Sustaining Medicinal Barks: Survival and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae), a Tropical Tree under Experimental Debarking
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Current Harvest Practices
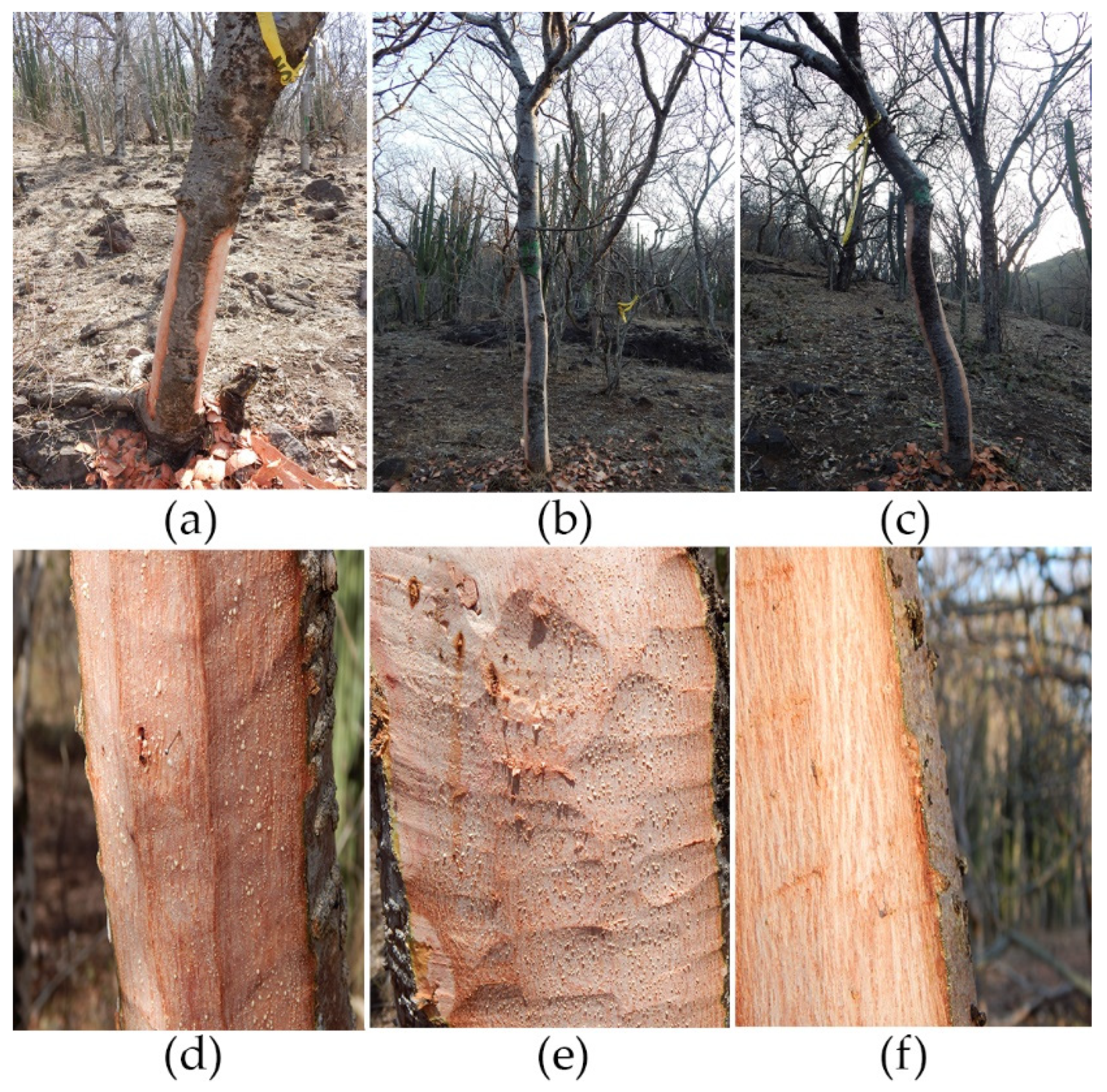
2.4. Experimental Debarking and Bark Regeneration Assessment
2.5. Data Analysis
3. Results
3.1. Qualitative Description of Bark Regeneration
3.2. Quantitative Evaluation of Bark Regeneration
3.3. Survival Rate of Amphipterygium adstringens
4. Discussion
4.1. Bark Regeneration and Tolerance of A. adstringens after Debarking
4.2. Factors That Affect Bark Regeneration of A. adstringens
4.3. Impact of Debarking on A. adstringens Survival
4.4. Sustaining Medicinal Barks: Challenges and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shackleton, S.; Shackleton, C.; Shanley, P. Non-Timber Forest Products in the Global Context, 1st ed.; Tropical Forestry Series; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological sustainability of non-timber forest product harvest: Principles and methods. In Ecological Sustainability for Non-Timber Forest Products. Dynamics and Case Studies of Harvesting, 1st ed.; Shackleton, C., Pandey, A., Ticktin, T., Eds.; People and Plants International-Conservation Series; Routledge: London, UK, 2015; Chapter 3; pp. 31–53. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Hall, P.; Bawa, K. Methods to assess the impact of extraction of Non-Timber Tropical Forest Products on Plant Populations. Econ. Bot. 1993, 47, 234–247. [Google Scholar] [CrossRef]
- Delvaux, C.; Sinsin, B.; Darchambeau, F.; Van Damme, P. Recovery from bark harvesting of 12 medicinal tree species in Benin, West Africa. J. Appl. Ecol. 2009, 46, 703–712. [Google Scholar] [CrossRef]
- Delvaux, C.; Sinsin, B.; Van Damme, P. Impact of season, stem diameter and intensity of debarking on survival and bark re-growth pattern of medicinal tree species, Benin, West Africa. Biol. Conserv. 2010, 143, 2664–2671. [Google Scholar] [CrossRef]
- Cunningham, A.; Campbell, B.; Luckert, M. Bark: Use, Management, and Commerce in Africa. Adv. Econ. Bot. 2014, 17, 1–288. [Google Scholar]
- Blancas, J.; Caballero, J.; Beltrán-Rodríguez, L.; Cortés, L. Non-Timber Forest Products of Mexico: An Overview, 1st ed.; Thematic Network Non-Timber Forest Products of the National Council of Science and Technology (CONACYT No. 280901); CONACYT: Mexico City, Mexico, 2017.
- Beltrán-Rodríguez, L.; Cristians, S.; Sierra-Huelsz, A.; Blancas, J.; Maldonado-Almanza, B.; Bye, R. Barks as Non-Timber Forest Products in Mexico: National Analysis and Recommendations for their Sustainable Use, 1st ed.; Instituto de Biología, Universidad Nacional Autónoma de México (UNAM): Mexico City, Mexico, 2020; ISBN 978-607-30-4054-9. [Google Scholar]
