Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries
Abstract
1. Introduction
2. Microalgae as a Renewable Industrial Feedstock
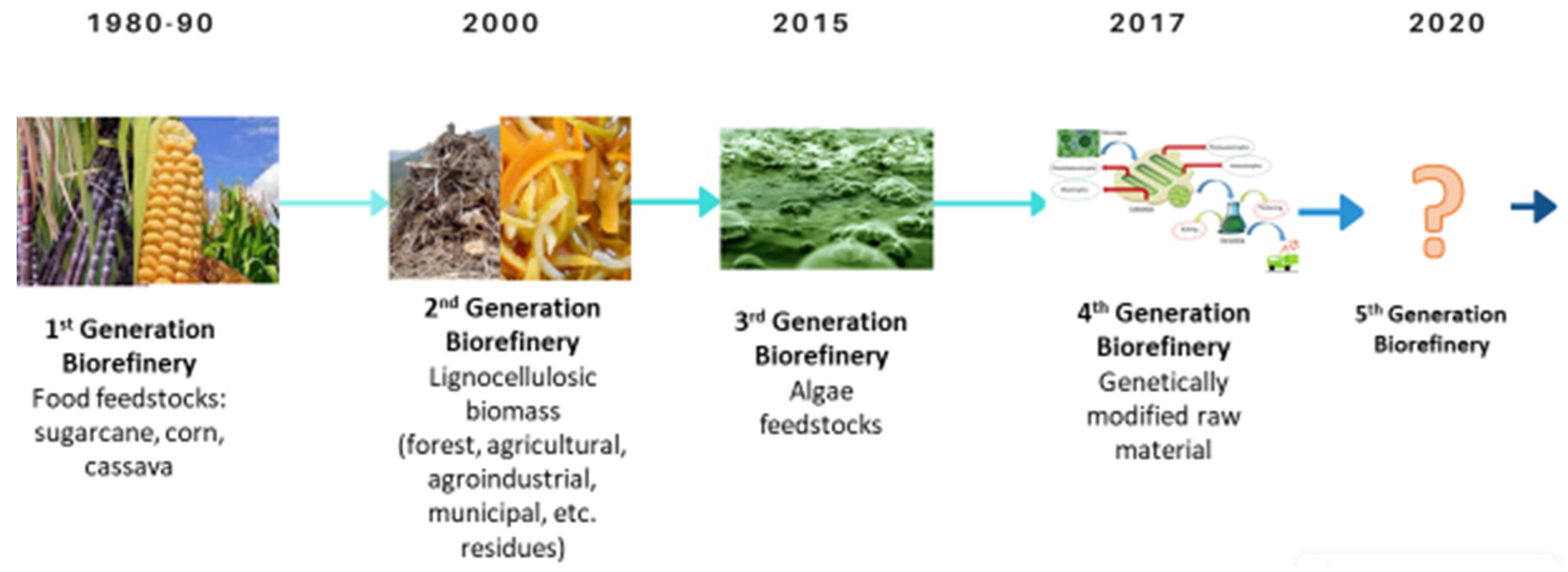
2.1. Biorefinery as the Basis of the Bioeconomy
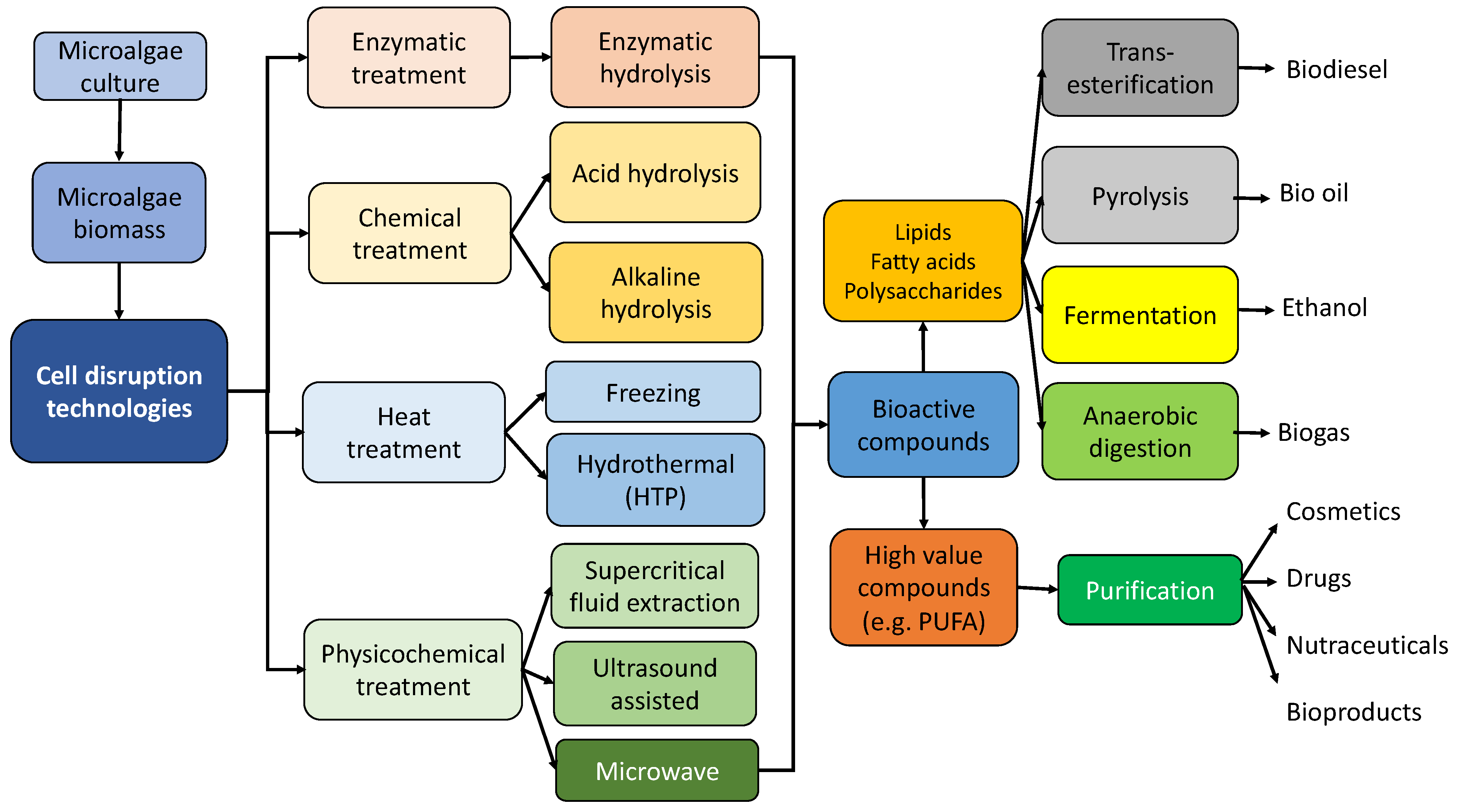
2.2. Microalgae Processing Technologies
3. Cellular Components of Interest and Their Role in Microalgal Metabolism
3.1. Proteins
3.2. Enzymes
3.3. Sugars and Polysaccharides
3.4. Fatty Acids and Lipids
3.5. Carotenoids and Pigments
3.6. Polyphenols
3.7. Vitamins
4. Health Properties of Microalgal Metabolites
4.1. Antioxidant Activity
4.2. Antitumor Activity
4.3. Antimicrobial Activity
4.4. Antifungal Activity
4.5. Anti-Inflammatory Activity
4.6. Antiviral Activity
4.7. UV Protection
5. Industrial Microalgae Applications
5.1. Pharmaceutical Industry
5.2. Nutraceutical, Cosmetics, and Personal Care Industries
5.3. Food Industry
5.4. Animal Feed and Aquaculture
5.5. Agricultural Fertilizers and Soil Amendments
5.6. Chemical Products
6. Environmental Algae Applications
6.1. Carbon Sequestration
6.2. Bioremediation
6.3. Wastewater Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Günerken, E.; Hondt, E.D.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Faiza, B.; Yusuf, C. Microalgae Cultivation Fundamentals. In Algae Biotechnology Products and Processes, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 1–19. ISBN 978-3-319-12333-2. [Google Scholar]
- Hu, I. Production of potential coproducts from microalgae. In Biomass, Biofuels and Biochemicals, 2nd ed.; Elsevier: Chennai, India, 2019; pp. 345–358. ISBN 9780444641922. [Google Scholar]
- Chandra, R.; Iqbal, M.N.; Vishal, G.; Lee, H.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2018, 278, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Naresh Kumar, A.; Chatterjee, S.; Hemalatha, M.; Avanthi, A.; Min, B.; Kim, S.-H.; Mohan, S.V. Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour. Technol. 2019, 296, 1–7. [Google Scholar] [CrossRef]
- Brasil, B.; Silva, F.; Siqueira, F. Microalgae biorefineries: The Brazilian scenario in perspective. New Biotechnol. 2017, 39, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Iasimone, F.; Seira, J.; Desmond-Le Quemener, E.; Panico, A.; De Felice, V.; Pirozzi, F.; Steyer, J.-P. Bioflocculation and settling studies of native wastewater filamentous cyanobacteria using different cultivation systems for a low-cost and easy to control harvesting process. J. Environ. Manag. 2019, 256, 109957. [Google Scholar] [CrossRef]
- Shokravi, Z.; Shokravi, H.; Chyuan, O.H.; Lau, W.J.; Koloor, S.S.R.; Petru, M.; Ismail, A.F. Improving Lipid Productivity in Microalgae by Bilateral Enhancement of Biomass and Lipid Contents: A Review. Sustainability 2020, 12, 9083. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Moraes, L.; Zaparoli, M.; Morais, M.G. Progress in the physicochemical treatment of microalgae biomass for value-added product recovery. Bioresour. Technol. 2020, 301, 122727. [Google Scholar] [CrossRef]
