Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products
Abstract
1. Introduction
2. Experiment Outline
2.1. Preparation of Activated Sludge and Cement Matrix
2.2. Preparation of the Concrete
2.3. Experiment Methods
2.3.1. Compressive Strength
2.3.2. X-Ray Diffraction (XRD) Analysis
2.3.3. Freeze–Thaw Resistance of Concrete
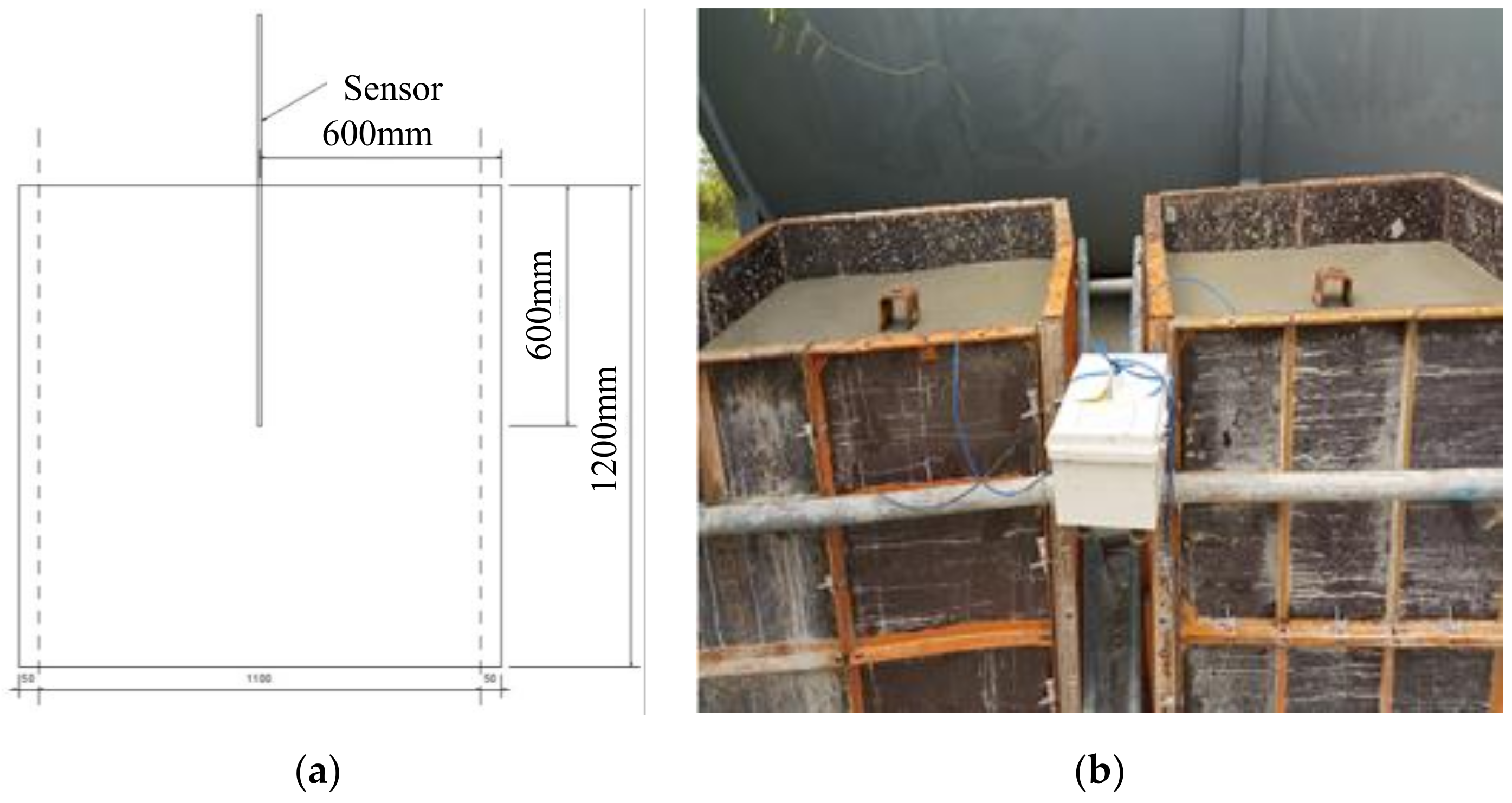
2.3.4. Carbonation Resistance of the Concrete
2.3.5. Heat of Hydration
