Vegetation Pattern and Regeneration Dynamics of the Progressively Declining Monotheca buxifolia Forests in Pakistan: Implications for Conservation
Abstract
1. Introduction
2. Materials and Methods
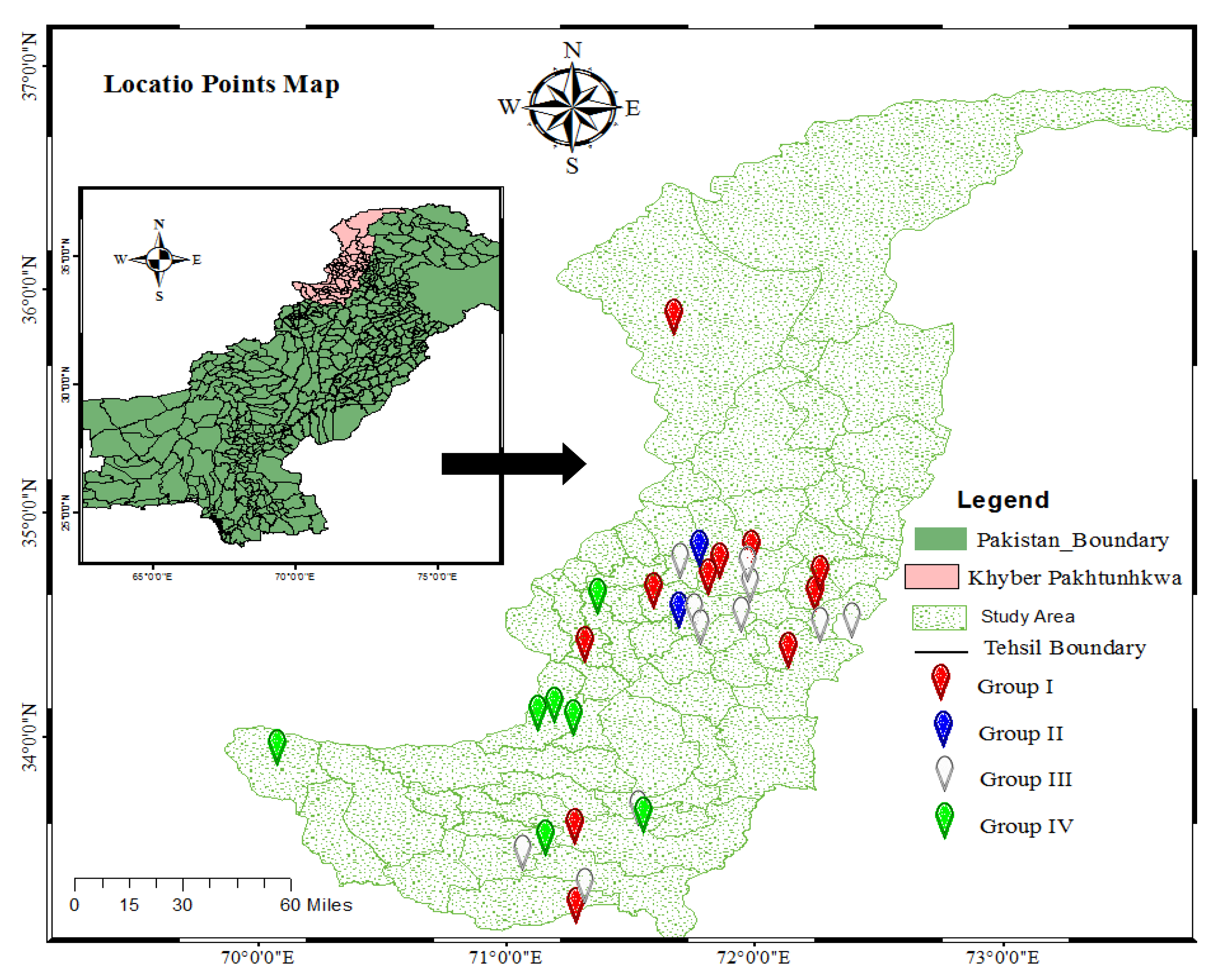
2.1. Study Area
2.2. Sampling Design Data Collection
2.3. Measurements
2.3.1. Floristic Composition
2.3.2. Environmental Factors
2.3.3. Regeneration Measurement
2.4. Data Analysis
3. Results
3.1. Floristic Composition
3.2. Chorological Affinities and Life-Forms
3.3. Communities–Environment Relationships
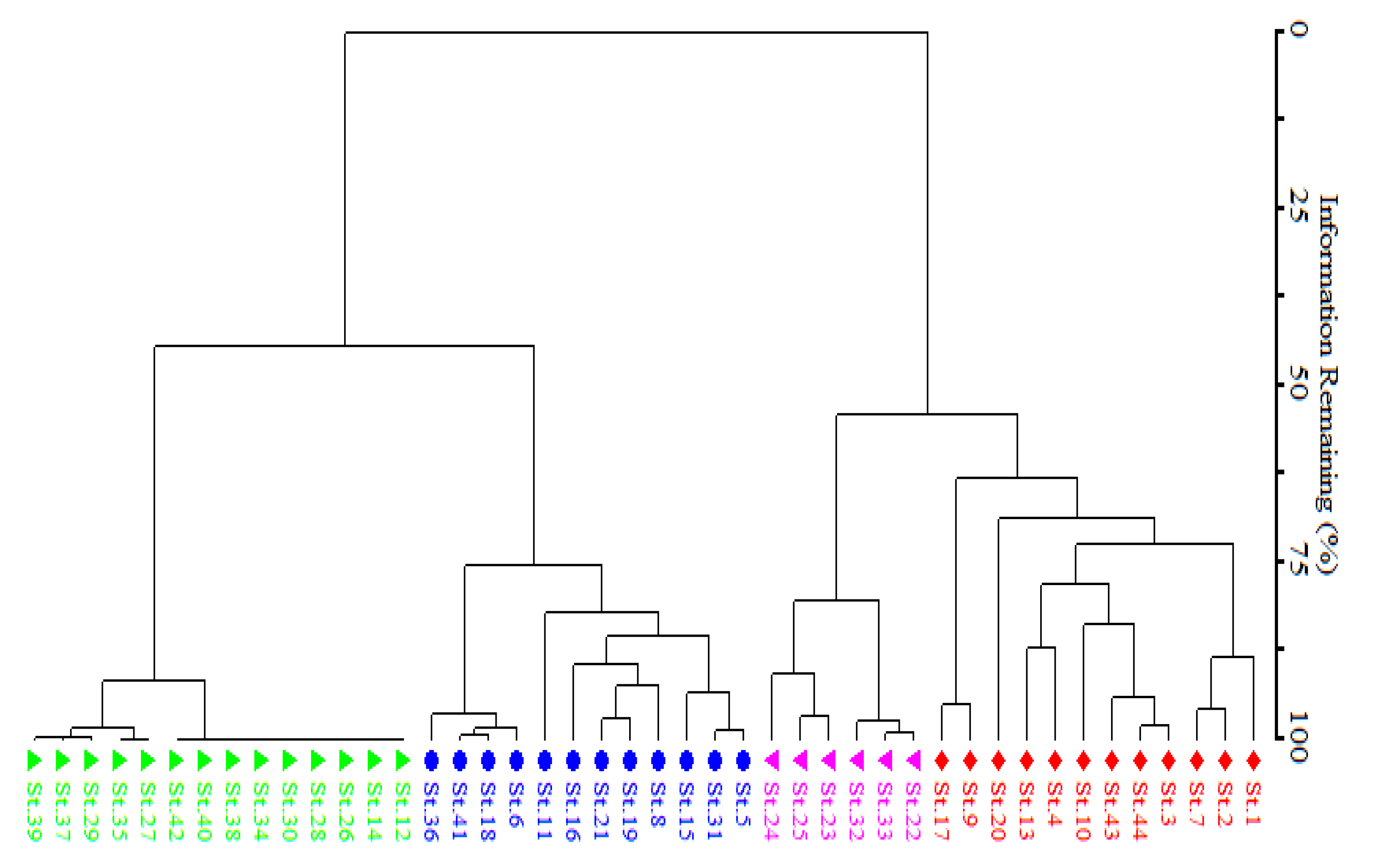
3.4. Description of the Ecological Species Groups
3.5. Stand Characteristics
3.6. Regeneration Dynamics of Monotheca
4. Discussion
4.1. Floristic Composition and Chorology
4.2. RDA
4.3. Classification of Monotheca Stands
4.4. Stand Structure
4.5. Size Classes and Regeneration
4.6. Implications for Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakhoul, J.; Fernandez, C.; Bousquet-Mélou, A.; Nemer, N.; Abboud, J.; Prévosto, B. Vegetation dynamics and regeneration of Pinus pinea forests in Mount Lebanon: Towards the progressive disappearance of pine. Ecol. Eng. 2020, 152, 105866. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Ali, K.; Khan, I. Influence of environmental variables on the distribution of woody species in Muslim graveyards of Malakand Division, Hindukush Range Mountains of Pakistan. Pak. J. Bot. 2017, 49, 2357–2366. [Google Scholar]
- Cutini, A.; Chianucci, F.; Giannini, T.; Manetti, M.C.; Salvati, L. Is anticipated seed cutting an effective option to accelerate transition to high forest in European beech (Fagus sylvatica L.) coppice stands? Ann. For. Sci. 2015, 72, 631–640. [Google Scholar] [CrossRef]
- Ameztegui, A.; Coll, L.; Benavides, R.; Valladares, F.; Paquette, A. Understory light predictions in mixed conifer mountain forests: Role of aspect-induced variation in crown geometry and openness. For. Ecol. Manag. 2012, 276, 52–61. [Google Scholar] [CrossRef]
- Lefrancois, M.L.; Beaudet, M.; Messier, C. Crown openness as influenced by tree and site characteristics for yellow birch, sugar maple, and eastern hemlock. Can. J. For. Res. 2008, 38, 488–497. [Google Scholar] [CrossRef]
- Augusto, L.; Dupouey, J.L.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Amin, M.; Gurmani, A.R.; Ali, F.; Khan, S.M.; Farid, A.; Shakur, M.; Khan, W. Investigation of multi-pesticide residues in Prunus persica L.(peach) cultivars of district Swat using gas chromatography-mass spectroscopy. Pol. J. Environ. Stud. 2022, 31, 1535–1542. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: Natural patterns and response to variable-retention harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Qian, H.; Klinka, K.; Økland, R.H.; Krestov, P.; Kayahara, G.J. Understorey vegetation in boreal Picea mariana and Populus tremuloides stands in British Columbia. J. Veg. Sci. 2003, 14, 173–184. [Google Scholar] [CrossRef]
