Development of Biosorbent Derived from the Endocarp Waste of Gayo Coffee for Lead Removal in Liquid Wastewater—Effects of Chemical Activators
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Activation of CE Adsorbent
2.3. Characterizations of the Sorbent
2.4. Adsorption of Simulated Lead Wastewater Experiment
2.5. Isotherm and Kinetics of Adsorption
3. Results
3.1. Characteristics of CE Activated Carbon
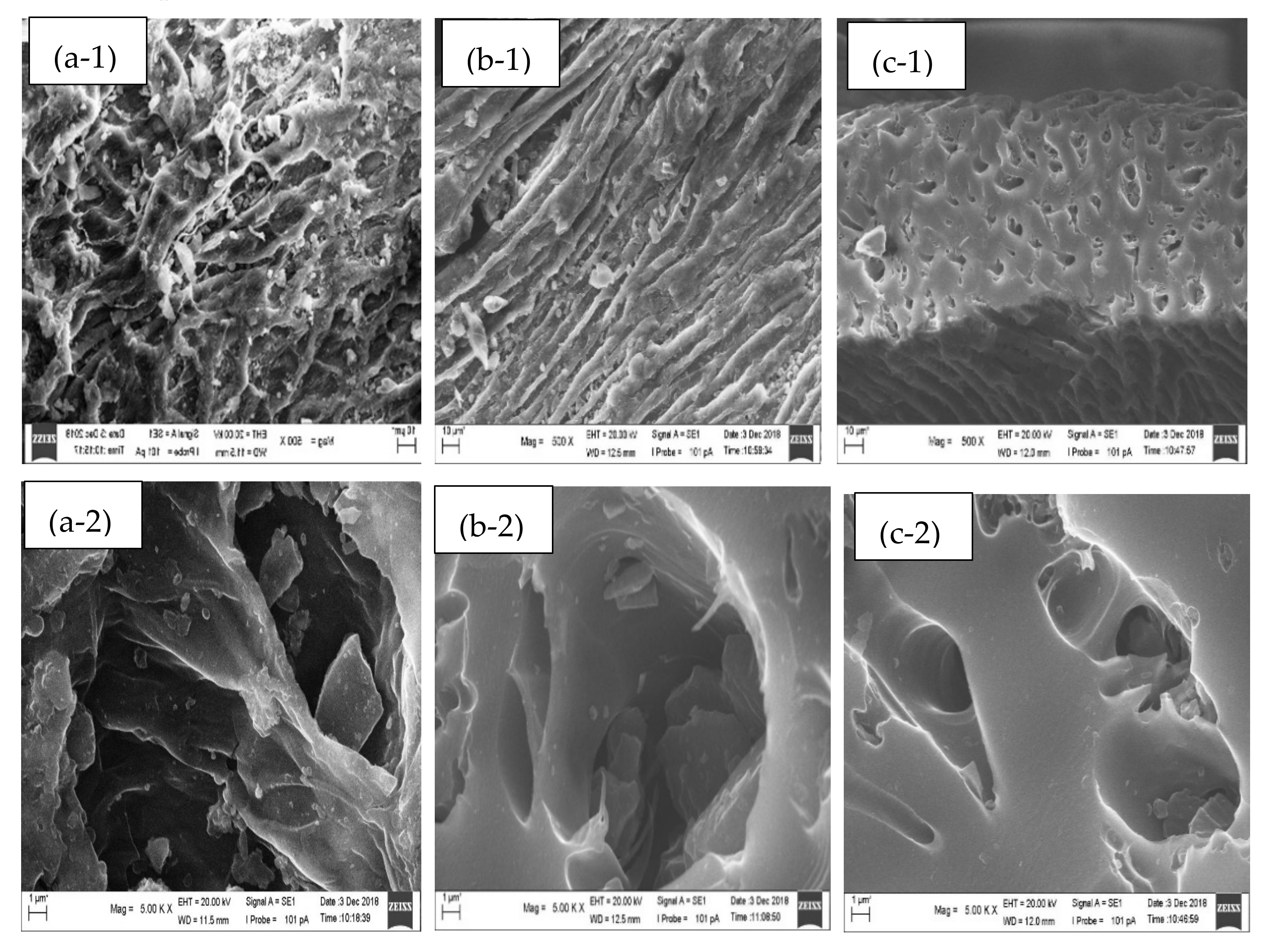
3.2. Morphological Analysis
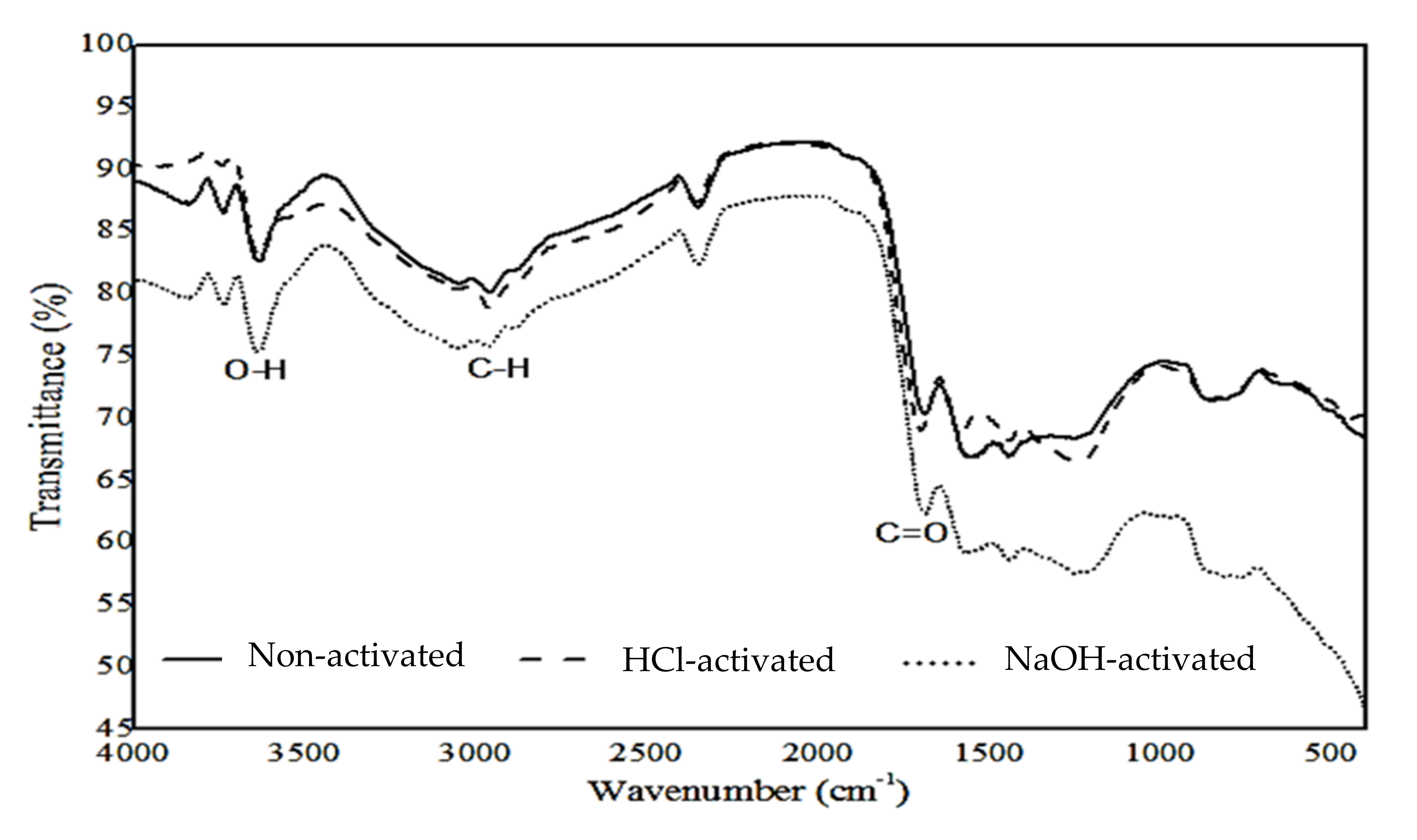
3.3. Chemical Functional Groups Analysis
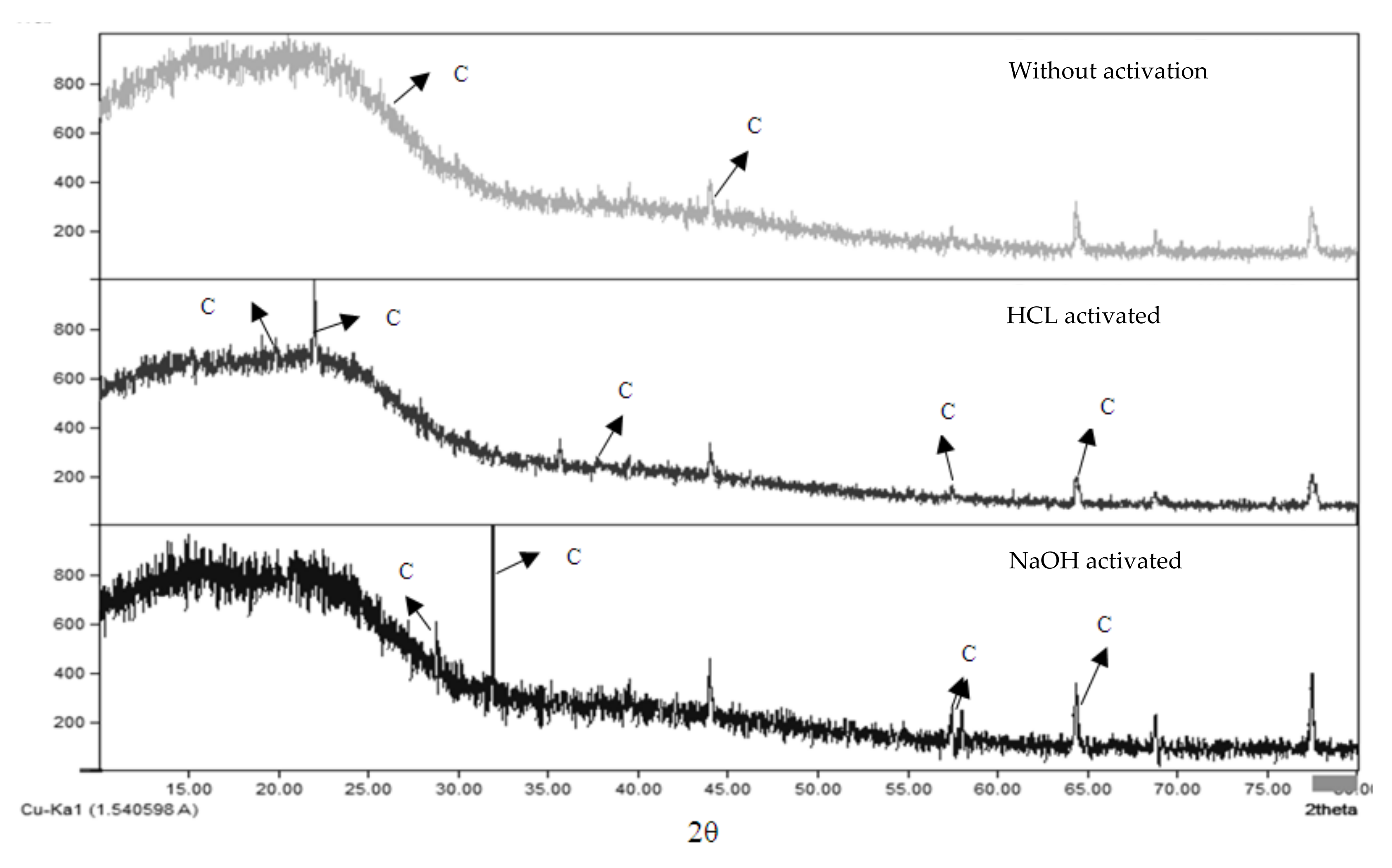
3.4. XRD and XRF Analysis
3.5. Surface Area Analyzer (SAA)
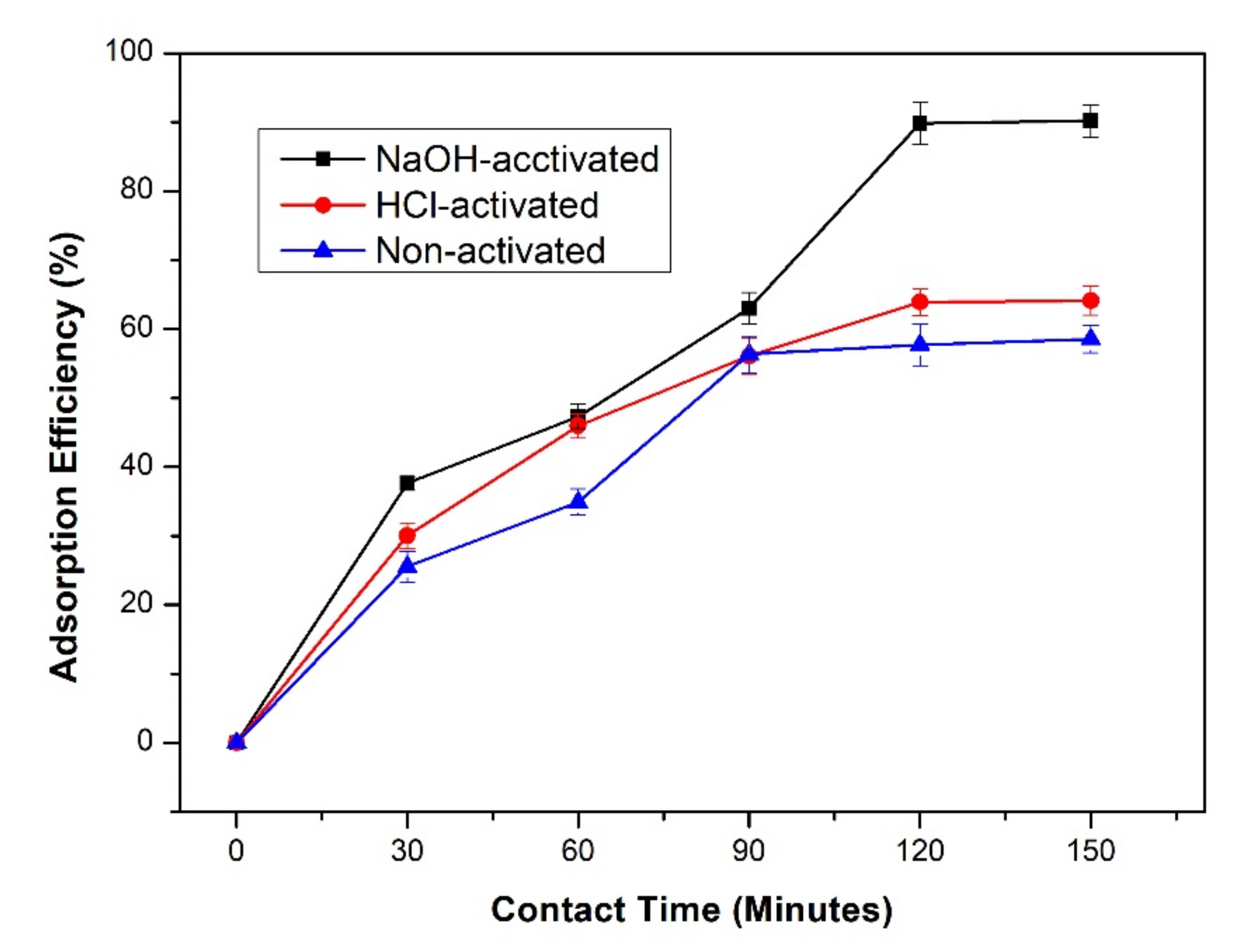
3.6. Effect of Contact Time on the Adsorption Efficiency and Capacity of Lead
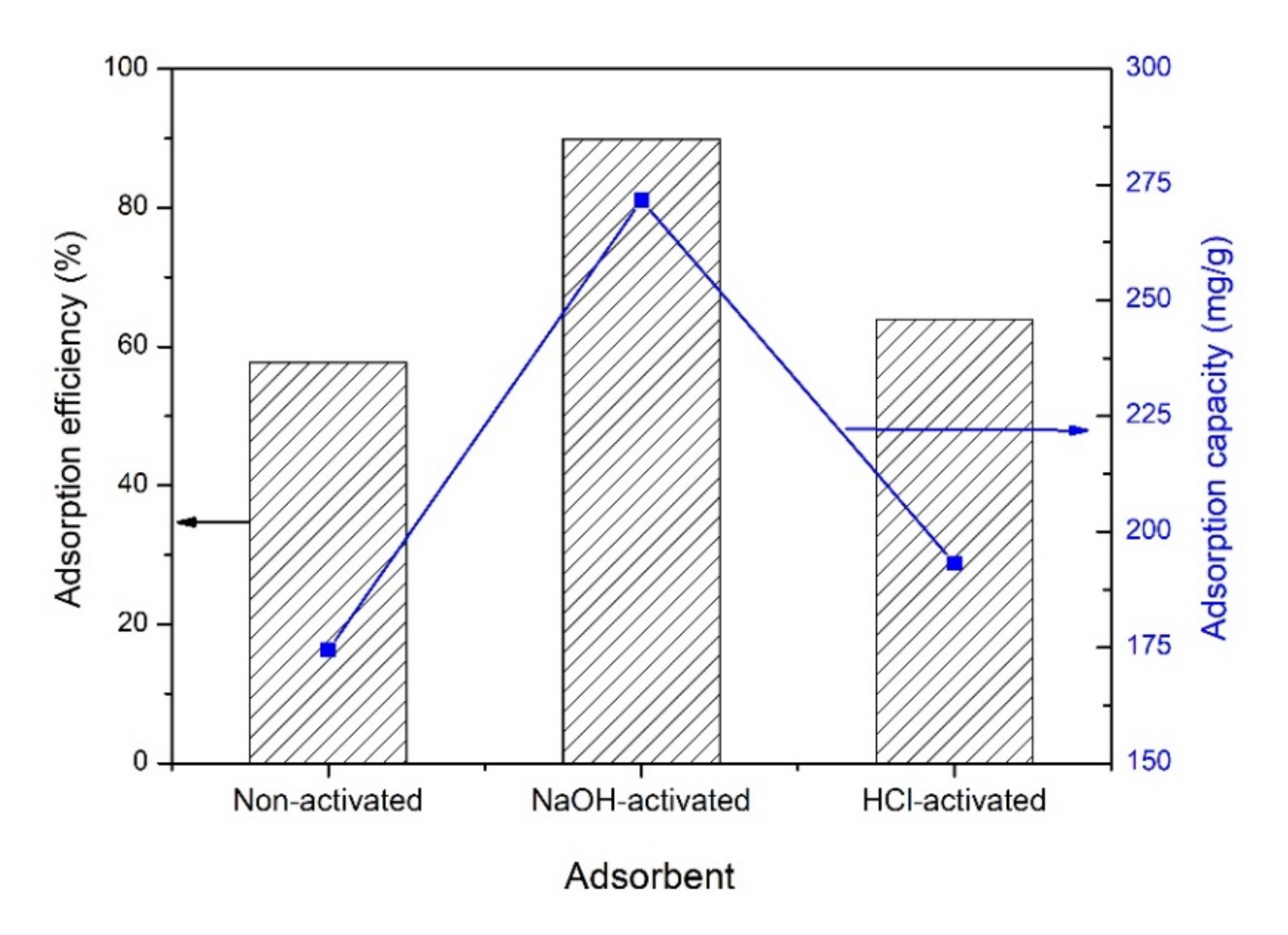
3.7. Effect of Activator Type on the Adsorption Efficiency and Capacity
3.8. Adsorption Isotherms
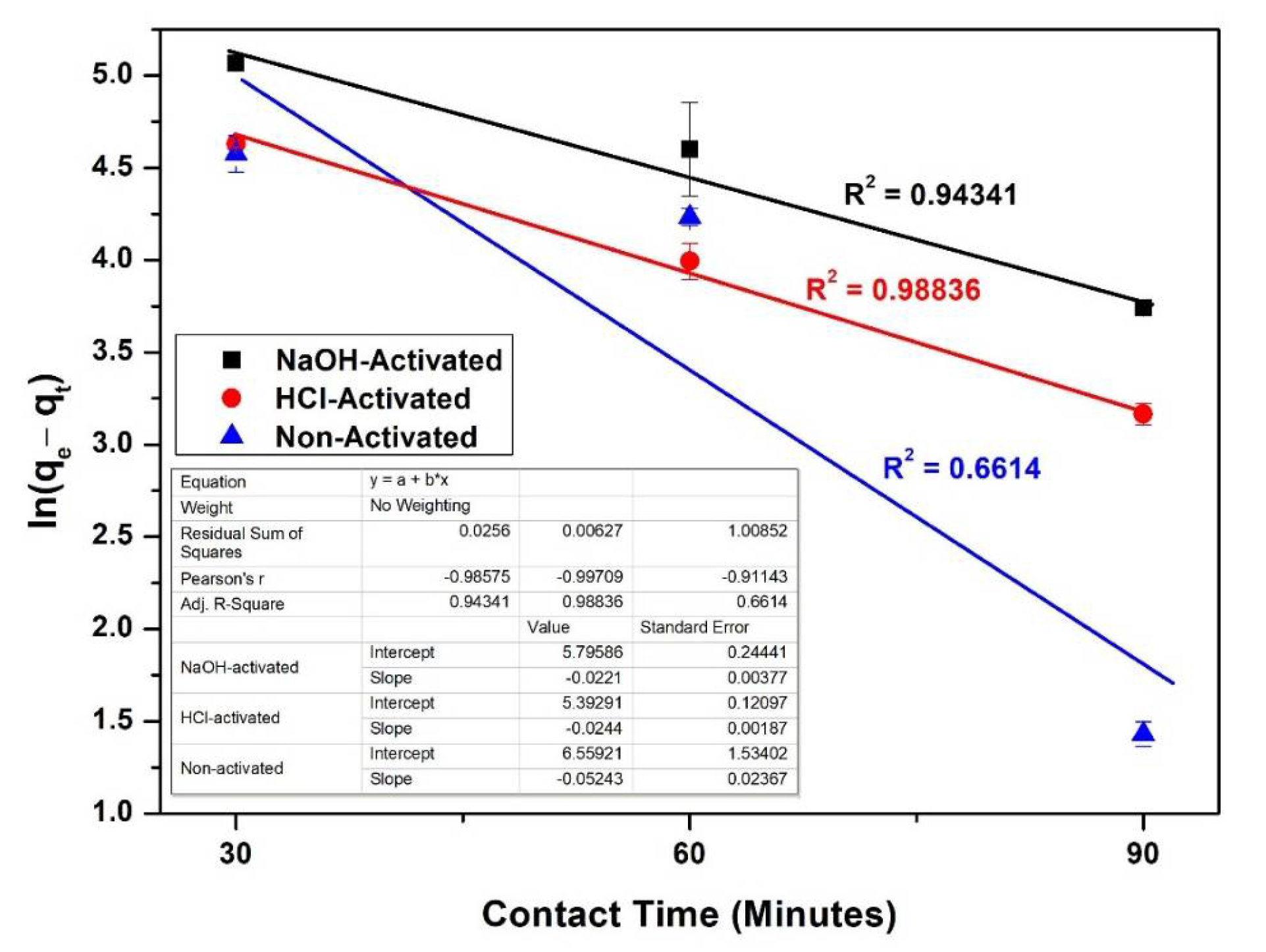
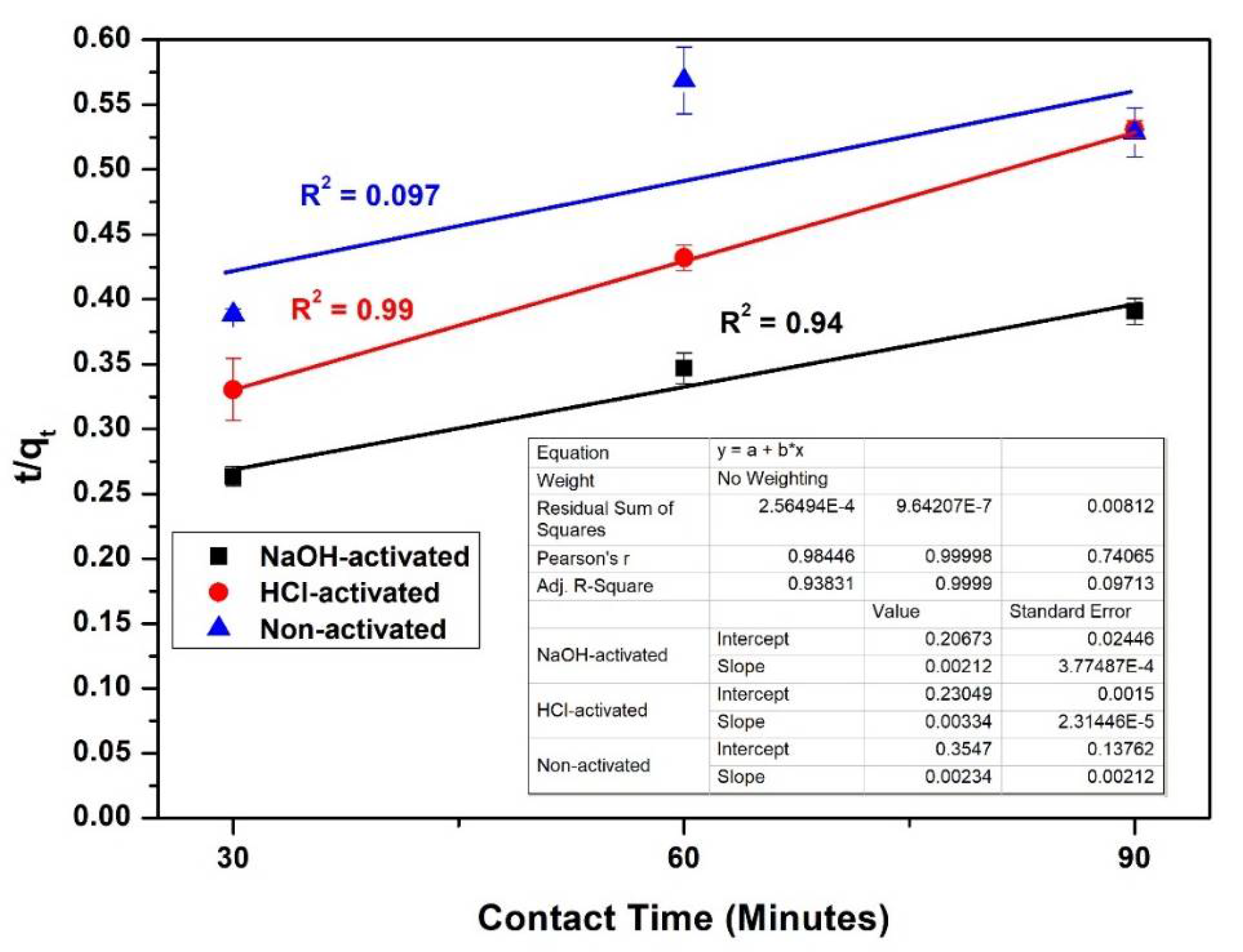
3.9. Kinetics of Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead (II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Ng, D.-Q.; Chen, C.-Y.; Lin, Y.-P. A new scenario of lead contamination in potable water distribution systems: Galvanic corrosion between lead and stainless steel. Sci. Total Environ. 2018, 637–638, 1423–1431. [Google Scholar] [CrossRef]
- Zietz, B.; Dassel de Vergara, J.; Kevekordes, S.; Dunkelberg, H. Lead contamination in tap water of households with children in Lower Saxony. Sci. Total Environ. 2001, 275, 19–26. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Bailón-Salas, A.M.; Ordaz-Díaz, L.A.; Cháirez-Hernández, I.; Alvarado-de la Peña, A.; Proal-Nájera, J.B. Lead and copper removal from groundwater by spherical agglomeration using a biosurfactant extracted from Yucca decipiens Trel. Chemosphere 2018, 207, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lalmi, A.; Bouhidel, K.