Evaluation of Changes in the Permeability Characteristics of a Geotextile–Polynorbornene Liner for the Prevention of Pollutant Diffusion in Oil-Contaminated Soils
Abstract
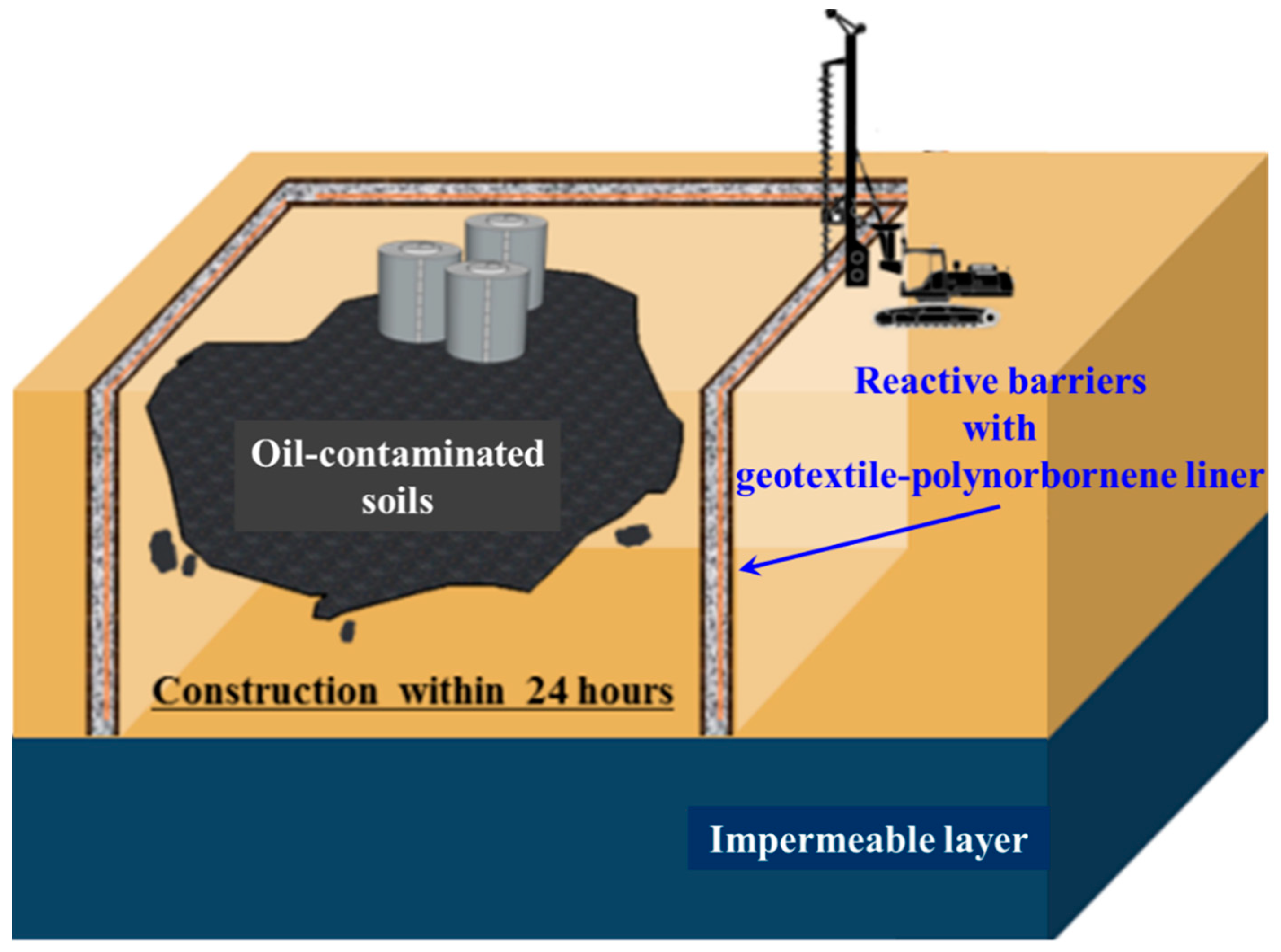
1. Introduction
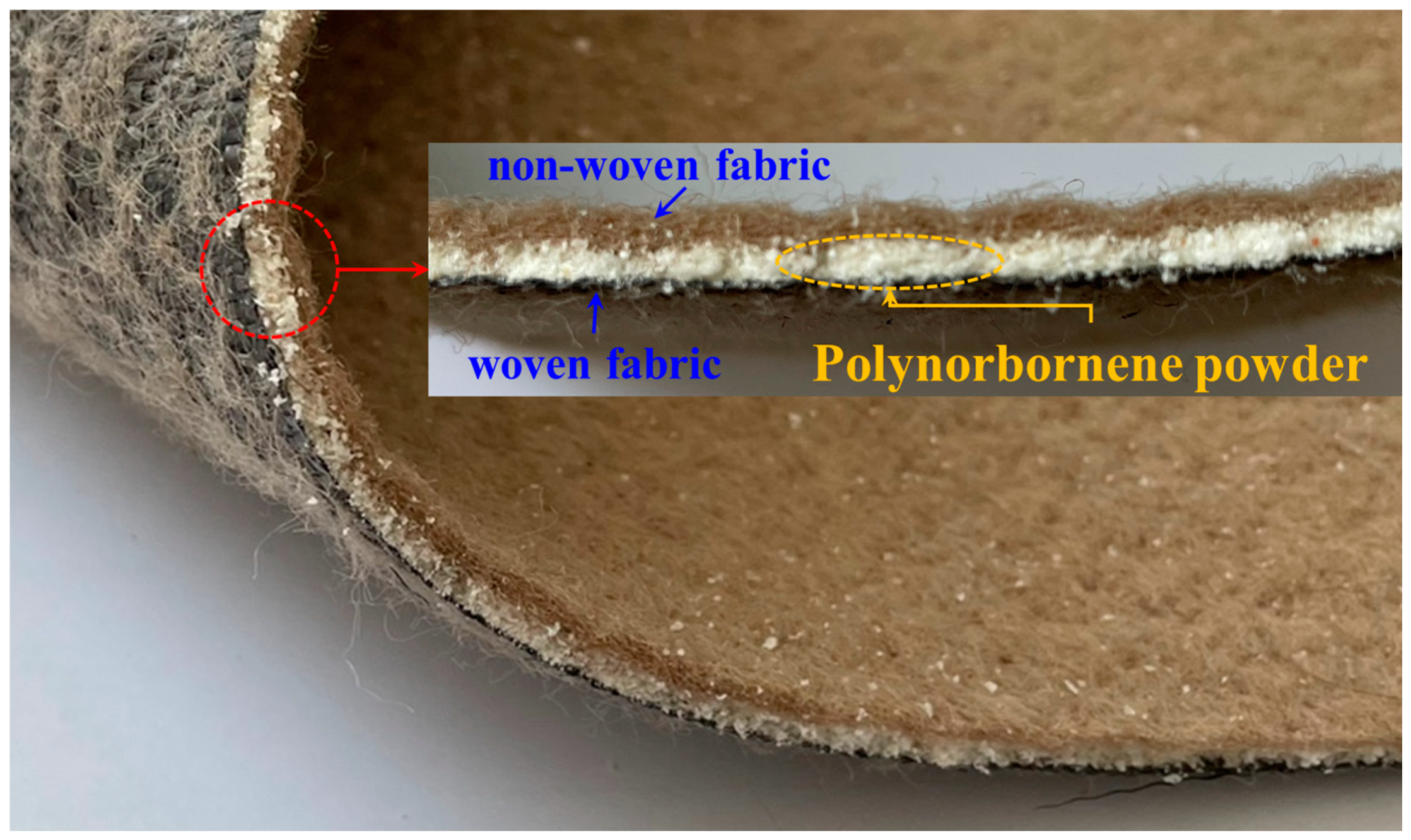
2. Overview of the Geotextile–Polynorbornene Liner
3. Materials and Methods
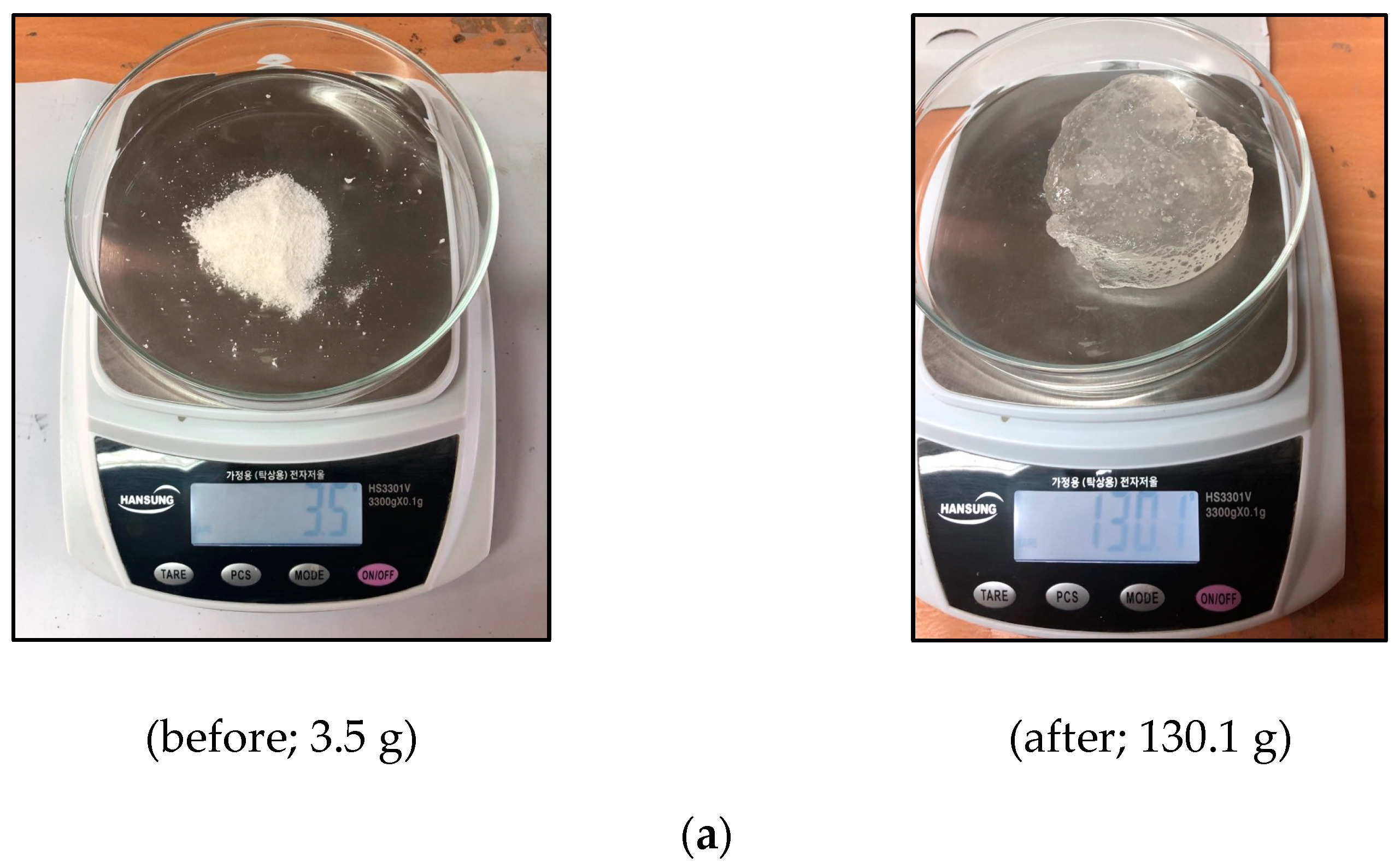
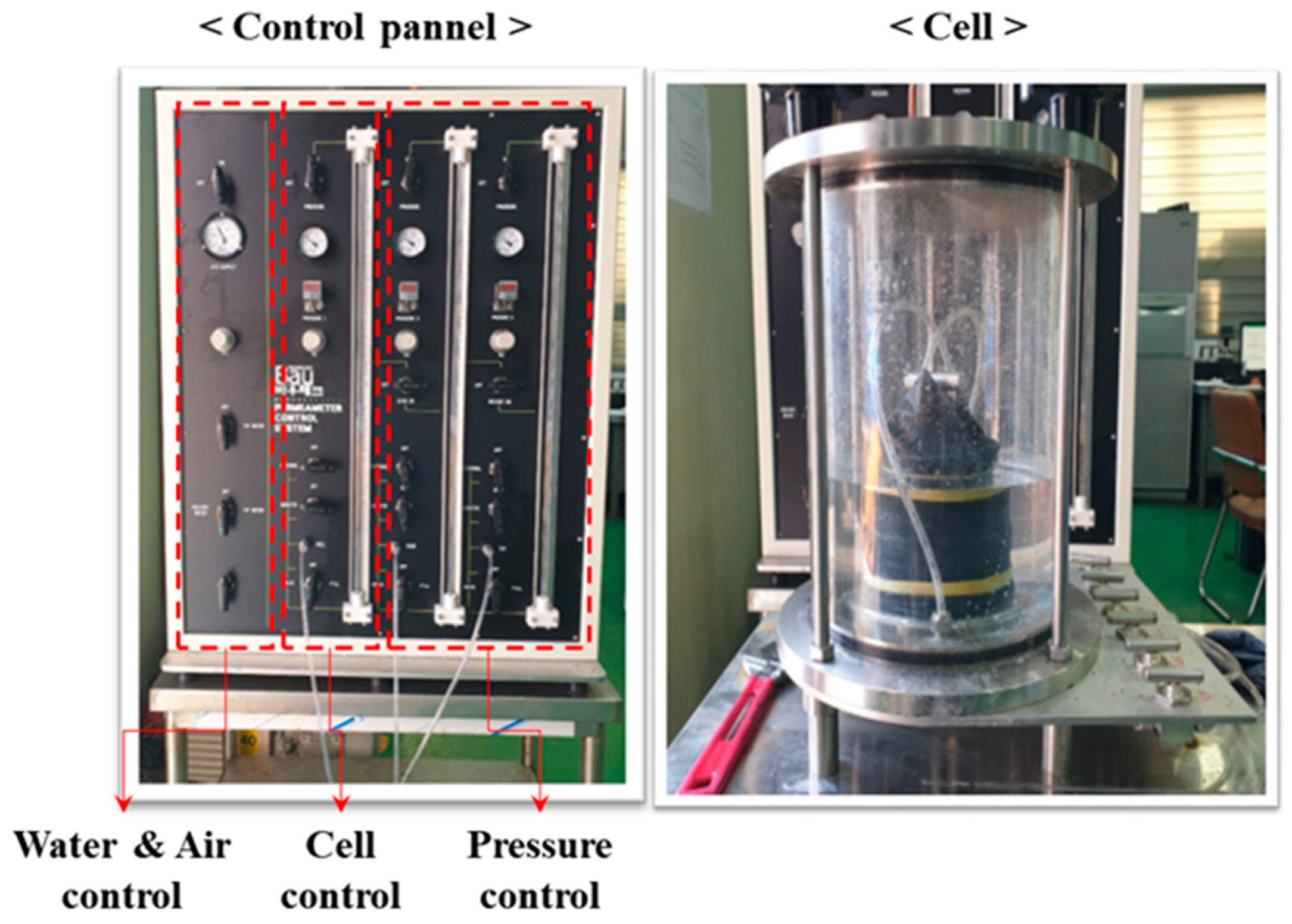
3.1. Test Apparatus and Materials
3.2. Test Procedure
4. Results and Discussion
4.1. Changes in the Permeability Characteristics of the Geotextile–Polynorbornene Liner over Time after Contacting the Pollutant
