New Classification Method to Evaluate Pollution Levels of Sewage Contaminated Lakes
Abstract
1. Introduction
2. Experimental Program
2.1. Description of Lakes
2.2. Field and Laboratory Tests
3. Discussion of Results
3.1. Water Quality of Bengaluru Lakes in Relation to Most Common Natural Concentrations (MCNC) of World Rivers
3.2. Comparison of Quality of Bengaluru Lakes with Other Indian Lakes
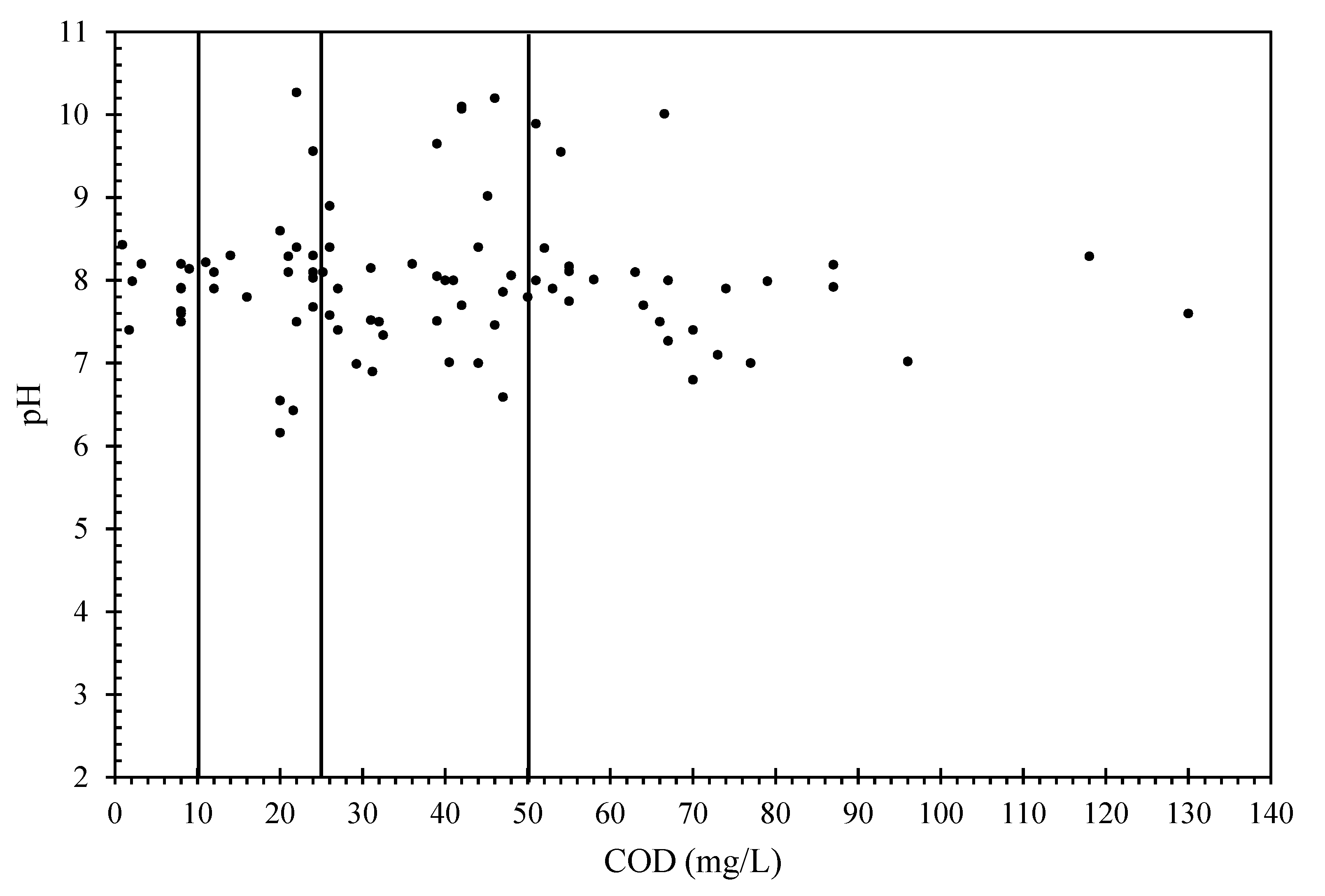
3.3. Proposed Method to Evaluate Pollution Level of Sewage Contaminated Lakes
3.3.1. Data from Present Study
3.3.2. Data from Other Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jumbe, A.S.; Nandini, K.; Tandon, S.; Sunitha, N. Bangalore lakes—Issues and perspective on pollution, restoration and management. In Proceedings of the Taal 2007: 12th World Lake Conference, Rajasthan, India, 28 October–2 November 2007; Sengupta, M., Dalwani, R., Eds.; ILEC: Jaipur, India, 2008; pp. 1699–1706. [Google Scholar]
- Shivakumar, K.V. Water quality monitoring of lakes in Bangalore. In Proceedings of the Taal 2007: 12th World Lake Conference, Rajasthan, India, 28 October–2 November 2007; Sengupta, M., Dalwani, R., Eds.; ILEC: Jaipur, India, 2008; pp. 1908–1915. [Google Scholar]
- Government of Karnataka, Department of Urban Development. Expert‘s Committee Report on Rejuvenation of Bellandur Lakes. Government of Karnataka. 2016. Available online: https://opencity.in/documents/expert-committee-report-on-rejuvenation-of-bellandur-lake-november-2016 (accessed on 14 February 2021).
- Ramachandra, T.V.; Sincy, V.; Asulabha, K.S.; Sudarshan, P.; Bhat, P.S.; Rahaman, M.F. Recurring Fish. Mortality Episodes in Bangalore Lakes: Sign of Irresponsible and Fragmented Governance; Envis Technical Report 105; Energy and Wetlands Research Group, Centre of Ecological Sciences, Indian Institute of Science: Bangalore, India, 2016; Available online: http://wgbis.ces.iisc.ernet.in/energy/water/paper/ETR105/index.html (accessed on 14 February 2021).
- Farnham, D.J.; Gibson, R.A.; Hsueh, D.Y.; McGillis, W.R.; Culligan, P.J.; Zain, N.; Buchanan, R. Citizen science-based water quality monitoring: Constructing a large database to characterize the impacts of combined sewer overflow in New York City. Sci. Total Environ. 2017, 580, 168–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Hu, M.; Luo, J.; Li, J.; Liang, Q. Combining citizen science and land use data to identify drivers of eutrophication in the Huangpu River system. Sci. Total Environ. 