- McCormick, K.; Kautto, N. The Bioeconomy in Europe: An Overview. Sustainability 2013, 5, 2589–2608. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- De Buck, V.; Polanska, M.; Van Impe, J. Modeling Biowaste Biorefineries: A Review. Front. Sustain. Food Syst. 2020, 4, 1–19. [Google Scholar] [CrossRef]
- Rajak, R.C.; Jacob, S.; Kim, B.S. A holistic zero waste biorefinery approach for macroalgal biomass utilization: A review. Sci. Total Environ. 2020, 716, 137067. [Google Scholar] [CrossRef]
- Alam, M.; Xu, J.; Wang, Z. Microalgae as a Mainstream Food Ingredient: Demand and Supply Perspective. In Microalgae Biotechnology for Food, 1st ed.; Springer: Singapore, 2020; pp. 29–79. ISBN 9789811501692. [Google Scholar]
- Hughes, S.; Gibbons, W.; Bryan, M.; Rich, J. Sustainable Multipurpose Biorefineries for Third-Generation Biofuels and Value-Added Co-Products. In Biofuels—Economy Environment Sustainability, 1st ed.; InTech: Rijeka, Croatia, 2013; pp. 245–267. ISBN 978-953-51-0950-1. [Google Scholar]
- Adekunle, A.; Orsat, V.; Raghava, V. Lignocellulosic bioethanol: A review and design conceptualization study of production from cassava peels. Renew. Sustain. Energy Rev. 2016, 64, 518–530. [Google Scholar] [CrossRef]
- Antizar-ladislao, B.; Kingdom, U. Second-generation biofuels and local bioenergy systems. Biofuels Bioprod. Biorefin. 2008, 2, 455–469. [Google Scholar] [CrossRef]
- Abila, N. Managing municipal wastes for energy generation in Nigeria. Renew. Sustain. Energy Rev. 2014, 37, 182–190. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Su, Y.; Song, K.; Zhang, P.Y.; Cheng, J.; Chen, X. Progress of microalgae biofuel’s commercialization. Renew. Sustain. Energy Rev. 2017, 74, 402–411. [Google Scholar] [CrossRef]
- Antunes, J.; Leão, P.; Vasconcelos, V. Marine biofilms: Diversity of communities and of chemical cues. Environ. Microbiol. Rep. 2019, 11, 287–305. [Google Scholar] [CrossRef]
- Schiano, G.; Spicer, A.; Chuck, C.J.; Allen, M.J. The Microalgae Biorefinery: A Perspective on the Current Status and Future Opportunities Using Genetic Modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, L.J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvent. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes f or microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Vingiani, G.M.; De Luca, P.; Ianora, A.; Dobson, D.W.; Lauritano, C. Microalgal enzymes with biotechnological applications. Mar. Drugs 2019, 17, 459. [Google Scholar] [CrossRef]
- Mishra, H.; Mazumde, A.; Prabuthas, P. Recent Developments on Algae as a Nutritional Supplement. In Algal Biorefinery: An Integrated Approach, 1st ed.; Springer: Kharagpur, India, 2015; pp. 219–233. ISBN 978-3-319-22813-6. [Google Scholar]
- Amorim, M.L.; Soares, J.; Vieira, B.B.; Batista-Silva, W.; Martins, M.A. Extraction of proteins from the microalga Scenedesmus obliquus BR003 followed by lipid extraction of the wet deproteinized biomass using hexane and ethyl acetate. Bioresour. Technol. 2020, 307, 123190. [Google Scholar] [CrossRef]