- Aikens, M.L.; Ellum, D.; McKenna, J.J.; Kelty, M.J.; Ashton, M.S. The effects of disturbance intensity on temporal and spatial patterns of herb colonization in a southern New England mixed-oak forest. For. Ecol. Manag. 2007, 252, 144–158. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- El-Bana, M.I.; Al-Mathnani, A. Vegetation-soil relationships in the Wadi Al-Hayat area of the Libyan Sahara. Aust. J. Basic Appl. Sci. 2009, 3, 740–747. [Google Scholar]
- Eilu, G.; Hafashimana, D.L.; Kasenene, J.M. Density and species diversity of trees in four tropical forests of the Albertine rift, western Uganda. Divers. Distrib. 2004, 10, 303–312. [Google Scholar] [CrossRef]
- Yang, J.; El-Kassaby, Y.A.; Guan, W. The effect of slope aspect on vegetation attributes in a mountainous dry valley, Southwest China. Sci. Rep. 2020, 10, 16465. [Google Scholar] [CrossRef] [PubMed]
- Ringrose, S.; Matheson, W.; Wolski, P.; Huntsman-Mapila, P. Vegetation cover trends along the Botswana Kalahari transect. J. Arid Environ. 2003, 54, 297–317. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Ahmad, A.; Khan, A.A. Structure and biomass carbon of Olea ferruginea forests in the foot hills of Malakand division, Hindukush range mountains of Pakistan. Acta Ecol. Sin. 2019, 39, 261–266. [Google Scholar] [CrossRef]
- Timilsina, N.; Ross, M.S.; Heinen, J.T. A community analysis of sal (Shorea robusta) forests in the western Terai of Nepal. For. Ecol. Manag. 2007, 241, 223–234. [Google Scholar] [CrossRef]
- Malik, Z.A.; Bhatt, A.B. Regeneration status of tree species and survival of their seedlings in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 2016, 57, 677–690. [Google Scholar]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1997, 52, 107–145. [Google Scholar] [CrossRef]
- Pandey, S.K.; Shukla, R.P. Regeneration strategy and plant diversity status in degraded sal forests. Curr. Sci. 2001, 81, 95–102. [Google Scholar]
- Dutta, G.; Devi, A. Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J. For. Res. 2013, 24, 715–720. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Fares, A.L.B.; Calvão, L.B.; Torres, N.R.; Gurgel, E.S.C.; Michelan, T.S. Environmental factors affect macrophyte diversity on Amazonian aquatic ecosystems inserted in an anthropogenic landscape. Ecol. Indic. 2020, 113, 106231. [Google Scholar] [CrossRef]
- Mavhura, E.; Mushure, S. Forest and wildlife resource-conservation efforts based on indigenous knowledge: The case of Nharira community in Chikomba district, Zimbabwe. For. Policy Econ. 2019, 105, 83–90. [Google Scholar] [CrossRef]