- Fierro, A.; Guerrero, C.; Hersch-Martínez, P.; Pérez, A. Some commercially important wild medicinal barks from the tropical deciduous forests in the Balsas River Basin: Effect of the harvest on its population density. In Agroforestry Systems in Latin America and the Tropical Deciduous Forest in Mexico, 1st ed.; Monroy, R., Colín, H., Boyas, C., Eds.; National Institute of Forestry, Agricultural and Livestock Research (INIFAP): Mexico City, Mexico; Autonomous University of the Morelos State (UAEM): Cuernavaca, Mexico, 2000; pp. 533–541. [Google Scholar]
- Linares, E.; Bye, R. Traditional Markets in Mesoamerica: A Mosaic of History and Traditions. In Ethnobotany of Mexico: Interactions of Peoples and Plants in Mesoamerica, 1st ed.; Lira, R., Casas, A., Blancas, J., Eds.; Springer: New York, NY, USA, 2016; Chapter 7; pp. 151–178. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L.; Manzo-Ramos, F.; Maldonado-Almanza, B.; Martínez-Ballesté, A.; Blancas, J. Wild Medicinal Species Traded in the Balsas Basin, Mexico: Risk Analysis and Recommendations for Their Conservation. J. Ethnobiol. 2017, 37, 743–764. [Google Scholar] [CrossRef]
- Monteiro, J.; Lins, E.; de Lima, E.; Amorim, E.; Albuquerque, U. Bark regeneration and tannin content in Myracrodruon urundeuva Allemão after simulation of extractive damages-Implications to management. Environ. Monit. Assess. 2011, 180, 31–39. [Google Scholar] [CrossRef]
- Soares, I.; Sobral, A.; Monteiro, J.; Lima, E.; Albuquerque, U. Impact of collection on bark regeneration from Stryphnodendron rotundifolium Mart. in northeastern Brazil. Environ. Monit. Assess. 2017, 189, 234. [Google Scholar] [CrossRef]
- Cunningham, A.; Mbenkum, F. Sustainability of Harvesting Prunus Africana Bark in Cameroon: A Medicinal Plant in International Trade, 1st ed.; People and Plants; UNESCO: Paris, France, 1993. [Google Scholar]
- Baldauf, C.; Maës dos Santos, F. The effect of management systems and ecosystems types on bark regeneration in Himatanthus drasticus (Apocynaceae): Recommendations for sustainable harvesting. Environ. Monit. Assess. 2014, 186, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Biggs, A. Phellogen regeneration in injured peach tree Bark. Ann. Bot. 1986, 57, 463–470. [Google Scholar] [CrossRef]
- Pandey, A.; Mandal, A. Sustainable Harvesting of Terminalia arjuna (Roxb.) Wight & Arnot (Arjuna) and Litsea glutinosa (Lour.) Robinson (Maida) Bark in Central India. J. Sustain. Forest. 2012, 31, 294–309. [Google Scholar] [CrossRef]
- Solares, F.; Jasso, J.; Vargas-Hernández, J.; Soto, M.; Rodríguez, C. Regeneration capacity in lateral thickness and in crust of Cuachalalate (Amphipterygium adstringens Schiede ex Schlect.) in the Morelos State. Ra Ximhai 2006, 2, 481–495. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L.; Romero-Manzanares, A.; Luna-Cavazos, M.; García-Moya, E. Architectural and morphological variation of Hintonia latiflora (Rubiaceae) in relation to bark harvest and environmental factors. Rev. Biol. Trop. 2017, 65, 900–916. [Google Scholar] [CrossRef][Green Version]