- Brasil, B.d.S.A.F.; de Siqueira, F.G.; Salum, T.F.C.; Zanette, C.M.; Spier, M.R. Microalgae and cyanobacteria as enzyme biofactories. Algal Res. 2017, 25, 76–89. [Google Scholar] [CrossRef]
- Otto, B.; Schlosser, D. First laccase in green algae: Purification and characterization of an extracellular phenol oxidase from Tetracystis aeria. Planta 2014, 240, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Buckle, K.; Camire, M.; Heymann, H.; Hutkins, R.; Lund, D.; Weaver, C.; Kinsella, J.; Taylor, S. Present and Future Prospects of Seaweeds in Developing Functional Foods. Adv. Food Nutr. Res. 2011, 64, 1–15. [Google Scholar]
- Ramawat, K.G.; Mérillon, J.M. Algal Polysaccharides and Health. In Polysaccharides: Bioactivity and Biotechnology, 1st ed.; Springer: Udaipur, India, 2015; pp. 109–144. ISBN 978-3-319-16298-0. [Google Scholar]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Alam, A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Qiu, S.; Ge, S. Determination of Microalgal Lipid Content and Fatty Acid for Biofuel Production. Biomed Res. Int. 2018, 2018, 1503126. [Google Scholar] [CrossRef]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of fatty acid content and composition in microalgae. J. Vis. Exp. 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Torzillo, G. Synthesis of Antioxidant Carotenoids in Microalgae in Response to Physiological Stress. In Carotenoids; IntechOpen: London, UK, 2017; pp. 143–157. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Recht, L.; Töpfer, N.; Batushansky, A.; Sikron, N.; Gibon, Y.; Fait, A.; Nikoloski, Z.; Boussiba, S.; Zarka, A. Metabolite profiling and integrative modeling reveal metabolic constraints for carbon partitioning under nitrogen starvation in the green algae Haematococcus pluvialis. J. Biol. Chem. 2014, 289, 30387–30403. [Google Scholar] [CrossRef]
- Jerez, I.; García, S.; Rodríguez, G.; Rico, M.; Afonso, C.; Gómez, J.L. Phenolic Profile and Antioxidant Activity of Crude Extracts from Microalgae and Cyanobacteria Strains. J. Food Qual. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects in vitro toward hepatotoxicity model. Afr. J. Pharm. Pharmacol. 2009, 3, 133–139. [Google Scholar]
- Goiris, K.; Van Colen, W.; Isabel, W.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Fact. 2020, 19, 1–23. [Google Scholar] [CrossRef]
- Krishna, A.; Wayne, K.; Rambabu, K.; Tao, Y.; Chu, D.; Show, P. Food Science and Human Wellness Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar]
