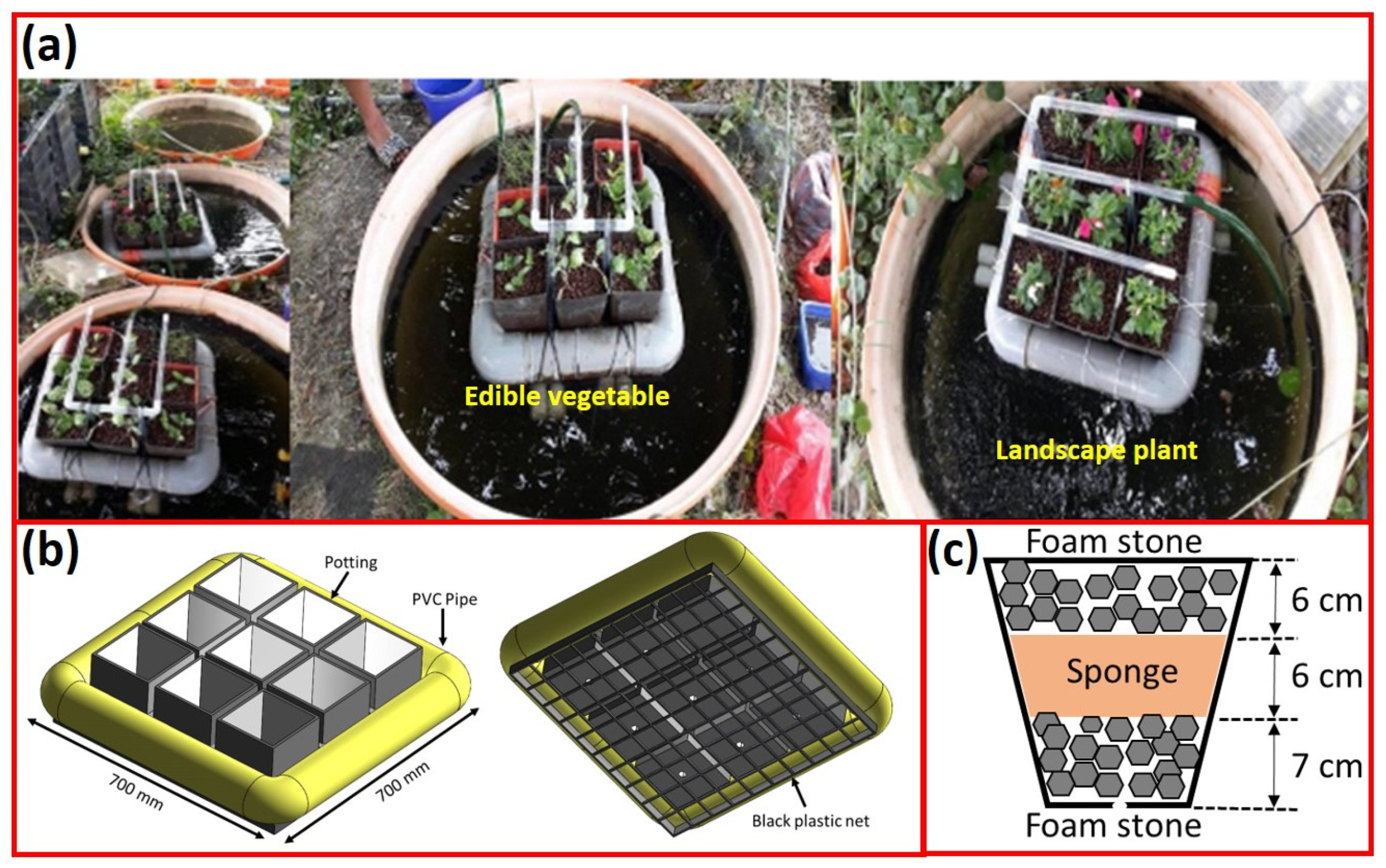
3.2.1. Water Temperature and Dissolved Oxygen
The water temperature of the control group, the vegetable group and the flower group varied with the seasons, but the three groups had coincidental trends. The vegetable group had the highest average water temperature, at 24.8 °C, while the flower group had the lowest average water temperature, at 24.5 °C. The average temperature of the water in the control group was 24.8 °C, as shown in
Figure 3a. The control group, which was free of floating islands, had water temperatures that were higher or lower than the water temperatures of the vegetable and flower groups, because the water was unshielded and directly influenced by the ambient environment.