- Ryniker, K.A.; Bush, J.K.; Van Auken, O.W. Structure of Quercus gambelii communities in the Lincoln National forest, New Mexico, USA. For. Ecol. Manag. 2006, 233, 69–77. [Google Scholar] [CrossRef]
- Al-Sherif, E.A.; Ayesh, A.M.; Rawi, S.M. Floristic composition, life form and chorology of plant life at Khulais region, Western Saudi Arabia. Pak. J. Bot. 2013, 45, 29–38. [Google Scholar]
- Ali, F.; Khan, N.; Abd_Allah, E.F.; Ahmad, A. Species Diversity, Growing Stock Variables and Carbon Mitigation Potential in the Phytocoenosis of Monotheca buxifolia Forests along Altitudinal Gradient across Pakistan. Appl. Sci. 2022, 12, 1292. [Google Scholar] [CrossRef]
- Khan, N.; Ahmed, M.; Wahab, M.; Ajaib, M.; Hussain, S.S. Studies along an altitudinal gradient in Monotheca buxifolia (falc.) ad, forest, District Lower Dir, Pakistan. Pak. J. Bot. 2010, 42, 3029–3038. [Google Scholar]
- Murad, W.; Azizullah, A.; Adnan, M.; Tariq, A.; Khan, K.U.; Waheed, S.; Ahmad, A. Ethnobotanical assessment of plant resources of Banda Daud Shah, district Karak, Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 77. [Google Scholar] [CrossRef]
- Gulzar, H.; Hazrat, A.; Gulzar, K.; Ali, F.; Khan, N.; Nisar, M.; Khan, I.; Ullah, A. Medicinal plants and their traditional uses in Thana Village, District Malakand, Khyber Pakhtunkhwa, Pakistan. Int. J. Endorsing Health Sci. Res. 2019, 7, 11–21. [Google Scholar] [CrossRef]
- Ahmed, M.; Husain, T.; Sheikh, A.H.; Hussain, S.S.; Siddiqui, M.F. Phytosociology and structure of Himalayan forests from different climatic zones of Pakistan. Pak. J. Bot. 2006, 38, 361. [Google Scholar]
- Khan, N.; Ali, F.; Ali, K.; Shahid, S. Composition, structure and regeneration dynamics of Olea ferruginea Royle forests from Hindukush range of Pakistan. J. Mt. Sci. 2015, 12, 647–658. [Google Scholar] [CrossRef]
- Irshad, M.; Khan, N.; Ali, K.; Muhammad, Z. The influence of environmental variables on Punica granatum L. assemblages in subtropical dry temperate woodland in the district of Lower Dir, Khyber Pakhtunkhwa, Pakistan. Turk. J. Bot. 2016, 40, 610–622. [Google Scholar] [CrossRef]
- Zahid, M.; Khan, N.; Ali, S.; Ullah, A.; Khan, S.M. Density and taxonomic diversity of understory vegetation in relation to site conditions in natural stands of Acacia modesta in Malakand Division, Khyber Pakhtunkhwa, Pakistan. Science 2016, 35, 26–34. [Google Scholar] [CrossRef][Green Version]
- Cheema, M.S.Z.A.; Qadir, S.A. Autecology of Acacia Senegal (L.) Willd. Vegetation 1973, 27, 131–162. [Google Scholar] [CrossRef]
- Beg, A.R.; Mirza, H.K. Some more plant communities and the future of dry oak forest zone in Swat valley. Pak. J. For. 1984, 34, 25–35. [Google Scholar]