4.2. Numerical Analysis
4.2.1. Finite Difference Analysis (FDA)
4.2.2. Changes in the Permeability Characteristics of the Geotextile–Polynorbornene Liner According to the Concentration of the Oil Pollutant
5. Conclusions
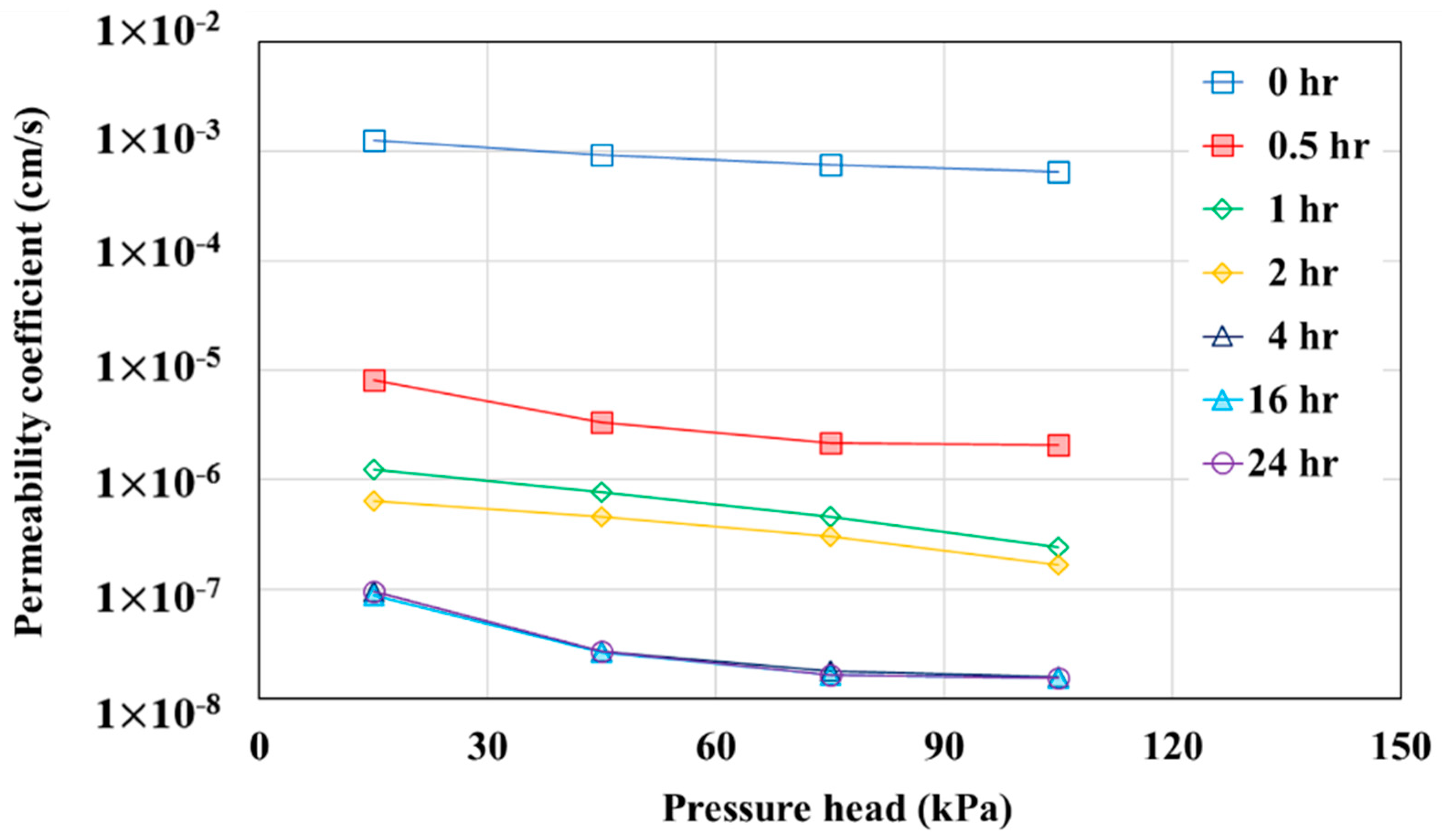
- When changes in the permeability coefficient were examined at different pressure heads and different pollutant contact times, the permeability coefficient decreased as the pressure head increased (the hydraulic gradient increased) regardless of the pollutant contact time. In addition, when