-E.; Sahraoui, B.; Anfif, C.E.H. Removal of lead from polluted waters using ion exchange resin with Ca(NO3)2 for elution. Hydrometallurgy 2018, 178, 287–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Xiang, Q.; Liu, F.; Ling, C.; Wang, F.; Li, Y.; Li, A. A high-flux and anti-interference dual-functional membrane for effective removal of Pb(II) from natural water. J. Hazard. Mater. 2020, 384, 121492. [Google Scholar] [CrossRef] [PubMed]
- S Samal, K.; Das, C.; Mohanty, K. Development of hybrid membrane process for Pb bearing wastewater treatment. J. Water Process Eng. 2016, 10, 30–38. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Křivčík, J.; Mikulášek, P. Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem. Eng. 2014, 256, 324–334. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Biela, R.; Šopíková, L. Efficiency of sorption materials on the removal of lead from water. Appl. Ecol. Environ. Res. 2017, 15, 1527–1536. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef]
- Herzog, T.H.; Jänchen, J. Adsorption Properties of Modified Zeolites for Operating Range Enhancement of Adsorption Heat Pumps through the Use of Organic Adsorptive Agents. Energy Procedia 2016, 91, 155–160. [Google Scholar] [CrossRef][Green Version]
- Bożęcka, A.; Orlof-Naturalna, M.; Sanak-Rydlewska, S. Removal of lead, cadmium and copper ions from aqueous solutions by using ion exchange resin C 160. Gospod. Surowcami Miner. 2016, 32, 129–140. [Google Scholar] [CrossRef]
- Abo-Farha, S.A.; Abdel-Aal, A.Y.; Ashour, I.A.; Garamon, S.E. Removal of some heavy metal cations by synthetic resin purolite C100. J. Hazard. Mater. 2009, 169, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Yakout, S.M. Removal of the hazardous, volatile, and organic compound benzene from aqueous solution using phosphoric acid activated carbon from rice husk. Chem. Cent. J. 2014, 8, 52. [Google Scholar] [CrossRef]