3. Results and Discussion
3.1. Compressive Strength
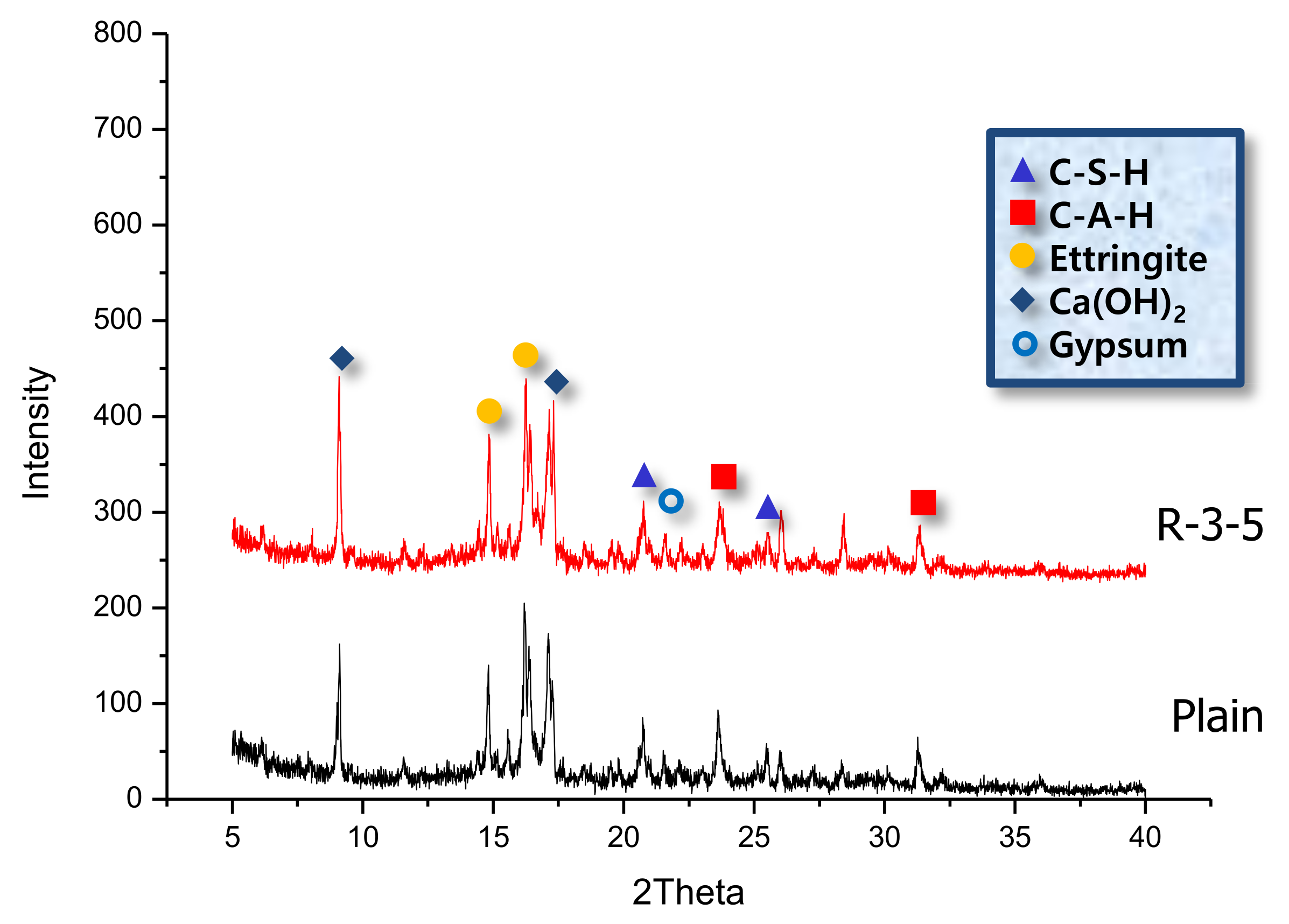
3.2. X-Ray Diffraction Analysis
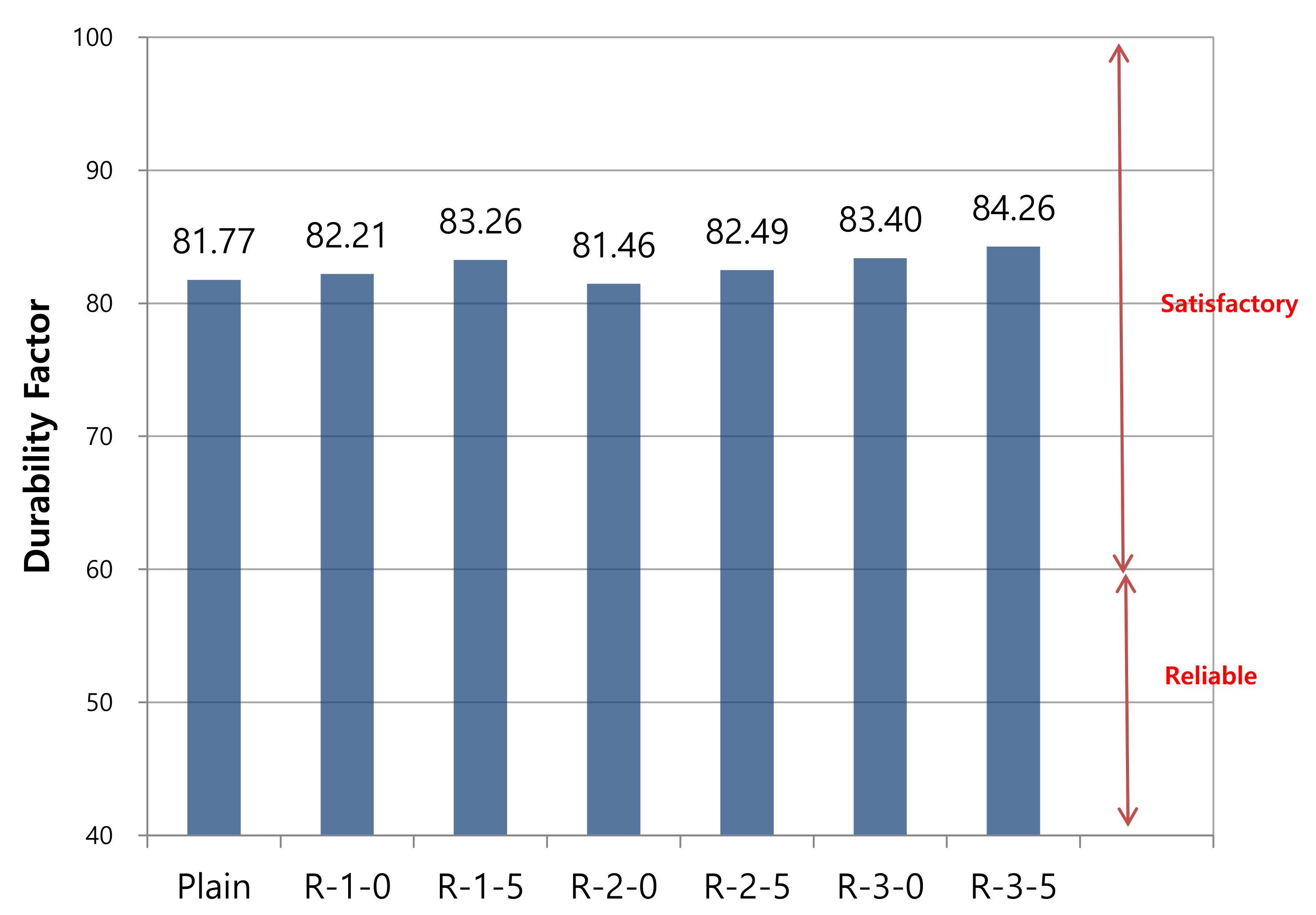
3.3. Evaluation of Freeze–Thaw Resistance
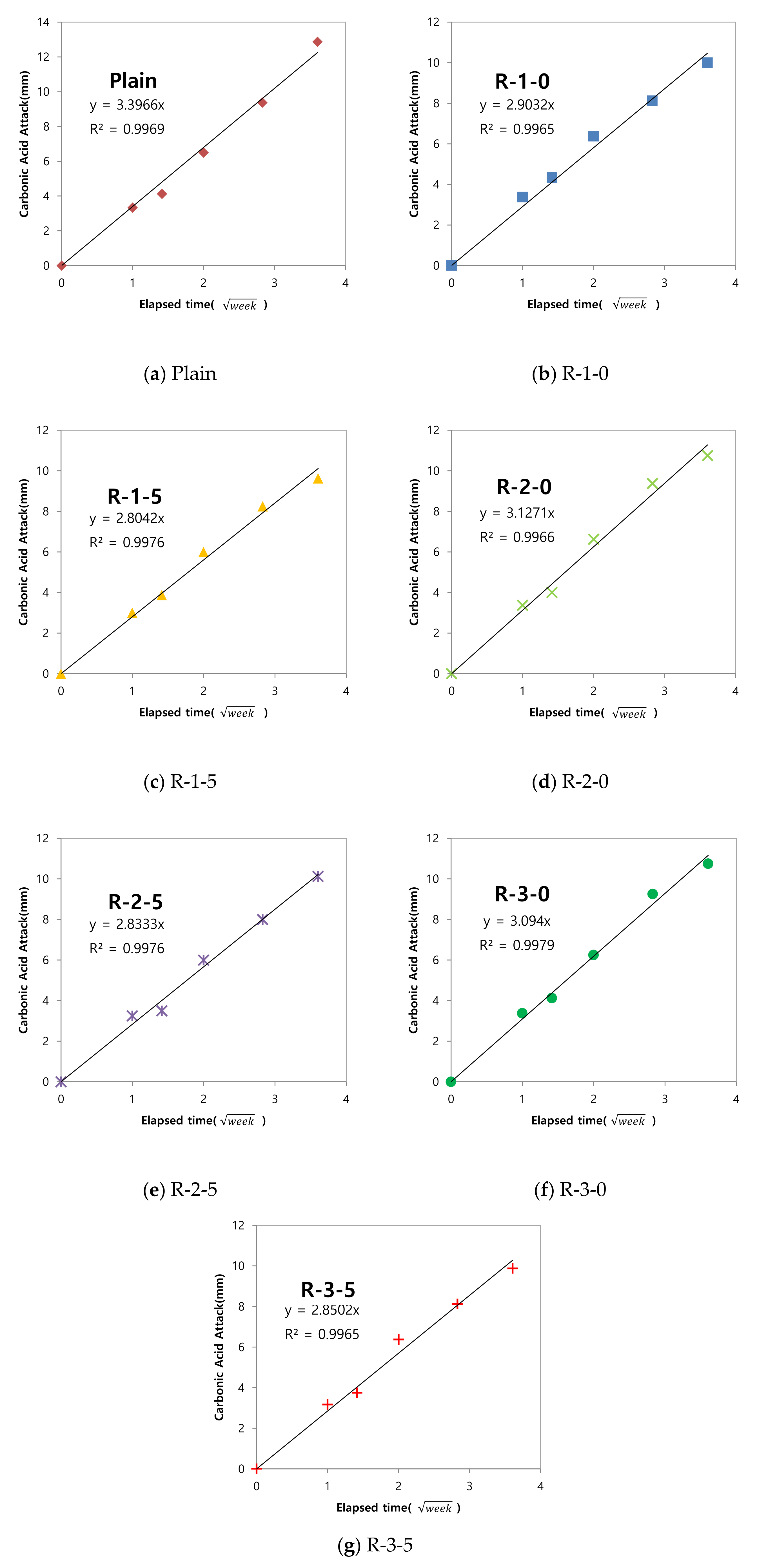
3.4. Evaluation of Carbonation Resistance
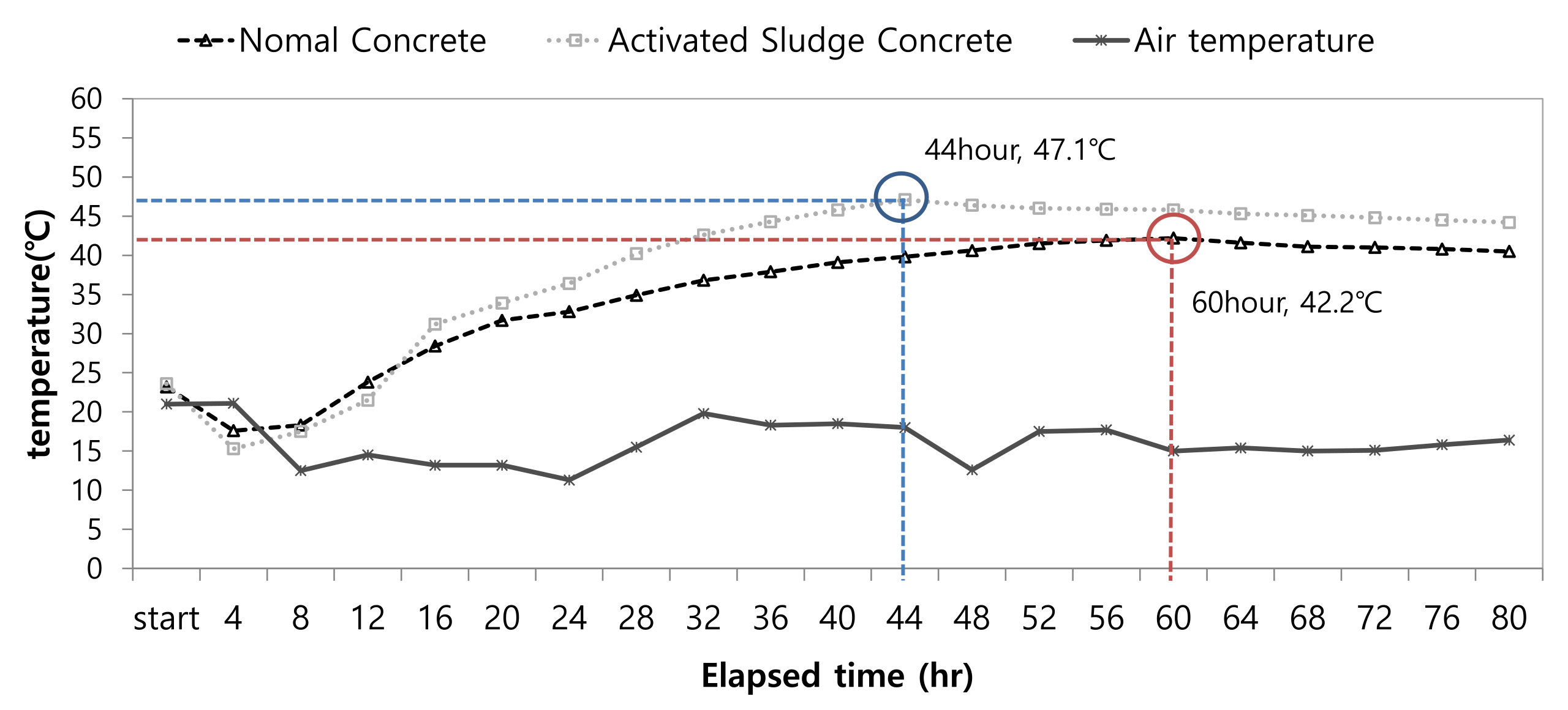
3.5. Heat of Hydration Evaluation for the Concrete
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Han, C. Vision of