- Solares, F.; Vázquez-Alvarado, J.; Gálvez-Cortés, M. Commercialization channels of cuachalalate (Amphipterigium adstringens Schiede ex Schlecht.) bark in Mexico. Rev. Mex. Cienc. For. 2012, 3, 29–42. [Google Scholar]
- Da Silva, J.; da Silva, L.; Albuquerque, U.; Cardoso, C. Bark and latex harvesting short-term impact on native tree species reproduction. Environ. Monit. Assess. 2018, 190, 744. [Google Scholar] [CrossRef]
- Solares, F. Bark Regeneration Capacity and Phytochemical Evaluation before and after Debarking in Cuachalalate. Master’s Thesis, Colegio de Postgraduados Campus Montecillo, Texcoco, Mexico, 1995. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (para Adaptarlo a las Condiciones de la República Mexicana), 16th ed.; Instituto de Geografía, Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2004. [Google Scholar]
- Servicio Meteorológico Nacional. Datos climáticos. Departamento de información estadística. Comisión Nacional del Agua. Available online: https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=mor (accessed on 18 May 2014).
- Beltrán-Rodríguez, L.; Valdez-Hernández, J.; Luna-Cavazos, M.; Romero-Manzanares, A.; Pineda-Herrera, E.; Maldonado-Almanza, B.; Borja de la Rosa, M.; Blancas-Vázquez, J. Structure and tree diversity of secondary deciduous tropical forests in the Sierra de Huautla Biosphere Reserve, Morelos. Rev. Mex. Biodivers. 2018, 89, 108–122. [Google Scholar] [CrossRef]
- Durand, L. Pensar positivo no basta. Actitudes en torno a la conservación en la Reserva de la Biosfera Sierra de Huautla, México. Interciencia 2010, 35, 430–436. [Google Scholar]
- López-Medellín, X.; Vázquez, L.; Valenzuela-Galván, D.; Wehncke, E.; Maldonado-Almanza, B.; Durand-Smith, L. Percepciones de los habitantes de la Reserva de la Biósfera Sierra de Huautla: Hacia el desarrollo de nuevas estrategias de manejo participativo. Interciencia 2017, 42, 8–16. [Google Scholar]
- Abad-Fitz, I.; Maldonado-Almanza, B.; Aguilar-Dorantes, K.; Sánchez-Méndez, L.; Gómez-Caudillo, L.; Casas, A.; Blancas, J.; García-Rodríguez, Y.; Beltrán-Rodríguez, L.; Sierra-Huelsz, J.; et al. Consequences of traditional management in the production and quality of copal resin (Bursera bipinnata Moc. & Sessé ex DC.) Engl.) in Mexico. Forests 2020, 11, 991. [Google Scholar] [CrossRef]
- Cuevas, X. A revision of the genus Amphipterygium (Julianiaceae). Ibugana 2005, 13, 27–47. [Google Scholar]
- Argueta, A. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Ciudad de México, México, 1994.
