Abstract
Metal–organic frameworks (MOFs) and their derivatives have delivered perfect answers in detection, separation, solving water and electromagnetic pollution and improving catalysis and energy storage efficiency due to their advantages including their highly tunable porosity, structure and versatility. Recently, MOF/biomass, bio-MOFs and their derivatives have gradually become a shining star in the MOF family due to the improvement in the application performance of MOFs using biomass and biomolecules. However, current studies lack a systematic summary of the synthesis and advancements of MOF/biomass, bio-MOFs and their derivatives. In this review, we describe their research progress in detail from the following two aspects: (1) synthesis of MOF/biomass using biomass as a template to achieve good dispersion and connectivity at the same time; (2) preparing bio-MOFs by replacing traditional organic linkers with biomolecules to enhance the connection stability between metal ions/clusters and ligands and avoid the formation of toxic by-products. This enables MOFs to possess additional unique advantages, such as improved biocompatibility and mechanical strength, ideal reusability and stability and lower production costs. Most importantly, this is a further step towards green and sustainable development. Additionally, we showcase some typical application examples to show their great potential, including in the fields of environmental remediation, energy storage and electromagnetic wave absorption.
1. Introduction
Metal–organic frameworks (MOFs), which are advanced porous coordination polymers, comprising metal ions/clusters and organic linkers by orderly self-assembly, have set off a research boom around the world in the 21st century [1,2]. Thanks to the high selectivity of metal ions/clusters and organic linkers, since first being reported, upwards of 90,000 MOFs have been created [3], and they have shown ultra-strong applicability in adsorption [2,4], separation [5,6], sensing [7,8], storage [9,10] and catalysis [11,12], which benefits from their high specific surface area, adjustable pore size and diverse morphology and structure [13,14,15]. Recently, MOFs have been applied as self-sacrificed templates/precursors to prepare porous carbon by in-situ pyrolysis; the carbon skeleton derived from organic linkers not only retains the morphology and porous structure of MOFs but also protects the metal/metal oxide formed by the carbothermic reduction, which greatly enhances the conductivity and stability [16,17,18]. Compared to traditional metal/carbon composites, MOF derivatives possess a more ordered pore structure and a more uniform distribution of metals/metal oxides.
However, there are two big obstacles for the further development of MOFs and their derivatives [19,20]: (1) MOFs and their derivatives tend to agglomerate, especially in the process of pyrolysis, which decreases the specific surface area and porosity, and even destroys the morphology of the derivatives, thus having a negative impact on the application performance. (2) MOFs with poor stability are prone to hydrolyzing in water or acid/alkaline environments due to the weak binding energy between the metal ions and organic ligands. At this time, toxic organic ligands will be released into the environment, which poses a threat to biological safety.
For the former, incorporating MOF precursors with a carbon template, such as graphene [21], carbon nanofibers [22] or carbon nanotubes [23], is a common and effective method to improve the dispersity and structure of MOF derivatives. Nevertheless, carbon nanomaterials consume a lot of fossil resources and funds. As a matter of fact, no other material is better as an ideal template than biomass. Biomass, an abundant, cheap, renewable natural material, is the best potential candidate to replace carbon nanomaterials. Inspiringly, the surface of biomass is rich in hydroxyl and carboxyl, which is helpful to form stable links between MOFs and biomass [24]. The combination of biomass and MOFs illuminates a low-cost and sustainable strategy to prepare advanced function materials with excellent performance [25,26,27].
For the latter, biomolecules collected from biomass, e.g., amino acids, peptides, bases and polysaccharides, can be used as linkers—replacing traditional organic linkers—to construct metal–biomolecule frameworks (bio-MOFs), in order to avoid the pollution caused by traditional organic linkers with toxicity [28,29,30]. For example, p-phthalic acid, the organic linker of MOF-5, is harmful to organisms; when exposed to humid air, MOF-5 with poor stability tends to decompose and dissolve into water [31]. Bio-MOFs have several significant advantages in comparison with MOFs [32]: (1) good biocompatibility, strong self-assembly ability, nontoxicity and easy recycling; (2) structural diversity, including rigid and flexible structures, and many different binding sites, forming a variety of coordination modes with metal ions; (3) bio-MOFs constructed with chiral biomolecules show unique recognition and separation capabilities. Due to the rich heteroatom content, bio-MOF derivatives possess more active sites, which is an advantage that can significantly strengthen the electrochemical performance.
To the best of our knowledge, many works on MOFs and their derivatives have been reported [33,34,35], but there is a lack of comprehensive reviews related to MOF/biomass, bio-MOFs and their derivatives. Thus, in order to highlight the advantages of MOF/biomass, bio-MOFs and their derivatives (Figure 1), the current review aims at recommending research on them, and introducing, in detail, the advancements in the application of them in the field of environmental remediation, energy storage and electromagnetic wave absorption.
Figure 1.
The advantages of MOF/biomass and bio-MOFs.
2. MOF/Biomass Composites
2.1. MOF/Biomass Extracts
To date, many biomass extracts have shown great promise for use as carbon sources to enhance the performance of MOFs. Recent research indicated that introducing biomass extracts into MOF precursors can not only improve the structure of MOF derivatives but also offset the lack of carbon content of organic linkers [36,37,38].
For instance, small glucose biomolecules can easily load on the surface or penetrate into the internal pores of MOFs (Figure 2a). Cao et al. used ZIF (zeolitic imidazolate framework)-7/glucose to propose nitrogen-doped porous carbon by in situ pyrolysis, which presented high electrocatalytic activity and excellent stability [39]. Glucose has a significant impact on the microstructure, composition and conductivity of ZIF-7 derivatives. After carbonization, rather than a cubic structure, the porous carbon showed a sheet-like or graphene-like structure of ZIF-7 (Figure 2b). The addition of glucose prevented the formation of macropores, because the glucose-derived graphite carbon could catalytically convert ZnO to the metal Zn; the metal Zn with a low boiling point evaporated from the carbon matrix, thus promoting the formation of micropores, and an improvement in the specific surface area (SSA) and porosity (Figure 2c). Cai et al. used glucose as a sealed reactor to wrap ZIF-8 and found that glucose effectively prevented the loss of pyrrole nitrogen species (Figure 2d), which had a positive effect on the RhB removal performance [40]. In another study, Gu et al. adjusted the structure of CoZn-MOF/glucose- derivatives (ZnO/Co3O4@NC) by regulating the loading ratio of glucose [41]. Zn2+ tends to combine with the hydroxy group of sugars [42]; hence, dense snake-like ZnO/C nanorods were induced to grow on the surface of ZnO/Co3O4@NC during pyrolysis. Instead, with the increase in the glucose content, the ZnO/C nanorods became greater in number and larger, which led to the agglomeration of the metallic oxide.
Maji et al. fabricated porous materials by carbonizing amine-functionalized IRMOF-3/sucrose, which achieved good carbon oxide storage by regulating the content of sucrose [43]. The authors concluded that sucrose formed good hydrogen bonding interactions with the MOFs within the pores and did not disrupt the crystal structure of the MOFs. Nitrogen absorption experiments showed that the SSA of the IRMOF-3/sucrose derivatives (nanoporous carbons, NPCs) was several times higher than that of IRMOF-3 and the sucrose derivative. When 300 mg of sucrose was added, the loading of sucrose in IRMOF-3 reached the limit. By this time, an encouraging gas storage capacity was obtained, with the highest SSA of 3119 m2/g and a 1.93 cm3/g pore volume. The experimental results supported that the NPCs could capture 5.1 wt% H2 and 64 wt% CO2, at most, under different conditions.
Recently, chitosan has been widely used for environmental remediation because of its biodegradability, water solubility and nontoxicity [44,45]. However, this substance exhibits poor chemical and mechanical stability, because it will completely dissolve in acid solutions or form a gel in alkaline solutions [46]. Studies have shown that there are strong interactions between pollutants and MOFs/chitosan, including complexation, hydrogen bonds, van der Waals forces and π–π interactions [47,48]. Therefore, the combination of chitosan and MOFs is a powerful alliance, which not only improves the stability of both but also brings about better performance. As Zhu et al. reported, the addition of chitosan improved the ability to adsorb tetracycline [49]. On the one hand, ZIF-8 was evenly distributed in the chitosan matrix, which ensured a higher adsorption capacity of ZIF-8/chitosan. On the other hand, chitosan has many amino and hydroxyl groups which are the active sites of tetracycline absorption. The results showed that each gram of ZIF-8/chitosan could absorb 495.04 mg of tetracycline at most; even after 10 adsorption/desorption cycles, the adsorption efficiency of ZIF-8/chitosan for tetracycline could still reach 90%, illustrating it is a green and reusable bio-adsorbent. Naeem et al. synthesized the dye adsorbent chitosan/Fe-MOF-235 with an absorption capacity of 2833 mg/g (methylene blue) and 2467 mg/g (methyl orange) [50]. After absorbing the dye, the XRD curve of chitosan/Fe-MOF-235 barely changed, which proved that chitosan/Fe-MOF-235 possessed superior stability. Moreover, chitosan/Fe-MOF-235 could achieve efficient absorption under acid/alkaline conditions or high temperature (10–200 °C) due to its good adaptability. Besides the above, other MOF/chitosan absorbents, such as PAN/chitosan/UiO-66-NH2 [48], HKUST-1/chitosan [49] and MIL-100(Fe)/chitosan [51], have shown great application prospects in the field of environmental remediation.
