Effect of the Quasi-Petal Heat Transfer Tube on the Melting Process of the Nano-Enhanced Phase Change Substance in a Thermal Energy Storage Unit
Abstract
1. Introduction
2. Methodology
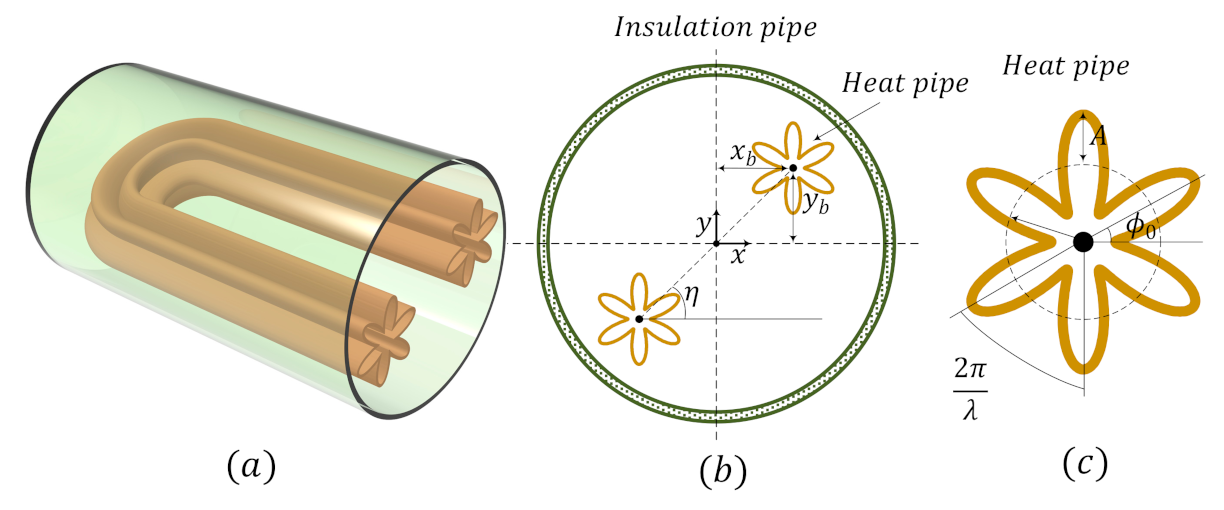
2.1. Physical Model
2.2. Governing Equations
3. Numerical Method and Model Verification
3.1. Numerical Approach
3.2. Grid-Independency Test
3.3. Model Verification
4. Results and Discussion
5. Conclusions
- The cross-sectional shape of the inner tube is an important parameter in heat transfer and in the energy storage of the PCM. Using a petal-shaped tube instead of a circular one increases the surface contact between the tube and the surrounding PCM, which, consequently, intensifies heat transfer and enhances melting. This effect is further emphasized when the number of petals Λ and/or their amplitude is increased. The average charging power can be increased by up to 45% when a petal-shaped tube with Λ = 8 is used instead of a circular one, and by 26% when the amplitude of the petals is increased by 3 times.
- The distance between the two branches of the inner tube rd has a slight effect on the thermal and flow behaviors in the heat exchanger. Shifting the inner tube towards the center by reducing rd or towards the outer shell by raising rd limits the zone of PCM melting and diminishes energy storage. The optimal value for PCM melting and energy storage is rd = 0.4 rs. Nonetheless, the impact of 0.4 rd remains relatively limited. A 4% reduction in the charging power is observed when rd is increased from 0.4 rs to 0.5 rs.
- The inclination angle of the inner tube impacts the convective effects in the cavity. Using a vertical tube instead of a horizontal one intensifies the convection of the melt and increases the stored energy. As a consequence, a vertical inner tube leads to a 5% increase in the charging power compared to a horizontal one.