2017, 584–585, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Vane, C.H.; Kim, A.W.; McGowan, S.; Leng, M.J.; Heaton, T.H.E.; Kendrick, C.P.; Coombs, P.; Yang, H.; Swann, G.E.A. Sedimentary records of sewage pollution using faecal markers in contrasting peri-urban shallow lakes. Sci. Total Environ. 2010, 409, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Sheela, A.M.; Letha, J.; Joseph, S. Environmental status of a tropical lake system. Environ. Monit. Assess. 2011, 180, 427–449. [Google Scholar] [CrossRef]
- Bunzel, K.; Kattwinkel, M.; Liess, M. Effects of organic pollutants from wastewater treatment plants on aquatic invertebrate communities. Water Res. 2013, 47, 597–606. [Google Scholar] [CrossRef]
- Taylor, J.M.; King, R.S.; Pease, A.A.; Winemiller, K.O. Nonlinear response of stream ecosystem structure to low-level phosphorus enrichment. Freshw. Biol. 2014, 59, 969–984. [Google Scholar] [CrossRef]
- Basílico, G.; Magdaleno, A.; Paz, M.; Moretton, J.; Faggi, A.; de Cabo, L. Sewage pollution: Genotoxicity assessment and phytoremediation of nutrients excess with Hydrocotyle ranunculoides. Environ. Monit. Assess. 2017, 189, 182. [Google Scholar] [CrossRef]
- Rao, S.M.; Anthony, P.; Mogili, N.V. Biochemical indicators of algal bloom in sewage-contaminated lakes. J. Hazard. Toxic Radioact. Waste 2019, 23, 04019019. [Google Scholar] [CrossRef]
- Zang, C.; Huang, S.; Wu, M.; Du, S.; Scholz, M.; Gao, F.; Lin, C.; Guo, Y.; Dong, Y. Comparison of relationships between pH, dissolved oxygen and chlorophyll a for aquaculture and non-aquaculture waters. Water Air Soil Pollut. 2011, 219, 157–174. [Google Scholar] [CrossRef]
- Chapman, D.V. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-04-1921-600-1. [Google Scholar]
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Adamovich, B.V.; Zhukova, T.V.; Mikheeva, T.M.; Kovalevskaya, R.Z.; Luk’yanova, E.V. Long-term variations of the trophic state index in the Narochanskie Lakes and its relation with the major hydroecological parameters. Water Resour. 2016, 43, 809–817. [Google Scholar] [CrossRef]
- Kumar, P.; Mahajan, A.K.; Meena, N.K. Evaluation of trophic status and its limiting factors in the Renuka lake of lesser Himalaya, India. Environ. Monit. Assess. 2019, 191, 105. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.E.; Simpson, J. A Coordinator’s Guide to Volunteer Lake Monitoring Methods; NALMS Report; North American Lake Management Society: Madison, WI, USA, 1996; Available online: https://www.nalms.org/product/a-coordinators-guide-to-volunteer-monitoring/ (accessed on 14 February 2021).
