occur with an Increase in Production Intensity? Aquac. Eng. 2021, 92, 102130. [Google Scholar] [CrossRef]
- Davis, R.P.; Boyd, C.E.; Davis, D.A. Resource Sharing and Resource Sparing, Understanding the Role of Production Intensity and Farm Practices in Resource Use in Shrimp Aquaculture. Ocean Coast. Manag. 2021, 207, 105595. [Google Scholar] [CrossRef]
- Engle, C.R.; Kumar, G.; van Senten, J. Resource-Use Efficiency in US Aquaculture: Farm-Level Comparisons across Fish Species and Production Systems. Aquac. Environ. Interact. 2021, 13, 259–275. [Google Scholar] [CrossRef]
- Rincón, M.A.P.; Hurtado, I.C.; Restrepo, S.; Bonilla, S.P.; Calderón, H.; Ramírez, A. Metodología Para La Medición de La Huella Hídrica En La Producción de Tilapia, Cachama y Trucha: Estudios de Caso Para El Valle Del Cauca (Colombia). Ing. Compet. 2017, 19, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Kiss, G. Statistical Report on Harvest Results in the Aquaculture Sector (2006–2019). 2020. Available online: repo.aki.gov.hu/3585/ (accessed on 14 January 2022). (In Hungarian)
- Eurostat. Production from Aquaculture Excluding Hatcheries and Nurseries. Available online: https://ec.europa.eu/eurostat/databrowser/view/fish_aq2a/default/table?lang=en (accessed on 9 May 2022).
- Tahar, A.; Kennedy, A.; Fitzgerald, R.D.; Clifford, E.; Rowan, N. Full Water Quality Monitoring of a Traditional Flow-through Rainbow Trout Farm. Fishes 2018, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Vrba, J.; Kaushik, S.J.; Mraz, J. Nutrient Footprint and Ecosystem Services of Carp Production in European Fishponds in Contrast to EU Crop and Livestock Sectors: European Carp Production and Environment. J. Clean. Prod. 2020, 270, 122268. [Google Scholar] [CrossRef]
- Gyalog, G.; Oláh, J.; Békefi, E.; Lukácsik, M.; Popp, J. Constraining Factors in Hungarian Carp Farming: An Econometric Perspective. Sustainability 2017, 9, 2111. [Google Scholar] [CrossRef] [Green Version]
- Raftowicz, M.; le Gallic, B. Inland Aquaculture of Carps in Poland: Between Tradition and Innovation. Aquaculture 2020, 518, 734665. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Maritime Affairs and Fisheries; Recirculation Aquaculture Systems; EUMOFA: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Gál, D.; Kucska, B.; Kerepeczki, É.; Gyalog, G. Feasibility of the Sustainable Freshwater Cage Culture in Hungary and Romania. AACL Bioflux 2011, 4, 598–605. [Google Scholar]
- Lasner, T.; Brinker, A.; Nielsen, R.; Rad, F. Establishing a Benchmarking for Fish Farming—Profitability, Productivity and Energy Efficiency of German, Danish and Turkish Rainbow Trout Grow-out Systems. Aquac. Res. 2017, 48, 3134–3148. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Pedersen, P.B. Solid and Suspended/Dissolved Waste (N, P, O) from Rainbow Trout (Oncorynchus Mykiss). Aquaculture 2011, 313, 92–99. [Google Scholar] [CrossRef]
- Bregnballe, J. A Guide to Recirculation Aquaculture: An Introduction to the New Environmentally Friendly and Highly Productive Closed Fish Farming Systems, 2015th ed.; Food and Agriculture Organization of the United Nations: Copenhagen, Denmark, 2015. [Google Scholar]
- Adámek, Z.; Mössmer, M.; Hauber, M. Current Principles and Issues Affecting Organic Carp (Cyprinus carpio) Pond Farming. Aquaculture 2019, 512, 734261. [Google Scholar] [CrossRef]
- Eljasik, P.; Panicz, R.; Sobczak, M.; Sadowski, J. Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes. Sustainability 2022, 14, 3724. [Google Scholar] [CrossRef]
- Varga, M.; Berzi-Nagy, L.; Csukas, B.; Gyalog, G. Long-Term Dynamic Simulation of Environmental Impacts on Ecosystem-Based Pond Aquaculture. Environ. Model. Softw. 2020, 134, 104755. [Google Scholar] [CrossRef]
- Rosa, R.; Marques, A.; Nunes, M.L. Impact of Climate Change in Mediterranean Aquaculture. Rev. Aquac. 2012, 4, 163–177. [Google Scholar] [CrossRef]
- Comte, A. Recent Advances in Climate Change Vulnerability/Risk Assessments in the Fisheries and Aquaculture Sector; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Boyd, C.E.; Effects of Weather and Climate on Aquaculture. Global Aquaculture Advocate. 2020. Available online: https://www.globalseafood.org/advocate/effects-of-weather-and-climate-on-aquaculture/ (accessed on 20 April 2022).