the pollutant contact time was 4 h or longer, the permeability coefficient of the geotextile–polynorbornene liner was 10−7 cm/s or less, which is defined as almost an impermeable layer.
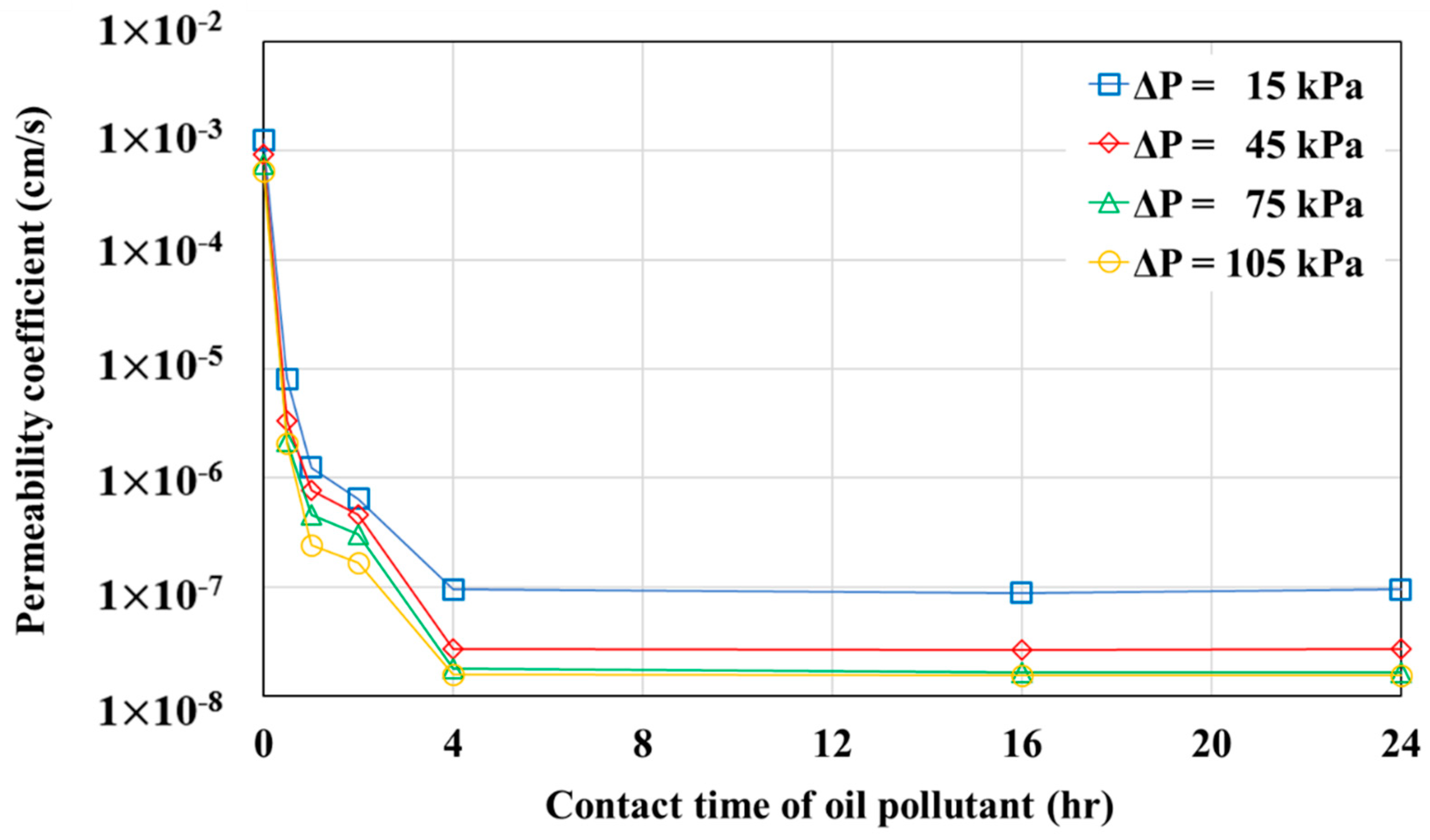
- Changes in the permeability coefficient were examined over time under different pressure head conditions. There was almost no change in the permeability coefficient starting from the pollutant contact time of 4 h. Thus, when the pollutant contact time reaches 4 h or more, the geotextile–polynorbornene liner has an impermeable layer that can block pollutants.