- Fujii, K.; Nakashima, H.; Hashidzume, Y. Isolation of folate-producing microalgae, from oligotrophic ponds in Yamaguchi. J. Appl. Microbiol. 2010, 108, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Woortman, D.V.; Fuchs, T.; Striegel, L.; Fuchs, M.; Weber, N.; Brück, T.B.; Rychlik, M. Microalgae a Superior Source of Folates: Quantification of Folates in Halophile Microalgae by Stable Isotope Dilution Assay. Front. Bioeng. Biotechnol. 2020, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from Algae and Cyanobacter. In Algal Green Chemistry, 1st ed.; Elsiever: Cambridge, MA, USA, 2017; pp. 65–89. ISBN 9780444640413. [Google Scholar]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Gheda, S.; El-sheekh, M.; Abou-zeid, A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pac. J. Trop. Med. 2018, 11, 583–589. [Google Scholar]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Nifantiev, N.E. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Moradian, A.; Abdolali, S.; Shadman, M.; Morowvat, M. Antifungal and Antibacterial Activity of the Microalgae Collected from Paddy Fields of Iran: Characterization of Antimicrobial Activity of Chroococcus dispersus. J. Biol. Sci. 2007, 7, 904–910. [Google Scholar] [CrossRef]
- Salem, O.M.A.; Hoballah, E.M.; Ghazi, S.M.; Hanna, S.N. Antimicrobial activity of microalgal extracts with special emphasize on Nostoc sp. Life Sci. J. 2014, 11, 752–758. [Google Scholar]
- Falaise, C.; François, C.; Travers, M.-A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y.; et al. Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs 2016, 14, 159. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Battah, M.; Ibrahim, H.; Naggar, M.; Gawad, F.; Amer, M. Antifungal agent from Spirulina maxima: Extraction and Characterization. Glob. J. Pharmacol. 2014, 8, 228–236. [Google Scholar]
- Amaro, H.; Guedes, A.; Malcata, X. Antimicrobial activities of microalgae: An invited review. Sci. Microb. Pathog. 2011, 3, 1272–1280. [Google Scholar]
- Martínez, E.; Escudero, C. Cyanobacteria and Microalgae in the Production of Valuable Bioactive Compounds; IntechOpen: London, UK, 2018; Volume 111, pp. 105–128. [Google Scholar]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J. Chapter 12—Anticancer, Antiviral, Antibacterial, and Antifungal Properties in Microalgae. In Microalgae in Health and Disease Prevention, 1st ed.; Academic Press: Orlando, FL, USA, 2018; pp. 235–261. ISBN 9780128114063. [Google Scholar]
- Vehapi, M.; Yilmaz, A.; Ozcimen, D. Antifungal Activities of Chlorella vulgaris and Chlorella minutissima Microalgae Cultivated in Bold Basal Medium, Wastewater and Tree Extract Water Against Aspergillus niger and Fusarium oxysporum. Rom. Biotechnol. Lett. 2018. [Google Scholar] [CrossRef]
- Wali, A.F.; Dhaheri, Y.A.; Pillai, J.R.; Mushtaq, A.; Rao, P.G.; Rabbani, S.; Firdous, A.; Elshikh, M.S.; Farraj, D.A. LC-MS Phytochemical Screening, In Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- Takahashi, S.; Yoshida, M.; Watanabe, M.M.; Isoda, H. Anti-Inflammatory Effects of Aurantiochytrium limacinum 4W-1b Ethanol Extract on Murine Macrophage RAW264 Cells. BioMed Res. Int. 2019, 2019, 3104057. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.; Bermejo, E.; Garbayo, I.; Cuaresma, M. Chemically-induced production of anti-inflammatory molecules in microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Macromolecules 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Selleck, S.B. Order Out of Chaos: Assembly of Ligand Binding Sites in Heparan Sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2012, 11, 2399–2419. [Google Scholar] [CrossRef]