- Dispersing conductive nanoparticles in the PCM increases its thermal conductivity, which improves its thermal transfer properties. However, the type of nanoparticles dispersed in the PCM has little effect on the melted volume and the stored energy, as similar results are obtained when either Cu or GO nanoparticles are used, with a little advantage for Cu. On the other hand, raising the concentration of the nanoparticles enhances PCM melting and the corresponding stored energy. Using an 8% nanoparticle, the charging power can be raised by 7% for GO and 11% for Cu nanoparticles than a pure PCM.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nižetić, S.; Jurčević, M.; Arıcı, M.; Arasu, A.V.; Xie, G. Nano-enhanced phase change materials and fluids in energy applications: A review. Renew. Sustain. Energy Rev. 2020, 129, 109931. [Google Scholar] [CrossRef]
- Reddy, K.; Mudgal, V.; Mallick, T. Review of latent heat thermal energy storage for improved material stability and effective load management. J. Energy Storage 2018, 15, 205–227. [Google Scholar] [CrossRef]
- Li, Z.; Ma, T.; Zhao, J.; Song, A.; Cheng, Y. Experimental study and performance analysis on solar photovoltaic panel integrated with phase change material. Energy 2019, 178, 471–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A review on phase change material application in building. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Kong, X.; Jie, P.; Yao, C.; Liu, Y. Experimental study on thermal performance of phase change material passive and active combined using for building application in winter. Appl. Energy 2017, 206, 293–302. [Google Scholar] [CrossRef]
- Abdolmaleki, L.; Sadrameli, S.; Pirvaram, A. Application of environmental friendly and eutectic phase change materials for the efficiency enhancement of household freezers. Renew. Energy 2020, 145, 233–241. [Google Scholar] [CrossRef]
- Liang, S.; Tian, C.; Zhu, Y.; Gu, Y.; Chen, K.; Wang, J. Phase-Change Energy-Storage Material Nanocapsule and Preparation Method Thereof. CN104449590A, 25 March 2015. [Google Scholar]
- Wang, X.; Jiang, F.; Wu, D. Magnetic Microencapsulated Phase Change Energy Storage Material and Preparation Method Thereof. CN103992774A, 20 August 2014. [Google Scholar]
- Li, Y.; Wang, F. High Temperature Resistant Type Phase-Change Material Micro-Capsule and Preparation Thereof. CN101376800B, 9 February 2011. [Google Scholar]
- Wang, J.; Xie, H.; Li, Y. Paraffinic Based Carbon Nano-Tube Compound Phase Transformation Heat Accumulating Material and Preparation Thereof. CN101407714A, 15 April 2009. [Google Scholar]
- Christ, M.U.; Ottinger, O.H.; Bacher, J.J. Latent Heat Storage Material and Process for Manufacture of the Latent Heat Storage Material. U.S. Patent US7923112B2, 12 April 2011. [Google Scholar]
- White, M.; Brehm, P. Systems, Apparatus and Methods for Thermal Energy Storage, Coupling and Transfer. U.S. Patent US8464535B2, 18 June 2013. [Google Scholar]
- Ho, C.; Gao, J. An experimental study on melting heat transfer of paraffin dispersed with Al2O3 nanoparticles in a vertical enclosure. Int. J. Heat Mass Transf. 2013, 62, 2–8. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khodadadi, J.; Al-Hattab, T.A.; Al-Mashat, S.M. Experimental and numerical investigation of melting of phase change material/nanoparticle suspensions in a square container subjected to a constant heat flux. Int. J. Heat Mass Transf. 2013, 66, 672–683. [Google Scholar] [CrossRef]
- Zeng, Y.; Fan, L.-W.; Xiao, Y.-Q.; Yu, Z.-T.; Cen, K.-F. An experimental investigation of melting of nanoparticle-enhanced phase change materials (NePCMs) in a bottom-heated vertical cylindrical cavity. Int. J. Heat Mass Transf. 2013, 66, 111–117. [Google Scholar] [CrossRef]
- Li, Z.-R.; Hu, N.; Liu, J.; Zhang, R.-H.; Fan, L.-W. Revisiting melting heat transfer of nano-enhanced phase change materials (NePCM) in differentially-heated rectangular cavities using thermochromic liquid crystal (TLC) thermography. Int. J. Heat Mass Transf. 2020, 159, 120119. [Google Scholar] [CrossRef]
- Parsazadeh, M.; Duan, X. Numerical and statistical study on melting of nanoparticle enhanced phase change material in a shell-and-tube thermal energy storage system. Appl. Therm. Eng. 2017, 111, 950–960. [Google Scholar] [CrossRef]
