The Use of Ethanol as an Alternative Fuel for Small Turbojet Engines
Abstract
1. Introduction
2. Materials and Methods
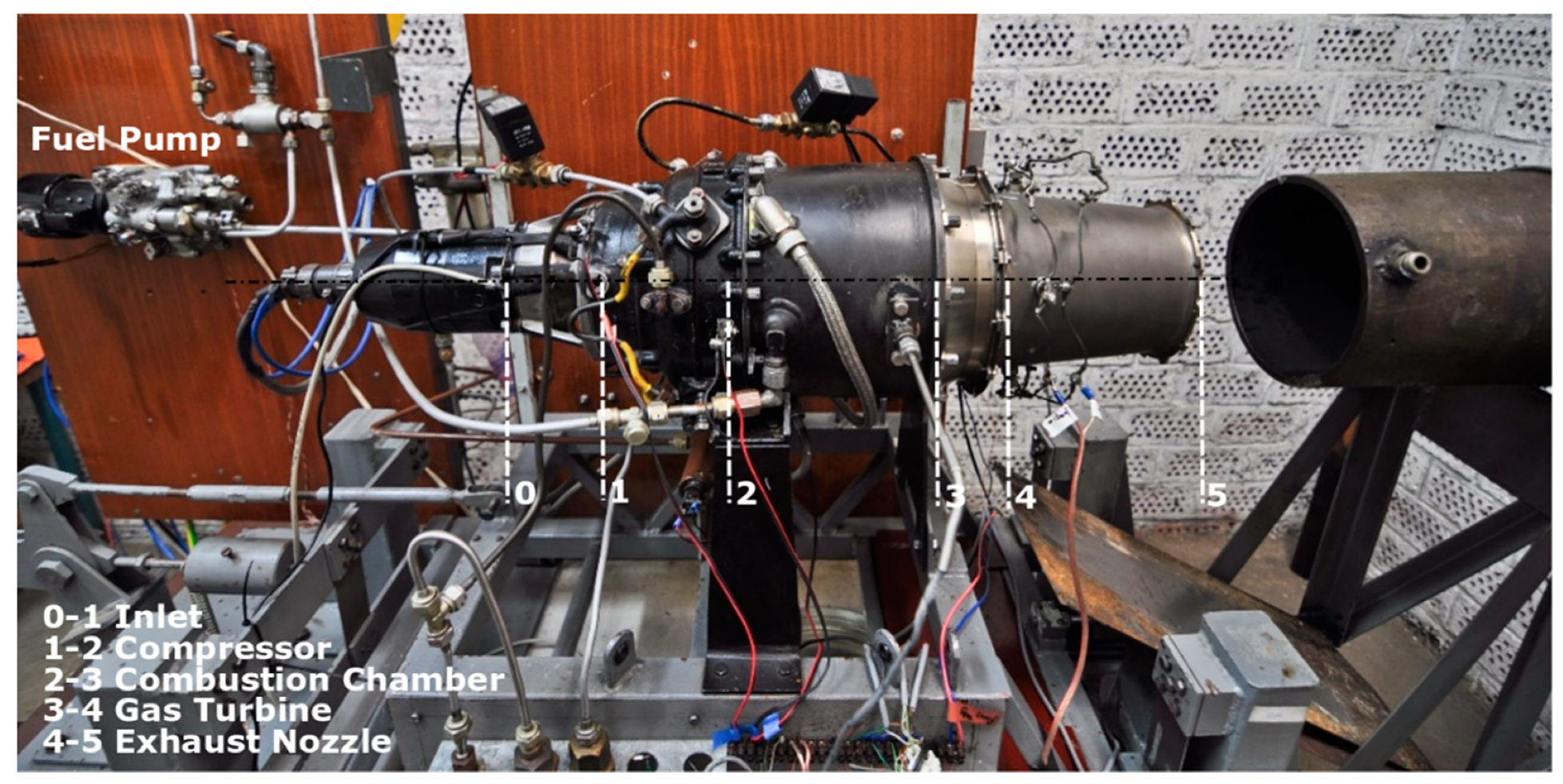
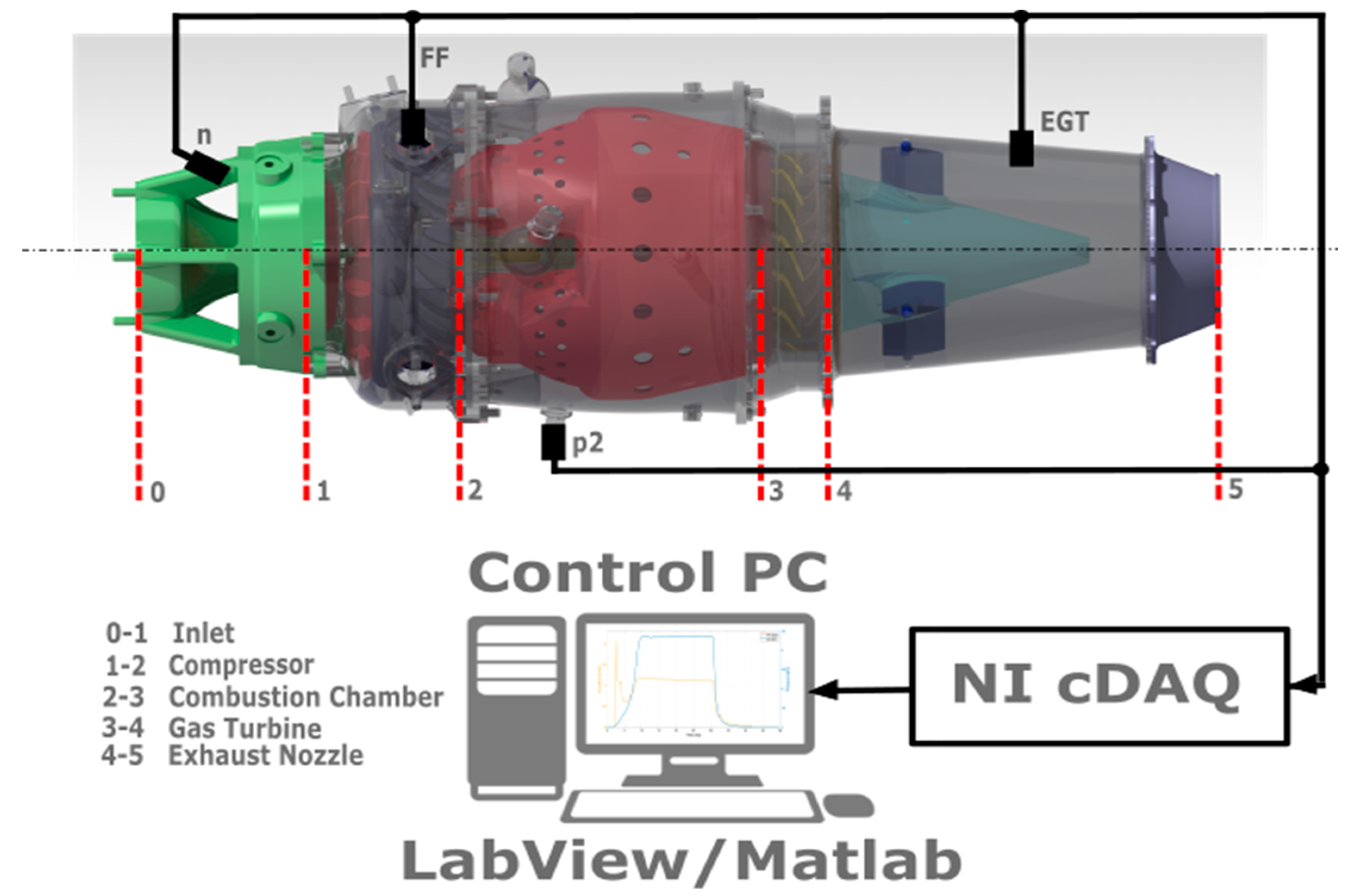
2.1. The Experimental Engine and Measurement System
- NI 9263—100 kHz Voltage Output Module, used for the control of analogue engine systems;
- NI 9472—8-Channel, Digital I/O Module, used for the control of digital engine systems;
- NI 9205—±10 V, 250 kHz, 16-Bit, 32-Channel C Series Voltage Input Module, used for the measurement of analogue sensors, in this case, QBE2002-P10;
- NI 9423—8 Channel Sinking Input, C Series Digital Module, used for the measurement of frequency signals, in this case, optical speed sensor;
- NI 9213—NI-9213, 16-channel, Thermocouple Input Module, used for the measurement of thermocouples, in this case, K-thermocouple EGT sensors.