As the most abundant renewable natural resource on the earth, cellulose can be obtained from lignocellulosic biomass, such as cotton, wood and bamboo [52,53,54]. Cellulose is easier to use for MOF modification/anchoring thanks to the greater number of hydroxyl groups on the surface in comparison to other biomolecules. Kadib proposed a strategy of preparing cellulose@MOF composites, which can be divided into the following [55]:
(1) Physical mixing. There are two fatal disadvantages, i.e., MOFs are unevenly dispersed and can easily leach from the bio-composites, which can only be alleviated by introducing more functional groups on the cellulose surface. Emam et al. oxidized the hydroxyl group of cellulose into a carboxyl group with stronger binding ability using hydrogen peroxide, in order to realize the good binding of Cu-BTC with cotton [56]. In the study of Zou et al. [57], they synthesized graphene nanoplate/C-ZIF-67/cellulose nanofiber films using a similar method with slight adjustments. Cellulose imparted outstanding mechanical properties and excellent electromagnetic interference (EMI) shielding performance to the composite films: the tensile strength was 46.33 MPa and the EMI shielding effectiveness reached 50.5 dB at a low thickness of 0.1 mm.
(2) In-situ growth. The preparation process is generally as follows: firstly, cellulose needs to be dispersed in the metal salt/organic linker solution and then immersed in the organic linker/metal salt solution, which enables better dispersion of the MOFs and tighter attachment between the MOFs and biomass. For example, in a typical experimental process, cotton was added to 1,2,4,5-benzenetetracarboxylic dianhydride solution, after aging for 3 h at 70 °C; then, zirconium tetrachloride was added into the solution which continued to be heated at 150 °C; finally, UiO-66-(COOH)2 was grown in the cotton (Figure 2e) [58]. After modifying with UiO-66-(COOH)2, the cotton turned from white to yellow. The simulation results showed that UiO-66-(COOH)2@cotton has the potential to remove creatinine from the blood, where a maximum absorption capacity of 212.8 mg/g was achieved, which was better than that of UiO-66-(COOH)2@cotton synthesized ex- situ (192.3 mg/g). The data of the MOF content sufficiently explained the difference. The loading of MOFs for in- situ composites was 153.26 mg/g, but it was 43.63 mg/g for ex- situ composites.
(3) Layer-by-layer growth. This is illustrated with examples: As seen in Figure 2f, Cu-BTC/cotton was prepared via alternating immersion in aqueous solutions containing copper (II) acetate and the benzene-1,3,5-tricarboxylic acid ligand precursor [59]. This method reduced the formation of free MOFs and realized a high loading rate of Cu-BTC on the surface of the cotton. The efficiency of catalyzing NO release from S-nitroso cysteamine increased by 7–9 times as compared to the catalyzer-free reaction.
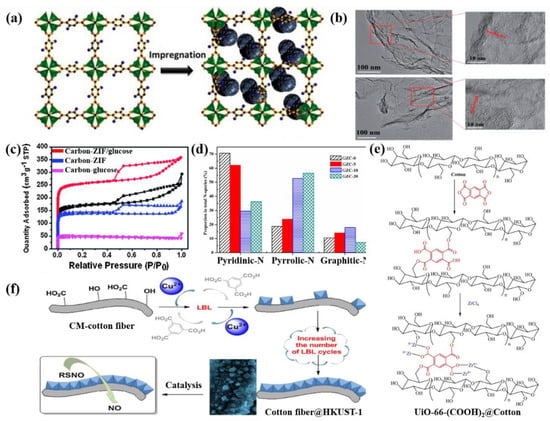
Figure 2.
(a) Mechanism diagram of biomass extract filling in MOFs. Reprinted with permission from Ref. [43]. Copyright 2016, Elsevier; (b) HRTEM images of ZIF-7/glucose derivatives; (c) nitrogen adsorption–desorption isotherms of ZIF-7/glucose, ZIF-7 and glucose derivatives. Reprinted with permission from Ref. [39]. Copyright 2013, Royal Society of Chemistry; (d) proportion of various N-species in the total nitrogen functional groups of carbons. Reprinted with permission from Ref. [40]. Copyright 2021, Elsevier; (e) the formation mechanism of UiO-66-(COOH)2 within cotton fabric to produce a composite. Reprinted with permission from Ref. [58]. Copyright 2018, Elsevier; (f) LBL method for the growth of MOFs by subsequently immersing the cotton fiber in an alternating bath of metal and ligand organic precursor solutions. Reprinted with permission from Ref. [55]. Copyright 2019, Elsevier.
2.2. MOF/Biomass
Based on its large number of electronegative groups (-OH, -COOH) from cellulose, hemicellulose and lignin, highly developed directional pore structure, excellent mechanical properties and good biocompatibility [60,61,62], biomass has been widely used as an ideal template for advanced functional materials to prepare electrodes, supercapacitors, catalyzers, adsorbents, absorbers, etc. [63,64,65,66,67]. In addition, biomass with great mechanical properties can serve as a mechanical support to ensure the mechanical stability of composite materials. These characteristics can also be perfectly reflected in MOF/biomass. Here, the application is discussed from the perspective of whether MOF/biomass composites are pyrolyzed or not.
As depicted in Figure 3a, Keplinger et al. synthesized ZIF-8/beech composites by in- situ growth of ZIF-8 within beech [68]. They first pretreated the beech with NaOH solution to deprotonate the carboxyl groups and etch the beech surface, which ensured that ZIF-8 would grow effectively. The hierarchical ZIF-8/beech presented an absorption selectivity function, because the pore aperture of ZIF-8 only allowed the transport of CO2 but hindered the diffusion of N2, and the carboxyl group of beech could absorb CO2 but not N2. Moreover, the adsorption and separation of gases are usually carried out under pressurized conditions, so good mechanical properties are the basis for ensuring the stable operation and high reusability of composite systems. ZIF-8/beech exhibited outstanding mechanical strength: the compressive strength and ultimate tensile stress reached up to 100 MPa and 74 MPa, respectively (Figure 3c,d), demonstrating the wood support template can offer a more significant improvement in the mechanical properties of MOFs involved composites in comparison with polymer templates [69,70]. Most importantly, the authors verified that this synthesis strategy was universal and could be applied to different MOFs and various woods (Figure 3b). In Wang and co-workers’ research, the natural pores of wood not only facilitated the load of MOFs but also limited organic pollutants’ transmission channels [71]. The water containing organic pollutants could only be transmitted along the open and elongated channels of the wood, which effectively improved the contact probability of organic pollutants with UiO-66. Then, UiO-66 in the channel adsorbed the organic pollutants through electrostatic interaction, in order to achieve a high removal efficiency (98%).
Biomass is vulnerable to fungi due to the high amounts of starch, polysaccharide and other nutrients [67,72], resulting in a decline in product performance. In such a context, Qin et al. explored the antibacterial properties of MOF-modified bamboo and wood [73]. The results showed that bamboo and wood exhibited significantly improved antibacterial properties, without affecting the stability of MOF-199, and fungi interacted with copper ions on the surface of MOF-199, thus resulting in cell dissolution (Figure 3e).