- Sarkheil, H.; Tavakoli, J.; Behnood, R. Oil by-product removal from aqueous solution using sugarcane bagasse as absorbent. Int. J. Emerg. Trends Sci. Technol. 2014, 2, 48–52. [Google Scholar]
- Ekpete, O.A.; Marcus, A.C.; Osi, V. Preparation and characterization of activated carbon obtained from plaintain Musa Paradisiaca fruit stem. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Hossain, M.; Ngo, H.; Guo, W.; Nguyen, T. Biosorption of Cu (II) from water by banana peel based biosorbent: Experiments and models of adsorption and desorption. J. Water Sustain. 2012, 2, 87–104. [Google Scholar]
- Olguin, M.; López-González, H.; Serrano-Gomez, J. Hexavalent chromium removal from aqueous solutions by Fe-modified peanut husk. Water Air Soil Pollut. 2013, 224, 1654. [Google Scholar] [CrossRef]
- Nashine, A.L.; Tembhurkar, A.R. Equilibrium, kinetic and thermodynamic studies for adsorption of As (III) on coconut (Cocos nucifera L.) fiber. J. Environ. Chem. Eng. 2016, 4, 3267–3273. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Koo, N.; Park, J.; Lee, S.-H.; Bak, G.-I.; Kim, J.-G. The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessments. J. Environ. Manag. 2014, 146, 124–130. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Kumar, S. Biosorption of lead ions from the aqueous solution by Sargassum filipendula: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2016, 4, 4587–4599. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhana, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Ningsih, D.A.; Said, I.; Ningsih, P. Adsorpsi Logam Timbal (Pb) Dari Larutannya Dengan Menggunakan Adsorben Dari Tongkol Jagung. J. Akad. Kim. 2016, 5, 55–60. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Yuan, X.; Wu, Z.; Wang, L.; Peng, X.; Leng, L.; Zeng, G. Removal of Basic Dye from Aqueous Solution using Cinnamomum camphora Sawdust: Kinetics, Isotherms, Thermodynamics, and Mass-Transfer Processes. Sep. Sci. Technol. 