2.2. The Experimental Fuel
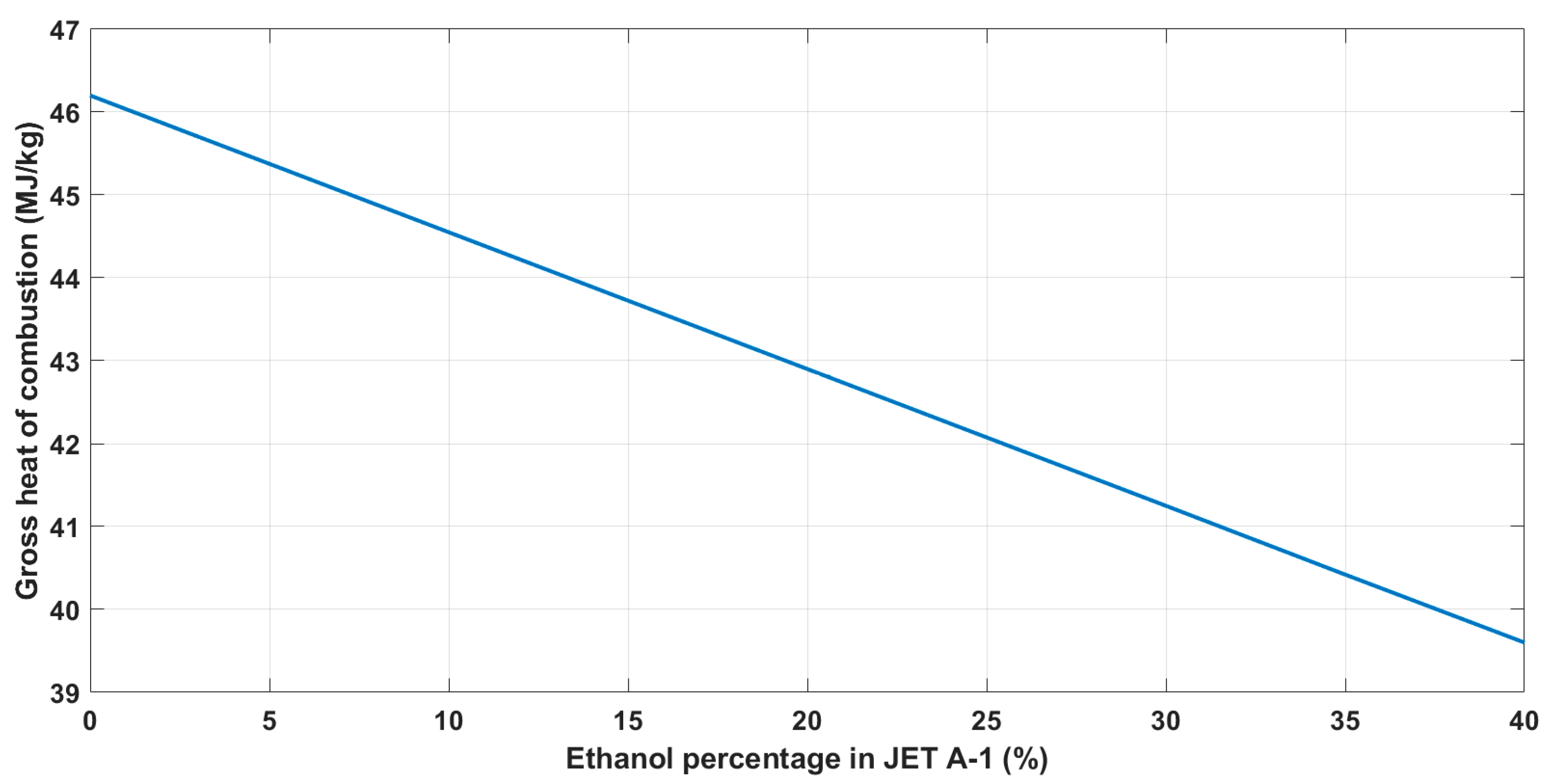
2.3. Calculation of the Gross Heat of the Combustion of Alternative Fuel Blends
- QPCS
- is the gross heat of combustion (MJ/kg);
- E
- is the water equivalent of the calorimeter, the bomb, accessories, and the water introduced into the bomb (MJ/kg);
- Ti
- is the initial temperature (K);
- Tm
- is the maximum temperature (K);
- b
- is the correction required for the combustion heat of the “fuels” used during the test, i.e., the firing wire, cotton thread, cigarette-making paper, and benzoic acid or combustion aid (MJ/kg);
- c
- is the temperature correction factor required for the exchange of heat with the outside, which is nil if an adiabatic jacket is used (K);
- m
- is the mass of the test specimen in kilograms (kg).