- Hidari, I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Commun. Biochem. Biophys. Res. 2008, 376, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral Effects of Sulfated Exopolysaccharide from the Marine Microalga Gyrodinium impudicum Strain KG03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, I.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoids sources. J. Appl. Phycol. 2012, 24, 731–741. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- De Mooij, T.; De Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Sydney, T.; Marshall-Thompson, J.A.; Kapoore, R.V.; Vaidyanathan, S.; Pandhal, J.; Fairclough, J.P.A. The effect of high-intensity ultraviolet light to elicit microalgal cell lysis and enhance lipid extraction. Metabolites 2018, 8, 65. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Vijay, D.; Shankar, J.; Singh, D.P. Photosynthetic performance, nutrient status and lipid yield of microalgae Chlorella vulgaris and Chlorococcum humicola under UV-B exposure. Curr. Res. Biotechnol. 2019, 1, 65–77. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Jha, D.; Jain, V.; Sharma, B.; Kant, A.; Garlapati, V.K. Microalgae-based Pharmaceuticals and Nutraceuticals: An Emerging Field with Immense Market Potential. ChemBioEng Rev. 2017, 4, 257–272. [Google Scholar] [CrossRef]
- Deniz, I.; García-Vaquero, M.; Imamoglu, E. Trends in red biotechnology: Microalgae for pharmaceutical applications. In Microalgae-Based Biofuels and Bioproducts, 1st ed.; Woodhead Publishing: Cambridge, MA, USA, 2017; pp. 429–460. ISBN 978-0-08-101023-5. [Google Scholar]
- Paniagua, M. Microalgal Nutraceutical. In Handbook of Marine Microalgae Biotechnology Advances Microalgae, 1st ed.; Academic Press: Waltham, MA, USA, 2015; pp. 255–267. ISBN 9780128007761. [Google Scholar]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hemantkumar, J.N.; Rahimbhai, M.I. Microalgae and Its Use in Nutraceuticals and Food Supplements. In Microalgae—From Physiology to Application, 1st ed.; IntechOpen: London, UK, 2019; pp. 1–11. ISBN 978-1-83880-036-9. [Google Scholar]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Fathi, E.; Allah, A. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2017, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Jochum, M.; Moncayo, L.P.; Jo, Y. Microalgal cultivation for biofertilization in rice plants using a vertical semi-closed airlift photobioreactor. PLoS ONE 2018, 13, e0203456. [Google Scholar] [CrossRef]
- Costa, A.J.; De Morais, M.G. An Open Pond System for Microalgal Cultivation. In Biofuels from Algae, 1st ed.; Elsevier: Oxford, UK, 2014; pp. 1–22. ISBN 978-0-444-59558-4. [Google Scholar]