By analyzing the example of MOF/biomass, the principle of the stable loading of MOF crystals on the biomass surface can be summarized as (1) the electrostatic interaction between the MOFs and carboxylate, (2) and the hydrogen bond formed with hydroxyl [74]. Therefore, the surface quality of biomass affects the crystallinity and size of MOFs. Fewer hydroxyl and carboxyl groups are exposed on the smooth surface, which leads to lower crystallinity and a larger crystal size [75]. However, even if the biomass surface is -pretreated with an activator, the loading ratio of MOFs in MOF/biomass is only less than 5% [68,71]. Consequently, it is extremely urgent to develop an innovative method to improve the loading ratio of MOFs. To break through the bottleneck, magnetic induction heating (MIH) has timely emerged [76,77]. Drawing on the experience of MIH, Li et al. synthesized UiO-66-NH2@Fe3O4-wood twice by in- situ growth [78]. Firstly, Fe3O4 nanoparticles were synthesized in situ in wood, and then MOFs were induced to grow for 8 h in the magnetic wood under a 27 mT magnetic field (Figure 3f). In the magnetic field, the Joule heat generated by magnetic induction gathers around the matrix, which not only makes MOFs more inclined to nucleate in the wood but also improves the crystallization rate and yield of MOFs. It is worth mentioning that the growth of MOF can be further regulated by the loading rate of magnetic particles and the magnetic field strength. Under the condition of oil heat, due to the uniform heat dispersion, a large number of free MOFs are produced (Figure 3g). Compared with MOF/biomass prepared by the oil- bath method, the yield of MOFs in UiO-66-NH2@Fe3O4-wood was dramatically increased to 49.6 wt%. The external magnetic field served as a dynamic switch that determines the uptake and release of methylene blue (MB). When UiO-66-NH2@Fe3O4-wood was exposed to a 7.8 mT magnetic field, MB absorption of 96.3% was achieved in 20 min, and no organic linkers were detected in the MB aqueous solution, which demonstrated its excellent hydrolytic stability. By virtue of similar protocols to the synthesis of UiO-66-NH2@Fe3O4-wood, the yield of MIL-100(Fe)@Fe3O4-wood and HKUST-1@Fe3O4-wood can also reach up to 58.4 wt% and 39.7 wt%.
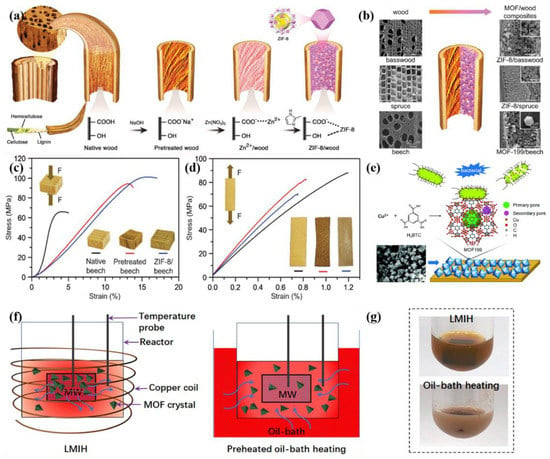
Figure 3.
(a) Schematic representation of the fabrication process to obtain ZIF-8/wood composites; (b) schematic representation of the versatility of the synthetic approach to other wood species and MOFs; (c) compressive and (d) tensile stress-strain curves of native beech, pretreated beech and ZIF-8/beech composite. Reprinted with permission from Ref. [68]. Copyright 2020, Wiley-VCH Verlag; (e) schematics of the fabrication of MOF-deposited woody materials and their antibacterial mechanism. Reprinted with permission from Ref. [73]. Copyright 2019, Royal Society of Chemistry; (f) schematic illustration of the distribution of heat and MOF crystals in reaction systems with LMIH (left) and pre-heated oil- bath heating (right); (g) typical photographs of the reaction systems after 8 h of LMIH and oil- bath heating. Reprinted with permission from Ref. [78]. Copyright 2021, Elsevier.
2.3. MOF/Biomass Derivatives
MOF-derived carbon materials have excellent properties and wide applications, but organic ligands are mostly heterocyclic molecules, which leads to the fact that MOF-derived carbon is mostly amorphous carbon with poor conductivity, greatly weakening its electrochemical performance [65,79]. Consequently, there is a need to introduce a material that not only improves the electrical conductivity but also acts as a bridge to facilitate the transport of electrons between mutually independent MOF derivative particles. Until now, many materials have been used to build continuous structures with MOF-derived carbon; as a result, biomass carbon stands out from many materials due to its natural -3D hierarchical structure, sustainability and light weight [80,81].
For example, in the field of EMW absorption, MOFs and biomass are both attractive raw materials for the preparation of absorbers [74,82,83,84]; combining MOFs and biomass is likely a win-win strategy and a desirable means to prepare state-of-the-art absorbers [85,86]. Che et al. added Co(NO3)2·6H2O to a modified cotton fiber pre-immersed in 2-mIM solution, and ZIF-67 grew in situ evenly and fully on the surface of the cotton fibers during aging, following which the cotton fiber@ZIF-67 was annealed at 900 °C under a mixed H2/Ar atmosphere [87]. The hollow cotton fibers connected Co/C nano-dodecahedron particles modified with villus-like carbon nanotubes to form a strong conductive network, which enhanced the conductivity loss (Figure 4a). Notably, the hollow cotton fibers not only prolonged the transmission path of the electromagnetic wave (EMW) but also induced multiple Debye dipolar relaxations through the oxygen-containing groups of their surface (Figure 4b). Compared with pure ZIF-67 derivatives [88], the reflection loss and effective absorption bandwidth (EAB) were remarkably optimized. In Huang and co-workers’ research [89], CoNi-MOF/bamboo fiber derivatives possessed a superior EMW absorption performance, and the reflection loss could reach −75.19 dB with a 2.66 mm thickness (Figure 4c,d). The authors concluded that the bamboo fibers greatly enhanced the EMW absorption capacity by optimizing the impedance matching and interface polarization. Ni/NC/C composites derived from Ni-MOF/loofah exhibited excellent EMW absorption performance, as reported by Li et al., and the minimum reflection loss was −63.10 dB at 2.0 mm with a 16% filling ratio; furthermore, the effective absorption bandwidth could reach up to 35.44 GHz [90]. On the one hand, -the 3D hollow structure of the loofahs provided a channel for the conduction of electrons and heat energy and enhanced the conductivity loss; on the other hand, the rich interface and defects of the Ni/NC/C composites improved the polarization probability (Figure 4e). In addition, the Ni/C derived from the Ni-MOF induced strong magnetic loss and optimized the impedance matching. In addition to loofahs, pine nutshells containing nitrogen [86] or highly wood aerogels [91] also posed a great influence on the EMW absorption performance of MOF/biomass-derived carbon.
MOF/biomass derivatives can not only be used for EMW absorption but can also be devoted to environmental remediation. For instance, Co@N-PC was prepared by pyrolyzing ZIF-67@poplar sawdust synthesized in situ, which demonstrated a strong organic dye removal performance [85]. Taking poplar sawdust as the carrier improved the utilization rate of the biomass and reduced the cost of water treatment, but the uniform -3D conductive structure composed of poplar sawdust and ZIF also promoted the material transfer and electron transfer in the process of dye removal. As shown in Figure 4f, Co@N-PC displayed a much better dye degradation efficiency than other catalysts. In another study, Li et al. studied the absorption capacity of an MB absorbent which combined Cu-BTC and activated carbon derived from agricultural waste; its maximum absorption capacity exceeded 400 mg/g [92], but it cannot be recycled and reused like Co@N-PC using magnetic recovery. MOF/biomass derivatives have also made achievements in the field of sensors. Co3O4/BC was prepared by the pyrolysis of ZIF-67 grown in situ on the surface of biomass (hemp stems) carbon. As illustrated in Figure 4h, Co3O4/BC exhibited good application prospects as a green, environmentally friendly NO2 gas sensor thanks to the excellent synergistic effect between the two components [93]. The pores of biomass carbon provided a natural channel for gas adsorption, analysis and diffusion. The catalytic effect of Co3O4 improved the graphitization degree of biomass carbon, which was conducive to the rapid transfer of electrons, in order to enhance the gas sensitivity (Figure 4g).
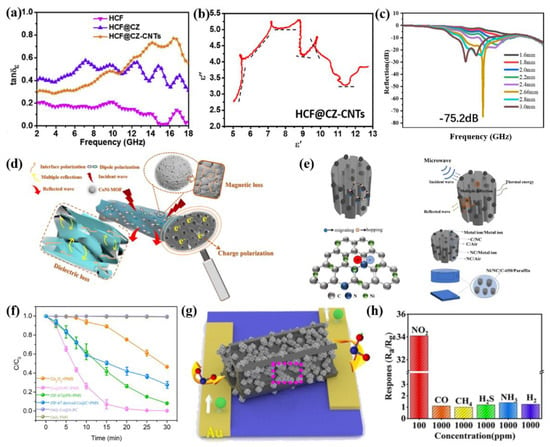
Figure 4.