the Ready Mixed Concrete Industry. Mag. Korea Concr. Inst. 2013, 25, 25–28. [Google Scholar]
- US RMC Production. Korea Ready Mixed Concrete Industry Association; US RMC Production: Houston, TX, USA, 2013. [Google Scholar]
- Japan’s Ready-Mixed Concrete Production. Korea Ready Mixed Concrete Industry Association; Japan’s Ready-Mixed Concrete Production: Tokyo, Japan, 2019. [Google Scholar]
- Statistics Yearbook of Remicon. Korea Federation of Ready Mixed Concrete Industry Co-Operatives; Ssangyong Remicon Co., Ltd.: Seoul, Korea, 2019. [Google Scholar]
- Shin, H.C. Reuse of Ready Mixed Concrete Waste Washed Water to Improve the Properties of Ground Granulated Blast Furnace Slag Concrete Doctoral Dissertation. Ph.D. Thesis, University of Hanyang, Seoul, Korea, 2002. [Google Scholar]
- Abunqira, I.J.M. Removal of Selected Heavy Metals from Wash Water Generated from Ready-Mix Concrete Trucks; United Arab Emirates University: Abu Dhabi, United Arab Emirates, 2008. [Google Scholar]
- Han, C. Control of Quality and Equipment of Re-Mi-Con Using Recycling Water; Korea Concrete Institute: Seoul, Korea, 2002; pp. 40–48. [Google Scholar]
- Yoon, G.W.R.H.G.; Han, C.G.; Bahn, H.Y. A Fundamental Study on the Reuse of Recycling Water of Ready Mixed Concrete such as Concrete Water; Architectural Institute of Korea: Seoul, Korea; pp. 409–412.