- Cinar, S.O.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic production from microalgae: A review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Chang, J.-S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar]
- Li, Y.; Song, S.; Xia, L.; Yin, H.; García Meza, J.V.; Ju, W. Enhanced Pb(II) removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem. Eng. J. 2019, 361, 167–179. [Google Scholar] [CrossRef]
- Çeçen, F.; Aktaş, Ö. Activated Carbon for Water and Wastewater Treatment; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, G.; Cao, X.; Wei, D. Molecular characterization of CO2 sequestration and assimilation in microalgae and its biotechnological applications. Bioresour. Technol. 2017, 244, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal Bioremediation: Current Practices and Perspectives Microalgal bioremediation. J. Biochem. Technol. 2017, 3, 299–304. [Google Scholar]
- Salama, E.-S.; Roh, H.-S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.-H. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [PubMed]
- Furuhashi, Y.; Honda, R.; Noguchi, M.; Hara-Yamamura, H.; Kobayashi, S.; Higashimine, K.; Hasegawa, H. Optimum conditions of pH, temperature and preculture for biosorption of europium by microalgae Acutodesmus acuminatu. Biochem. Eng. J. 2019, 143, 58–64. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Afkar, E.; Ababna, H.; Fathi, A.A. Toxicological response of the green alga Chlorella vulgaris, to some heavy metals. Am. J. Environ. Sci. 2010, 6, 230–237. [Google Scholar] [CrossRef]
- Ummalyma, S.; Pandey, A.; Sukumaran, R.; Sahoo, D. Bioremediation by Microalgae: Current and Emerging Trends for Effluents Treatments for Value Addition of Waste Streams. In Energy, Environment, and Sustainability, 1st ed.; Springer: Singapore, 2018; pp. 355–375. ISBN 978-981-10-7433-2. [Google Scholar]
- Benítez, M.B.; Champagne, P.; Ramos, A.; Torres, A.F.; Ochoa-Herrera, V. Wastewater treatment for nutrient removal with Ecuadorian native microalgae. Environ. Technol. 2019, 40, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Neveux, N.; Magnusson, M.; Mata, L.; Whelan, A.; de Nys, R.; Paul, N.A. The treatment of municipal wastewater by the macroalga Oedogonium sp. and its potential for the production of biocrude. Algal Res. 2016, 13, 284–292. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Sial, A.; Zhang, B.; Zhang, A.; Liu, K.; Imtiaz, S.A.; Yashir, N. Microalgal–Bacterial Synergistic Interactions and Their Potential Influence in Wastewater Treatment: A Review. BioEnergy Res. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.; Qi, Y.; Song, C.; Chen, G. The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour. Technol. 2019, 292, 122017. [Google Scholar] [CrossRef]

| Microalgal Biomaterial | Main Compound | Property | Commercial Application | Microalgae Source | References |
|---|---|---|---|---|---|