2014, 49, 2689–2699. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Mulana, F.; Ismail, T.A.; Hafdiansyah, M.F. Activation and characterization of waste coffee grounds as bio-sorbent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012029. [Google Scholar]
- Al-Essa, K. Activation of Jordanian Bentonite by Hydrochloric Acid and Its Potential for Olive Mill Wastewater Enhanced Treatment. J. Chem. 2018, 2018, 8385692. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Wee, J.-H.; Yang, C.-M.; Yang, K.S. Efficient removals of Hg and Cd in aqueous solution through NaOH-modified activated carbon fiber. Chem. Eng. 2020, 392, 123768. [Google Scholar] [CrossRef]
- Mariana, M.; Mahidin, M.; Mulana, F.; Agam, T.; Hafdiansyah, F. Reactivity improvement of Ca(OH)2 sorbent using diatomaceous earth (DE) from Aceh Province. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012001. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Enhancing adsorption of Pb(II) from aqueous solution by NaOH and EDTA modified Artocarpus odoratissimus leaves. J. Environ. Chem. Eng. 2018, 6, 7172–7184. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. 2008 Introduction to Spectroscopy (Chapter 2); Cengage Learning: Weinheim, Germany, 2002. [Google Scholar]
- Nasution, Z.A.; Rambe, S.M. Karakterisasi dan identifikasi gugus fungsi dari karbon cangkang kelapa sawit dengan metode methano-pyrolysis. J. Din. Penelit. Ind. 2013, 24, 108–113. [Google Scholar]
- Şencan, A.; Kılıç, M. Investigation of the Changes in Surface Area and FT-IR Spectra of Activated Carbons Obtained from Hazelnut Shells by Physicochemical Treatment Methods. J. Chem. 2015, 2015, 651651. [Google Scholar] [CrossRef]