(a) Dielectric loss tangent of HCF, HCF@CZ and HCF@CZ-CNTs; (b) Cole–Cole semicircle plots of HCF@CZ-CNTs. Reprinted with permission from Ref. [94]. Copyright 2020, Elsevier; (c) reflection loss curve of CN-ABF; (d) EMW absorption mechanism diagram of CN-ABF. Reprinted with permission from Ref. [89]. Copyright 2021, Academic Press Inc.; (e) mechanism of the absorption of electromagnetic waves by the Ni/NC/C composite. Reprinted with permission from Ref. [90]. Copyright 2022, Elsevier; (f) degradation efficiency of MB in different reaction systems within 30 min. Reprinted with permission from Ref. [85]. Copyright 2021, Elsevier; (g) gas sensing detection schematic diagram of CoBC-700; (h) selective testing of the response of the CoBC-700 sensor to various gases. Reprinted with permission from Ref. [93]. Copyright 2021, Elsevier.
3. Bio-MOFs
3.1. Biomolecules for Bio-MOFs
Organic linkers are one of the key factors to determine the topology of MOFs, which indirectly determines the application properties of MOFs. However, at present, the high price of commercial organic linkers increases the cost of industrial production. In addition, toxic organic solvents need to be used in the synthesis of MOFs, and the decomposition of MOFs leads to the outflow of toxic organic ligands, which will cause subtle damage to the environment [87]. Biomolecules, including amino acids, nucleobases, peptides, cyclodextrins and saccharides, can serve as organic linkers for MOF synthesis due to their rich resources, -nontoxicity, good biocompatibility and structural diversity [20]. Therefore, they have been successfully applied to separation, detection, catalysis, antimicrobials, biomedicine, biosensing, etc. [28].
α-Amino acids, which belong to amphoteric organic compounds, have many carboxyl and amino groups which are ideal binding sites for metal ions. There are three main types of bio-MOFs constructed by α-amino acids [95]: (1) direct chelation; (2) addition of additional polydentate organic ligands and bridging anions; and (3) chemically modified amino acids. Dae-Won Park’s group synthesized the 3D water-soluble zinc-glutamate-MOF (ZnGlu) from natural L-glutamate, which is an eco-friendly catalyst for CO2 conversion [96]. Compared with conventional MOF-based catalysts, it has the advantages of a high catalytic selectivity, high thermochemical stability and easy separation. Peptides are biomolecules in which amino acids are connected by peptide bonds, and they can be used to synthesize functional 2D and 3D bio-MOFs due to their inherent chirality and recognition properties. Chiral and rigid ladder-type building units, Co-L-GG, are constructed by the chiral dipeptide glycine-L(S)-glutamic acid, which can be used to prepare reticulated peptide MOFs [97]. The yield, thermal stability and porosity of peptidyl MOFs are decided by the type and size of the building units, and the enantioselective adsorption performance depends heavily on the pore size of the MOFs. Other studies have proved that the conformation and stability of peptide-based MOFs are affected by the flexible/rigid properties of peptides and will determine the biological application performance of MOFs [87,98].
Nucleobases are rich in oxygen- and nitrogen-containing groups and aromatic rings; all of them can interact with metal ions through coordination, π–π stacking or hydrogen bonding [30,99]. There are two types of bases: purine and pyrimidine. Purine bases have more coordination sites, so they are more widely used. Bio-MOF-1 is the first purine-derived 3D porous MOF consisting of a Zn (II)-ade octahedral cage with an anionic nature that allows it to be used for the storage of cationic drug molecules; the pores can also be modified by a cation exchange strategy for special applications [100]. With this in mind, Nathaniel L. Rosi’s group changed the structure and function of MOFs by controlling the coordination mode, where the specific surface area reached 4300 m2/g and the pore volume was 4.3 cm3/g [101].
Carbohydrates, including monosaccharides, disaccharides, oligosaccharides and polysaccharides [102], need to be oxidized before being used as linkers. The most common are α-CD, β-CD and γ-CD [29,103]. Among them, γ-CD has been extensively studied because of its better structural symmetry. CD-MOF-2 ([(γ-CD)(MOH)2], where M is K+ or Rb+) synthesized with γ-CD has good thermal stability and architectural stability; inspiringly, there was no obvious weight loss or pore change even at 200 °C [104].
There are other biomolecules that naturally exist in humans, animals or plants, that contain several groups that can coordinate with metal ions and that can also be used as bio-MOF linkers. Table 1 lists the -bio-MOF building units and -bio-MOF structures with unique characteristics that cannot be obtained with traditional ligands. For example, 3D [Fe3O(MeOH)3(fum)3(CO2CH3)]·4.5MeOH formed by the coordination of Fe3+ and fumaric acid and medi-MOF-1 formed by the coordination of Zn2+ and curcumin both show great application potential.

Table 1.
Bio-organic ligand building blocks and characterization of bio-MOFs. Adapted with permission from Ref. [105]. Copyright 2021, American Chemical Society.
3.2. Bio-MOF Derivatives
Bio-MOFs are attractive precursors for the preparation of functional carbon materials. Firstly, they have a high permanent porosity; secondly, the rich nitrogen and active functional groups in the biomolecules can still be inherited by the derived carbon materials after carbonization, which are beneficial to improve the physicochemical properties and play a role in enhancing the catalytic and adsorption properties.
Bio-MOF-1 is an MOF crystal formed by the interaction of Zn2+ with adenine (C5H5N5) and biphenyl dicarboxylate. The flow chart of preparing derivative carbon materials with bio-MOF-1 is shown in Figure 4a. In a high-temperature N2 atmosphere, for N-rich bio-MOFs, CNTs and a large number of active centers were induced, which endow the material with excellent electrocatalytic activity [116]. This is because nitrogen in the carbon structure would lead to a pulsed effect, which promotes the formation of CNTs [117,118]. Tian et al. went a further step to study the effect of temperature on the growth of CNTs [119]. When the temperature was higher than 800 °C, the amount of CNTs increased significantly, which resulted in an increase in the SSA. Additionally, the pyrolysis time is also an important factor affecting the properties of bio-MOF derivatives [120,121,122]. An appropriate extension of the pyrolysis time could evaporate more Zn, increase the proportion of micropores and improve the graphitization degree and conductivity (the more graphitic N, the higher the conductivity; graphitic N is exhibited in Figure 5b), which is conducive to enhancing the adsorption and catalytic properties of bio-MOF derivatives. Moreover, Qiu et al. systematically investigated the effect of K+-doped on the structure of bio-MOF-1-derived carbon [121]. K+ acted as an activator during the pyrolysis process; compared with the bio-MOF-1-derived carbon dominated by mesopores, many new micropores were produced in the K+@bio-MOF-1-derived carbon (KBM). Consequently, KBM not only showed selective adsorption of carbon dioxide but also had excellent electrochemical cycle stability.
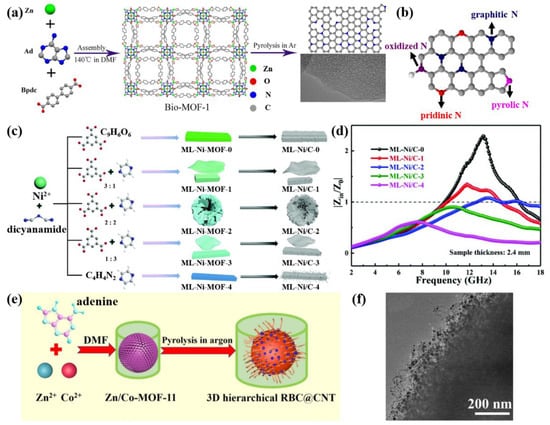
Figure 5.
(a) Schematic illustration of the synthesis of bio-MOF-1 derivatives; (b) schematic representation of various N types in nitrogen-doped carbon materials. Reprinted with permission from Ref. [121]. Copyright 2019, Academic Press Inc.; (c) schematic illustration of the preparation of ML-Ni/C composites; (d) comparison of the relative input impedance (|Zin/Z0|) at a thickness of 2.4 mm for ML-Ni/C composites. Reprinted with permission from Ref. [122]. Copyright 2021, Royal Society of Chemistry; (e) synthetic scheme for the preparation of 3D hierarchical RBC@CNT composites; (f) TEM images of RBC@CNT composites. Reprinted with permission from Ref. [123]. Copyright 2020, Elsevier.