- Huang, J.; Ji, M.; Xie, Y.; Wang, S.; He, Y.; Ran, J. Global semi-arid climate change over last 60 years. Clim. Dyn. 2016, 46, 1131–1150. [Google Scholar] [CrossRef]
- Hussain, F.; Nabi, G.; Wu, R.S. Spatiotemporal Rainfall Distribution of Soan River Basin, Pothwar Region, Pakistan. Adv. Meteorol. 2021, 12, 973. [Google Scholar] [CrossRef]
- Hart, J.L.; Clark, S.L.; Torreano, S.J.; Buchanan, M.L. Composition, structure, and dendroecology of an old-growth Quercus forest on the tablelands of the Cumberland Plateau, USA. For. Ecol. Manag. 2012, 266, 11–24. [Google Scholar] [CrossRef]
- Khan, N.; Ahmed, M.; Shaukat, S.S.; Wahab, M.; Siddiqui, M.F. Structure, diversity, and regeneration potential of Monotheca buxifolia (Falc.) A. DC. dominated forests of Lower Dir District, Pakistan. Front. Agric. China 2011, 5, 106–121. [Google Scholar] [CrossRef]
- Jaja, N. Understanding the Texture of Your Soil for Agricultural Productivity; Virginia State University: Petersburg, VA, USA, 2016; pp. 1–6. [Google Scholar]
- ISO 10390:2005; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10390:ed-2:v1:fr (accessed on 1 March 2022).
- Rigg, L.S.; Enright, N.J.; Jaffré, T. Stand structure of the emergent conifer Araucaria laubenfelsii in maquis and rainforest, Mont Do, New Caledonia. Aust. Ecol. 1998, 23, 528–538. [Google Scholar] [CrossRef]
- Mueller Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; No. 581.5 M8; John Wiley and Sons: New York, NY, USA, 1974; pp. 1–547. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Rahman, I.U.; Khan, N.; Ali, K.; Ahmad, S. Vegetation–environment relationship in Pinus wallichiana forests of the Swat Hindukush range of Pakistan. J. For. Res. 2020, 31, 185–195. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, N.; Ali, K. Classification and ordination of understory vegetation using multivariate techniques in the Pinus wallichiana forests of Swat Valley, northern Pakistan. Sci. Nat. 2017, 104, 24. [Google Scholar] [CrossRef] [PubMed]
- Javad, M.; Mehdi, H.; Prevosto, B. Effects of vegetation patterns and environmental factors on woody regeneration in semi-arid oak-dominated forests of western Iran. J. Arid Land 2017, 9, 368–378. [Google Scholar] [CrossRef]
- Hong, W.; Wang, X.G.; Wu, C.Z. Life table and spectral analysis of endangered plant Taxus chinensis var. mairei population. Chin. J. Appl. Ecol. 2004, 15, 1109–1112. [Google Scholar]
- Wu, J.; Zhang, X.; Dengi, C.; Liu, G.; Li, H. Characteristics and dynamics analysis of Populus euphratica populations in the middle reaches of Tarim River. J. Arid Land 2010, 2, 250–256. [Google Scholar] [CrossRef]
- Malik, Z.H. Comparative Study of the Vegetation of Ganga Chotti and Bedori Hill Dist. Bagh Azad Jammu and Kashmir. Ph.D. Thesis, University of Peshawar, Peshawar, Pakistan, 2005. [Google Scholar]