| Proteins | Phycobiliproteins, glycoproteins | Antitumor, antimicrobial, anti-inflammatory, UV protection | Nutraceuticals, medicines, cosmetics, dyes, food products, animal feed | Spirulina sp., Chlorella vulgaris, Dunaliella salina, Dunaliella sp., Haematococcus pluvialis, Porphyridium sp. | [31,32,55,81,87] |
| Enzymes | Acyl-CoA, diacylglycerol, acyltransferase 2, ∆6-desaturase, L-asparaginase | Antitumor | Production of biofuels, bioremediation, cosmetics, medicines, cleaning products and other chemical products | Chlamydomonas reinhardtii, Chlorella elipsoidea, Phaeodactylum tricornutum, Chlorella vulgaris, Chlamidomonas sp. | [30,33,34] |
| Sugars and Polysaccharides | Calcium spirulan, glycosoaminoglycanxylose, glucose, galactose, mannose, cellulose, alginate, agar | Antitumor, anti-inflammatory, antiviral | Nutraceuticals, drugs, cosmetics, food additives, industrial products, biofuel | Chlorella vulgaris, Porphyridium sp., Porphyridium cruentum, Spirulina sp. | [31,37,55,69,73] |
| Fatty acids and Lipids | Phospholipids, glycolipids, sterols, polyunsaturated fatty acids (PUFAs), decahexaenoic acids (DHA), eicosapentaenoic acid (EPA), chlorellin | Antitumor, antimicrobial, antifungal, anti-inflammatory, antiviral | Nutraceuticals, drugs, food, animal feed, biofuel production | Chlorella sp., Spirulina sp., Spirulina maxima, Dunaliella salina | [11,55,61,68,69,81] |
| Carotenoids and Pigments | Astaxanthin, β-carotene, lutein, fucoxanthin | Antioxidant, antitumor, antifungal, anti-inflammatory, UV protection | Nutraceuticals, medicines, food products, food additives, colorants, manufacture of chemical products | Haematococcus pluvialis, Dunaliella salina, Scenedesmus sp. | [3,42,52,68,69,81,87] |
| Polyphenols | Salicylic, transcinnamic, chlorogenic, caffeic acids | Antioxidant, antifungal, anti-inflammatory | Nutraceuticals, food products, drugs and cosmetics | Spirulina maxima, Phaeodactylum tricornutum, Tetraselmis suecica | [46,47,68,71] |
| Vitamins | A, C, E, thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folic acid, cobalamin | Antioxidant, antifungal, antiviral | Nutraceuticals, food, food additives, animal feed ingredient, cosmetics | Chlamydomonas sp. Chlorococcum sp. Chlorella vulgaris, Chlorella sp., Spirulina sp. | [48,50,51,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability 2021, 13, 3063. https://doi.org/10.3390/su13063063
Orejuela-Escobar L, Gualle A, Ochoa-Herrera V, Philippidis GP. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability. 2021; 13(6):3063. https://doi.org/10.3390/su13063063
Chicago/Turabian StyleOrejuela-Escobar, Lourdes, Arleth Gualle, Valeria Ochoa-Herrera, and George P. Philippidis. 2021. "Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries" Sustainability 13, no. 6: 3063. https://doi.org/10.3390/su13063063
APA StyleOrejuela-Escobar, L., Gualle, A., Ochoa-Herrera, V., & Philippidis, G. P. (2021). Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability, 13(6), 3063. https://doi.org/10.3390/su13063063