Judging from the above, it is feasible to apply bio-MOF derivatives to solve EM pollution. For example, a new rod-shaped Ni-MOF was formed by the coordination of Ni+ and pyrazine. Even when calcined in a non-reducing atmosphere, a large number of CNTs can be grown in situ on the surface of its derivative Ni/C [122]. However, its finite performance cannot meet the requirements of advanced EMW absorbers. For this reason, Lin and co-workers further used a mixed ligand strategy to optimize the performance of Ni/C. In the process of the coordination of pyrazine and Ni+, trimesic acid was introduced to change the topological structure and color of Ni-MOF. As observed in Figure 5c, the rod-shaped structure of ML-Ni/C-0 became a regular flower but without CNTs. When the molar ratio of trimesic acid/pyrazine was 1:1, ML-Ni/C-2 showed a stronger absorption capacity as a result of the improved impedance matching (Figure 5d), and the maximum reflection loss was −65.3 dB with a 5.1 GHz EAB. As depicted in Figure 5e, a rambutan-like bimetallic/carbon@CNT (RBC@CNT) 3D hierarchical composite was designed by pyrolyzing bimetallic Co/Zn-MOF-11 [123], which achieved a stronger EMW dissipation capacity than Co/Zn-ZIF-derived composites [124]. The special structure led to multiple reflections and scattering, and the improved interfacial polarization, conductivity loss due to the intertwined CNTs (Figure 5f) and magnetic loss of the metallic cobalt jointly promoted the enhancement of the EMW absorption capability.
4. Conclusions and Prospects
The combination of biomass and MOFs provides a new idea for researchers to prepare high-performance functional materials. In MOF/biomass, the biomass provides mechanical support for the MOFs and imparts a higher porosity and larger specific surface area. In addition, the biomass not only solves the problem of MOF aggregation but also connects independent MOF crystals, which significantly improves the performance of MOFs. Bio-MOFs with biomolecules as ligands show stronger attraction in comparison with traditional MOFs. Firstly, biomolecules have multiple coordination sites, which can form a variety of coordination modes with metal ions, meaning they have higher structural adjustability. Secondly, bio-MOFs have special chiral properties, which enables them to have a specific recognition function, good biocompatibility and -nontoxic properties, which broaden their applications in biology. In brief, biomass and biomolecules are a low-cost, abundant renewable resource; in today’s extreme pursuit of sustainable development, rational and efficient utilization of biomass and biomolecules is in line with the requirements of green development.
Along with all the advantages mentioned above, MOF/biomass and bio-MOFs still suffer many challenges, including, but not limited to, the following:
- (1)
- The low loading ratio of MOFs in biomass. As the main phase of functional materials, the loading ratio of MOFs directly determines the properties of MOF/biomass composites. Therefore, in most experiments, the biomass needs to be pretreated to create more binding sites for the MOFs. However, the synthetic strategies, such as physical mixing, in- situ growth or layer-by-layer growth, cannot make MOFs nucleate quickly and efficiently on the surface of the biomass.
- (2)
- The interaction between the composite systems is relative. In the process of in- situ synthesis, the competition between the surface functional groups of the biomass and organic linkers affects the crystallinity and morphology of the MOFs.
- (3)
- The stability of the biomass. The premise of improving the stability of MOFs is to ensure the stability of the biomass. Biomass is easily affected by the environment, such as ultraviolet light and fungi, which will accelerate its degradation. Meanwhile, modifying the biomass with a chemical method makes the preparation process of MOF/biomass composites complicated.
Keeping these pros and cons in mind, MOF/biomass and bio-MOFs still have considerable prospects. First of all, we need to move forward with research on MOF/biomass and bio-MOFs: for example, we need to continue to seek more suitable biomasses and test more biomolecules to prepare new bio-MOFs. Secondly, we should deeply study the composite mechanism between MOFs and biomass to reduce the influence of the biomass surface quality on the morphology and crystallinity of MOFs. Moreover, the purpose of developing new functional materials is for application, so more advanced technology needs to be developed for industrialization. In particular, it is necessary to reduce the energy consumption in the preparation of MOF/biomass and bio-MOF derivatives.
Author Contributions
J.L.: writing and conceptualization; Y.L.: investigation; Z.L.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangsu Agricultural Science and Technology Independent Innovation Fund (CX(20)3041), the National Natural Science Foundation of China (No. 31971740), the Research Project of Jiangxi Forestry Bureau (No. 202134) and the Nanping Science and Technology Planning Project (N2020Z001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 6149. [Google Scholar] [CrossRef] [Green Version]
- Pétuya, R.; Durdy, S.; Antypov, D.; Gaultois, M.W.; Berry, N.G.; Darling, G.R.; Katsoulidis, A.P.; Dyer, M.S.; Rosseinsky, M.J. Machine-Learning Prediction of Metal–Organic Framework Guest Accessibility from Linker and Metal Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202114573. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Ammonia Adsorption and Its Effects on Framework Stability of MOF-5 and MOF-177. J. Colloid Interface Sci. 2010, 348, 615–620. [Google Scholar] [CrossRef]
- Kadioglu, O.; Keskin, S. Efficient Separation of Helium from Methane Using MOF Membranes. Sep. Purif. Technol. 2018, 191, 192–199. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent Metal–Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A Route to High Surface Area, Porosity and Inclusion of Large Molecules in Crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [Green Version]
- Dai, F.; Luo, J.; Zhou, S.; Qin, X.; Liu, D.; Qi, H. Porous Hafnium-Containing Acid/Base Bifunctional Catalysts for Efficient Upgrading of Bio-Derived Aldehydes. J. Bioresour. Bioprod. 2021, 6, 243–253. [Google Scholar] [CrossRef]
- Zhu, G.; Li, H.; Qiang, M.; Li, S.; Kang, K.; Xu, K.; Deng, S. Application and research progress of metal-organic framework materials in biomass and its derived chemicals. J. For. Eng. 2021, 6, 23–34. [Google Scholar] [CrossRef]
- Zhu, H.; Jiao, Q.; Fu, R.; Su, P.; Yang, C.; Feng, C.; Li, H.; Shi, D.; Zhao, Y. Cu/NC@Co/NC Composites Derived from Core-Shell Cu-MOF@Co-MOF and Their Electromagnetic Wave Absorption Properties. J. Colloid Interface Sci. 2022, 613, 182–193. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, W.; Han, X.; Zhou, L.; Zhen, H.; Wu, C.; Yu, Q.; Xiu, G. B,N-Decorated Carbocatalyst Based on Fe-MOF/BN as an Efficient Peroxymonosulfate Activator for Bisphenol A Degradation. J. Hazard. Mater. 2022, 430, 127832. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Bandal, H.A.; Ramakrishna, S.; Kim, H. Critical Review, Recent Updates on Zeolitic Imidazolate Framework-67 (ZIF-67) and Its Derivatives for Electrochemical Water Splitting. Adv. Mater. 2022, 34, 2107072. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, H.; Xue, H.; Braunstein, P.; Pang, H. Pillared-Layer Ni-MOF Nanosheets Anchored on Ti3C2 MXene for Enhanced Electrochemical Energy Storage. J. Colloid Interface Sci. 2022, 614, 130–137. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.-H. Green Synthesis of Metal–Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Li, R.; Lou, Z.; Gu, S.; Wang, Q.; Liu, J.; Li, Y. Preparation of magnetic carbon with microwave absorption property using bamboo powder. J. For. Eng. 2021, 6, 112–120. [Google Scholar] [CrossRef]