- Qadri, M.Z.H. Phytosociological Study on the Vegetation of Kotli Hill, Azad Kashmir. Doctoral Dissertation; Botany Department, Peshawar, University: Peshawar, Pakistan, 1986. [Google Scholar]
- Zhang, X.; Wang, M.; She, B.; Xiao, Y. Quantitative classification and ordination of forest communities in Pangquangou National Nature Reserve. Acta Ecol. Sin. 2006, 26, 754–761. [Google Scholar] [CrossRef]
- Heydari, M.; Omidipour, R.; Abedi, M.; Baskin, C. Effects of fire disturbance on alpha and beta diversity and on beta diversity components of soil seed banks and aboveground vegetation. Plant Ecol. Evol. 2017, 150, 247–256. [Google Scholar] [CrossRef]
- Small, C.J.; McCarthy, B.C. Relationship of understory diversity to soil nitrogen, topographic variation, and stand age in an eastern oak forest, USA. For. Ecol. Manag. 2005, 217, 229–243. [Google Scholar] [CrossRef]
- Adel, M.N.; Pourbabaei, H.; Dey, D.C. Ecological species group—Environmental factors relationships in unharvested beech forests in the north of Iran. Ecol. Eng. 2014, 69, 1–7. [Google Scholar] [CrossRef]
- Bigelow, S.W.; Canham, C.D. Community organization of tree species along soil gradients in a north-eastern USA forest. J. Ecol. 2002, 90, 188–200. [Google Scholar] [CrossRef]
- Amorim, P.K.; Batalha, M.A. Soil-vegetation relationships in hyperseasonal cerrado, seasonal cerrado, and wet grassland in Emas National Park (central Brazil). Acta Oecol. 2007, 32, 319–327. [Google Scholar] [CrossRef]
- Vellend, M.; Harmon, L.J.; Lockwood, J.L.; Mayfield, M.M.; Hughes, A.R.; Wares, J.P.; Sax, D.F. Effects of exotic species on evolutionary diversification. Trends Ecol. Evol. 2007, 22, 481–488. [Google Scholar] [CrossRef]
- Khan, S.M.; Page, S.; Ahmad, H.; Harper, D. Identifying plant species and communities across environmental gradients in the Western Himalayas: Method development and conservation use. Ecol. Inform. 2013, 14, 99–103. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Y.; Zhou, X. Application of geographically weighted regression in estimating the effect of climate and site conditions on vegetation distribution in Haihe Catchment, China. Plant Ecol. 2010, 209, 349–359. [Google Scholar] [CrossRef]
- Coblentz, D.; Keating, P.L. Topographic controls on the distribution of tree islands in the high Andes of south-western Ecuador. J. Biogeogr. 2008, 35, 2026–2038. [Google Scholar] [CrossRef]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sfair, J.C.; de Bello, F.; de França, T.Q.; Baldauf, C.; Tabarelli, M. Chronic human disturbance affects plant trait distribution in a seasonally dry tropical forest. Environ. Res. Lett. 2018, 13, 025005. [Google Scholar] [CrossRef]