The dissolved oxygen in the water was influenced by the temperature and the contents in the water. Bubble generators were placed in the control, flower, and vegetable groups to increase the oxygen content in the water and to allow the influential conditions to be coincidental. As shown in
Figure 3a, the three groups had similar trend variations for the dissolved oxygen in the water, and these trends were influenced by the temperature. The higher the ambient temperature, the higher the water temperature would be, and the lower the dissolved oxygen in the water. In contrast, the dissolved oxygen increased as the temperature fell, as shown in
Figure 3a.
The year-round average dissolved oxygen level in the vegetable and flower groups was 4~8 mg/L. The dissolved oxygen of the vegetable and control groups measured on one day could have a difference as large as 18 times. Among them, the DO of the flower group reached 6.69 mg/L on 25 August, which was 23% higher than that of the vegetable group, while the control group was only 0.05 mg/L. It can be seen that an Intelligence Aquaponics System has the function of aeration and can stabilize the DO in the water, which is helpful because it grows in aquatic organisms. In the mid-stage of the experiment, the dissolved oxygen level of the control group fluctuated due to eutrophication, and the excessive growth of algae induced the fluctuation of the dissolved oxygen level. The leaves of the vegetable and flower groups fell in spring and the flowers and fruit sank to the bottom, causing the dissolved oxygen level to decrease. It recovered to 2~6 mg/L after the aquaponics system was set up and became five or six times that of the control group. In the mid- and late-stages, the dissolved oxygen at the bottom of control group remained at 0~1 mg/L. Because the fish school died, the amount of organic matter and the number of aquatic microorganisms increased, causing the dissolved oxygen in the water to be consumed and to remain at a low value.
3.2.2. ORP & EC
Natural microbes can purify the various organics and inorganics in sewage by a redox reaction. The cleanliness of a water sample can be obtained from the ORP, and the oxidation-reduction power of a water body can be known.
The higher the ORP value of a water sample is, the higher the amount of dissolved oxygen in the water. A high level of dissolved oxygen in water can promote microbes and bacteria to decompose dead tissue and pollutants effectively. Generally, the higher the ORP value is, the stronger the capacity of decomposition in the water body, which means that the water body is healthier.
Figure 4a shows that the annual average ORP of the vegetable group in the water with an artificial floating island was 85.5 mV and the annual average ORP of the flower group was 100 mV. When the vegetables or flowers were planted on the floating island, the ORP remained positive and the dissolved oxygen in the water could be increased effectively. The redox reaction of the water was enhanced, and the domestic sewage from the campus lake, the fish school excrement, and the uneaten feed could be decomposed effectively.
On 27 October, the ORP of the control group reached the lowest point of −173.9 mv, while the values of the vegetable and flower groups were 77.4 mv and 76 mv, respectively, which is quite close, with only a 1.9% difference. It can be seen that an Intelligence Aquaponics System has a stable function for the ORP. Under the same test conditions, the planting of flowers on the artificial floating island was more effective in purifying the water than the planting of vegetables, so that the water quality is maintained within a certain range in order for organisms to live, and the ORP is relatively stable (
Figure 4a).
The water in the control group (without an artificial floating island) was taken from the domestic sewage in the campus lake. The contaminants in the water, namely the Carassius auratus, which all died in the first month, the uneaten feed and the fish school excrement were all deposited on the water bottom. Bacteria and microbes decomposed the organics in the deposits and consumed a large amount of oxygen, thus causing the dissolved oxygen in the water to decrease. There were no plants in the water, therefore the redox reaction could not be performed effectively to purify the water. The ORP was negative, and the annual average was −28.1 mV. The negative ORP indicated that the water in the control group was quite dirty, making it unsuitable for the growth of organisms and plants.