- Zhou, X.; Han, H.; Wang, Y.; Zhang, C.; Lv, H.; Lou, Z. Silicon-Coated Fibrous Network of Carbon Nanotube/Iron towards Stable and Wideband Electromagnetic Wave Absorption. J. Mater. Sci. Technol. 2022, 121, 199–206. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Zhou, X.; Kara, U.I.; Mamtani, R.S.; Lv, H.; Zhang, M.; Yang, Z.; Li, Y.; Wang, C.; et al. An Angle-Insensitive Electromagnetic Absorber Enabling a Wideband Absorption. J. Mater. Sci. Technol. 2022, 113, 33–39. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Zhang, Y.; Zhou, X.; Li, R.; Liu, J.; Li, Y.; Lv, H. In-Situ Formation of Low-Dimensional, Magnetic Core-Shell Nanocrystal for Electromagnetic Dissipation. Compos. Part B 2021, 214, 108744. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, J.; Wang, X.; Li, W.; Wu, Y.; Jiang, Z. Biomass@MOF-Derived Carbon Aerogels with a Hierarchically Structured Surface for Treating Organic Pollutants. Ind. Eng. Chem. Res. 2020, 59, 17529–17536. [Google Scholar] [CrossRef]
- Bian, W.; Chen, J.; Chen, Y.; Xu, W.; Jia, J. A Novel Waste Paper Cellulose-Based Cu-MOF Hybrid Material Threaded by PSS for Lithium Extraction with High Adsorption Capacity and Selectivity. Cellulose 2021, 28, 3041–3054. [Google Scholar] [CrossRef]
- Sun, P.-P.; Li, Y.-M.; Zhang, Y.-H.; Shi, H.; Shi, F.-N. Preparation and Application of Ce-Cu Based Metal Organic Framework/Biomass Carbon Composites in Energy Storage. J. Alloys Compd. 2022, 896, 163081. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martínez, M.; An, J.; Solé-Font, I.; Rosi, N.L.; Maspoch, D. Metal–Biomolecule Frameworks (MBioFs). Chem. Commun. 2011, 47, 7287. [Google Scholar] [CrossRef]
- Zulys, A.; Yulia, F.; Muhadzib, N. Nasruddin Biological Metal–Organic Frameworks (Bio-MOFs) for CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 37–51. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological Metal–Organic Frameworks: Structures, Host–Guest Chemistry and Bio-Applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishaq, S.; Tamime, R.; Bilad, M.R.; Khan, A.L. Mixed Matrix Membranes Comprising of Polysulfone and Microporous Bio-MOF-1: Preparation and Gas Separation Properties. Sep. Purif. Technol. 2019, 210, 442–451. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, L.; Chang, Y.; Liu, M. Gas Sensing Based on Metal-Organic Frameworks: Concepts, Functions, and Developments. J. Hazard. Mater. 2022, 429, 128321. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, Q.; Pan, Z.; Li, L.; Li, C.; Ling, Y.; Wang, Z.; Chen, M.; Wang, Z.; Yao, Y.; et al. Freestanding Metal–Organic Frameworks and Their Derivatives: An Emerging Platform for Electrochemical Energy Storage and Conversion. Chem. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent Progress in Metal-Organic Framework-Based Supercapacitor Electrode Materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Zhao, G.; Fang, Y.; Dai, W.; Ma, N. Copper-Containing Porous Carbon Derived from MOF-199 for Dibenzothiophene Adsorption. RSC Adv. 2017, 7, 21649–21654. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Bai, X.; Liang, M.; Ma, J. Recent Progress on Metal-Organic Framework-Derived Porous Carbon and Its Composite for Pollutant Adsorption from Liquid Phase. Chem. Eng. J. 2021, 405, 126960. [Google Scholar] [CrossRef]
- Zhou, X.; Han, H.; Yan, H.; Wang, Y.; Zhang, C.; Lv, H.; Lou, Z. Multi-Interface Self-Assembling on MXenes Skeleton towards Wideband Electromagnetic Dissipation. Mater. Today Phys. 2022, 24, 100685. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Xiang, Z.; Shen, Z.; Yun, J.; Cao, D. ZIF-Derived in Situ Nitrogen-Doped Porous Carbons as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Energy Environ. Sci. 2013, 7, 442–450. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liang, Y.; Zhou, J.; Liu, L.; Huang, S.; Cai, J. Nitrogen-Doped Carbons from in-Situ Glucose-Coated ZIF-8 as Efficient Adsorbents for Rhodamine B Removal from Wastewater. Microporous Mesoporous Mater. 2021, 310, 110662. [Google Scholar] [CrossRef]
- Tong, R.; Ning, L.; Li, H.; Zhang, Z.; Gu, W.; Liu, X. Snake-like ZnO Nanorods Encapsulated by Carbon Shell Self-Assembled on the Porous N-Doped Carbon Nanocages with Co3O4 and ZnO Nanoparticles Embedded for Superior Lithium Storage. Electrochim. Acta 2020, 359, 136924. [Google Scholar] [CrossRef]
- Revathi, T.; Thambidurai, S. Immobilization of ZnO on Chitosan-Neem Seed Composite for Enhanced Thermal and Antibacterial Activity. Adv. Powder Technol. 2018, 29, 1445–1454. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Datta, K.K.R.; Shiva, K.; Bhattacharyya, A.J.; Eswaramoorthy, M.; Maji, T.K. Controlled Synthesis of Tunable Nanoporous Carbons for Gas Storage and Supercapacitor Application. Microporous Mesoporous Mater. 2015, 206, 127–135. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Advances in the Application of Chitosan-Based Metal Organic Frameworks as Adsorbents for Environmental Remediation. Carbohydr. Polym. 2022, 283, 119153. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Maurya, S.; Rathod, V.K. Polysaccharide Based Metal Organic Frameworks (Polysaccharide–MOF): A Review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan Modifications for Adsorption of Pollutants–A Review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of Three Selected Pharmaceuticals and Personal Care Products (PPCPs) onto MIL-101(Cr)/Natural Polymer Composite Beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/Chitosan Nanofibers for Adsorption and Membrane Filtration of Pb(II), Cd(II) and Cr(VI) Ions from Aqueous Solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H.; Tian, Y.; Zhu, G. Uniform and Stable Immobilization of Metal-Organic Frameworks into Chitosan Matrix for Enhanced Tetracycline Removal from Water. Chem. Eng. J. 2020, 382, 122893. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Din, I.U.; Farooq, M.; Khan, I.W.; Hamayun, M.; Malik, T. Synthesis of Chitosan Composite of Metal-Organic Framework for the Adsorption of Dyes; Kinetic and Thermodynamic Approach. J. Hazard. Mater. 2022, 427, 127902. [Google Scholar] [CrossRef]
- Xiong, N.; Wan, P.; Zhu, G.; Xie, F.; Xu, S.; Zhu, C.; Hursthouse, A.S. Sb(III) Removal from Aqueous Solution by a Novel Nano-Modified Chitosan (NMCS). Sep. Purif. Technol. 2020, 236, 116266. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A Critical Review on Cellulose: From Fundamental to an Approach on Sensor Technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Joseph, B.; K, S.V.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the Recent Developments in All-Cellulose Nanocomposites: Properties and Applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef]
- El Hankari, S.; Bousmina, M.; El Kadib, A. Biopolymer@Metal-Organic Framework Hybrid Materials: A Critical Survey. Prog. Mater. Sci. 2019, 106, 100579. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Elshahat, M.; Emam, H.E. Cu–BTC@cotton Composite: Design and Removal of Ethion Insecticide from Water. RSC Adv. 2016, 6, 42324–42333. [Google Scholar] [CrossRef]
- Yuan, M.; Fei, Y.; Zhang, H.; Qiu, B.; Shen, L.; He, X.; Liang, M.; Zhou, S.; Chen, Y.; Zou, H. Electromagnetic Asymmetric Films Comprise Metal Organic Frameworks Derived Porous Carbon for Absorption-Dominated Electromagnetic Interference Shielding. Compos. Part B 2022, 233, 109622. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Rehan, M.; Emam, H.E. Figuration of Zr-Based MOF@cotton Fabric Composite for Potential Kidney Application. Carbohydr. Polym. 2018, 195, 460–467. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Harding, J.L.; Reynolds, M.M. Immobilization of Metal–Organic Framework Copper(II) Benzene-1,3,5-Tricarboxylate (CuBTC) onto Cotton Fabric as a Nitric Oxide Release Catalyst. ACS Appl. Mater. Interfaces 2015, 7, 26742–26750. [Google Scholar] [CrossRef]