- Central Pollution Control Board. Guidelines for Water Quality Monitoring; MINARS/27/2007-08; Central Pollution Control Board: Delhi, India, 2008; Available online: https://cpcb.nic.in/openpdffile.php?id=UmVwb3J0RmlsZXMvTmV3SXRlbV85N19ndWlkZWxpbmVzb2Z3YXRlcnF1YWxpdHltYW5hZ2VtZW50LnBkZg (accessed on 14 February 2021).
- Department of Environment Malaysia. National Water Quality Standards for Malaysia. Available online: https://www.doe.gov.my/portalv1/wp-content/uploads/2019/05/Standard-Kualiti-Air-Kebangsaan.pdf (accessed on 14 February 2021).
- Peletz, R.; Kisiangani, J.; Bonham, M.; Ronoh, P.; Delaire, C.; Kumpel, E.; Marks, S.; Khush, R. Why do water quality monitoring programs succeed or fail? A qualitative comparative analysis of regulated testing systems in sub-Saharan Africa. Int. J. Hyg. Environ. Health 2018, 221, 907–920. [Google Scholar] [CrossRef]
- Loiselle, S.A.; Frost, P.C.; Turak, E.; Thornhill, I. Citizen scientists supporting environmental research priorities. Sci. Total Environ. 2017, 598, 937. [Google Scholar] [CrossRef]
- Quinlivan, L.; Chapman, D.V.; Sullivan, T. Validating citizen science monitoring of ambient water quality for the United Nations sustainable development goals. Sci. Total Environ. 2020, 699, 134255. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Belmont, P.; Alvarado, J.; Vázquez-Salvador, N.; Rodríguez, E.; Valiente, E.; Díaz, J. Water quality monitoring in the Xochimilco peri-urban wetland: Experiences engaging in citizen science. Freshw. Sci. 2019, 38, 342–351. [Google Scholar] [CrossRef]
- Lottig, N.R.; Wagner, T.; Norton Henry, E.; Spence Cheruvelil, K.; Webster, K.E.; Downing, J.A.; Stow, C.A. Long-term citizen-collected data reveal geographical patterns and temporal trends in lake water clarity. PLoS ONE 2014, 9, e95769. [Google Scholar] [CrossRef]
- Lévesque, D.; Cattaneo, A.; Deschamps, G.; Hudon, C. In the eye of the beholder: Assessing the water quality of shoreline parks around the Island of Montreal through citizen science. Sci. Total Environ. 2017, 579, 978–988. [Google Scholar] [CrossRef]
- Miguel-Chinchilla, L.; Heasley, E.; Loiselle, S.; Thornhill, I. Local and landscape influences on turbidity in urban streams: A global approach using citizen scientists. Freshw. Sci. 2019, 38, 303–320. [Google Scholar] [CrossRef]
- Yardi, K.D.; Bharucha, E.; Girade, S. Post-restoration monitoring of water quality and avifaunal diversity of Pashan Lake, Pune, India using a citizen science approach. Freshw. Sci. 2019, 38, 332–341. [Google Scholar] [CrossRef]
- Buytaert, W.; Zulkafli, Z.; Grainger, S.; Acosta, L.; Alemie, T.C.; Bastiaensen, J.; De Bièvre, B.; Bhusal, J.; Clark, J.; Dewulf, A.; et al. Citizen science in hydrology and water resources: Opportunities for knowledge generation, ecosystem service management, and sustainable development. Front. Earth Sci. 2014, 2, 26. [Google Scholar] [CrossRef]
- Perelló, J.; Klimczuk, A.; Land-Zandstra, A.; Vohland, K.; Wagenknecht, K.; Narraway, C.; Lemmens, R.; Ponti, M. The Recent Past and Possible Futures of Citizen Science: Final Remarks. In The Science of Citizen Science; Springer International Publishing: Cham, Switzerland, 2021; pp. 517–529. [Google Scholar]
- Meybeck, M.; Helmer, R. The quality of rivers: From pristine stage to global pollution. Glob. Planet Chang. 1989, 1, 283–309. [Google Scholar] [CrossRef]
- World Population Review: Bangalore Population. Available online: https://worldpopulationreview.com/world-cities/bangalore-population (accessed on 14 February 2021).