- Khalil, H.; Jawaid, M.; Firoozian, P.; Rashid, U.; Islam, A.; Akil, H.M. Activated carbon from various agricultural wastes by chemical activation with KOH: Preparation and characterization. J. Biobased Mater. Bioenergy 2013, 7, 708–714. [Google Scholar] [CrossRef]
- Das, D.; Samal, D.P.; Meikap, B. Preparation of activated carbon from green coconut shell and its characterization. J. Chem. Eng. Technol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Zhou, P. Regenstein, Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. J. Food Sci. 2005, 70, c392–c396. [Google Scholar] [CrossRef]
- Mariana, M.; Mulana, F.; Sofyana, S.; Dian, N.P.; Lubis, M.R. Characterization of adsorbent derived from Coconut Husk and Silica (SiO2). IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012022. [Google Scholar] [CrossRef]
- Tan, K.A.; Morad, N.; Teng, T.T.; Norli, I.; Panneerselvam, P. Removal of Cationic Dye by Magnetic Nanoparticle (Fe3O4) Impregnated onto Activated Maize Cob Powder and Kinetic Study of Dye Waste Adsorption. APCBEE Procedia 2012, 1, 83–89. [Google Scholar] [CrossRef]
- Thi, T.T.L.; Le Van, K. Adsorption behavior of Pb (II) in aqueous solution using coffee husk-based activated carbon modified by nitric acid. Am. J. Eng. Res. 2016, 5, 120–129. [Google Scholar]
- Ngapa, Y.D. Kajian Pengaruh Asam-Basa Pada Aktivasi Zeolit dan Karakterisasinya sebagai Adsorben Pewarna Biru Metilena. J. Kim. Pendidik. Kim. 2017, 2, 90–96. [Google Scholar] [CrossRef]
- Weng, C.-H.; Lin, Y.-T.; Hong, D.-Y.; Sharma, Y.C.; Chen, S.-C.; Tripathi, K. Effective removal of copper ions from aqueous solution using base treated black tea waste. Ecol. Eng. 2014, 67, 127–133. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Citric acid modified agricultural by-products as copper ion adsorbents. Adv. Environ. Res. 2000, 4, 1–7. [Google Scholar] [CrossRef]
- Gaya, U.I.; Otene, E.; Abdullah, A.H. Adsorption of aqueous Cd (II) and Pb (II) on activated carbon nanopores prepared by chemical activation of doum palm shell. SpringerPlus 2015, 4, 458. [Google Scholar] [CrossRef]