- Burgert, I.; Cabane, E.; Zollfrank, C.; Berglund, L. Bio-Inspired Functional Wood-Based Materials–Hybrids and Replicates. Int. Mater. Rev. 2015, 60, 431–450. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Yuan, T.; Wang, Q.; Wu, X.; Hu, S.; Hao, X.; Liu, X.; Li, Y. Fabrication of Crack-Free Flattened Bamboo and Its Macro-/MicroMorphological and Mechanical Properties. J. Renew. Mater. 2021, 9, 959. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.D.; Alessi, D.S.; Komárek, M.; Ok, Y.S.; et al. Multifunctional Iron-Biochar Composites for the Removal of Potentially Toxic Elements, Inherent Cations, and Hetero-Chloride from Hydraulic Fracturing Wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Seow, J.Z.Y.; Zhao, H.; Xu, Z.J.; Ji, G. A Flexible and Lightweight Biomass-Reinforced Microwave Absorber. Nano-Micro Lett. 2020, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, Q.; Kara, U.I.; Mamtani, R.S.; Zhou, X.; Bian, H.; Yang, Z.; Li, Y.; Lv, H.; Adera, S.; et al. Biomass-Derived Carbon Heterostructures Enable Environmentally Adaptive Wideband Electromagnetic Wave Absorbers. Nano-Micro Lett. 2021, 14, 11. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Sun, W.; Liu, J.; Yan, H.; Han, H.; Bian, H.; Li, Y. Regulating Lignin Content to Obtain Excellent Bamboo-Derived Electromagnetic Wave Absorber with Thermal Stability. Chem. Eng. J. 2022, 430, 133178. [Google Scholar] [CrossRef]
- Lou, Z.; Han, X.; Liu, J.; Ma, Q.; Yan, H.; Yuan, C.; Yang, L.; Han, H.; Weng, F.; Li, Y. Nano-Fe3O4/Bamboo Bundles/Phenolic Resin Oriented Recombination Ternary Composite with Enhanced Multiple Functions. Compos. Part B 2021, 226, 109335. [Google Scholar] [CrossRef]
- Tu, K.; Puértolas, B.; Adobes-Vidal, M.; Wang, Y.; Sun, J.; Traber, J.; Burgert, I.; Pérez-Ramírez, J.; Keplinger, T. Green Synthesis of Hierarchical Metal–Organic Framework/Wood Functional Composites with Superior Mechanical Properties. Adv. Sci. 2020, 7, 1902897. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhang, M.; Nie, J.; Tan, J.; Yang, B.; Song, S. Design of Double-Component Metal–Organic Framework Air Filters with PM2.5 Capture, Gas Adsorption and Antibacterial Capacities. Carbohydr. Polym. 2019, 203, 415–422. [Google Scholar] [CrossRef]
- Zhao, H.; Miao, Q.; Huang, L.; Zhou, X.; Chen, L. Preparation of long bamboo fiber and its reinforced polypropylene membrane composites. J. For. Eng. 2021, 6, 96–103. [Google Scholar] [CrossRef]
- Guo, R.; Cai, X.; Liu, H.; Yang, Z.; Meng, Y.; Chen, F.; Li, Y.; Wang, B. In Situ Growth of Metal–Organic Frameworks in Three-Dimensional Aligned Lumen Arrays of Wood for Rapid and Highly Efficient Organic Pollutant Removal. Environ. Sci. Technol. 2019, 53, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lou, Z. Progress of bamboo flatten technology research. J. For. Eng. 2021, 6, 14–23. [Google Scholar] [CrossRef]
- Su, M.; Zhang, R.; Li, H.; Jin, X.; Li, J.; Yue, X.; Qin, D. In Situ Deposition of MOF199 onto Hierarchical Structures of Bamboo and Wood and Their Antibacterial Properties. RSC Adv. 2019, 9, 40277–40285. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, X.; Wang, L.; Wei, Y.; Zhao, Z.; Du, K.; Chen, D.; Li, X.; Zhou, C.; Liu, G.; et al. ZIF-67-Derived Co@N-PC Anchored on Tracheid Skeleton from Sawdust with Micro/Nano Composite Structures for Boosted Methylene Blue Degradation. Sep. Purif. Technol. 2021, 278, 119489. [Google Scholar] [CrossRef]
- Su, M.; Zhang, R.; Li, J.; Jin, X.; Zhang, X.; Qin, D. Tailoring Growth of MOF199 on Hierarchical Surface of Bamboo and Its Antibacterial Property. Cellulose 2021, 28, 11713–11727. [Google Scholar] [CrossRef]
- Sadiq, M.M.; Suzuki, K.; Hill, M.R. Towards Energy Efficient Separations with Metal Organic Frameworks. Chem. Commun. 2018, 54, 2825–2837. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, G.; Li, H.; Hill, M.R. Magnetic Metal–Organic Framework Composites: Solvent-Free Synthesis and Regeneration Driven by Localized Magnetic Induction Heat. ACS Sustain. Chem. Eng. 2019, 7, 13627–13632. [Google Scholar] [CrossRef]
- Huang, G.; Huang, C.; Tao, Y.; Li, H. Localized Heating Driven Selective Growth of Metal-Organic Frameworks (MOFs) in Wood: A Novel Synthetic Strategy for Significantly Enhancing MOF Loadings in Wood. Appl. Surf. Sci. 2021, 564, 150325. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Xie, H.; Zhao, Y. Graphene-Carbon Nanotube @ Cobalt Derivatives from ZIF-67 for All-Solid-State Asymmetric Supercapacitor. Appl. Surf. Sci. 2021, 568, 150929. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Derived from Non-Customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2021, 7, 109–115. [Google Scholar] [CrossRef]
- Ren, S.; Yu, H.; Wang, L.; Huang, Z.; Lin, T.; Huang, Y.; Yang, J.; Hong, Y.; Liu, J. State of the Art and Prospects in Metal-Organic Framework-Derived Microwave Absorption Materials. Nano-Micro Lett. 2022, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.-C.; Yang, X.-Y.; Zhang, X.-R.; Huang, X.-Y.; Cao, M.-S.; Li, L.; Yang, H.-J.; Cao, W.-Q. Tailoring MOF-Based Materials to Tune Electromagnetic Property for Great Microwave Absorbers and Devices. Carbon 2020, 162, 157–171. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.P.; Ji, G.; Xu, Z.J. Biomass-Derived Porous Carbon-Based Nanostructures for Microwave Absorption. Nano-Micro Lett. 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Wang, Q.; Wu, X.; Pang, J.; Jiang, Z.; Chen, G.; Dong, C.; Wang, L.; Gong, C. Biomass Derived Porous Carbon (BPC) and Their Composites as Lightweight and Efficient Microwave Absorption Materials. Compos. Part B 2021, 207, 108562. [Google Scholar] [CrossRef]
- Di, X.; Wang, Y.; Lu, Z.; Cheng, R.; Yang, L.; Wu, X. Heterostructure Design of Ni/C/Porous Carbon Nanosheet Composite for Enhancing the Electromagnetic Wave Absorption. Carbon 2021, 179, 566–578. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Y.; Li, Y.; Sun, X.; Wang, S.; Liang, L.; Ning, Y.; Li, J.; Yin, W.; Che, R.; et al. Dramatically Enhanced Electromagnetic Wave Absorption of Hierarchical CNT/Co/C Fiber Derived from Cotton and Metal-Organic-Framework. Carbon 2020, 161, 517–527. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically Derived Metal Organic Frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yan, J.; Huang, Y.; Liu, X.; Ding, L.; Zong, M.; Liu, P.; Li, T. Magnetic Porous CoNi@C Derived from Bamboo Fiber Combined with Metal-Organic-Framework for Enhanced Electromagnetic Wave Absorption. J. Colloid Interface Sci. 2021, 595, 78–87. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, W.; Ni, C.; Yan, S.; Yu, L.; Li, X. “Tree Blossom” Ni/NC/C Composites as High-Efficiency Microwave Absorbents. Chem. Eng. J. 2022, 430, 132621. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Yang, C.; Sun, Q.; Xu, X. Implanting FeCo/C Nanocages with Tunable Electromagnetic Parameters in Anisotropic Wood Carbon Aerogels for Efficient icrowave Absorption. J. Mater. Chem. A 2020, 8, 18863–18871. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Lamine, A.B.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of Methylene Blue from Aqueous Solution on Activated Carbons and Composite Prepared from an Agricultural Waste Biomass: A Comparative Study by Experimental and Advanced Modeling Analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Chen, J.; Lv, H.; Bai, X.; Liu, Z.; He, L.; Wang, J.; Zhang, Y.; Sun, B.; Kan, K.; Shi, K. Synthesis of Hierarchically Porous Co3O4/Biomass Carbon Composites Derived from MOFs and Their Highly NO2 Gas Sensing Performance. Microporous Mesoporous Mater. 2021, 321, 111108. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Getachew, N.; Díaz, K.; Díaz-García, M.; Chebude, Y.; Díaz, I. Synthesis of Metal–Organic Frameworks in Water at Room Temperature: Salts as Linker Sources. Green Chem. 2015, 17, 1500–1509. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, W.; Tan, Z.; Tian, W.; Guo, Y.; Wang, H.; Fu, J.; Jian, X. Ultra-Small Co/CNTs Nanohybrid from Metal Organic Framework with Highly Efficient Microwave Absorption. Compos. Part B 2018, 152, 316–323. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Babu, R.; Roshan, R.K.; Lee, H.; Kim, H.; Tharun, J.; Suresh, E.; Park, D.-W. An Lcy-Topology Amino Acid MOF as Eco-Friendly Catalyst for Cyclic Carbonate Synthesis from CO2: Structure-DFT Corroborated Study. J. Mater. Chem. A 2015, 3, 22636–22647. [Google Scholar] [CrossRef]