- Sealey, B.; Phillips, P.S.; Hill, G. Waste management issues for the UK ready-mixed concrete industry. Resour. Conserv. Recycl. 2001, 32, 321–331. [Google Scholar] [CrossRef]
- Paolini, M.; Khurana, R. Admixtures for recycling of waste concrete. Cem. Concr. Compos. 1998, 20, 221–229. [Google Scholar] [CrossRef]
- Chini, A.R.; Muszynski, L.C.; Ellis, B.S. Recycling Process Water in Ready-Mixed Concrete Operations; Technical Report No. WPI 0510863; School of Building Construction: Gainesville, FL, USA, 1999. [Google Scholar]
- Kwon, C.; Kong, T.; Lee, H.; Son, H.; Ryu, S. Property of a Change on Slump & Air Content According Elapsed Time in Concrete Using Recycled Water of Ready mixed Concrete. J. Korea Concr. Inst. 2014, 26, 605–606. [Google Scholar]
- Yang, E.-I.; Park, J.-H.; Kim, K.-R.; Jo, G.-J. Durability and Strength Characteristics of Concrete Using Sludge Water above Specification. J. Korea Concr. Inst. 2006, 18, 199–204. [Google Scholar] [CrossRef]
- Mun, H.Y.S.; Choi, J.J. A Study on the Recycling of Wastewater from Red Mix Concrete Plant. J. Korean Remicon Ind. Soc. 1989, 21–29. [Google Scholar]
- Mun, H.Y. Research on the Development of Concrete Waste Sludge from Ready-Mixed Concrete Plant; Korea Science Foundation: Seoul, Korea, 2001. [Google Scholar]
- Hatanaka, S.; Tanigawa, Y. State of the Arts on the Study of Concrete Sludge. Concr. J. (Jpn.) 1995, 33, 14–24. [Google Scholar] [CrossRef]
- Lee, S.D. An Experimental Study on the Reuse of Recycling Water of Ready Mixed Concrete as Concrete Water; University of Cheongju: Cheongju, Korea, 1994. [Google Scholar]
- Yoon, G.W.R.H.G.; Han, C.G.; Bahn, H.Y.; Kim, G.C. A Study on the Mortar for the Reuse of Recycling Water of Ready Mixed Concrete as Concrete Water. J. Archit. Inst. Korea 1995, 11, 261–267. [Google Scholar]
- Yoon, G.W.R.H.G.; Han, C.G.; Bahn, H.Y.; Kim, G.C. A Study on the Concrete for the Reuse of Recycling Water of Ready Mixed Concrete as Concrete Water. J. Archit. Inst. Korea 1995, 11, 201–207. [Google Scholar]
- Kim, K.J.K.G.H.; Lee, M.H.; Lee, S.H.; Han, C.G. A Study on the Engineering Properties of Concrete Using Stabilizing Agent of Recycling Water; Architectural Institute of Korea: Seoul, Korea, 2003; pp. 503–506. [Google Scholar]
- Lee, M.-H.; Lee, S.-H.; Park, Y.-S.; Park, J.-M. Properties of retard type stabilizing agent for reuse of sludge water of ready mixed concrete. J. Korea Concr. Inst. 2005, 17, 105–112. [Google Scholar] [CrossRef]
- Lee, M.H.L.S.H.; Park, Y.S.; Park, J.M. Effects of Mixing Basin Facilities for Stable Reuse of Sludge Water of Ready Mixed Concrete. J. Archit. Inst. Korea Struct. Constr. 2005, 21, 71–78. [Google Scholar]
- Chatveera, B.; Lertwattanaruk, P.; Makul, N. Effect of sludge water from ready-mixed concrete plant on properties and durability of concrete. Cem. Concr. Compos. 2006, 28, 441–450. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Dawneerangen, A. Evaluation of recycled water recovered from a ready-mix concrete plant for reuse in concrete. J. South Afr. Inst. Civ. Eng. 2010, 52, 77–82. [Google Scholar]