- Mulana, F.; Muslim, A.; Alam, P.N. Adsorption of Pb (II) Heavy Metals from Wastewater Using Modified Rice Husk as Adsorbent. In Proceedings of the Annual International Conference, Syiah Kuala University-Life Sciences and Engineering, Banda Aceh, Indonesia, 9–11 September 2015; Syiah Kuala University: Aceh, Indonesia, 2015; Volume 5, pp. 12–17. [Google Scholar]
- Faisal, M. Efisiensi penyerapan logam Pb2+ dengan menggunakan campuran bentonit dan enceng gondok. J. Tek. Kim. 2015, 4, 20–24. [Google Scholar] [CrossRef]
- Muslim, A. Australian pine cones-based activated carbon for adsorption of copper in aqueous solution. Int. J. Eng. Sci. Technol. 2017, 12, 280–295. [Google Scholar]
- Muslim, A.; Aprilia, S.; Suha, T.; Fitri, Z. Adsorption of Pb (II) ions from aqueous solution using activated carbon prepared from areca catechu shell: Kinetic, isotherm and thermodynamic studies. J. Korean Chem. Soc. 2017, 61, 89–96. [Google Scholar]
- Muslim, A.; Ellysa, E.; Said, S.D. Cu (II) Ions Adsorption Using Activated Carbon Prepared from Pithecellobium Jiringa (Jengkol) Shells With Ultrasonic Assistance: Isotherm, Kinetic and Thermodynamic Studies. J. Eng. Technol. Sci. 2017, 49, 472–490. [Google Scholar] [CrossRef][Green Version]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)–Grafted Chitosan Adsorbents with Different Cross-Linking for Pb (II) and Cd (II) Uptake. Langmuir 2014, 30, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Karatas, M. Removal of Pb (II) from water by natural zeolitic tuff: Kinetics and thermodynamics. J. Hazard. Mater. 2012, 199–200, 383–389. [Google Scholar] [CrossRef]
- Theivarasu, C.; Mylsamy, S. Removal of Malachite Green from Aqueous Solution by Activated Carbon Developed from Cocoa (Theobroma Cacao) Shell–A Kinetic and Equilibrium Studies. E-J. Chem. 2018, 8, 714808. [Google Scholar] [CrossRef]