The redox corresponds to the EC, and the higher the EC, the lower the ORP. The EC has a considerable influence on plant irrigation. The EC is related to the total ionic concentration in water, as well as to the mobility, valence number, relative concentration and water temperature. Most salts can be ionized, therefore a higher salt content represents a lower fitness for irrigation. The EC is less than 750 mho/cm for the irrigation water quality in Taiwan. Among the final values of EC on October 27, the vegetable group performed best at 410 mho/cm, followed by the flower group at 346.2 mho/cm, and the control group performed the worst at only 328.1 mho/cm, and the difference between the vegetable and flower groups was about 16.6%, which is speculated to be caused by fish disturbing the suspended particles in the water (
Figure 4b).
The EC of the vegetable and flower groups was 300 to 470, which was suitable for plant irrigation. The EC of the control group was quite close to that of the flower group at the beginning; however, in the mid- and late-stages, as the control group did not have any equipment installed and there were no fish to disturb the water, so the suspended particles in the water settled to the bottom. The difference between the EC values at 5 cm and 85 cm below the surface was more than twice as much in May. The bottom salinity was relatively high and unstable. The upper and lower layers of the water in the vegetable and flower groups showed convection and there was no difference in the EC. It remained within a stable range and tended to decrease, which meant that the artificial floating island with plants in the water could absorb the nutritive salt in the water and maintain the water stability.
There is little difference in the EC between the upper and lower water layers of the two groups of vegetables and flowers, but the values of the control group are quite different. It can be seen that the Intelligence Aquaponics System has a certain effect. Taking EC on 12 May as an example, the upper and lower water layers of the vegetable group were 423 mho/cm and 422.2 mho/cm, which differed by only 0.2%, while the upper and lower water layers of the flower group were 356.6 mho/cm and 356.9 mho/cm, respectively, with a difference of only 0.1%, and the upper and lower water layers of the control group were 299.7 mho/cm and 518 mho/cm, which differed by as much as 42%. It can be seen that the EC of the upper and lower water layers of the control group is unstable and different. It is speculated that the climate turns hot in May, and the growth of algae in the water will affect the water quality, as shown in
Figure 5.
3.2.3. Total Phosphorus in the Water Body
The water used in the control, vegetable, and flower groups came from the campus lake of the MingDao University and consisted of wastewater from daily life. The most familiar daily non-fecal drainage is the water used for cleaning, which often contains cleansers, therefore the acid and soda concentrations and total phosphorus content in the water were measured. The total phosphorus content was measured because cleansers are usually mixed with phosphate compounds to enhance the cleaning effect.
The factors influencing the pH of the water included the NO2 exhaled from the fish, the leftover feed, excrement, plants in the water body, and the ambient fallen leaves and fruits in the water, which released tannic acid and changed the pH value of water; therefore, the fallen leaves and fruits in the water needed to be removed periodically.
The water bodies used in this study were taken from the water outlet of Li-tze Lake in November 2018. In the beginning, the pH value of the three water bodies was about PH 6.5, indicating that the water was neutral. The pH value changed steadily within a range of PH 6~8 during the research process. However, the pH value of the control group fluctuated largely in the mid-stage as the fish died and, as there was no fish disturbance in the late-stage, the pH of the upper layer became quite different from that of the bottom layer. The pH value of the water bodies of the vegetables and flowers changed steadily, in comparison to the water body of the control group without plants, as shown in
Figure 6a, as there was circulation inside the water bodies, and the pH differences between the upper and lower water layers were small.