- Rao, S.; Mogili, N.V.; Priscilla, A.; Lydia, A. Aqueous chemistry of anthropogenically contaminated Bengaluru lakes. Sustain. Environ. Res. 2020, 30, 8. [Google Scholar] [CrossRef]
- Huang, W.; Mao, J.; Zhu, D.; Lin, C. Impacts of land use and land cover on water quality at multiple buffer-zone scales in a lakeside city. Water 2019, 12, 47. [Google Scholar] [CrossRef]
- Raj, K.G.; Trivedi, S.; Ramesh, K.S.; Sudha, R.; Subramoniam, S.R.; Ravishankar, H.M.; Vidya, A. Assessment of vegetation cover of Bengaluru city, India, using geospatial techniques. J. Indian Soc. Remote Sens. 2020. [Google Scholar] [CrossRef]
- Fu, B.; Burgher, I. Riparian vegetation NDVI dynamics and its relationship with climate, surface water and groundwater. J. Arid Environ. 2015, 113, 59–68. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- Schouw, N.L.; Danteravanich, S.; Mosbaek, H.; Tjell, J.C. Composition of human excreta—A case study from Southern Thailand. Sci. Total Environ. 2002, 286, 155–166. [Google Scholar] [CrossRef]
- Boutin, C.; Eme, C. Domestic wastewater characterization by emission source. In Proceedings of the 13th IWA Specialized Conference on Small Water and Wastewater Systems, Athens, Greece, 14–16 September 2016; pp. 1–8. [Google Scholar]
- Kuradagi, A.; Gadag, R.B. Water quality analysis of Bhishma lake at Gadag city. Int. J. Res. Eng. Technol. 2016, 5, 320–327. [Google Scholar] [CrossRef]
- Reddy, M.V.; Babu, K.S.; Balaram, V.; Satyanarayanan, M. Assessment of the effects of municipal sewage, immersed idols and boating on the heavy metal and other elemental pollution of surface water of the eutrophic Hussainsagar Lake (Hyderabad, India). Environ. Monit. Assess. 2012, 184, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; US Geological Survey: Alexandria, VA, USA, 1985; ISBN 978-14-1022-308-1. [Google Scholar]
- Kresic, N. Hydrogeology and Groundwater Modeling, 2nd ed.; CRC Press: New York, NY, USA, 2007; ISBN 978-08-4933-348-4. [Google Scholar]
- Kumar, S.; Ghosh, N.C.; Singh, R.P.; Sonkusare, M.M.; Singh, S.; Mittal, S. Assessment of water quality of lakes for drinking and irrigation purposes in Raipur city, Chhattisgarh, India. Int. J. Eng. Res. Appl. 2015, 5, 42–49. [Google Scholar]
- Rajamanickam, R.; Nagan, S. A study on water quality status of major lakes in Tamil Nadu. Int. J. Res. Environ. Sci. 2016, 2, 9–21. [Google Scholar] [CrossRef]