| Parameter | SNI No. 06-3730-1995 Standard | Activator | Results |
|---|---|---|---|
| Water content | Max. 15% | NaOH 0.5 M | 1% ± 0.72 |
| HCl 0.5 M | 4% ± 0.03 | ||
| - | 4% ± 0.12 | ||
| Ash content | Max. 10% | NaOH 0.5 M | 3.5% ± 0.22 |
| HCl 0.5 M | 3.5% ± 0.37 | ||
| - | 4% ± 0.53 | ||
| Iodine number | Min. 750 mg/g | NaOH 0.5 M | 1628.6 mg/g ± 1.67 |
| HCl 0.5 M | 1226.7 mg/g ± 1.29 | ||
| - | 994.05 mg/g ± 2.44 |
| Component | % w/w | ||
|---|---|---|---|
| Non-Activated | HCl-Activated | NaOH-Activated | |
| SiO2 | 47.32 | 56.72 | 60.0 |
| Al2O3 | 12.46 | 8.9 | 6.8 |
| Fe2O3 | 9.98 | 8.21 | 8.11 |
| MgO | 9.84 | 6.96 | 7.52 |
| SO3 | 0.16 | 0.23 | 0.21 |
| K2O | 0.45 | 0.36 | 0.35 |
| CaO | 15.54 | 13.9 | 13 |
| Na2O | 0.07 | 0.11 | 0.9 |
| P2O5 | 0.161 | 0.161 | 0.161 |
| TiO2 | 0.377 | 0.362 | 0.355 |
| Mn2O3 | 0.163 | 0.146 | 0.139 |
| CrO3 | 0.078 | 0.135 | 0.112 |
| SrO | 0.26 | 0.29 | 0.26 |
| BaO | 0.05 | 0.07 | 0.05 |
| ZnO | 0.03 | 0.07 | 0.03 |
| CuO | 0.079 | 0.089 | 0.079 |
| MnO | 0.402 | 0.5 | 0.34 |
| K2O | 2.16 | 1.79 | 1.16 |
| Eu2O3 | 0.32 | 0.9 | 0.32 |
| Re2O7 | 0.1 | 0.1 | 0.1 |
| Adsorbent Type | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) | Surface Area to Pore Volume Ratio |
|---|---|---|---|---|
| Non-activated | 1.8405 | 0.001836 | 39.9030 | 1002.451 |
| HCl-activated | 1.4057 | 0.001262 | 42.8215 | 1113.866 |
| NaOH-activated | 0.9707 | 0.000689 | 47.8671 | 1408.853 |
| Adsorbent Material | Maximum Capacity of Adsorption (mg/g) | Reference |
|---|---|---|
| Arabica Gayo CE (NaOH-activated) | 272.56 | This work |
| Peanut shell | 39 | [1] |
| Rice husk | 5.08 | [50] |
| Bentonite-water hyacinth | 0.987 | [51] |
| Australian Pine Cones | 151.515 | [52] |
| Areca Catechu Shell | 55.25 | [53] |
| Oil Palm Empty Fruit Bunches | 9.319 | [54] |