The total phosphorus is measured by measuring the amount of orthophosphate, compound phosphate and organophosphorus in the water. The phosphor in sewage generally exists as orthophosphate and compound phosphate. If the total phosphorus concentration in a water sample is high, the water may be polluted by industrial wastewater, domestic sewage, cleansers or fertilizers. Phosphor is an important nutrient for plant growth; however, when excessive phosphor enters a water body, the algae will reproduce and die in large quantities. Their decomposition consumes a large amount of oxygen and causes the dissolved oxygen in water to be exhausted, thus forming eutrophication. However, phosphor can promote the differentiation of flower buds and budding, making the flowers colorful, vigorous and plump, and it can promote plant growth. The application of phosphor in the late stage of plant growth and development is the most effective.
The total phosphorus of the control group was high in February. The dissolved oxygen in the water rose suddenly and an algae bloom occurred, but in March, a lot of the algae died and the dissolved oxygen dropped suddenly, as shown in
Figure 6b. On 2 October, the TP of the flower group reached 6.8 mg/L, while that in the vegetable group also reached 2 mg/L, and the control group had almost no TP. It is speculated that the decayed leaves of the flower and vegetable groups increased the TP, after the dead algae decomposed, the amount of phosphor in the control group increased; however, in June, duckweed began growing in large quantities in the upper layer. The duckweed absorbed the phosphor and caused the total phosphorus to decrease to 0~0.3, as shown in
Figure 6b.
The total phosphorus content of the vegetable and flower groups increased continuously from November 2018 to March 2019, but there was no sudden rise, compared to the total phosphorus content of the control group. The vegetables and flowers grew obviously during this period. Phosphor is quite important for the primary growth of vegetable plant seedlings, and insufficient phosphor will influence a differentiation in the growth. The total phosphorus content in the water body of the vegetable group was lower than that in the flower group. The plants of the vegetable group were replanted after a typhoon occurred in June. It could be observed in
Figure 7 that the growth rate of the plants in the vegetable group that were replanted after the typhoon, was higher than that before the typhoon, and the total phosphorus content was lower than that in the water of the flower group. The planting of either flowers or vegetables in the water could effectively reduce the total phosphorus content in the water and reduce the mass breeding of algae.
3.2.4. Analysis of Nitrogenous Substances in the Water Body
Nitrogen is the most abundant element in the atmosphere and it is an essential element of all organisms. Nitrogen fertilizer is used in large quantities in the crop growing process, as it promotes plant growth. However, nitrogenous fertilizer flows into rivers and lakes as runoff and contaminates the water. The nitrate and nitrite in water are requisites for the growth of plankton in water. When the nitrogen content in water is high, the water becomes eutrophicated, inducing the mass mortality of fish.
The total nitrogen content in water is an important index for measuring water quality and contributes to the evaluation of the pollution level and cleanliness of a water body. The total nitrogen is the total amount of different forms of inorganic nitrogen and organic nitrogen. Inorganic nitrogen includes NH3-N, NO2-N, and NO3-N. In this part of the study, the water quality was analyzed according to the NH3-N, NO2-N, and NO3-N in the water body.
The nitrogen content in water is one of the primary indicators of eutrophication. It was found that the total nitrogen rose sharply due to the mass mortality of fish during the mid-stage, and that the total nitrogen index was less than the standard 12 mg/L of the daily emissions from public sewers. Only the total nitrogen of the control group increased to almost 12 mg/L in April, at which time the fish in the control group died. In comparison to the experimental group, the total amount of nitrogen in the two groups was lower than 10 mg/L; however, that of the flower group increased during this period, due to the petals and leaves falling into the water. Taking 2 April as an example, the TN of the flower group was 2 mg/L, that of the vegetable group was 0 mg/L, and that of the control group was 12.9 mg/L; the difference was 6.5 times. The death of fish and algae in the control group was the main cause of the increase in TN, as shown in
Figure 8a.