- Olivera, A.G.; Soto, M.; Martínez, M.; Terrazas, T.; Solares, F. Phytochemical study of cuachalalate (Amphiptherygium adstringens Schiede ex Schlecht). J. Ethnopharmacol. 1999, 68, 109–113. [Google Scholar] [CrossRef]
- Oviedo-Chavez, I.; Ramírez-Apan, T.R.; Soto-Hernández, M.; Martínez-Vázquez, M. Principles of the bark of Amphipterygium adstringens (Julianaceae) with anti-inflammatory activity. Phytomedicine 2004, 11, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, A.; Mata, R. Monografía Científica de Plantas Medicinales de México. Cuachalalate-Amhipterygium adstringens-. Pruebas de Control de Calidad (Identidad y Composición), Eficacia y Seguridad, 1st ed.; Sentido Giratorio: Ciudad de México, Mexico, 2009. [Google Scholar]
- Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos. Farmacopea Herbolaria de los Estados Unidos Mexicanos, 2nd ed.; Secretaría de Salud: Ciudad de México, Mexico, 2013.
- Orduño, A. Bark Anatomy of Four Species of the Tropical Deciduous Forest of the State of Morelos: Origin, Development and Regeneration. Master’s Thesis, Colegio de Postgraduados Campus Montecillo, Texcoco, Mexico, 1998. [Google Scholar]
- Hersch-Martínez, P. Destino Común: Los Recolectores y su Flora Medicinal. El Comercio de Flora Medicinal Silvestre Desde el Suroccidente Poblano, 1st ed.; Instituto Nacional de Antropología e Historia: Ciudad de México, Mexico, 1999. [Google Scholar]
- Rodríguez, T. Management and Conservation of Commercial Medicinal Plants in the Municipality of Copalillo, Guerrero. Master’s Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2003. [Google Scholar]
- Solares, F.; Gálves, M. Manual para una Producción Sustentable de Corteza de Cuachalalate (Amphipterygium Adstringens Schiede ex Schlecht); Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP): Zacatepec, Morelos, 2002.
- Delvaux, C.; Sinsin, B.; Van Damme, P.; Beeckman, H. Wound reaction after bark harvesting: Microscopic and macroscopic phenomena in ten medicinal tree species (Benin). Trees 2010, 24, 941–951. [Google Scholar] [CrossRef]
- Mead, R.; Curnow, R.; Hasted, A. Statistical Methods in Agriculture and Experimental Biology, 2nd ed.; Springer Science & Business Media: New Delhi, India, 1993. [Google Scholar]
- Stemmler, M. Person-Centered Methods: Configural Frequency Analysis (CFA) and Other Methods for the Analysis of Contingency Tables, 1st ed.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- IBM Corp Released. IBM SPSS Statistics for Windows; Version 21.0; IBM Corp: New York, NY, USA, 2012. [Google Scholar]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2007. [Google Scholar]
- Firth, D. Bias reduction, the Jefreys prior and GLIM. In Advances in GLIM and Statistical Modelling, 1st ed.; Fahrmeir, L., Francis, B., Gilchrist, R., Tutz, G., Eds.; Springer: New York, NY, USA, 1992; pp. 91–100. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Vermeulen, W.; Geldenhuys, C. Experimental Protocols and Lessons from Strip Harvesting of Bark for Medicinal use in the Southern Cape Forests; Wild Resources Limited FRP-DFID Project R8305 Report; Department for International Development: London, UK, 2004.