- Yamuna, S.M.; Balasubramanian, A. Water quality variations in the lakes of Mysore district, Karnataka. In Proceedings of the International Symposium on Restoration of Lakes and Wetlands, Bengaluru, India, 27–29 November 2000; Ramachandra, T.V., Murthy, C.R., Ahalya, N., Eds.; Centre of Ecological Sciences, Indian Institute of Science: Bangalore, India, 2000. [Google Scholar]
- Kangabam, R.D.; Bhoominathan, S.D.; Kanagaraj, S.; Govindaraju, M. Development of a water quality index (WQI) for the Loktak Lake in India. Appl. Water Sci. 2017, 7, 2907–2918. [Google Scholar] [CrossRef]
- Singh, O.; Rai, S.P.; Kumar, V.; Sharma, M.K.; Choubey, V.K. Water quality and eutrophication status of some lakes in the Western Himalayan region (India). In Proceedings of the Taal 2007: 12th World Lake Conference, Rajasthan, India, 28 October–2 November 2007; Sengupta, M., Dalwani, R., Eds.; ILEC: Jaipur, India; pp. 286–291. [Google Scholar]
- Sonal, T.; Kataria, H.C. Physico-Chemical Studies of Water Quality of Shahpura Lake, Bhopal (M.P) with Special Reference to Pollution Effects on Ground Water of its Fringe Areas. Curr. World Environ. 2012, 7, 139–144. [Google Scholar] [CrossRef][Green Version]




| Sample | EC (μS/cm) | pH | CO32− (mg/L) | HCO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | NO3−, mg/L | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | DO, mg/L | COD, mg/L | N+P (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PU1 | 533.0 | 6.2 | 0 | 236.7 | 66.0 | 236.0 | 4.0 | 57.0 | 29.0 | 29.0 | 13.0 | 4.8 | 20.0 | 4.2 |
| PU2 | 813.0 | 6.5 | 0 | 344.0 | 97.0 | 319.0 | 9.0 | 90.0 | 40.0 | 92.0 | 16.0 | 4.2 | 20.0 | 3.3 |
| PU3a * | 603.0 | 9.9 | 17.0 | 104.0 | 81.7 | 7.0 | 0.22 | 41.2 | 16.8 | 76.5 | 15.3 | 13.0 | 51.0 | 1.0 |
| PU4a | 611.0 | 9.6 | 19.0 | 101.0 | 81.3 | 7.5 | 0.02 | 26.0 | 12.8 | 68.2 | 12.9 | 15.0 | 39.0 | 0.8 |
| PU5a | 616.0 | 10.2 | 21.0 | 92.0 | 84.5 | 7.5 | 0.02 | 24.7 | 13.9 | 73.9 | 14.4 | 13.2 | 46.0 | 0.9 |
| PU6a | 760.0 | 9.5 | 3.1 | 212.0 | 82.4 | 31.0 | 0.43 | 25.0 | 13.5 | 70.5 | 14.0 | 12.5 | 54.0 | 1.2 |
| PU7a | 622.0 | 9.0 | 18.0 | 109.0 | 101.8 | 18.0 | 0.43 | 17.0 | 13.5 | 68.1 | 13.3 | 12.5 | 45.1 | 1.0 |
| PU8a | 573.0 | 10.0 | 22.0 | 91.0 | 82.9 | 7.0 | 0.46 | 18.5 | 12.8 | 66.7 | 12.1 | 14.0 | 66.5 | 1.4 |
| PU9a | 545.0 | 10.1 | 24.0 | 80.0 | 86.2 | 7.0 | 0.02 | 24.5 | 12.9 | 66.6 | 13.1 | 14.0 | 42.0 | 0.8 |