- Panicz, R.; Całka, B.; Cubillo, A.; Ferreira, J.G.; Guilder, J.; Kay, S.; Kennerley, A.; Lopes, A.; Lencart e Silva, J.; Taylor, N.; et al. Impact of Climate-driven Temperature Increase on Inland Aquaculture: Application to Land-based Production of Common Carp (Cyprinus carpio L.). Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Cruz-Casallas, P.E.; Medina-Robles, V.M.; Velasco-Santamaría, Y.M. Fish Farming of Native Species in Colombia: Current Situation and Perspectives. Aquac. Res. 2011, 42, 823–831. [Google Scholar] [CrossRef]
- Blanchet, M.A.; Primicerio, R.; Smalås, A.; Arias-Hansen, J.; Aschan, M. How Vulnerable Is the European Seafood Production to Climate Warming? Fish. Res. 2019, 209, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.J.; Delaporte, A.; Phillips, M.J.; Beveridge, M.; O’keefe, M. Blue Frontiers: Managing the Environmental Costs of Aquaculture; WorldFish: Penang, Malaysia, 2011. [Google Scholar]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental Performance of Blue Foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.F.; Mamun-Ur-Rashid, M. Quantifying Greenhouse Gas Emissions from Global Aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef]
- Biermann, G.; Geist, J. Life Cycle Assessment of Common Carp (Cyprinus carpio L.)—A Comparison of the Environmental Impacts of Conventional and Organic Carp Aquaculture in Germany. Aquaculture 2019, 501, 404–415. [Google Scholar] [CrossRef]
- Waite, R.; Beridge, M.; Brummett, R.; Castine, S. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Washington, DC, USA, 2014. [Google Scholar]
- Liu, Y.; Rosten, T.W.; Henriksen, K.; Hognes, E.S.; Summerfelt, S.; Vinci, B. Comparative Economic Performance and Carbon Footprint of Two Farming Models for Producing Atlantic Salmon (Salmo Salar): Land-Based Closed Containment System in Freshwater and Open Net Pen in Seawater. Aquac. Eng. 2016, 71, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Turchini, G.M. Recirculating Aquaculture Systems (RAS): Environmental Solution and Climate Change Adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Beusen, A.H.W.; Overbeek, C.C.; Bureau, D.P.; Pawlowski, M.; Glibert, P.M. Hindcasts and Future Projections of Global Inland and Coastal Nitrogen and Phosphorus Loads Due to Finfish Aquaculture. Rev. Fish. Sci. 2013, 21, 112–156. [Google Scholar] [CrossRef]
- van Rijn, J. Waste Treatment in Recirculating Aquaculture Systems. Aquac. Eng. 2013, 53, 49–56. [Google Scholar] [CrossRef]
- Abate, T.G.; Nielsen, R.; Tveterås, R. Stringency of Environmental Regulation and Aquaculture Growth: A Cross-Country Analysis. Aquac. Econ. Manag. 2016, 20, 201–221. [Google Scholar] [CrossRef]
- Gál, D.; Pekár, F.; Kerepeczki, É. A Survey on the Environmental Impact of Pond Aquaculture in Hungary. Aquac. Int. 2016, 24, 1543–1554. [Google Scholar] [CrossRef]
- Aubin, J.; Baizeau, V.; Jaeger, C.; Roucaute, M.; Gamito, S. Modeling Trophic Webs in Freshwater Fishpond Systems Using Ecopath: Towards Better Polyculture Management. Aquac. Environ. Interact. 2021, 13, 311–322. [Google Scholar] [CrossRef]
- Bhari, B.; Visvanathan, C. Sustainable Aquaculture: Socio-Economic and Environmental Assessment. In Sustainable Aquaculture, Applied Environmental Science and Engineering for a Sustainable Future; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 63–93. [Google Scholar] [CrossRef]
- Jayanthi, M.; Thirumurthy, S.; Samynathan, M.; Manimaran, K.; Duraisamy, M.; Muralidhar, M. Assessment of Land and Water Ecosystems Capability to Support Aquaculture Expansion in Climate-Vulnerable Regions Using Analytical Hierarchy Process Based Geospatial Analysis. J. Environ. Manag. 2020, 270, 110952. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, J.; González-Bravo, R.; Loge, F.J. Water-Energy-Food Security: A Nexus Perspective of the Current Situation in Latin America and the Caribbean. Energy 2020, 194, 116824. [Google Scholar] [CrossRef]