- Hosseinizadeh, S.; Darzi, A.R.; Tan, F. Numerical investigations of unconstrained melting of nano-enhanced phase change material (NEPCM) inside a spherical container. Int. J. Therm. Sci. 2012, 51, 77–83. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Xu, X.; Zhao, Y. Experimental study on the storage and release characteristics of phase change materials with different nanomaterials as addictives. Heat Mass Transf. 2020, 56, 2769–2777. [Google Scholar] [CrossRef]
- Ji, C.; Qin, Z.; Low, Z.; Dubey, S.; Choo, F.H.; Duan, F. Non-uniform heat transfer suppression to enhance PCM melting by angled fins. Appl. Therm. Eng. 2018, 129, 269–279. [Google Scholar] [CrossRef]
- Xie, J.; Lee, H.M.; Xiang, J. Numerical study of thermally optimized metal structures in a Phase Change Material (PCM) enclosure. Appl. Therm. Eng. 2019, 148, 825–837. [Google Scholar] [CrossRef]
- Mostafavi, A.; Parhizi, M.; Jain, A. Semi-analytical thermal modeling of transverse and longitudinal fins in a cylindrical phase change energy storage system. Int. J. Therm. Sci. 2020, 153, 106352. [Google Scholar] [CrossRef]
- Pourakabar, A.; Darzi, A.A.R. Enhancement of phase change rate of PCM in cylindrical thermal energy storage. Appl. Therm. Eng. 2019, 150, 132–142. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical modeling of nano enhanced PCM solidification in an enclosure with metallic fin. J. Mol. Liq. 2018, 259, 424–438. [Google Scholar] [CrossRef]
- Nguyen-Thoi, T.; Bhatti, M.; Ali, J.A.; Hamad, S.M.; Sheikholeslami, M.; Shafee, A.; Haq, R.U. Analysis on the heat storage unit through a Y-shaped fin for solidification of NEPCM. J. Mol. Liq. 2019, 292, 111378. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Jafaryar, M.; Shafee, A.; Li, Z. Simulation of nanoparticles application for expediting melting of PCM inside a finned enclosure. Phys. A Stat. Mech. Appl. 2019, 523, 544–556. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E.C. Simultaneous energy storage and recovery in the triplex-tube heat exchanger with PCM, copper fins and Al2O3 nanoparticles. Energy Convers. Manag. 2019, 180, 949–961. [Google Scholar] [CrossRef]
- Zhang, X.; Sheikholeslami, M.; Yan, W.-M.; Shafee, A.; Selimefendigil, F.; Babazadeh, H. Energy storage analysis for discharging of nanoparticle enhanced phase change material within a triplex-tube thermal storage. J. Energy Storage 2020, 31, 101640. [Google Scholar] [CrossRef]
- Alizadeh, M.; Hosseinzadeh, K.; Mehrzadi, H.; Ganji, D. Investigation of LHTESS filled by Hybrid nano-enhanced PCM with Koch snowflake fractal cross section in the presence of thermal radiation. J. Mol. Liq. 2019, 273, 414–424. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Haq, R.-U.; Shafee, A.; Li, Z. Heat transfer behavior of nanoparticle enhanced PCM solidification through an enclosure with V shaped fins. Int. J. Heat Mass Transf. 2019, 130, 1322–1342. [Google Scholar] [CrossRef]
- Li, Z.; Sheikholeslami, M.; Ayani, M.; Shamlooei, M.; Shafee, A.; Waly, M.I.; Tlili, I. Acceleration of solidification process by means of nanoparticles in an energy storage enclosure using numerical approach. Phys. A Stat. Mech. Appl. 2019, 524, 540–552. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical simulation for solidification in a LHTESS by means of nano-enhanced PCM. J. Taiwan Inst. Chem. Eng. 2018, 86, 25–41. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M.; Ahmadlouydarab, M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020, 115305. [Google Scholar] [CrossRef]
- Hussanan, A.; Salleh, M.Z.; Khan, I.; Shafie, S. Convection heat transfer in micropolar nanofluids with oxide nanoparticles in water, kerosene and engine oil. J. Mol. Liq. 2017, 229, 482–488. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Biwole, P.H. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in Porous Media; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Buongiorno, J. Convective transport in nanofluids. J. Heat Transf. 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Shamlooei, M.; Moradi, R. Fe3O4-Ethylene glycol nanofluid forced convection inside a porous enclosure in existence of Coulomb force. J. Mol. Liq. 2018, 249, 429–437. [Google Scholar] [CrossRef]
- Selvaraj, V.; Morri, B.; Nair, L.M.; Krishnan, H. Experimental investigation on the thermophysical properties of beryllium oxide-based nanofluid and nano-enhanced phase change material. J. Therm. Anal. Calorim. 2019, 137, 1527–1536. [Google Scholar] [CrossRef]