| PU10a | 610.0 | 10.1 | 19.0 | 88.0 | 83.1 | 7.0 | 0 | 24.5 | 12.9 | 66.6 | 13.1 | 14.0 | 42.0 | 0.8 |
| UL1a | 269.0 | 8.9 | 2.0 | 114.7 | 36.0 | 116.0 | 4.0 | 18.0 | 14.0 | 28.0 | 10.0 | 5.9 | 26.0 | 1.7 |
| UL2a | 266.0 | 9.6 | 2.0 | 114.7 | 36.0 | 100.4 | 5.0 | 19.0 | 15.0 | 28.0 | 10.0 | 6.2 | 24.0 | 1.9 |
| UL3a | 446.0 | 7.0 | 0 | 169.0 | 24.0 | 61.0 | 27.0 | 36.0 | 7.7 | 39.3 | 7.2 | 0.4 | 96.0 | 14.7 |
| UL4a | 331.0 | 8.1 | 0 | 97.0 | 43.0 | 40.0 | 3.5 | 18.7 | 6.9 | 32.1 | 10.2 | 13.0 | 48.0 | 1.8 |
| UL5a | 331.0 | 8.4 | 3.0 | 95.0 | 43.0 | 23.0 | 3.8 | 17.4 | 6.9 | 33.2 | 10.4 | 13.0 | 52.0 | 1.9 |
| UL6a | 333.0 | 8.0 | 0 | 104.0 | 40.0 | 42.0 | 2.6 | 20.3 | 7.9 | 33.5 | 13.4 | 8.0 | 58.0 | 1.8 |
| NG1a | 1124.0 | 8.6 | 2.0 | 158.6 | 62.0 | 208.0 | 6.0 | 26.0 | 30.0 | 47.0 | 12.0 | 10.1 | 20.0 | 2.1 |
| NG2a | 1117.0 | 10.3 | 3.0 | 124.4 | 62.0 | 188.0 | 7.8 | 24.0 | 12.0 | 57.0 | 13.0 | 12.2 | 22.0 | 2.2 |
| SK1 | 1100.0 | 7.8 | 0 | 219.6 | 50.0 | 51.0 | 7.0 | 44.0 | 30.0 | 20.0 | 8.0 | 5.8 | 16.0 | 2.0 |
| SK2 | 1091.0 | 7.9 | 0 | 222.0 | 50.0 | 63.0 | 6.0 | 40.0 | 30.0 | 39.0 | 8.0 | 5.9 | 12.0 | 1.7 |
| Parameter | Mixed Wastewater | |||
|---|---|---|---|---|
| Minimum | Maximum | Median | Standard Deviation | |
| pH | 7.1 | 7.9 | 7.2 | 0.42 |
| HCO3− (mg/L) | 108.6 | 551.0 | 305.0 | 221.6 |
| Cl− (mg/L) | 95.4 | 101.6 | 99.8 | 3.2 |
| SO42− (mg/L) | 6.3 | 20.2 | 10.0 | 7.2 |
| NO3− (mg/L) | 0.50 | 2.5 | 0.70 | 1.1 |
| Ca2+ (mg/L) | 46.0 | 78.3 | 52.6 | 17.1 |
| Mg2+ (mg/L) | 19.7 | 24.1 | 19.8 | 2.5 |
| Na+ (mg/L) | 73.4 | 95.4 | 86.6 | 10.7 |
| K+ (mg/L) | 16.7 | 20.2 | 18.9 | 1.8 |
| NH4+ (mg/L) | 15.7 | 50.3 | 28.5 | 17.5 |
| TDS (mg/L) | 590.9 | 811.0 | 682.5 | 110.6 |
| COD (mg/L) | 54.4 | 99.2 | 83.0 | 22.7 |
| DO (mg/L) | 0.10 | 0.48 | 0.42 | 0.20 |
| Parameter (1) | Lake Samples | Median Composition of World Rivers [31] (6) | ||||
|---|---|---|---|---|---|---|
| Minimum (2) | Median (3) | Maximum (4) | Standard Deviation (5) | |||
| pH | 6.2 | 9.0 | 10.3 | 1.23 | ------ | |
| HCO3− (mg/L) | 80.0 | 109.0 | 344.0 | 71.1 | 31.0 | 3.5 |
| Cl− (mg/L) | 36.0 | 66.0 | 101.8 | 21.6 | 3.9 | 16.9 |
| SO42− (mg/L) | 7.0 | 40.0 | 319.0 | 93.3 | 9.2 | 4.3 |
| NO3− (mg/L) | 0.0 | 3.5 | 9.0 | 3.0 | 0.44 | 8.0 |
| Ca2+ (mg/L) | 17.0 | 24.5 | 90.0 | 18.1 | 8.0 | 3.1 |
| Mg2+ (mg/L) | 6.9 | 13.5 | 40.0 | 9.4 | 2.4 | 5.6 |
| Na+ (mg/L) | 20.0 | 57.0 | 92.0 | 21.3 | 3.7 | 15.4 |