- Mariot, A.; Mantovani, A.; Dos Reis, M. Bark harvesting systems of Drymis brasiliensis Miers in the Brazilian Atlantic Rainforest. Ann. Acad. Bras. Cienc. 2014, 86, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Guedje, N.; Tchamou, N.; Lejoly, J. Tree response to bark harvest: The case of a medicinal species, Garcinia lucida, as source of raw materials for plant-based drug development. J. Appl. Biosci. 2016, 99, 9476–9491. [Google Scholar] [CrossRef]
- Romero, C.; Bolker, B. Effects of stem anatomical and structural traits on responses of stem damage: And experimental study in the Bolivian Amazon. Can. J. For. Res. 2008, 38, 611–618. [Google Scholar] [CrossRef]
- Shigo, A. Compartmentalization: A conceptual framework for understanding how trees grow and defend themselves. Annu. Rev. Phytopathol 1984, 22, 189–214. [Google Scholar] [CrossRef]
- Stobbe, H.; Schmitt, U.; Eckstein, D.; Dujesiefken, D. Developmental stages and fine structure of surface callus formed after debarking of living Lime Trees (Tilia sp.). Ann. Bot. 2002, 89, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Mwange, K.; Hou, H.; Cui, K. Relationship between endogenous indole-3acetic acid and abscisic changes and bark recovery in Eucommia ulmoides Oliv. after girdling. J. Exp. Bot. 2003, 54, 1889–1907. [Google Scholar] [CrossRef]
- Ngubeni, N.; Jacobs, S.; Seydack, A.; Vermeulen, W.; Sass, G.; Seifert, T. Trade-off relationships between tree growth and defense: A comparison of Ocotea bullata and Curtisia dentata following bark harvesting in an evergreen moist South African Forest. Trees 2017, 31, 339–348. [Google Scholar] [CrossRef]
- Geldenhuys, C.; Syampungani, S.; Meke, G.; Vermeulen, W. Response of different species to bark harvesting for traditional medicine in Southern Africa. In Multiple use Management of Natural Forests and Woodlands: Policy Refinement and Scientific Progress, 1st ed.; Bester, J., Seydack, A., Vorster, T., Van der Merwe, I., Dzivhani, S., Eds.; Department of Water Affairs and Forestry: Pretoria, South Africa, 2007; pp. 55–62. [Google Scholar]
- Zimmermann, M.; Brown, C. Trees: Structure and Function, 1st ed.; Springer: New York, NY, USA, 1971. [Google Scholar]
- Neely, D. Wound closure rates on trees. Arboric. Urban For. 1988, 14, 250–254. [Google Scholar]
- Romero, C. Bark: Structure and Functional Ecology. Adv. Econ. Bot. 2014, 17, 5–26. [Google Scholar]
- Fisher, J. Wound healing by exposed secondary xylem in Adansonia (Bombacaceae). IAWA Bull. 1981, 2, 193–199. [Google Scholar] [CrossRef]
- Romero, C.; Dovie, D.; Gambiza, J.; Luoga, E.; Schmitt, S.; Grundy, I. Effect of commercial bark harvesting on Adansonia digitata (Baobab) in the Save-Odzi Valley, Zimbabwe, with considerations for its management. Adv. Econ. Bot. 2014, 17, 95–114. [Google Scholar]
- Guedje, N.; Tchamou, N. Strategies towards sustainable bark sourcing as raw material for plant-based drug development: A case study on Garcinia lucida tree species. J. Appl. Biosci. 2017, 115, 11502–11512. [Google Scholar] [CrossRef][Green Version]
- Rosell, J. Bark in Woody Plants: Understanding the Diversity of a Multifunctional Structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef]