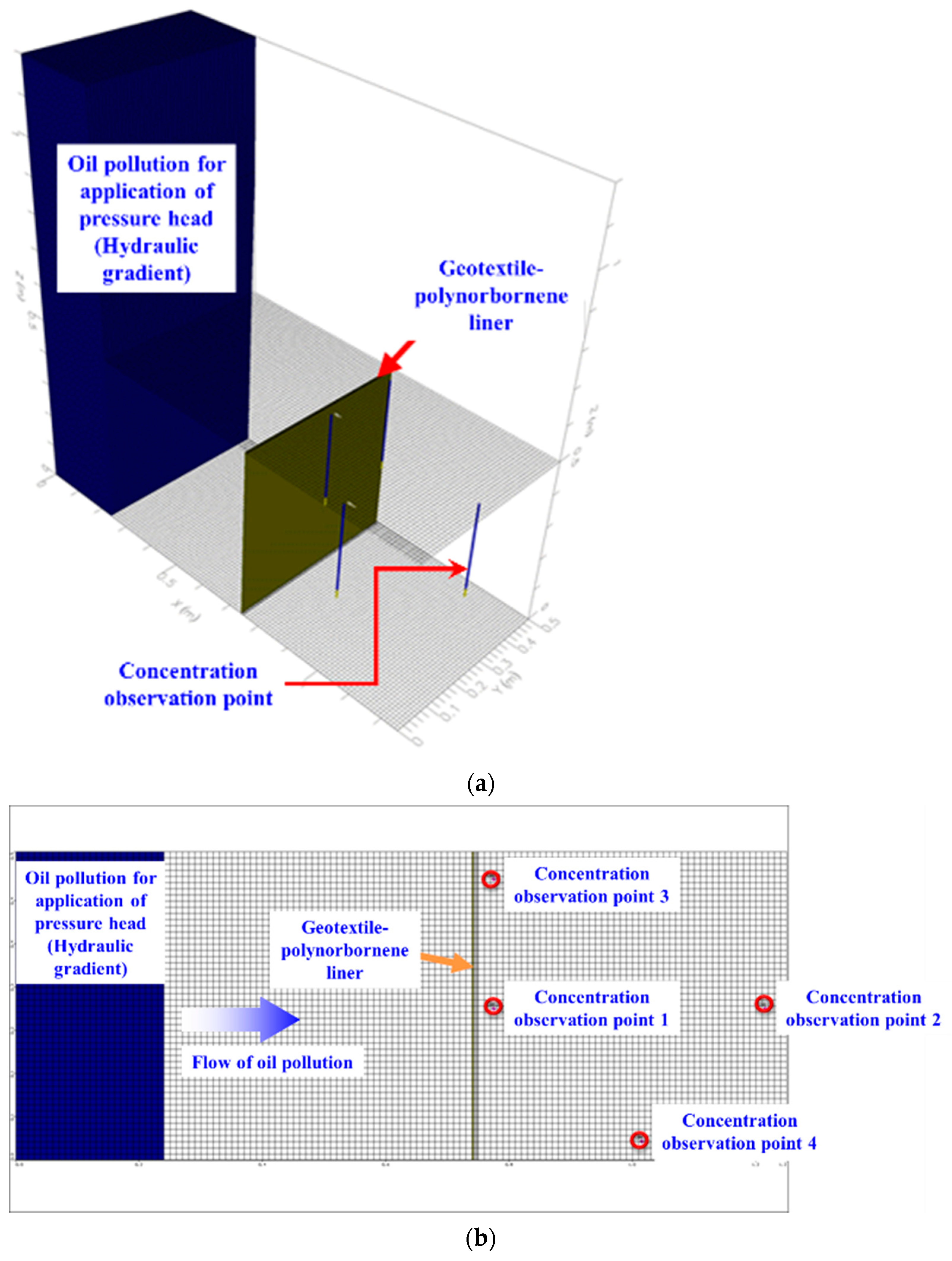
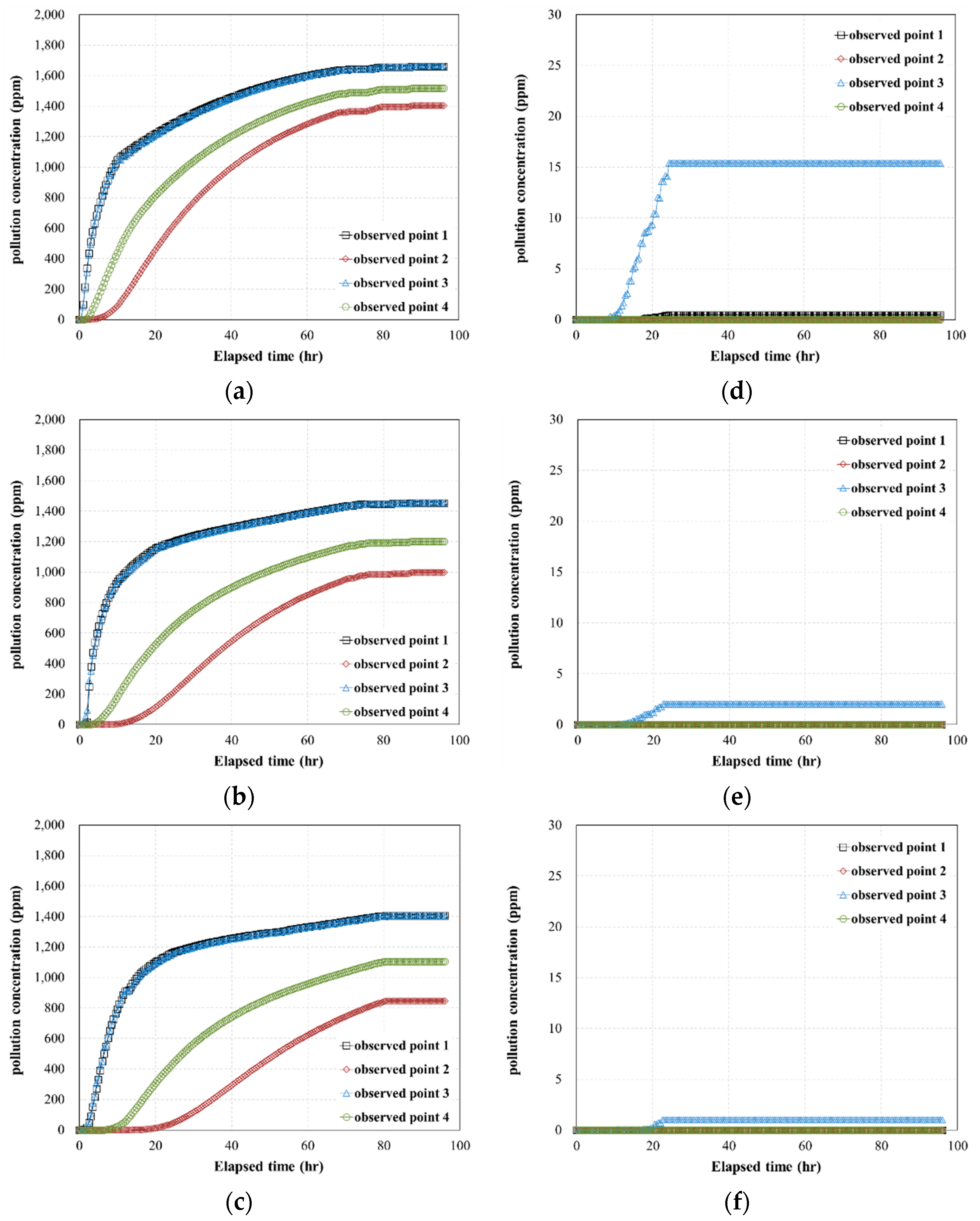
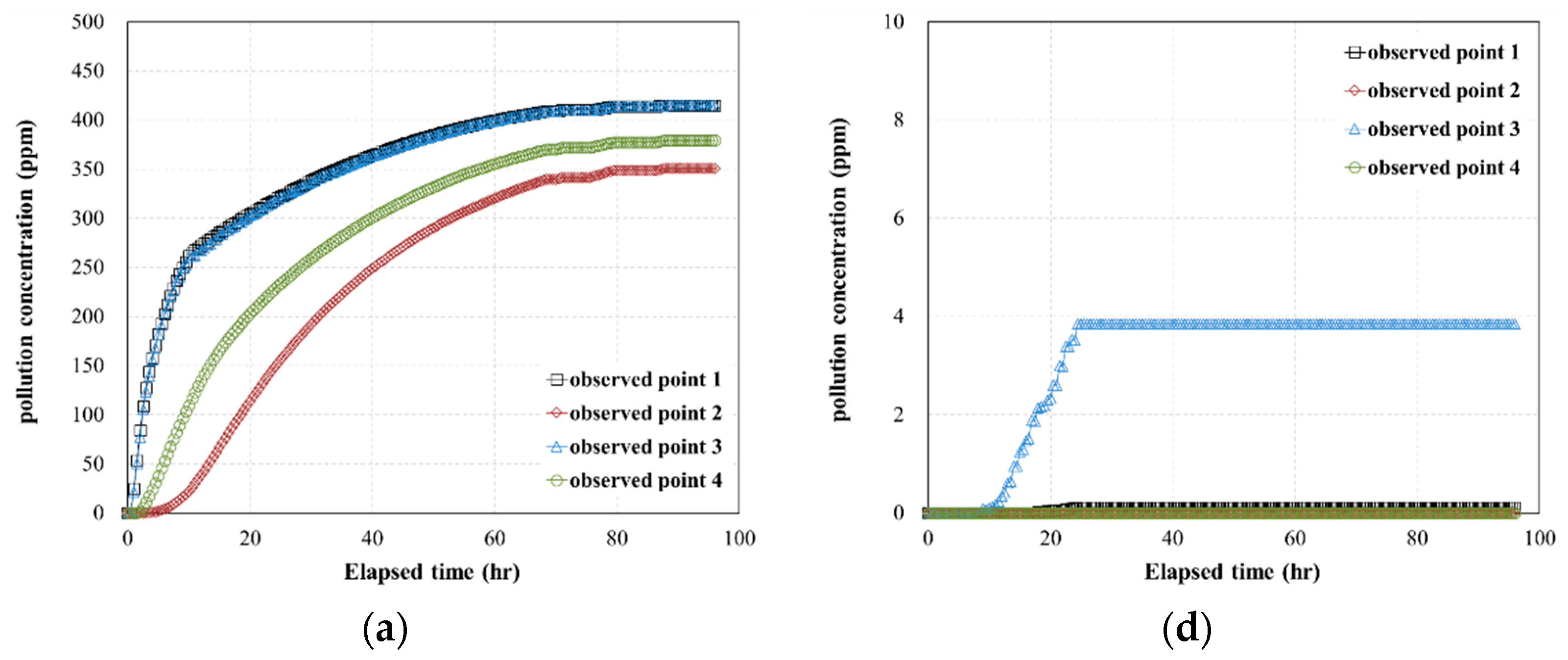
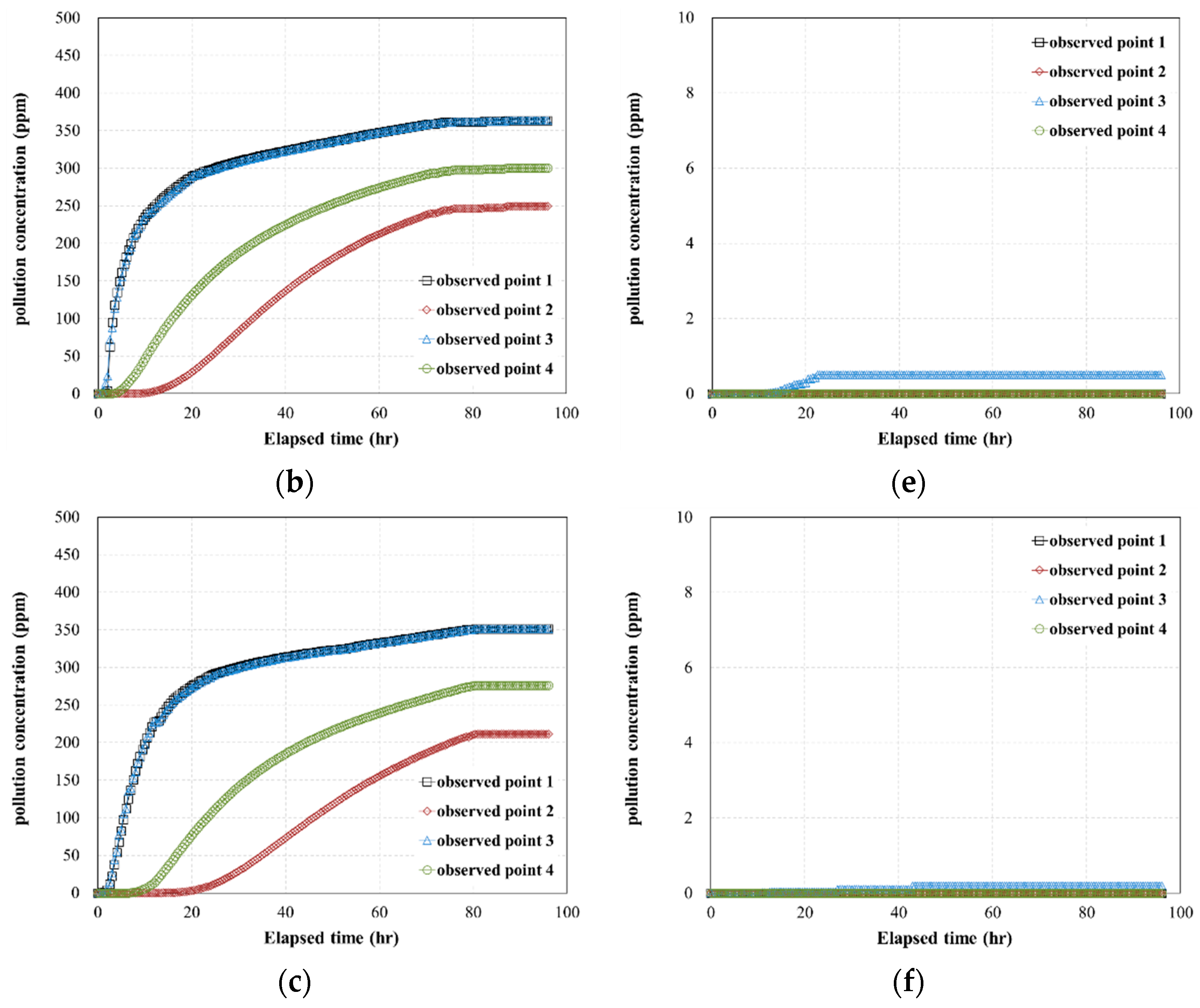
- The results of the 3D pollutant diffusion analysis showed that, for a pollutant contact time of 4 h, the maximum concentration of the pollutant that permeated through the geotextile–polynorbornene liner was less than approximately 0.8% compared to the initial pollutant concentration. Therefore, the numerical analysis results confirm that the geotextile–polynorbornene liner has a pollutant blocking effect over time.
- The test and numerical analysis results confirm the impermeability performance of the geotextile–polynorbornene liner against oil pollutants. Therefore, it has potential as an application for the prevention of pollutant diffusion.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Environment, Purification of Pollution Guideline; Environment of Korea: Sejong-si, Korea, 2007.