| Langmuir Isotherm | Freundlich Isotherm | Dubinin–Radushkevich Isotherm | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | qs (mg/g) | Kad (mol2/kJ2) | R2 | |
| 434.78 | 0.04 | 0.99 | 3.1 | 0.28 | 0.85 | 5.86 | −0.003 | 0.96 | This work |
| 16.81 | 0.30 | 0.99 | 1.5 | 2.88 | 0.82 | 6.8 | 0.064 | 0.83 | [56] |
| 9.32 | 1.55 | 0.99 | 1.4 | −0.13 | 0.33 | - | - | - | [54] |
| 54.95 | 0.37 | 0.99 | 1.5 | 0.03 | 0.91 | - | - | - | [52] |
| 500 | 0.004 | 0.99 | 1 | 0.02 | 1 | - | - | - | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariana, M.; Mulana, F.; Juniar, L.; Fathira, D.; Safitri, R.; Muchtar, S.; Bilad, M.R.; Shariff, A.H.M.; Huda, N. Development of Biosorbent Derived from the Endocarp Waste of Gayo Coffee for Lead Removal in Liquid Wastewater—Effects of Chemical Activators. Sustainability 2021, 13, 3050. https://doi.org/10.3390/su13063050
Mariana M, Mulana F, Juniar L, Fathira D, Safitri R, Muchtar S, Bilad MR, Shariff AHM, Huda N. Development of Biosorbent Derived from the Endocarp Waste of Gayo Coffee for Lead Removal in Liquid Wastewater—Effects of Chemical Activators. Sustainability. 2021; 13(6):3050. https://doi.org/10.3390/su13063050
Chicago/Turabian StyleMariana, Mariana, Farid Mulana, Lisa Juniar, Dinda Fathira, Risna Safitri, Syawaliah Muchtar, Muhammad Roil Bilad, Amir Husni Mohd Shariff, and Nurul Huda. 2021. "Development of Biosorbent Derived from the Endocarp Waste of Gayo Coffee for Lead Removal in Liquid Wastewater—Effects of Chemical Activators" Sustainability 13, no. 6: 3050. https://doi.org/10.3390/su13063050
APA StyleMariana, M., Mulana, F., Juniar, L., Fathira, D., Safitri, R., Muchtar, S., Bilad, M. R., Shariff, A. H. M., & Huda, N. (2021). Development of Biosorbent Derived from the Endocarp Waste of Gayo Coffee for Lead Removal in Liquid Wastewater—Effects of Chemical Activators. Sustainability, 13(6), 3050. https://doi.org/10.3390/su13063050