- Delvaux, C.; Sinsin, B.; Van Damme, P.; Beeckman, H. Size of conducting phloem: The “key” factor for bark recovery of 12 tropical medicinal tree species. Flora 2013, 208, 111–117. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L. Structure, Population Dynamics and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae) in the Ejido El Limón, Morelos, México. Ph.D. Thesis, Colegio de Postgraduados Campus Montecillo, Texcoco, Mexico, 2018. [Google Scholar]
- Fasola, T.; Egunyomi, A. Bark extractivism and uses of some medicinal plants. Niger. J. Bot. 2002, 15, 26–36. [Google Scholar]
- Luna-Nieves, A.; Meave, J.; Morellato, L.; Ibarra-Manríquez, G. Reproductive phenology of useful seasonally dry tropical forest trees: Guiding patterns for seed collection and plant propagation in nurseries. For. Ecol. Manag. 2017, 393, 52–62. [Google Scholar] [CrossRef]
- Guariguata, M.; Gilbert, G. Interspecific variation rates of trunk wound closure in a Panamanian Lowland Forest. Biotropica 1996, 28, 23–29. [Google Scholar] [CrossRef]
- Bañuelos, M.J.; Obeso, J.R. Resource allocation in the dioecious shrub Rhamnus alpinus: The hidden costs of reproduction. Evol. Ecol. Res. 2004, 6, 397–413. [Google Scholar]
- Montesinos, D.; De Luis, M.; Verdú, M.; Raventos, J.; García-Fayos, P. When, how and how much: Gender-specific resource-use strategies in the dioecious tree Juniperus thurifera. Ann. Bot. 2006, 98, 885–889. [Google Scholar] [CrossRef]
- Iszkulo, G.; Boratynski, A. Initial period of sexual maturity determines the greater growth rate of male over female in the dioecious tree Juniperus communis subsp. communis. Acta Oecol. 2011, 37, 99–102. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Gaylord, M.L.; Martinson, S.; Wagner, M.R. Attraction to monoterpenes and beetle-produced compounds by syntopic Ips and Dendroctonus bark beetles and their predators. Agric. For. Entomol. 2012, 14, 207–215. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.; Allen, C.; Breshears, D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2012, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Mass, M.; Burgos, A. Water Dynamics at the Ecosystem Level in Seasonally Dry Tropical Forests. In Seasonally Dry Tropical Forests. Ecology and Conservation, 1st ed.; Dirzo, R., Young, H., Mooney, H., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; Chapter 9; pp. 141–156. [Google Scholar] [CrossRef]
- Poorter, L.; McNeil, A.; Hurtado, V.; Prins, H.; Putz, F. Bark traits and life-history strategies of tropical dry- and moist forest trees. Funct. Ecol. 2014, 28, 232–242. [Google Scholar] [CrossRef]
- Devereux, S.; Sabates-Wheeler, R.; Longhurst, R. Seasonality, Rural Livelihoods and Development, 1st ed.; Routledge: London, UK, 2014. [Google Scholar]
- Hersch-Martínez, P.; González-Chévez, L.; Alvarez, A. Endogenous knowledge and practice regarding the environment in a Nahua community in Mexico. Agr. Hum. Val. 2004, 21, 127–137. [Google Scholar] [CrossRef]
- NOM-005-RECNAT. NORMA Oficial Mexicana NOM-005-RECNAT-1997, Que Establece los Procedimientos, Criterios y Especificaciones para Realizar el Aprovechamiento, Transporte y Almacenamiento de Corteza, Tallos y Plantas Completas de Vegetación Forestal; Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT): Mexico City, Mexico, 1997.
- De La O-Toris, J.; Maldonado-Almanza, B.; Martínez-Garza, C. Efecto de la perturbación en la comunidad de herbáceas nativas y ruderales de una elva estacional mexicana. Bot. Sci. 2012, 90, 469–480. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Osorio-Beristain, M.; Valenzuela-Galván, D.; Nicolás-Medina, A. Intra and inter-annual variation in seed rain in a secondary dry tropical forest excluded from chronic disturbance. For. Ecol. Manag. 2011, 262, 2207–2218. [Google Scholar] [CrossRef]