| K+ (mg/L) | 8.0 | 13.0 | 16.0 | 2.3 | 1.06 | 12.3 |
| NH4+ (mg/L) | 0.0 | 0.0 | 3.7 | 0.85 | 0.02 | |
| TDS (mg/L) | 172.7 | 396.1 | 729.9 | 188.2 | ------ | |
| COD (mg/L) | 12.0 | 42.0 | 66.6 | 16.3 | ------ | |
| DO (mg/L) | 4.2 | 12.5 | 18.8 | 3.8 | ------ | |
| Lake | No. of Samples | Cl/Na | HCO3/Na | SO4/Na | NO3/Na | COD (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | ||
| Present study | 19 | 0.68 | 1.62 | 0.81 | 0.45 | 4.14 | 1.13 | 0.02 | 2.03 | 0.31 | 0.00 | 0.13 | 0.04 | 12.0 | 66.6 | 42.0 |
| Raipur [44] | 27 | 0.50 | 1.09 | 0.76 | 0.42 | 1.79 | 0.80 | 0.00 | 0.28 | 0.05 | 0.00 | 0.09 | 0.01 | 8.0 | 118.0 | 39.0 |
| Ooty [45] | 4 | 0.83 | 1.29 | 1.18 | 1.29 | 1.83 | 1.55 | 0.04 | 0.12 | 0.08 | 0.00 | 0.15 | 0.01 | 46.0 | 70.4 | 60.8 |
| Yercaud [45] | 4 | 0.87 | 0.95 | 0.92 | 1.12 | 1.89 | 1.21 | 0.03 | 0.08 | 0.05 | 0.00 | 0.01 | 0.01 | 26.0 | 63.4 | 39.0 |
| Kodai [45] | 4 | 0.82 | 1.78 | 1.20 | 0.73 | 1.64 | 1.26 | 0.12 | 0.30 | 0.19 | 0.01 | 0.05 | 0.01 | 37.0 | 55.4 | 51.3 |
| Mysore district [46] | 22 | 0.21 | 23.6 | 0.70 | 0.42 | 7.54 | 0.90 | 0.00 | 0.24 | 0.04 | 0.01 | 0.19 | 0.01 | 12.0 | 248.0 | 28.0 |
| Class | Lake Pollution Status | K+ (mg/L) | COD (mg/L) | EC (μS/cm) | pH | DO (mg/L) | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|
| 1 | Non-polluted | 0–4 | 0–10 | 200–800 | 7.0–8.5 | 7.5 | ≤20 |
| 2 | Moderate pollution | 4–25 | 10–25 | 200–1200 | 6.0–11.0 | 5.5–7.5 | 1–120 |
| 3 | Severe pollution | 4–25 | 25–50 | 200–2000 | 6.0–11.0 | 4.0–5.5 | 1–120 |
| 4 | Mixed wastewater | 10–25 | >50 | 200–2000 | 6.0–11.0 | 0.9–4.0 | 5–200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.M.; Mogili, N.V. New Classification Method to Evaluate Pollution Levels of Sewage Contaminated Lakes. Sustainability 2021, 13, 3677. https://doi.org/10.3390/su13073677
Rao SM, Mogili NV. New Classification Method to Evaluate Pollution Levels of Sewage Contaminated Lakes. Sustainability. 2021; 13(7):3677. https://doi.org/10.3390/su13073677
Chicago/Turabian StyleRao, Sudhakar M., and Nitish Venkateswarlu Mogili. 2021. "New Classification Method to Evaluate Pollution Levels of Sewage Contaminated Lakes" Sustainability 13, no. 7: 3677. https://doi.org/10.3390/su13073677
APA StyleRao, S. M., & Mogili, N. V. (2021). New Classification Method to Evaluate Pollution Levels of Sewage Contaminated Lakes. Sustainability, 13(7), 3677. https://doi.org/10.3390/su13073677