- Awad, Y.M.; Kim, S.C.; Abd EI-Azeem, S.A.M.; Kim, K.H.; Kim, K.R.; Kim, K.J.; Jeon, C.; Lee, S.S. Veterinary antibiotics contamination in water, sediment, and soil near a swine manure composting facility. Environ. Earth Sci. 2014, 71, 1433–1440. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Huang, Q.; Yang, Y.; Nie, Z.; Cheng, J.; Yang, J.; Wang, Y.; Chao, M. Contamination and risk of heavy metals in soils and sediments from a typical plastic waste recycling area in North China. Ecotoxicol. Environ. Saf. 2015, 112, 343–351. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Teefy, D.A. Remediation technologies screening matrix and reference guide: Version III. Remediat. J. 1997, 8, 115–121. [Google Scholar] [CrossRef]
- Ministry of Environment, Guidelines for Cleaning Contaminated. 2007. (In Korean). Available online: http://www.me.go.kr/home/web/policy_data/read.do;jsessionid=U8n8W4UDhpcnTttwLejCuHvj.mehome1?pagerOffset=2990&maxPageItems=10&maxIndexPages=10&searchKey=&searchValue=&menuId=10261&orgCd=&condition.deleteYn=N&seq=3297 (accessed on 13 April 2007).
- Jeong, J.; Kim, H.; Lee, S.; Jeong, S.W. Effects of Diesel Dose and Soil Texture on Variation in the Concentration of Total Petroleum Hydrocarbon in the Diesel-Contaminated Soil. J. Korean Soc. Environ. Eng. 2017, 37, 69–72. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Jung, W.C.; Park, M.; Kim, M.S.; Lee, S.J.; Park, H. Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies. Sustainability 2020, 12, 10078. [Google Scholar] [CrossRef]
- Cho, K.; Myung, E.; Kim, H.; Purev, O.; Park, C.; Choi, N. Removal of Total Petroleum Hydrocarbons from Contaminated Soil through Microwave Irradiation. Int. J. Environ. Res. Public Health 2020, 17, 5952. [Google Scholar] [CrossRef]
- Sayed, K.; Baloo, L.; Sharma, N.K. Bioremediation of Total Petroleum Hydrocarbons (TPH) by Bioaugmentation and Biostimulation in Water with Floating Oil Spill Containment Booms as Bioreactor Basin. Int. J. Environ. Res. Public Health 2021, 18, 2226. [Google Scholar] [CrossRef]
- Han, S. Remediation Study of Total Petroleum Hydrocarbons Contaminated Soil Using Biopile. Master’s Thesis, Hankuk University of Foreign Studies, Seoul, Korea, 2017. [Google Scholar]
- Feng, D.; Lorenzen, L.; Aldrich, C.; Mar, P.W. Exsitu diesel contaminated soil washing with mechanical methods. Miner. Eng. 2001, 14, 1093–1100. [Google Scholar] [CrossRef]
- Lee, C.D.; Yoo, J.C.; Yang, J.S.; Kong, J.; Baek, K. Extraction of Total Petroleum Hydrocarbons from Petroleum Oil-Contaminated Sandy Soil by Soil Washing. J. Soil Groundw. Environ. 2013, 18, 18–24. [Google Scholar] [CrossRef]
- Khalladi, R.; Benhabiles, O.; Bentahar, F.; Moulai-Mostefa, N. Surfactant remediation of diesel fuel polluted soil. J. Hazard. Mater. 2009, 164, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, S.; Maes, A. Remediation of Diesel Contaminated, Sandy-Loam Soil Using Low Concentrated Surfactant Solutions. J. Soils Sediments 2005, 5, 240–244. [Google Scholar] [CrossRef]
- Hernández-Espriú, A.; Sánchez-León, E.; Martnez-Santos, P.; Torres, L.G. Remediation of a diesel-contaminated soil from a pipeline accidental spill: Enhanced biodegradation and soil washing processes using natural gums and surfactants. J. Soils Sediments 2013, 13, 152–165. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, K.J.; Song, J.Y.; An, S.G.; Jang, B.A. An Experimental Study to Improve Permeability and Cleaning Efficiency of Oil Contaminated Soil by Plasma Blasting. J. Eng. Geol. 2020, 30, 557–575. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L. In-Situ remediation of chromate contaminated groundwater using permeable reactive walls. Environ. Sci. Technol. 1997, 31, 3348–3357. [Google Scholar] [CrossRef]
- USEPA. In-Situ Remediation Technology Status Report, Treatment Walls; 542-K-94-004; USEPA: Washington, DC, USA, 1995.
- USEPA. Permeable Reactive Barrier Technologies for Contaminant Remediation; 600-R-98-125; USEPA: Washington, DC, USA, 1998.
- USEPA. Cost Analyses for Selected Groundwater Cleanup Projects; Pump and Treat System and Permeable Reactive Barriers, Solid Waste and Emergency Response (5102G); 542-R-00-013; USEPA: Washington, DC, USA, 2001.
- Boni, M.R.; Sbaffoni, S. The potential of compost-based biobarriers for Cr(VI) removal from contaminated groundwater, Column test. J. Hazard. Mater. 2009, 166, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.J.; Wu, H.N.; Chai, J.C.; Arulrajah, A. State-Of-The-Art Review of Geosynthetic Clay Liners. Sustainability 2017, 9, 2110. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Meier, A.; Sample-Lord, K. Limiting membrane and diffusion behavior of a geosynthetic clay liner. Geotext. Geomembr. 2016, 44, 707–718. [Google Scholar] [CrossRef]
- Liu, Y.; Bouazza, A.; Gates, W.P.; Rowe, R.K. Hydraulic performance of geosynthetic clay liners to sulfuric acid solutions. Geotext. Geomembr. 2015, 43, 14–23. [Google Scholar] [CrossRef]