- Stockdale, M.; López-Binnqüist, C. Manejo Comunitario Sustentable de Productos Forestales No Maderables. Un Manual para América Latina, 1st ed.; NTFP-EP: Maynila, Philippines; Centro de Investigaciones Tropicales de la Universidad Veracruzana (CITRO-UV): Xalapa, Mexico; Red-PFNM: Mexico City, Mexico; People and Plants International: Bristol, TN, USA, 2019. [Google Scholar]
- Hersch-Martínez, P. Commercialization of wild medicinal plants from southwest Puebla, Mexico. Econ. Bot. 1995, 49, 197–206. [Google Scholar] [CrossRef]
- Hersch-Martínez, P. Medicinal plants and regional traders in Mexico: Physiographic differences and conservational challenge. Econ. Bot. 1997, 51, 107–120. [Google Scholar] [CrossRef]


| Sex | Debarking Season | Diameter Category | Treatment | Mean (cm) | Standard Error | Variation Coefficient | Median | Variance |
|---|---|---|---|---|---|---|---|---|
| Male | Dry | DBH1 | T1 | 0.15 | 0.01 | 60.02% | 0.15 | 0.008 |
| DBH1 | T2 | 0.14 | 0.02 | 95.23% | 0.09 | 0.01 | ||
| DBH1 | T3 | 0.13 | 0.01 | 70.80% | 0.1 | 0.008 | ||
| DBH2 | T1 | 0.13 | 0.01 | 61.12% | 0.12 | 0.006 | ||
| DBH2 | T2 | 0.13 | 0.01 | 62.14% | 0.13 | 0.007 | ||
| DBH2 | T3 | 0.1 | 0.01 | 91.50% | 0.1 | 0.008 | ||
| Wet | DBH1 | T1 | 0.7 | 0.03 | 36.05% | 0.74 | 0.063 | |
| DBH1 | T2 | 0.76 | 0.03 | 27.47% | 0.81 | 0.043 | ||
| DBH1 | T3 | 0.74 | 0.03 | 27.75% | 0.78 | 0.043 | ||
| DBH2 | T1 | 0.41 | 0.05 | 99.20% | 0.39 | 0.171 | ||
| DBH2 | T2 | 0.76 | 0.03 | 27.39% | 0.79 | 0.043 | ||
| DBH2 | T3 | 0.78 | 0.03 | 27.69% | 0.8 | 0.04 | ||
| Female | Dry | DBH1 | T1 | 0.23 | 0.03 | 91.08% | 0.17 | 0.04 |
| DBH1 | T2 | 0.05 | 0.01 | 164.23% | 0.01 | 0.007 | ||
| DBH1 | T3 | 0.15 | 0.02 | 97.21% | 0.14 | 0.023 | ||
| DBH2 | T1 | 0.3 | 0.03 | 61.53% | 0.34 | 0.035 | ||
| DBH2 | T2 | 0.16 | 0.02 | 82.18% | 0.17 | 0.019 | ||
| DBH2 | T3 | 0.26 | 0.03 | 66.53% | 0.22 | 0.03 | ||
| Wet | DBH1 | T1 | 0.65 | 0.06 | 70.11% | 0.87 | 0.208 | |
| DBH1 | T2 | 0.86 | 0.03 | 24.40% | 0.89 | 0.044 | ||
| DBH1 | T3 | 0.71 | 0.05 | 56.15% | 0.87 | 0.161 | ||
| DBH2 | T1 | 0.78 | 0.02 | 26.48% | 0.82 | 0.043 | ||
| DBH2 | T2 | 0.79 | 0.02 | 25.63% | 0.82 | 0.041 | ||
| DBH2 | T3 | 0.87 | 0.03 | 27.34% | 0.95 | 0.05 |
| Origin | Type III Sums of Squares | f.d. | Squared Mean | F | p Value | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Corrected model | 10.195 a | 23 | 0.443 | 7.183 | 0 | 0.775 |
| Intercept | 27.355 | 1 | 27.355 | 443.241 | 0 | 0.902 |
| Season | 8.08 | 1 | 8.08 | 130.924 | 0 | 0.732 |
| Sex | 0.084 | 1 | 0.084 | 1.362 | 0.249 | 0.028 |
| Diameter | 0.039 | 1 | 0.039 | 0.635 | 0.429 | 0.013 |
| Treatment | 0.062 | 2 | 0.031 | 0.504 | 0.607 | 0.021 |
| Season:Sex | 0 | 1 | 0 | 0.006 | 0.94 | 0 |
| Season:Diameter | 0.009 | 1 | 0.009 | 0.151 | 0.699 | 0.003 |
| Season:Treatment | 0.463 | 2 | 0.232 | 3.751 | 0.031 | 0.135 |
| Sex:Diameter | 0.507 | 1 | 0.507 | 8.21 | 0.006 | 0.146 |
| Sex:Treatment | 0.141 | 2 | 0.07 | 1.14 | 0.328 | 0.045 |
| Diameter:Treatment | 0.118 | 2 | 0.059 | 0.956 | 0.392 | 0.038 |
| Season:Sex:Diameter | 0.064 | 1 | 0.064 | 1.031 | 0.315 | 0.021 |
| Season:Sex:Treatment | 0.089 | 2 | 0.045 | 0.724 | 0.49 | 0.029 |
| Season:Diameter:Treatment | 0.142 | 2 | 0.071 | 1.149 | 0.325 | 0.046 |