The NO
2-N level decreased to 1 mg/L in March and increased to 13 mg/L in July, after which it decreased greatly. The NO
3-N level decreased to 0.03 mg/L in July. The nitrogenous substances of the control group fluctuated greatly, possibly because the dissolved oxygen in the water of control group decreased greatly in March. Taking 2 July as an example, the NO
2 of the flower group was 3 mg/L, that of the vegetable group was 2 mg/L, that of the control group was 13 mg/L; the difference was 6.5 times, as shown in
Figure 3b. When water is deficient in oxygen, anaerobic ammoxidation occurs. Nitric acid is converted into nitrous acid by bacteria and microbes, and then into NO, which is combined with oxygen into NO
2 or N
2, and then it diffuses into the atmosphere. As shown in
Figure 8b,d the NO
3-N decreased and the NO
2-N increased. On the other hand, NH
3-N and nitrous acid exist simultaneously and turn into N
2 and water in an oxygen-deficient state. The equation is NH
4+ + NO
2− = N
2 + H
2O. The amount of dissolved oxygen in the water decreased greatly in July, as shown in
Figure 8b. The NH
3-N and nitrous acid levels also decreased greatly, as shown in
Figure 8c,d. The water of the control group only contained fish and the amount of nitrogenous substances in the water fluctuated greatly; therefore, the water was susceptible to anaerobic ammoxidation. Another reason for the reduction of NH
3-N is that duckweed often grew in large quantities on the water’s surface, which helped to absorb the NH
3-N waste and reduce the NH
3-N content.
The water bodies of the flower group and the vegetable group were steadier than the water body of the control group. The oxygen content in the water was sufficient, the nitrogen substances could perform nitrification to convert NH3-N into nitrous acid and could then nitrify it into nitric acid, through an aerobic reaction, which eventually became N2 and was released to the atmosphere. The nitrogen substances absorbed by the plants were mostly NH3-N and NO3-N, therefore the plants in the water body contributed to the stabilization of the NH3-N and NO3-N, compared to the water body of the control group.
The NO
2-N in the water body of the control group suddenly increased to 13 mg/L in July, causing the fish to die in quick succession. The NO
2-N of the vegetable and flower groups fluctuated; however, the concentration of NO
2-N for killing fish is 10~20 mg/L, therefore when the amount of dissolved oxygen in the water was sufficient, the nitrite could be converted quickly and the harm to the fish schools could be reduced, as shown in
Figure 8b. The nitrite content in the water with an aquaponics system was less than 10 mg/L in the late stage, therefore the conditions for growth were good.
NH
3-N comes mainly from the decomposition of animal excrement, residual feed and dead animals and plants. Amino acid is formed during the first stage of decomposition, and then NH
3-N, NO
2-N, and NO
3-N are gradually formed. The standard for the content of NH
3-N in the discharge water is lower than 10 mg/L in Taiwan. The NH
3-N content of the vegetable and flower groups was lower than 0.5 mg/L as a result of this system. The control group did not have an aquaponics system and the eutrophication was severe. The eutrophication increased suddenly to 2.75 mg/L during March to April, indicating there was moderate pollution, due to decomposing organisms in that period, which led to fish being killed in June. As the dead fish decomposed, the NH
3-N level increased again. The NH
3-N of the water body in the control group rose in March, and then decreased to 0 mg/L after September. Taking 2 April as an example, the NH
3-N of the flower group was 0.04 mg/L, that of the vegetable group was 0.01 mg/L, that of the control group was 2.75 mg/L; the difference was 68.7 times. In the control group, the death of fish and algae is the main cause of the increase in NH
3-N (
Figure 8c).
NO
3-N is the final product of nitrification in the nitrogen cycle, so nitrate nitrogen can indicate the degree of pollution in the water body. When the nitrate nitrogen content in rivers, lakes, or reservoirs is too high, it causes algae to multiply easily, which causes eutrophication in the water body. According to Taiwan’s tap water standard, it should be lower than 10 mg/L, and the discharge water standard should be lower than 50 mg/L. Taking 2 July as an example, the NH
3-N of the flower group was 1.7 mg/L, that of the vegetable group was 1.1 mg/L, and that of the control group was 0.03 mg/L, which were all under the tap water standard of less than 10 mg/L (
Figure 8d).