- Nizami, S.M. The inventory of the carbon stocks in sub-tropical forests of Pakistan for reporting under Kyoto Protocol. J. For. Res. 2012, 23, 377–384. [Google Scholar] [CrossRef]
- Jorge, L.A.B.; Pereira, V.R. Anthropogenic disturbances and the natural vegetation regeneration: A case study of a forest fragment located in a Cuesta Relief area, State of São Paulo, Brazil. Open J. For. 2015, 5, 621. [Google Scholar] [CrossRef]


| Axis 1 | Axis 2 | Axis 3 | p-Value | |
|---|---|---|---|---|
| Eigenvalue | 2.85 | 1.613 | 1.411 | 0.025 |
| % of variance explained | 10.6 | 6.0 | 5.2 | - |
| Cumulative % explained | 10.6 | 16.5 | 21.8 | - |
| Pearson Correlation, Spp-Envt | 0.893 | 0.902 | 0.896 | 0.118 |
| Kendall (Rank) Corr., Spp-Envt | 0.5 | 0.61 | 0.636 | - |
| Group-I Mono-Acacia | Group-II Mono-Olea | Group-III Mono-Eugl | Group-IV Mono | F | P | |
|---|---|---|---|---|---|---|
| Richness | 23 a (4.08 ± 0.59) | 09 b (3.33 ± 0.95) | 17 c (4.08 ± 0.89) | 07 b (3.43 ± 0.57) | 0.286 | 0.834 |
| Average no of individuals | 72.75 ± 3.2 | 68.5 ± 5.7 | 80.3 ± 5.76 | 72.78 ± 5.85 | 0.718 | 0.546 |
| Total no of individuals | 853 | 411 | 964 | 1019 | × | × |
| Margalef’s Index | 0.71 ± 0.13 | 0.54 ± 0.21 | 0.68 ± 0.19 | 0.56 ± 0.12 | 0.25 | 0.856 |
| Simpson’s Index | 1.45 ± 0.15 | 1.38 ± 0.19 | 1.57 ± 0.17 | 1.49 ± 0.11 | 0.228 | 0.875 |
| Shannon-Wiener Index | 0.62 ± 0.09 | 0.52 ± 0.25 | 0.68 ± 0.13 | 0.55 ± 0.09 | 0.290 | 0.832 |
| Pielou’s Index | 0.46 ± 0.05 | 0.29 ± 0.11 | 0.49 ± 0.06 | 0.48 ± 0.06 | 1.102 | 0.359 |
| Acroyms | Mono-Acacia | Mono-Olea | Mono-Eugl | Mono | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Mobu | 59.38 ± 1.6 a | 69.22 ± 2.44 b | 80.88 ± 1.29 c | 97.49 ± 0.97 d | 143.61 | 1.89 × 10−21 |
| Eugl | 0.72 ± 0.4 a | 0 ± 0 a | 3.83 ± 1.49 b | 0 ± 0 a | 42.219 | 0.002 |
| Quba | 1.44 ± 1.19 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.305 | 0.285 |
| Ceeu | 1.56 ± 1.28 | 0 ± 0 | 0.87 ± 0.59 | 0 ± 0 | 1.923 | 0.141 |
| Aial | 4.78 ± 1.5 a | 0.39 ± 0.39 b | 0.77 ± 0.77 b | 0 ± 0 b | 6.120 | 0.0015 |
| Moal | 1.89 ± 0.71 | 1.87 ± 1.34 | 0.61 ± 0.61 | 0.36 ± 0.36 | 1.483 | 0.233 |
| Fipa | 1.17 ± 0.54 | 1.4 ± 0.91 | 1.1 ± 0.75 | 0.27 ± 0.27 | 0.731 | 0.539 |
| Olfe | 2.61 ± 0.69 a | 19.58 ± 3.3 b | 1.51 ± 1.02 c | 0.34 ± 0.34 c | 42.377 | 1.72 × 10−12 |
| Meaz | 1.26 ± 0.63 | 0.77 ± 0.77 | 0 ± 0 | 0 ± 0 | 2.608 | 0.064 |
| Brpa | 0.43 ± 0.30 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.807 | 0.161 |
| Piro | 3.01 ± 2.21 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.631 | 0.197 |
| Dasi | 1.59 ± 1.08 | 0 ± 0 | 0.27 ± 0.27 | 0 ± 0 | 1.563 | 0.213 |
| Zaar | 0.44 ± 0.31 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.780 | 0.166 |
| Pige | 0.25 ± 0.25 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.881 | 0.458 |