- Nielsen, R.; Asche, F.; Nielsen, M. Restructuring European Freshwater Aquaculture from Family-Owned to Large-Scale Firms—Lessons from Danish Aquaculture. Aquac. Res. 2015, 47, 3852–3866. [Google Scholar] [CrossRef]
- Raftowicz, M.; le Gallic, B.; Kalisiak-mędelska, M.; Rutkiewicz, K.; Konopska-struś, E. Effectiveness of Public Aid for Inland Aquaculture in Poland—The Relevance of Traditional Performance Ratios. Sustainability 2021, 13, 5155. [Google Scholar] [CrossRef]
- Lasner, T.; Mytlewski, A.; Nourry, M.; Rakowski, M.; Oberle, M. Carp Land: Economics of Fish Farms and the Impact of Region-Marketing in the Aischgrund (DEU) and Barycz Valley (POL). Aquaculture 2020, 519, 734731. [Google Scholar] [CrossRef]




| Species, Production System | Direct Water Use 1 | Source and Further Information on Indirect Water Use for Upstream and Downstream Segments |
|---|---|---|
| Rainbow trout, pond culture (Colombia) | 16.9 m3/kg | Source: [43] The study calculated blue, grey, and green water footprint (WF) for the hatching and on-growing phases: feed and electricity (input) production. Calculated WFs were 19.8, 5.5, and 6.1 m3/kg for trout, tilapia, and chachama, respectively. |
| Tilapia, pond culture (Colombia) | 2.7 m3/kg | |
| Cachama, pond culture (Colombia) | 3.9 m3/kg | |
| Nile tilapia, extensive reservoir culture 2 (Mexico) | 2.8 m3/kg | Source: [30]. The study calculated blue, grey, and green WFs for the following stages: broodstock keeping, on-growing, fish processing, transport, feed, and fertilizer (input) production. Calculated WFs were 4.0, 37.8, and 68.2 m3/kg for extensive, semi-intensive, and intensive culture, respectively (on a live weight basis). |
| Nile tilapia, semi-intensive pond culture 3 (Mexico) | 8.7 m3/kg | |
| Nile tilapia, intensive pond culture 4 (Mexico) | 39.1 m3/kg | |
| Nile tilapia, semi-intensive pond culture 5 (Brazil) | 17–34 m3/kg | Source: [31] Water dependency analysis focused only on blue water use during on-growing stage. |
| Nile tilapia, Intensive cage culture in reservoir 6 (Brazil) | <0.01 m3/kg | Water dependency analysis focused only on blue water use during on-growing stage. Source: [33] |
| African catfish, intensive RAS culture 7 (Netherland) | 0.1 m3/kg | Analysis scoped system-associated (blue) water use during on-growing. If feed-associated (green) water use was added, water use would amount to 0.5 m3/kg. Source: [37] |
| Carp, semi-intensive pond culture 8 (Hungary) | 21.1 m3/kg | Calculated system-associated (blue) water use based on data from country-level statistical report for 2019 [44]. If feed-associated water use was added, water use would amount to 24.8 m3/kg. |
| Trout farmed in flow-through raceway tanks 9 (France) | 54.2 m3/kg | Water dependency analysis focused only on blue water use during on-growing stage. Source: [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyalog, G.; Cubillos Tovar, J.P.; Békefi, E. Freshwater Aquaculture Development in EU and Latin-America: Insight on Production Trends and Resource Endowments. Sustainability 2022, 14, 6443. https://doi.org/10.3390/su14116443
Gyalog G, Cubillos Tovar JP, Békefi E. Freshwater Aquaculture Development in EU and Latin-America: Insight on Production Trends and Resource Endowments. Sustainability. 2022; 14(11):6443. https://doi.org/10.3390/su14116443
Chicago/Turabian StyleGyalog, Gergő, Julieth Paola Cubillos Tovar, and Emese Békefi. 2022. "Freshwater Aquaculture Development in EU and Latin-America: Insight on Production Trends and Resource Endowments" Sustainability 14, no. 11: 6443. https://doi.org/10.3390/su14116443
APA StyleGyalog, G., Cubillos Tovar, J. P., & Békefi, E. (2022). Freshwater Aquaculture Development in EU and Latin-America: Insight on Production Trends and Resource Endowments. Sustainability, 14(11), 6443. https://doi.org/10.3390/su14116443