- Stylianou, K.C.; Gómez, L.; Imaz, I.; Verdugo-Escamilla, C.; Ribas, X.; Maspoch, D. Engineering Homochiral Metal-Organic Frameworks by Spatially Separating 1D Chiral Metal-Peptide Ladders: Tuning the Pore Size for Enantioselective Adsorption. Chem. Eur. J. 2015, 21, 9964–9969. [Google Scholar] [CrossRef]
- Rabone, J.; Yue, Y.-F.; Chong, S.Y.; Stylianou, K.C.; Bacsa, J.; Bradshaw, D.; Darling, G.R.; Berry, N.G.; Khimyak, Y.Z.; Ganin, A.Y.; et al. An Adaptable Peptide-Based Porous Material. Science 2010, 329, 1053–1057. [Google Scholar] [CrossRef]
- Martí-Gastaldo, C.; Warren, J.E.; Stylianou, K.C.; Flack, N.L.O.; Rosseinsky, M.J. Enhanced Stability in Rigid Peptide-Based Porous Materials. Angew. Chem. Int. Ed. 2012, 51, 11044–11048. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc-Adeninate Metal-Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-Adeninate Vertices for the Construction of an Exceptionally Porous Metal-Organic Framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A Review on the Recent Development of Cyclodextrin-Based Materials Used in Oilfield Applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; You, C.; Xiong, R.; Wang, F. Research progress of natural polysaccharide in the application of biomedical materials. J. For. Eng. 2021, 6, 1–8. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal-Organic Frameworks from Edible Natural Products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef] [PubMed]
- Keykhaee, M.; Razaghi, M.; Dalvand, A.; Salehian, F.; Soleimani, H.; Samzadeh-Kermani, A.; Shamsollahi, H.R.; Foroumadi, A.; Ramazani, A.; Khoobi, M.; et al. Magnetic Carnosine-Based Metal-Organic Framework Nanoparticles: Fabrication, Characterization and Application as Arsenic Adsorbent. J. Environ. Health Sci. Eng. 2020, 18, 1163–1174. [Google Scholar] [CrossRef]
- Mugaka, B.P.; Zhang, S.; Li, R.-Q.; Ma, Y.; Wang, B.; Hong, J.; Hu, Y.-H.; Ding, Y.; Xia, X.-H. One-Pot Preparation of Peptide-Doped Metal–Amino Acid Framework for General Encapsulation and Targeted Delivery. ACS Appl. Mater. Interfaces 2021, 13, 11195–11204. [Google Scholar] [CrossRef]
- Gładysiak, A.; Nguyen, T.N.; Anderson, S.L.; Boyd, P.G.; Palgrave, R.G.; Bacsa, J.; Smit, B.; Rosseinsky, M.J.; Stylianou, K.C. Shedding Light on the Protonation States and Location of Protonated N Atoms of Adenine in Metal–Organic Frameworks. Inorg. Chem. 2018, 57, 1888–1900. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, J.; Liu, J.; Dong, L.; Xin, Z.; Teng, Y.; Lan, Y. Adenine Components in Biomimetic Metal–Organic Frameworks for Efficient CO2 Photoconversion. Angew. Chem. Int. Ed. 2019, 58, 5226–5231. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Guo, J.; He, Z.; Guo, T.; Liu, Y.; Zhang, Y.; Zhang, J.; Feng, N. Activation of a Gamma–Cyclodextrin–Based Metal–Organic Framework Using Supercritical Carbon Dioxide for High–Efficient Delivery of Honokiol. Carbohydr. Polym. 2020, 235, 115935. [Google Scholar] [CrossRef]
- Hajra, S.; Sahu, M.; Padhan, A.M.; Lee, I.S.; Yi, D.K.; Alagarsamy, P.; Nanda, S.S.; Kim, H.J. A Green Metal–Organic Framework-Cyclodextrin MOF: A Novel Multifunctional Material Based Triboelectric Nanogenerator for Highly Efficient Mechanical Energy Harvesting. Adv. Funct. Mater. 2021, 31, 2101829. [Google Scholar] [CrossRef]
- Son, H.-J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q.; et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Mukherjee, S.; Warnan, J.; Li, W.; Wannapaiboon, S.; Hou, S.; Rodewald, K.; Rieger, B.; Weidler, P.G.; Wöll, C.; et al. Porphyrin Based Metal–Organic Framework Films: Nucleation and Growth. J. Mater. Chem. A 2020, 8, 25941–25950. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y.; Kim, S.-J.; Kwon, T.-H.; Huh, S.; Park, S. Bio-Functionalization of Metal–Organic Frameworks by Covalent Protein Conjugation. Chem. Commun. 2011, 47, 2904–2906. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Xu, G.; Zhang, L.; Xu, G.; Yang, L.; Sun, Z.; Jia, D. Efficient Co–N/PC@CNT Bifunctional Electrocatalytic Materials for Oxygen Reduction and Oxygen Evolution Reactions Based on Metal–Organic Frameworks. Nanoscale 2018, 10, 9077–9086. [Google Scholar] [CrossRef]
- Meng, J.; Niu, C.; Xu, L.; Li, J.; Liu, X.; Wang, X.; Wu, Y.; Xu, X.; Chen, W.; Li, Q.; et al. General Oriented Formation of Carbon Nanotubes from Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8212–8221. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Han, X.; Gao, X.; Liu, P. Metal Organic Framework (ZIF-67)-Derived Hollow CoS2/N-Doped Carbon Nanotube Composites for Extraordinary Electromagnetic Wave Absorption. Compos. Part B 2019, 163, 67–76. [Google Scholar] [CrossRef]
- Liu, W.; Ning, L.-M.; Li, S.-Q.; Liu, W.-X.; Zhang, Q.; Shao, J.; Tian, J.-L. N-Rich MOFs Derived N-Doped Carbon Nanotubes Encapsulating Cobalt Nanoparticles as Efficient and Magnetic Recoverable Catalysts for Nitro Aromatics Reduction. J. Alloys Compd. 2021, 862, 158333. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Adsorptive Removal of Wide Range of Pharmaceuticals and Personal Care Products from Water Using Bio-MOF-1 Derived Porous Carbon. Microporous Mesoporous Mater. 2018, 270, 102–108. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Lee, J.K.; Cho, C.-W.; Jhung, S.H. Remarkably Efficient Adsorbent for the Removal of Bisphenol A from Water: Bio-MOF-1-Derived Porous Carbon. Chem. Eng. J. 2018, 343, 225–234. [Google Scholar] [CrossRef]
- Yang, L.; Xu, G.; Ban, J.; Zhang, L.; Xu, G.; Lv, Y.; Jia, D. Metal-Organic Framework-Derived Metal-Free Highly Graphitized Nitrogen-Doped Porous Carbon with a Hierarchical Porous Structure as an Efficient and Stable Electrocatalyst for Oxygen Reduction Reaction. J. Colloid Interface Sci. 2019, 535, 415–424. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Mu, S.; Wang, Y.; Jiang, C.; Liu, Q.; Fang, Q.; Xue, M.; Qiu, S. Cation Exchanged MOF-Derived Nitrogen-Doped Porous Carbons for CO2 Capture and Supercapacitor Electrode Materials. J. Mater. Chem. A 2017, 5, 9544–9552. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Cheng, Y.; Bai, X.; Wen, B.; Lin, Y. Constructing a Nitrogen-Doped Carbon and Nickel Composite Derived from a Mixed Ligand Nickel-Based a Metal–Organic Framework toward Adjustable Microwave Absorption. Nanoscale 2021, 13, 9204–9216. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wen, B.; Wang, L. Carbon Nanotubes Modified CoZn/C Composites with Rambutan-like Applied to Electromagnetic Wave Absorption. Appl. Surf. Sci. 2020, 509, 145336. [Google Scholar] [CrossRef]
- Pan, J.; Xia, W.; Sun, X.; Wang, T.; Li, J.; Sheng, L.; He, J. Improvement of Interfacial Polarization and Impedance Matching for Two-Dimensional Leaf-like Bimetallic (Co, Zn) Doped Porous Carbon Nanocomposites with Broadband Microwave Absorption. Appl. Surf. Sci. 2020, 512, 144894. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).