- Wu, H.N.; Shen, S.L.; Liao, S.M.; Yin, Z.Y. Longitudinal structural modelling of shield tunnels considering shearing dislocation between segmental rings. Tunn. Undergr. Space Technol. 2015, 50, 317–323. [Google Scholar] [CrossRef]
- Wu, H.N.; Shen, S.L.; Yang, J. Identification of tunnel settlement caused by land subsidence in soft deposit of Shanghai. J. Perform. Constr. Facil. 2017, 31, 4017092. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, Q.; Liu, L. Impact of High Concentration Solutions on Hydraulic Properties of Geosynthetic Clay Liner Materials. Materials 2012, 5, 2326–2341. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, J.Y. A Feasibility Study on the Complex Media Permeable Reactive Barrier using Waste Resources. J. Korea Soc. Waste Manag. 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Ji, S.W.; Cheong, Y.W. Experiment of Reactive Media Selection for the Permeable Reactive Barrier Treating Groundwater contaminated by Acid Mine Drainage. Econ. Environ. Geol. 2005, 38, 237–245. [Google Scholar]
- Chung, S.L.; Lee, D.H. Remediation of PCE-contaminated Groundwater Using Permeable Reactive Barrier System with M0M-Bentonite. J. Soil Groundw. Environ. 2012, 17, 73–80. [Google Scholar] [CrossRef][Green Version]
- Guerin, T.F.; Hornerb, S.; McGovernb, T.; Davey, B. An application of permeable reactive barrier technology to petroleum hydrocarbon contaminated groundwater. Water Res. 2002, 36, 15–24. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Choi, N.C.; Park, C.Y. The Characterization of Pyrophyllite Based Ceramic Reactive Media for Permeable Reactive Barriers. J. Mineral. Soc. Korea 2019, 31, 227–234. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lim, D.H.; Lee, J.Y. A Feasibility Study on the Deep Soil Mixing Barrier to Control Contaminated Groundwater. J. Soil Groundw. Environ. 2001, 6, 53–59. [Google Scholar]
- Yun, S.Y.; An, H.K.; Oh, M.; Lee, J.Y. A study on the Evaluation of Permeability and Structure for Calcium Bentonite-Sand Mixtures. J. Korean Geosynth. Soc. 2019, 18, 1–10. [Google Scholar]
- Lee, S.K.; Kim, S.H.; Lee, K.J.; Shin, K.I. Functional Treatment of Recycled Ultrafine Fibrous Nonwovens. Text. Sci. Eng. 2005, 42, 370–375. [Google Scholar]
- Lee, Y.H.; Kim, J.S.; Kim, D.H.; Shin, M.S.; Jung, Y.J.; Lee, D.J.; Kim, H.D. Effect of Blend Ratio of PP/kapok Blend Nonwoven Fabrics on Oil Sorption Capacity. Environ. Technol. 2013, 34, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, Y.; Wang, A. Effect of Kapok Fiber Treated with Various Solvents on Oil Absorbency. Ind. Crop. Prod. 2012, 40, 178–184. [Google Scholar] [CrossRef]
- Shin, H.S.; Yoo, J.H.; Jin, L. A Study on Oil Absorption Rate and Oil Absorbency of Melt-blown Nonwoven. Text. Color. Finish. 2010, 22, 257–263. [Google Scholar] [CrossRef][Green Version]
- Rengasamy, R.S.; Das, D.; Karan, C.P. Study of Oil Sorption Behavior of Filled and Structured Fiber Assemblies Made from Polypropylene, Kapok and Milkweed Fibers. J. Hazard. Mater. 2011, 186, 526–532. [Google Scholar] [CrossRef]
- Lelaune, R.D.; Lindau, C.W.; Jugsujinda, A. Effectiveness of Nochar Solidifier Polymer in Removing Oil from Open Water in Coastral Wetlands. Spill Sci. Technol. Bull. 1999, 5, 357–359. [Google Scholar]
- Atta, A.; Arndt, K.F. Swelling and Network Parameters of High Oil Absorptive Network Based on 1-Octene and Isodecyl Acrylate Copolymers. J. Appl. Polym. Sci. 2005, 97, 80–91. [Google Scholar] [CrossRef]
- Shin, Y.S.; Cha, H.Y.; Park, S.S.; Woo, J.W.; Choi, J.S. Oil-absorption Capacity of poly(2-ethylhexylacrylate) Polymer in the Presence of Ultrasonic Wave. J. Korean Ind. Eng. Chem. 2002, 13, 326–329. [Google Scholar]
- Chang, S.C.; Chun, S.H.; Lee, M.S.; Lee, K.B.; Choi, J.S. Oil-absorption Behaviors and Kinetics of Poly(dodecyl acrylate). Appl. Chem. 1999, 3, 141–143. [Google Scholar]
- Jeong, H.J. Oil Absorptive Properties of Polypropylene Knit Fabric Treated with Oleophilic Acrylic Resin. J. Korea Acad. Ind. Coop. Soc. 2016, 17, 528–535. [Google Scholar]
- Yun, S.Y.; Choi, J.W.; Oh, M.; Lee, J.Y. Characteristic of Permeability with the Sand, Calcium Bentonite and Solidifier Mixtures according to Selective Reaction of TCE. J. Korean Geosynth. Soc. 2020, 19, 25–33. [Google Scholar]
- Nguyen, D.C.; Bui, T.T.; Cho, Y.B.; Kim, Y.S. Highly Hydrophobic Polydimethylsiloxane-Coated Expanded Vermiculite Sorbents for Selective Oil Removal from Water. Nanomaterials 2021, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.M.; Toth, C.R.A.; Collins, V.; Mussone, P.; Gieg, L.M. The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater. Microorganisms 2021, 9, 90. [Google Scholar] [CrossRef] [PubMed]



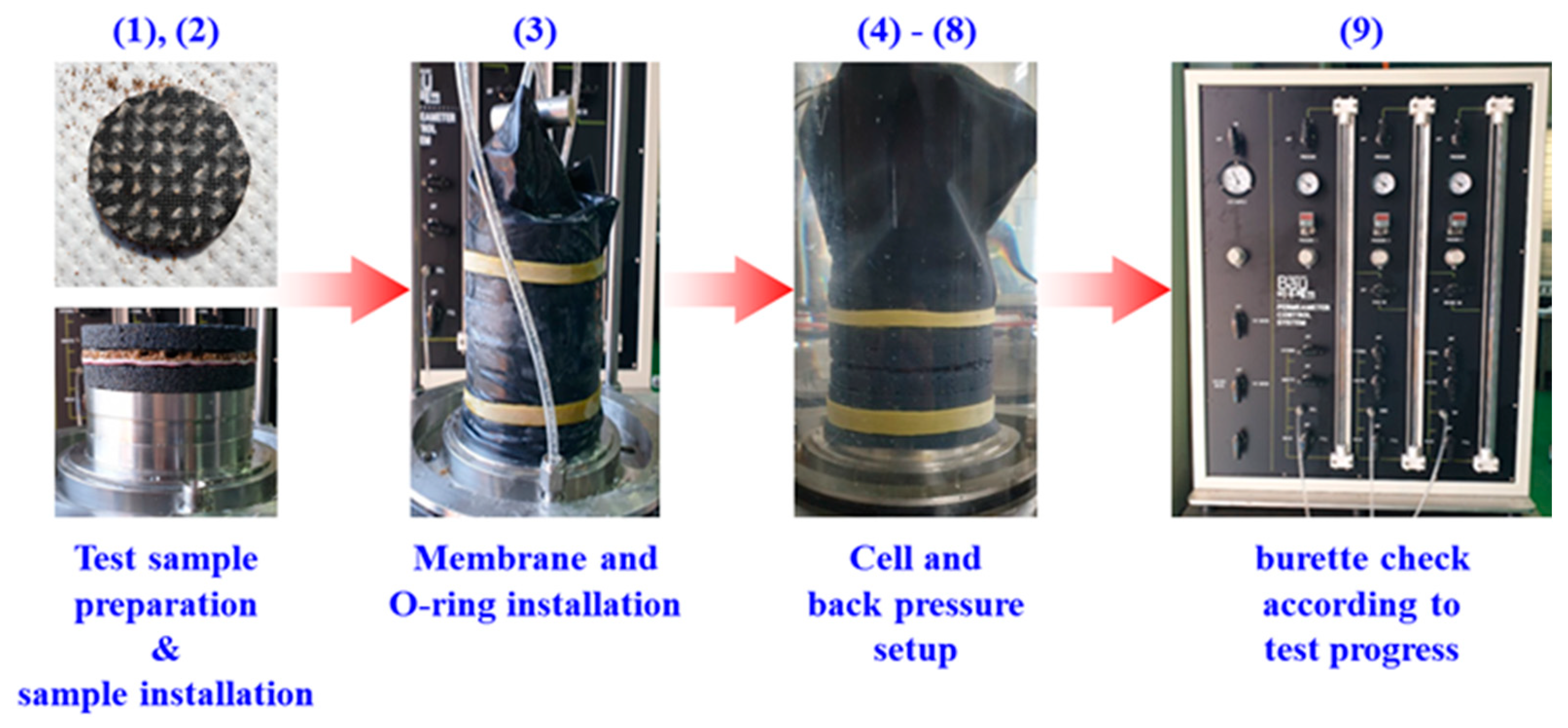

| Contact Time of Oil Pollutant | Pressure Head (ΔP, kPa) | Total Head (Δh, m) | Discharge Time (t, s) | Flow Rate (Q, cm3/s) | Specimen Area (A, cm2) | Specimen Length (L, cm) |
|---|---|---|---|---|---|---|
| 0 h | 15 | 1.53 | 8.2 | 40 | 50.27 | 2 |
| 45 | 4.59 | 3.8 | ||||
| 75 | 7.65 | 2.8 | ||||
| 105 | 10.71 | 2.3 | ||||
| 0.5 h | 15 | 1.53 | 16 | 0.5 | ||