| Sex:Diameter:Treatment | 0.209 | 2 | 0.105 | 1.696 | 0.194 | 0.066 |
| Season:Sex:Diameter:Treatment | 0.187 | 2 | 0.094 | 1.518 | 0.229 | 0.06 |
| Error | 2962 | 48 | 0.062 | - | - | - |
| Total | 40.513 | 72 | - | - | - | - |
| Total corrected | 13.158 | 71 | - | - | - | - |
| Coefficient | Standard Error | Lower Limit (95%) | Upper Limit (95%) | X2 | p Value | |
|---|---|---|---|---|---|---|
| Intercept | 0.845 | 1.087 | −1.019 | 3.277 | 0.767 | 0.381 |
| Season (dry) | 1.892 | 1.749 | −1.125 | 8.845 | 1.368 | 0.242 |
| Sex (male) | 0.584 | 1.422 | −2.188 | 3.681 | 0.185 | 0.667 |
| Diameter (≥20 cm) | 0.584 | 1.422 | −2.188 | 3.681 | 0.185 | 0.667 |
| Treatment (50%) | 0.793 | 1.675 | −2.368 | 5.842 | 0.241 | 0.624 |
| Treatment (75%) | 0.075 | 1.442 | −2.898 | 2.939 | 0.003 | 0.956 |
| Season (dry):Sex (male) | −0.039 | 1.602 | −6.039 | 3.993 | 0 | 0.985 |
| Season (dry):Diameter (≥20 cm) | −0.039 | 1.602 | −6.039 | 3.993 | 0 | 0.985 |
| Season (dry): Treatment 50% | −4.424 | 2.093 | −12.586 | −0.832 | 6.258 | 0.012 |
| Season (dry): Treatment 75% | −2.363 | 1.75 | −8.013 | 0.739 | 2.166 | 0.141 |
| Sex (male):Diameter (≥20 cm) | −2.041 | 1.361 | −4.789 | 0.477 | 2.528 | 0.112 |
| Sex (male):Treatment (50%) | 1.448 | 1.991 | −3.314 | 8.481 | 0.365 | 0.546 |
| Sex (male):Treatment (75%) | 0.821 | 1.751 | −3.307 | 6.371 | 0.178 | 0.673 |
| Diameter (≥ 20cm):Treatment (50%) | 1.448 | 1.991 | −3.314 | 8.481 | 0.365 | 0.546 |
| Diameter (≥ 20cm):Treatment (75%) | 0.821 | 1.751 | −3.307 | 6.371 | 0.178 | 0.673 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Rodríguez, L.; Valdez-Hernández, J.I.; Saynes-Vásquez, A.; Blancas, J.; Sierra-Huelsz, J.A.; Cristians, S.; Martínez-Ballesté, A.; Romero-Manzanares, A.; Luna-Cavazos, M.; Borja de la Rosa, M.A.; et al. Sustaining Medicinal Barks: Survival and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae), a Tropical Tree under Experimental Debarking. Sustainability 2021, 13, 2860. https://doi.org/10.3390/su13052860
Beltrán-Rodríguez L, Valdez-Hernández JI, Saynes-Vásquez A, Blancas J, Sierra-Huelsz JA, Cristians S, Martínez-Ballesté A, Romero-Manzanares A, Luna-Cavazos M, Borja de la Rosa MA, et al. Sustaining Medicinal Barks: Survival and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae), a Tropical Tree under Experimental Debarking. Sustainability. 2021; 13(5):2860. https://doi.org/10.3390/su13052860
Chicago/Turabian StyleBeltrán-Rodríguez, Leonardo, Juan Ignacio Valdez-Hernández, Alfredo Saynes-Vásquez, José Blancas, José Antonio Sierra-Huelsz, Sol Cristians, Andrea Martínez-Ballesté, Angélica Romero-Manzanares, Mario Luna-Cavazos, Ma. Amparo Borja de la Rosa, and et al. 2021. "Sustaining Medicinal Barks: Survival and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae), a Tropical Tree under Experimental Debarking" Sustainability 13, no. 5: 2860. https://doi.org/10.3390/su13052860
APA StyleBeltrán-Rodríguez, L., Valdez-Hernández, J. I., Saynes-Vásquez, A., Blancas, J., Sierra-Huelsz, J. A., Cristians, S., Martínez-Ballesté, A., Romero-Manzanares, A., Luna-Cavazos, M., Borja de la Rosa, M. A., Pineda-Herrera, E., Maldonado-Almanza, B., Ángeles-Pérez, G., Ticktin, T., & Bye, R. (2021). Sustaining Medicinal Barks: Survival and Bark Regeneration of Amphipterygium adstringens (Anacardiaceae), a Tropical Tree under Experimental Debarking. Sustainability, 13(5), 2860. https://doi.org/10.3390/su13052860