| Acmo | 11.35 ± 3.43 a | 4.89 ± 1.95 b | 2.69 ± 1.53 b | 0 ± 0 c | 5.776 | 0.002 |
| Pugr | 2.55 ± 1.56 | 0 ± 0 | 0 ± 0 | 0.42 ± 0.42 | 1.893 | 0.146 |
| Prar | 0.79 ± 0.54 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.836 | 0.156 |
| Acni | 0.25 ± 0.25 | 0 ± 0 | 1.07 ± 0.72 | 0 ± 0 | 1.519 | 0.224 |
| Grop | 0.7 ± 0.7 | 0 ± 0 | 0.96 ± 0.96 | 0 ± 0 | 0.566 | 0.640 |
| Poni | 0 ± 0 | 0 ± 0 | 0.50 ± 0.50 | 0 ± 0 | 0.881 | 0.458 |
| Zimo | 1.81 ± 1.31 | 1.25 ± 1.25 | 1.95 ± 1.61 | 0.64 ± 0.64 | 0.278 | 0.840 |
| Saol | 0.1 ± 0.1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.881 | 0.458 |
| Alle | 0.28 ± 0.28 | 0 ± 0 | 0.35 ± 0.35 | 0 ± 0 | 0.559 | 0.644 |
| Cade | 0 ± 0 | 0 ± 0 | 1.23 ± 0.99 | 0 ± 0 | 1.344 | 0.273 |
| Taap | 0 ± 0 | 0 ± 0 | 1.22 ± 1.22 | 0 ± 0 | 0.881 | 0.458 |
| Phda | 0 ± 0 | 0 ± 0 | 0.25 ± 0.25 | 0.47 ± 0.47 | 0.482 | 0.696 |
| Jure | 0.52 ± 0.52 | 0.64 ± 0.64 | 0 ± 0 | 0 ± 0 | 0.942 | 0.429 |
| Mono-Acacia | Mono-Olea | Mono-Eugl | Mono | F | P | |
|---|---|---|---|---|---|---|
| XLat | 34.06 ± 0.40 | 33.89 ± 0.19 | 33.97 ± 0.27 | 31.18 ± 2.12 | 0.699 | 0.558 |
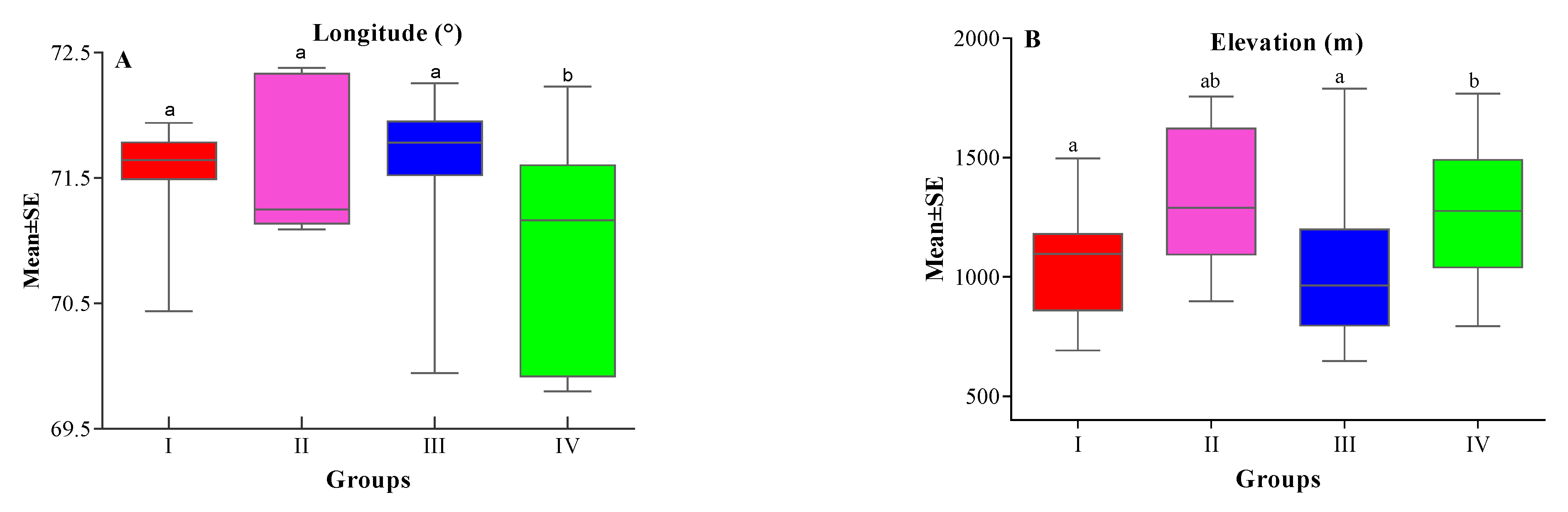
| XLong | 71.50 ± 2.13 a | 71.57 ± 0.24 a | 71.65 ± 0.16 a | 70.88 ± 0.22 b | 3.758 | 0.0181 |
| XEle | 1038.03 ± 68.8 a | 1328.1 ± 126.9 ab | 1031.72 ± 90 a | 1275.3 ± 80.68 b | 2.887 | 0.0473 |
| XSlope | 21.08 ± 2.85 | 28.5 ± 1.99 | 23.25 ± 2.92 | 26.79 ± 2.6 | 1.214 | 0.316 |
| XAsp | 225.75 ± 29.85 | 203.3 ± 49.88 | 219.3 ± 24 | 254.93 ± 19.35 | 0.581 | 0.630 |
| XClay | 11.9 ± 1.09 | 11.67 ± 1.97 | 11.8 ± 1.12 | 13.9 ± 1.01 | 0.935 | 0.432 |
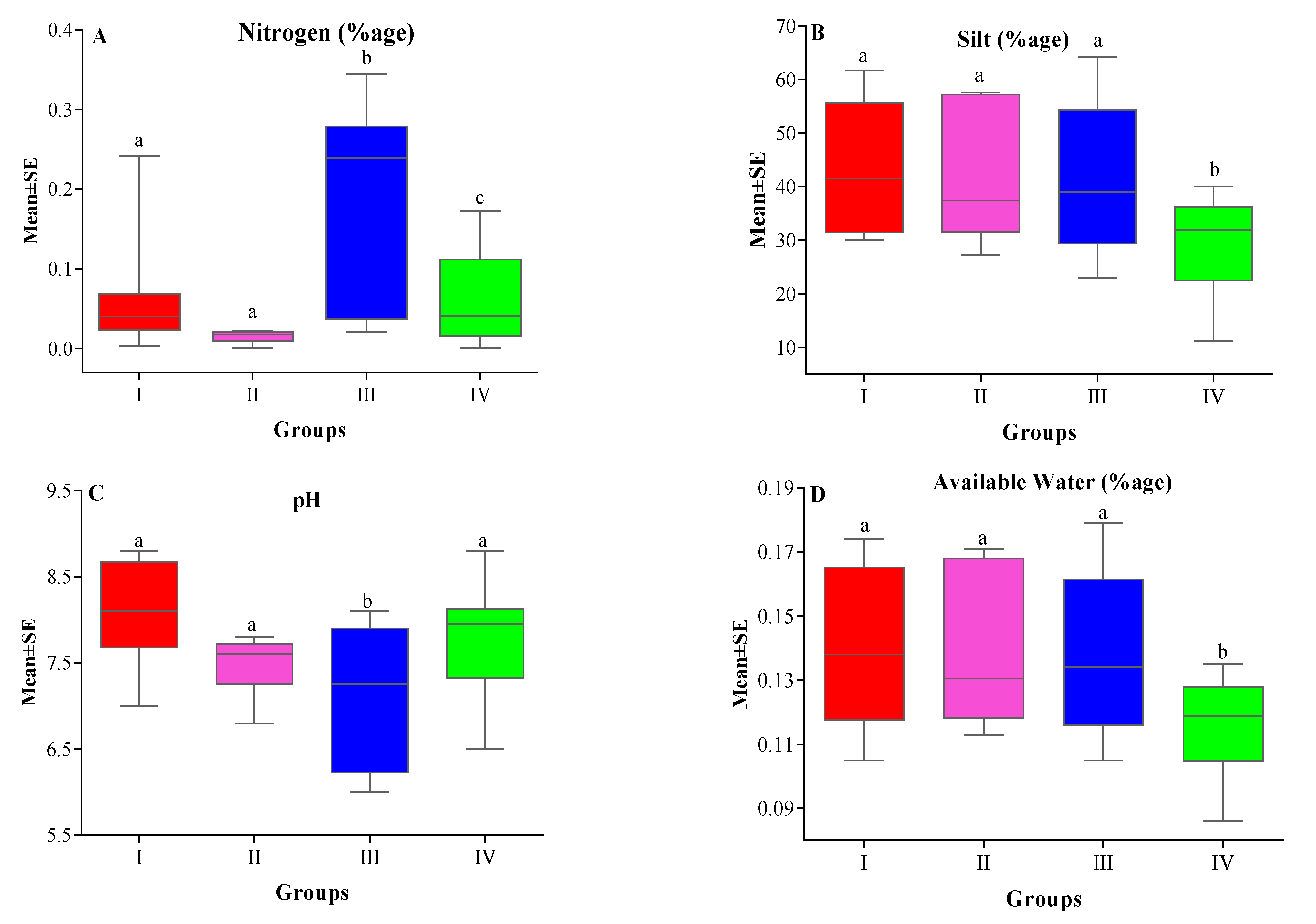
| XSilt | 43.18 ± 3.41 a | 41.62 ± 5.29 a | 41.49 ± 4.01 a | 29.63 ± 2.43 b | 3.643 | 0.020 |
| XSand | 44.94 ± 3.77 | 46.65 ± 4.6 | 46.80 ± 4.6 | 56.58 ± 2.45 | 2.261 | 0.096 |
| XTexture | 2 ± 0.21 | 2 ± 0.25 | 2.08 ± 0.22 | 2.36 ± 0.17 | 0.696 | 0.559 |
| XpH | 7.78 ± 0.15 a | 7.98 ± 0.21 a | 7.97 ± 0.11 b | 7.66 ± 0.18 a | 0.892 | 0.053 |
| X% OM | 1.03 ± 0.37 | 1.82 ± 0.72 | 2.15 ± 0.66 | 1.53 ± 0.4 | 0.890 | 0.4541 |
| X% Lime | 2.16 ± 0.44 | 3.04 ± 0.85 | 2.87 ± 1.06 | 2.73 ± 0.29 | 0.280 | 0.8391 |
| X% N | 0.05 ± 0.02 a | 0.10 ± 0.03 a | 0.11 ± 0.03 b | 0.07 ± 0.01 c | 8.001 | 0.0002 |
| XP (mg/kg) | 13.98 ± 1.42 | 16.8 ± 1.31 | 15.87 ± 1.34 | 14.38 ± 1.7 | 0.555 | 0.647 |
| XK (mg/kg) | 144.25 ± 14.35 | 149.8 ± 25.76 | 146.17 ± 18.3 | 156 ± 15.6 | 0.103 | 0.9577 |
| XFC | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.008 | 0.23 ± 0.006 | 1.187 | 0.3268 |
| XBD (g/cm3) | 1.46 ± 0.02 | 1.46 ± 0.03 | 1.46 ± 0.01 | 1.49 ± 0.01 | 0.956 | 0.4227 |
| XAW (%) | 0.14 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.01 a | 0.12 ± 0.003 b | 3.312 | 0.0295 |