- Reddy, J.N.; Gartling, D.K. The Finite Element Method in Heat Transfer and Fluid Dynamics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zienkiewicz, O.C.; Taylor, R.L.; Nithiarasu, P. The Finite Element Method for Fluid Dynamics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6. [Google Scholar]
- De Los Reyes, J.C.; González Andrade, S. A combined BDF-semismooth Newton approach for time-dependent Bingham flow. Numer. Methods Partial Differ. Equ. 2012, 28, 834–860. [Google Scholar] [CrossRef]
- Schenk, O.; Gärtner, K. Solving unsymmetric sparse systems of linear equations with PARDISO. Future Gener. Comput. Syst. 2004, 20, 475–487. [Google Scholar] [CrossRef]
- Wriggers, P. Nonlinear Finite Element Methods; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Verbosio, F.; De Coninck, A.; Kourounis, D.; Schenk, O. Enhancing the scalability of selected inversion factorization algorithms in genomic prediction. J. Comput. Sci. 2017, 22, 99–108. [Google Scholar] [CrossRef]
- Kumar, L.; Manjunath, B.S.; Patel, R.J.; Markandeya, S.G.; Agrawal, R.G.; Agrawal, A.; Kashyap, Y.; Sarkar, P.S.; Sinha, A.; Iyer, K.N.; et al. Experimental investigations on melting of lead in a cuboid with constant heat flux boundary condition using thermal neutron radiography. Int. J. Therm. Sci. 2012, 61, 15–27. [Google Scholar] [CrossRef]
- Bertrand, O.; Binet, B.; Combeau, H.; Couturier, S.; Delannoy, Y.; Gobin, D.; Lacroix, M.; Le Quéré, P.; Médale, M.; Mencinger, J.; et al. Melting driven by natural convection A comparison exercise: First results. Int. J. Therm. Sci. 1999, 38, 5–26. [Google Scholar] [CrossRef]
- Gau, C.; Viskanta, R. Melting and solidification of a pure metal on a vertical wall. J. Heat Transfer. 1986, 108, 174–181. [Google Scholar] [CrossRef]
- Brent, A.; Voller, V.R.; Reid, K. Enthalpy-porosity technique for modeling convection-diffusion phase change: Application to the melting of a pure metal. Numer. Heat Transf. Part A Appl. 1988, 13, 297–318. [Google Scholar]
- Kashani, S.; Ranjbar, A.; Abdollahzadeh, M.; Sebti, S. Solidification of nano-enhanced phase change material (NEPCM) in a wavy cavity. Heat Mass Transf. 2012, 48, 1155–1166. [Google Scholar] [CrossRef]
- Kuehn, T.H.; Goldstein, R.J. An experimental and theoretical study of natural convection in the annulus between horizontal concentric cylinders. J. Fluid Mech. 1976, 74, 695. [Google Scholar] [CrossRef]




















| Properties | Cu | GO | Capric Acid |
|---|---|---|---|
| Density (kg m−3) | 8933 | 1800 | Solid: 1018 Liquid: 888 |
| Latent heat (kJ kg−1) | NA | NA | 152.7 |
| Thermal expansion coefficient (K−1) | 1.67 × 10−5 | 28.4 × 10−5 | 1 × 10−3 |
| Fusion temperature (°C) | NA | NA | 32 |
| Thermal conductivity (Wm−1 K−1) | 401 | 5000 | Solid: 0.372 Liquid: 0.153 |
| Specific heat (kJ kg−1 K−1) | 0.385 | 0.717 | Solid: 1.9 Liquid: 2.4 |
| Kinematic viscosity (m2 s−1) | NA | NA | 3 × 10−6 |
| Grid Case | 1 | 2 * | 3 | 4 |
|---|---|---|---|---|
| elements | 7926 | 12,385 | 17,761 | 23,854 |
| computing time | 118 | 184 | 215 | 247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghalambaz, M.; Mehryan, S.A.M.; Feeoj, R.K.; Hajjar, A.; Younis, O.; Talebizadehsardari, P.; Yaïci, W. Effect of the Quasi-Petal Heat Transfer Tube on the Melting Process of the Nano-Enhanced Phase Change Substance in a Thermal Energy Storage Unit. Sustainability 2021, 13, 2871. https://doi.org/10.3390/su13052871
Ghalambaz M, Mehryan SAM, Feeoj RK, Hajjar A, Younis O, Talebizadehsardari P, Yaïci W. Effect of the Quasi-Petal Heat Transfer Tube on the Melting Process of the Nano-Enhanced Phase Change Substance in a Thermal Energy Storage Unit. Sustainability. 2021; 13(5):2871. https://doi.org/10.3390/su13052871
Chicago/Turabian StyleGhalambaz, Mohammad, Seyed Abdollah Mansouri Mehryan, Reza Kalantar Feeoj, Ahmad Hajjar, Obai Younis, Pouyan Talebizadehsardari, and Wahiba Yaïci. 2021. "Effect of the Quasi-Petal Heat Transfer Tube on the Melting Process of the Nano-Enhanced Phase Change Substance in a Thermal Energy Storage Unit" Sustainability 13, no. 5: 2871. https://doi.org/10.3390/su13052871
APA StyleGhalambaz, M., Mehryan, S. A. M., Feeoj, R. K., Hajjar, A., Younis, O., Talebizadehsardari, P., & Yaïci, W. (2021). Effect of the Quasi-Petal Heat Transfer Tube on the Melting Process of the Nano-Enhanced Phase Change Substance in a Thermal Energy Storage Unit. Sustainability, 13(5), 2871. https://doi.org/10.3390/su13052871