- Korean Standards Association. KS F 4009. Ready-Mixed Concrete; Korean Standards Association: Seoul, Korea, 2016. [Google Scholar]
- International, A. C94-21, Standard Specification for Ready-Mixed Concrete. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Ghrair, A.M.; Heath, A.; Paine, K.; Al Kronz, M. Waste Wash-Water Recycling in Ready Mix Concrete Plants. Environments 2020, 7, 108. [Google Scholar] [CrossRef]
- Korea Ready Mixed Concrete Industry Association. A Study on the Properties of Concrete Considered to Sewage Sludge Ratio of Sludge Water, 2010 R&D Report; Korea Ready Mixed Concrete Industry Association: Seoul, Korea, 2011; pp. 5–51. [Google Scholar]
- Kim, H.; Yoon, Y.; Lee, H.; Kim, S. Study of Desulfurization of Limestone and Crystal Habit of Gypsum by adding Dibasic Acid as Buffer Additives. J. Korean Ind. Eng. Chem. 2002, 13, 468–475. [Google Scholar]
- Park, W.C.M.K.J.; So, S.Y.; Soh, Y.S.; Kwon, W.T. Manufacturing of Cement Admixture Using FGD Gypsum; Korea Concrete Institute: Daegu, Korea, 2015; pp. 979–982. [Google Scholar]
- Park, J.; Chu, Y.; Lee, J.; Sung, H.; Seo, S.; La, Y. Physical Properties of ALC used Desulfurization Gypsum. Proc. Korea Concr. Inst. 2014, 26, 423–424. [Google Scholar]
- Elseknidy, M.H.; Salmiaton, A.; Nor Shafizah, I.; Saad, A.H. A Study on Mechanical Properties of Concrete Incorporating Aluminum Dross, Fly Ash, and Quarry Dust. Sustainability 2020, 12, 9230. [Google Scholar] [CrossRef]
- Musa, M.F.; Mohammad, M.F.; Mahbub, R.; Yusof, M.R. Enhancing the quality of life by adopting sustainable modular industrialised building system (IBS) in the Malaysian construction industry. Proc. Soc. Behav. Sci. 2014, 153, 79–89. [Google Scholar] [CrossRef]
- Bae, J.; Jang, Y.; Lee, B.; Park, W.; Kim, Y. Study on Hydration Heat of Cementless Mortar Containing FGD Gypsum. Proc. Korea Inst. Struct. Maint. Insp. 2013, 17, 516–517. [Google Scholar]
- Hyun, J.-Y.; Jeong, S.-B.; Chae, Y.-B.; Kim, B.-S. Manufacture and Application of anhydrous calcium sulfate from flue gas desulfurization gypsum. J. Korean Inst. Resour. Recycl. 2005, 14, 10–18. [Google Scholar]
- Son, H.J.K.Y.Y.; Seo, I.H.; Kwon, C.W.; Kong, T.W.; Ryu, S.L. Basic Studies for the Development of Ready-Mixed Concrete Recycling Water Sewage Sludge Ratio Measuring Technology Which Applied Optical Technology. In Proceedings of the Korean Concrete Institute Conference, Boryeong-si, Korea, 15–17 October 2014. [Google Scholar]
- International, A. C150-20, Standard Specification for Portland Cement. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- International, A. C109-20, Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Korean Standards Association. KS F 2403. Standard Test Method for Making Concrete Specimens; Korean Standards Association: Seoul, Korea, 2019. [Google Scholar]
- Korean Standards Association. KS F 2405. Standard Test Method for Compressive Strength Concrete; Korean Standards Association: Seoul, Korea, 2010. [Google Scholar]
- International, A. C666-15, Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- CPC-18 Measurement of hardened concrete carbonation depth. Mater. Struct. 1988, 21, 453–455. [CrossRef]