| XEc(µs/cm) | 282.4 ± 25 | 332 ± 31.9 | 316.25 ± 25.2 | 325.07 ± 21.1 | 0.743 | 0.5326 |
| XTDS | 155.33 ± 13.7 | 182.6 ± 17.54 | 173.96 ± 13.8 | 178.79 ± 11.63 | 0.7437 | 0.5323 |
| S.no | Categories | Community Types | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mono-Acacia | Mono-Olea | Mono-Eugl | Monotheca | ||||||
| D/ha | BA | D/ha | BA | D/ha | BA | D/ha | BA | ||
| 1 | Cut Stumps | 33.33 | × | 22.22 | × | 30.37 | × | 20.95 | × |
| 2 | Seedlings <3 cm | 11.48 | × | 7.41 | × | 15.93 | × | 15.55 | × |
| 3 | Saplings <5 cm | 9.25 | × | 5.92 | × | 15.93 | × | 14.28 | × |
| 4 | Small trees 5–30 cm | 78.51 | 2.69 | 89.63 | 3.58 | 57.41 | 2.38 | 83.49 | 3.34 |
| 5 | Medium trees 31–55 cm | 114.81 | 17.01 | 140 | 17.39 | 128.15 | 14.66 | 136.51 | 16.51 |
| 6 | Large trees56–80 cm | 50.37 | 17.12 | 26.65 | 10.31 | 64.44 | 23.49 | 49.2 | 11.2 |
| 7 | Mature trees < 80 cm | 11.85 | 8.66 | 2.961 | 1.56 | 30 | 22.07 | 3.8 | 3.55 |
| Total | 309.6 | 45.48 | 294.79 | 32.84 | 342.23 | 62.7 | 46.25 | 34.62 | |
| Dbh Classes | ax | Ix | dx | qx | Lx | tx | ex | kx |
|---|---|---|---|---|---|---|---|---|
| Seedlings | 113 | 1000 | 230.0885 | 0.231 | 615.044 | 14,172.56 | 14.17 | 0 |
| Saplings | 87 | 769.911 | −5495.58 | −7.137 | −2362.831 | 13,557.52 | 17.61 | 0.113559 |
| 5–30 | 708 | 6265.48 | −3460.18 | −0.552 | 1402.654 | 15,920.35 | 2.54 | −0.91051 |
| 31–55 | 1099 | 9725.66 | 6530.97 | 0.671 | 8128.318 | 14,517.69 | 1.49 | −0.19096 |
| 56–80 | 361 | 3194.69 | 1451.32 | 0.454 | 2323.001 | 6389.38 | 2 | 0.48349 |
| <80 | 197 | 1743.36 | 1743.36 | 1 | 1743.362 | 4066.37 | 2.33 | 0.263041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Vegetation Pattern and Regeneration Dynamics of the Progressively Declining Monotheca buxifolia Forests in Pakistan: Implications for Conservation. Sustainability 2022, 14, 6111. https://doi.org/10.3390/su14106111
Ali F, Khan N, Ali K, Khan MEH, Jones DA. Vegetation Pattern and Regeneration Dynamics of the Progressively Declining Monotheca buxifolia Forests in Pakistan: Implications for Conservation. Sustainability. 2022; 14(10):6111. https://doi.org/10.3390/su14106111
Chicago/Turabian StyleAli, Fayaz, Nasrullah Khan, Kishwar Ali, Muhammad Ezaz Hasan Khan, and David Aaron Jones. 2022. "Vegetation Pattern and Regeneration Dynamics of the Progressively Declining Monotheca buxifolia Forests in Pakistan: Implications for Conservation" Sustainability 14, no. 10: 6111. https://doi.org/10.3390/su14106111
APA StyleAli, F., Khan, N., Ali, K., Khan, M. E. H., & Jones, D. A. (2022). Vegetation Pattern and Regeneration Dynamics of the Progressively Declining Monotheca buxifolia Forests in Pakistan: Implications for Conservation. Sustainability, 14(10), 6111. https://doi.org/10.3390/su14106111