| 45 | 4.59 | 13 | ||||
| 75 | 7.65 | 12 | ||||
| 105 | 10.71 | 9 | ||||
| 1 h | 15 | 1.53 | 540 | |||
| 45 | 4.59 | 94 | ||||
| 75 | 7.65 | 34 | ||||
| 105 | 10.71 | 15 | ||||
| 2 h | 15 | 1.53 | 780 | |||
| 45 | 4.59 | 110 | ||||
| 75 | 7.65 | 57 | ||||
| 105 | 10.71 | 29 | ||||
| 4 h | 15 | 1.53 | 8242 | |||
| 45 | 4.59 | 2438 | ||||
| 75 | 7.65 | 967 | ||||
| 105 | 10.71 | 195 | ||||
| 16 h | 15 | 1.53 | 8402 | |||
| 45 | 4.59 | 2631 | ||||
| 75 | 7.65 | 990 | ||||
| 105 | 10.71 | 210 | ||||
| 24 h | 15 | 1.53 | 8420 | |||
| 45 | 4.59 | 2638 | ||||
| 75 | 7.65 | 970 | ||||
| 105 | 10.71 | 195 |
| Contact Time of Oil Pollutant | Pressure Head (ΔP, kPa) | Hydraulic Gradient | Flux (F, cm3/cm2·s) | Permeability Coefficient (k, cm/s) |
|---|---|---|---|---|
| 0 h | 15 | 77 | 9.68 × 10−2 | 1.26 × 10−3 |
| 45 | 230 | 2.11 × 10−1 | 9.17 × 10−4 | |
| 75 | 383 | 2.88 × 10−1 | 7.53 × 10−4 | |
| 105 | 536 | 3.46 × 10−1 | 6.46 × 10−4 | |
| 0.5 h | 15 | 77 | 6.22 × 10−4 | 8.12 × 10−6 |
| 45 | 230 | 7.65 × 10−4 | 3.33 × 10−6 | |
| 75 | 383 | 8.29 × 10−4 | 2.17 × 10−6 | |
| 105 | 536 | 1.11 × 10−3 | 2.06 × 10−6 | |
| 1 h | 15 | 77 | 1.84 × 10−5 | 1.24 × 10−6 |
| 45 | 230 | 1.06 × 10−4 | 7.64 × 10−7 | |
| 75 | 383 | 2.93 × 10−4 | 4.61 × 10−7 | |
| 105 | 536 | 6.63 × 10−4 | 2.41 × 10−7 | |
| 2 h | 15 | 77 | 1.28 × 10−5 | 6.40 × 10−7 |
| 45 | 301 | 9.04 × 10−5 | 4.56 × 10−7 | |
| 75 | 383 | 1.74 × 10−4 | 3.01 × 10−7 | |
| 105 | 536 | 3.43 × 10−4 | 1.67 × 10−7 | |
| 4 h | 15 | 77 | 1.21 × 10−6 | 9.52 × 10−8 |
| 45 | 230 | 4.08 × 10−6 | 2.69 × 10−8 | |
| 75 | 383 | 1.03 × 10−5 | 1.78 × 10−8 | |
| 105 | 536 | 5.10 × 10−5 | 1.58 × 10−8 | |
| 16 h | 15 | 77 | 1.18 × 10−6 | 8.84 × 10−8 |
| 45 | 230 | 3.78 × 10−6 | 2.63 × 10−8 | |
| 75 | 383 | 1.00 × 10−5 | 1.65 × 10−8 | |
| 105 | 536 | 4.74 × 10−5 | 1.55 × 10−8 | |
| 24 h | 15 | 77 | 1.18 × 10−6 | 9.52 × 10−8 |
| 45 | 230 | 3.77 × 10−6 | 2.68 × 10−8 | |
| 75 | 383 | 1.03 × 10−5 | 1.64 × 10−8 | |
| 105 | 536 | 5.10 × 10−5 | 1.54 × 10−8 |
| Classification | Oil Tank | Soils |
|---|---|---|
| Porosity | 0.9 | 0.25 |
| Horizontal permeability coefficient (cm/s) | 1 | 10−4 |
| Vertical permeability coefficient (cm/s) | 1 | 10−4 |
| Specific storativity (m−1) | 10−5 | 10−5 |
| Specific yield | 0.9 | 0.15 |
| Contact time of oil pollutant (h) | 96 | |
| Analysis Cases | Pollutant (TPH) Concentration (ppm) | Contact Time of Pollutant (h) | Pressure Head (ΔP, kPa) | Permeability Coefficient of Geotextile–Polynorbornene Liner (cm/s) |
|---|---|---|---|---|
| Case HC-1 | 6000 | 0.5 | 45 | 3.33 × 10−6 |
| Case HC-2 | 75 | 2.17 × 10−6 | ||
| Case HC-3 | 105 | 2.06 × 10−6 | ||
| Case HC-4 | 4 | 45 | 2.69 × 10−8 | |
| Case HC-5 | 75 | 1.78 × 10−8 | ||
| Case HC-6 | 105 | 1.58 × 10−8 | ||
| Case MC-1 | 2000 | 0.5 | 45 | 3.33 × 10−6 |
| Case MC-2 | 75 | 2.17 × 10−6 | ||
| Case MC-3 | 105 | 2.06 × 10−6 | ||
| Case MC-4 | 4 | 45 | 2.69 × 10−8 | |
| Case MC-5 | 75 | 1.78 × 10−8 | ||
| Case MC-6 | 105 | 1.58 × 10−8 | ||
| Case LC-1 | 500 | 0.5 | 45 | 3.33 × 10−6 |
| Case LC-2 | 75 | 2.17 × 10−6 | ||
| Case LC-3 | 105 | 2.06 × 10−6 | ||
| Case LC-4 | 4 | 45 | 2.69 × 10−8 | |
| Case LC-5 | 75 | 1.78 × 10−8 | ||
| Case LC-6 | 105 | 1.58 × 10−8 |
| Analysis Cases | Pollutant (TPH) Concentration (ppm) | Contact Time of Pollutant (h) | Pressure Head (ΔP, kPa) | Maximum Concentration of Observed Point 1 (ppm) |
|---|---|---|---|---|
| Case HC-1 | 6000 | 0.5 | 45 | 4985.8 |
| Case HC-2 | 75 | 4379.6 | ||
| Case HC-3 | 105 | 4200.9 | ||
| Case HC-4 | 4 | 45 | 46.1 | |
| Case HC-5 | 75 | 6.0 | ||
| Case HC-6 | 105 | 3.2 | ||
| Case MC-1 | 2000 | 0.5 | 45 | 1660.5 |
| Case MC-2 | 75 | 1453.2 | ||
| Case MC-3 | 105 | 1405.3 | ||
| Case MC-4 | 4 | 45 | 15.4 | |
| Case MC-5 | 75 | 2.0 | ||
| Case MC-6 | 105 | 1.0 | ||
| Case LC-1 | 500 | 0.5 | 45 | 415.13 |
| Case LC-2 | 75 | 363.31 | ||
| Case LC-3 | 105 | 351.32 | ||
| Case LC-4 | 4 | 45 | 3.8 | |
| Case LC-5 | 75 | 0.5 | ||
| Case LC-6 | 105 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J. Evaluation of Changes in the Permeability Characteristics of a Geotextile–Polynorbornene Liner for the Prevention of Pollutant Diffusion in Oil-Contaminated Soils. Sustainability 2021, 13, 4797. https://doi.org/10.3390/su13094797
Park J. Evaluation of Changes in the Permeability Characteristics of a Geotextile–Polynorbornene Liner for the Prevention of Pollutant Diffusion in Oil-Contaminated Soils. Sustainability. 2021; 13(9):4797. https://doi.org/10.3390/su13094797
Chicago/Turabian StylePark, Jeongjun. 2021. "Evaluation of Changes in the Permeability Characteristics of a Geotextile–Polynorbornene Liner for the Prevention of Pollutant Diffusion in Oil-Contaminated Soils" Sustainability 13, no. 9: 4797. https://doi.org/10.3390/su13094797
APA StylePark, J. (2021). Evaluation of Changes in the Permeability Characteristics of a Geotextile–Polynorbornene Liner for the Prevention of Pollutant Diffusion in Oil-Contaminated Soils. Sustainability, 13(9), 4797. https://doi.org/10.3390/su13094797