- Abo-El-Enein, S.A.; Ata, A.A.; Hassanien, A.; Mikhai, R. Kinetics and mechanism of slag cement hydration. J. Chem. Technol. Biotechnol. 1982, 32, 939–945. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E. Waste wash water recycling in ready-mixed concrete plants. Cem. Concr. Res. 2001, 3, 485–489. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H. Influence of thermally treated flue gas desulfurization (FGD) gypsum on performance of the slag powder concrete. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 1122–1127. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vazquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Jiang, L.; Li, C.; Wang, C.; Xu, N.; Chu, H. Utilization of flue gas desulfurization gypsum as an activation agent for high-volume slag concrete. J. Clean. Prod. 2018, 205, 589–598. [Google Scholar] [CrossRef]
- Wang, S.-D.; Pu, X.-C.; Scrivener, K.; Pratt, P. Alkali-activated slag cement and concrete: A review of properties and problems. Adv. Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Moon, H. Properties of cement matrix mixed with ready-mixed concrete sludge and residual water. Hanyang Univ. Korea ICCMC/IBST 2001, 519–525. [Google Scholar]





| Flue Gas Desulfurization Gypsum | Oxides Content (Wt%) | ||||||||
| CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | Na2O | K2O | LOI | |
| 60.5 | 4.78 | 0.86 | 25.6 | 0.46 | 0.81 | 0.04 | 0.25 | 6.50 | |
| Oxides | Content (Wt.%) | ||
|---|---|---|---|
| Cement | Fly Ash | Blast-Furnace Slag | |
| CaO | 62.0 | 3.0 | 41.7 |
| SiO2 | 20.8 | 56.4 | 31.7 |
| Al2O3 | 6.3 | 23.4 | 14.5 |
| SO3 | 2.2 | 0.4 | 2.1 |
| Fe2O3 | 3.2 | 7.9 | 0.7 |
| MgO | 3.3 | 1.5 | 5.4 |
| LOI | 1.3 | 1.2 | 2.6 |
| Specimen ID | W/B * (%) | S/a * (%) | Kg/m3 | SP * (%) | AE * (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C * | W * | FA * | BS * | RW * | DG * | G * | S * | |||||
| Plain | 50.8 | 49.5 | 253 | 172 | 51 | 34 | - | - | 846 | 934 | C× 0.5% | C× 0.1% |
| R-1-0 | 253 | 17.2 | - | |||||||||
| R-2-0 | 253 | 34.4 | ||||||||||
| R-3-0 | 253 | 51.6 | - | |||||||||
| R-1-5 | 240.35 | 17.2 | 12.65 | |||||||||
| R-2-5 | 240.35 | 34.4 | 12.65 | |||||||||
| R-3-5 | 240.35 | 51.6 | 12.65 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-Y.; Yang, H.-M.; Lee, H.-S. Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products. Sustainability 2021, 13, 4671. https://doi.org/10.3390/su13094671
Kim Y-Y, Yang H-M, Lee H-S. Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products. Sustainability. 2021; 13(9):4671. https://doi.org/10.3390/su13094671
Chicago/Turabian StyleKim, Young-Yeop, Hyun-Min Yang, and Han-Seung Lee. 2021. "Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products" Sustainability 13, no. 9: 4671. https://doi.org/10.3390/su13094671
APA StyleKim, Y.-Y., Yang, H.-M., & Lee, H.-S. (2021). Preparation and Properties of Sustainable Concrete Using Activated Sludge of Industrial By-Products. Sustainability, 13(9), 4671. https://doi.org/10.3390/su13094671