2.4. Experimental Design
- E0: Five experimental runs and a single run between each blend
- E10, E20, E30, E35, E40: Three experimental runs for each concentration
3. Results
- Peak time (tP): the time for which the maximum value of variables (peak values) was measured.
- Peak value: the measured value at the peak time.
- Settling time (tS): the time required for the measured (response) curve to reach and stay within a range of ±2% of the steady state (final) value. The settling time is, in our case, defined by Condition (3).
- Integral of value (A): In our case, the areas under the curves were computed using the midpoint rule of the numerical integral:
- Overshoot value (percentage overshoot PO):
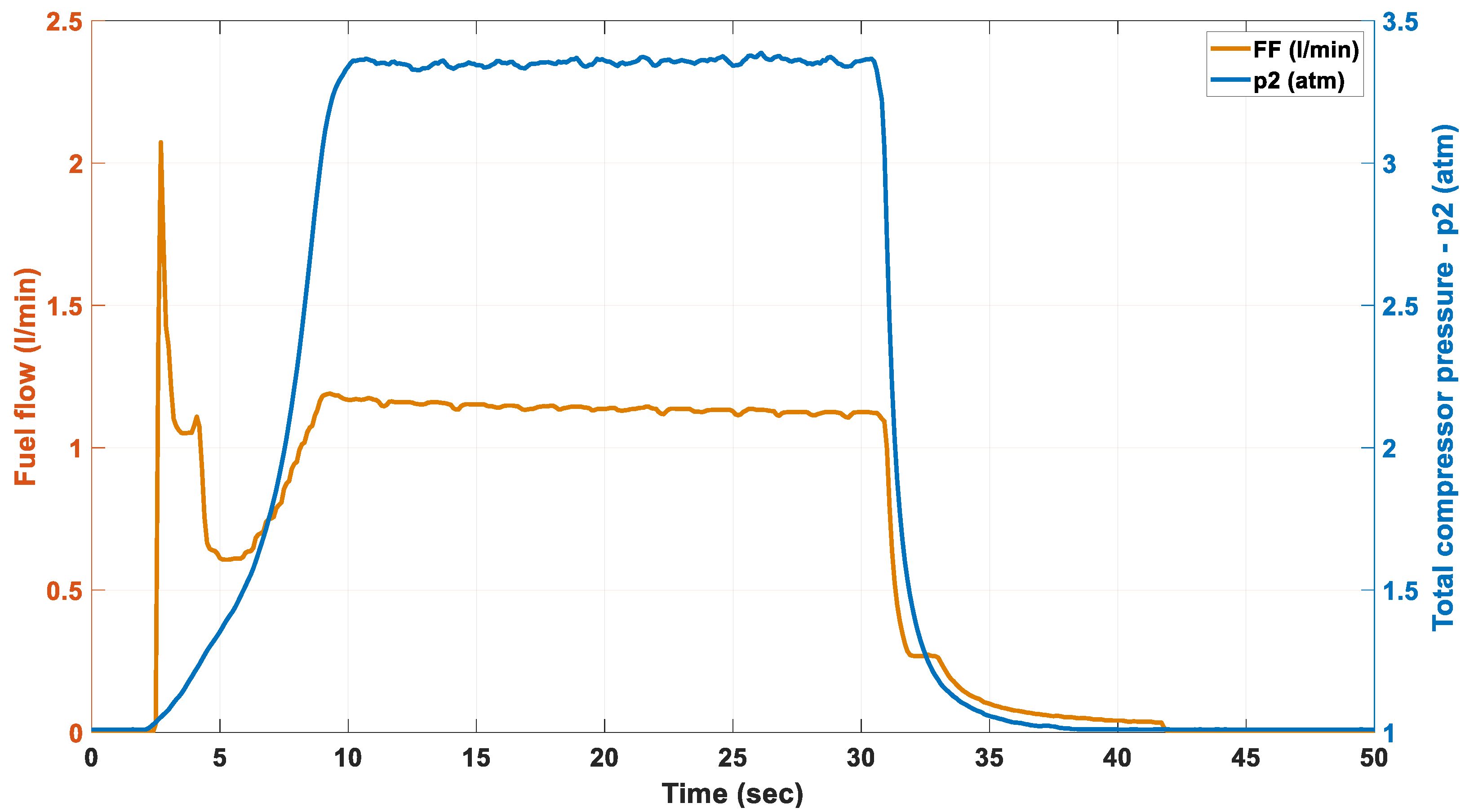
3.1. The Independent Variable—Fuel Flow
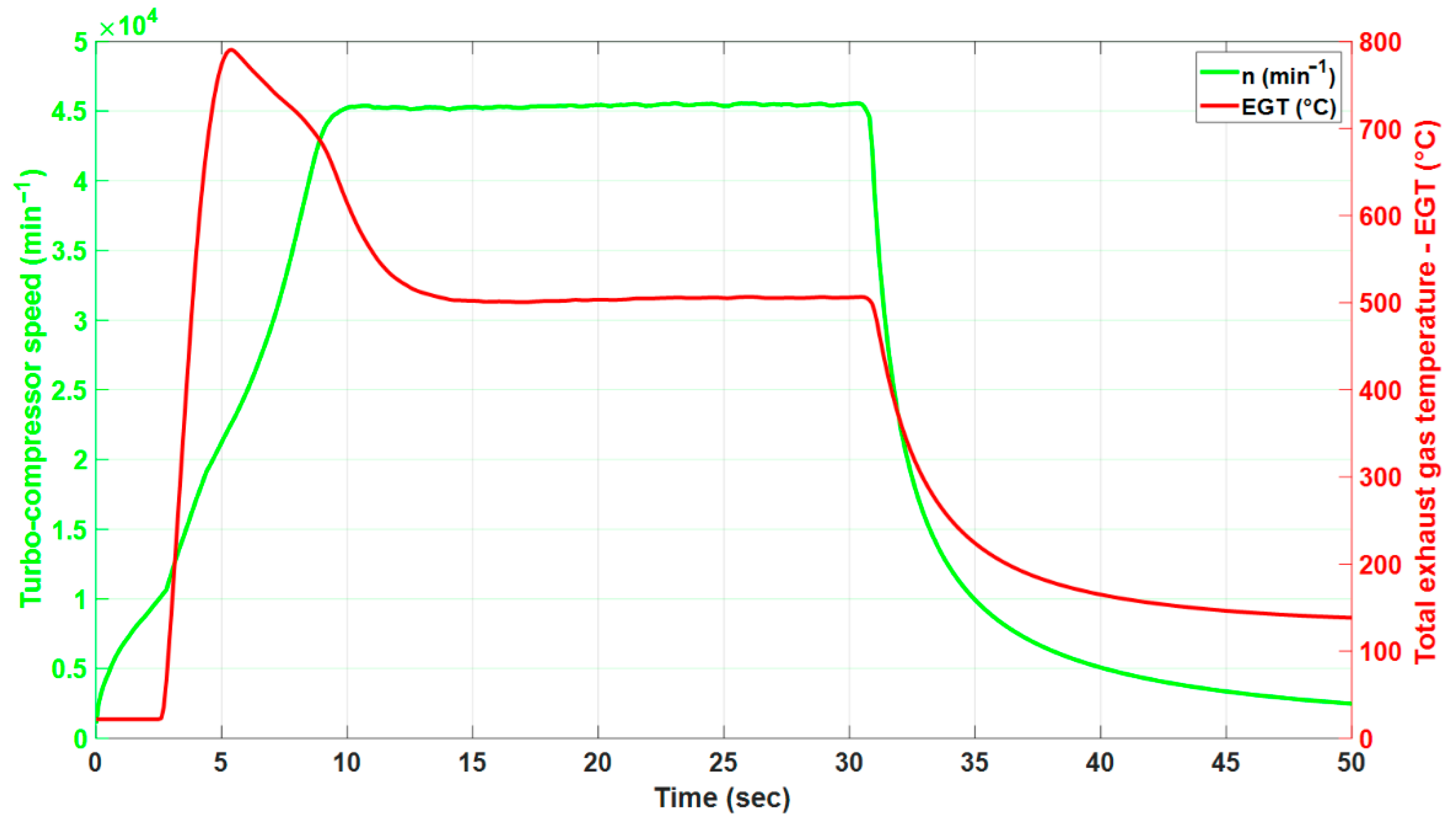
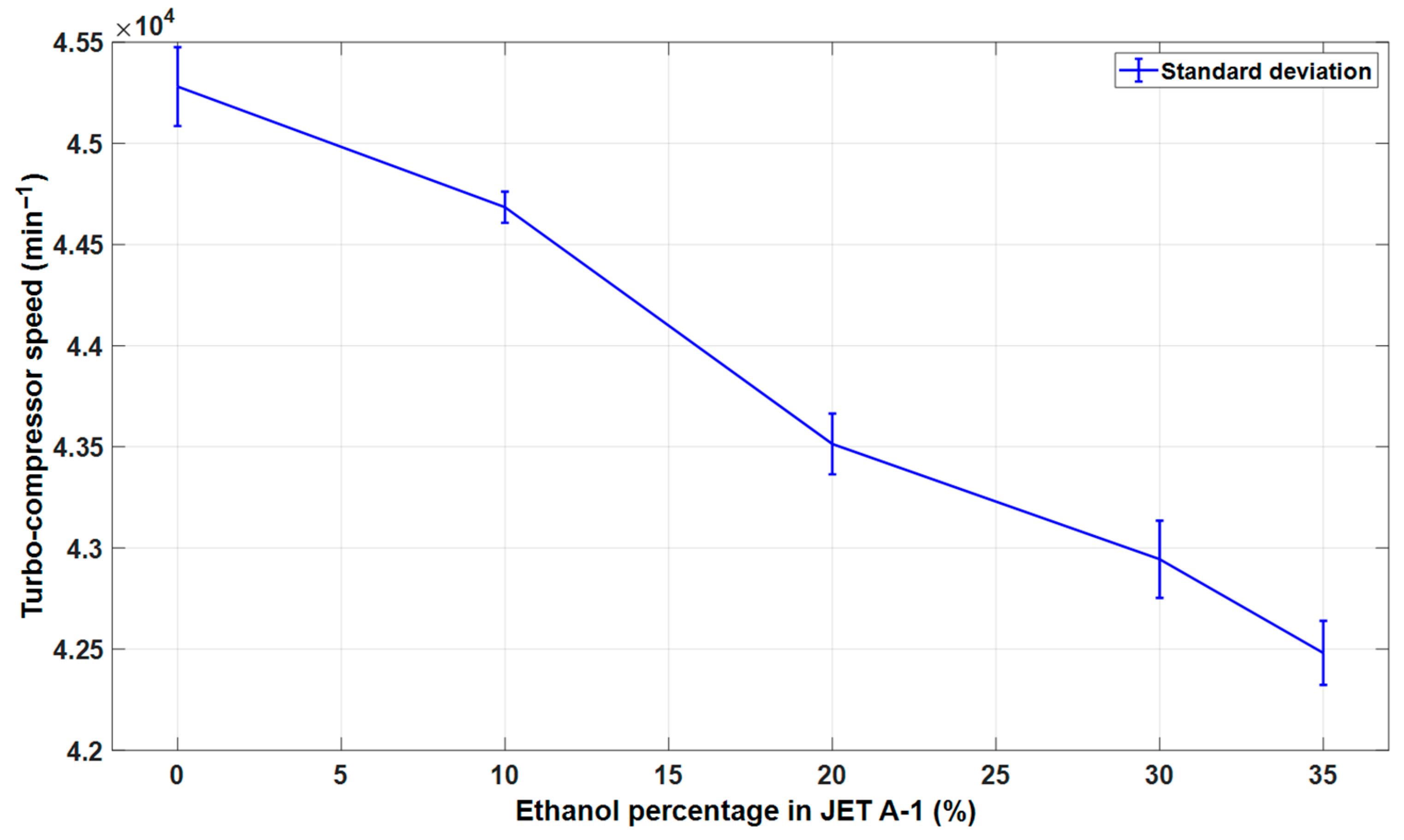
3.2. The Effect of Alternative Fuel on the Stable Speed of the Engine
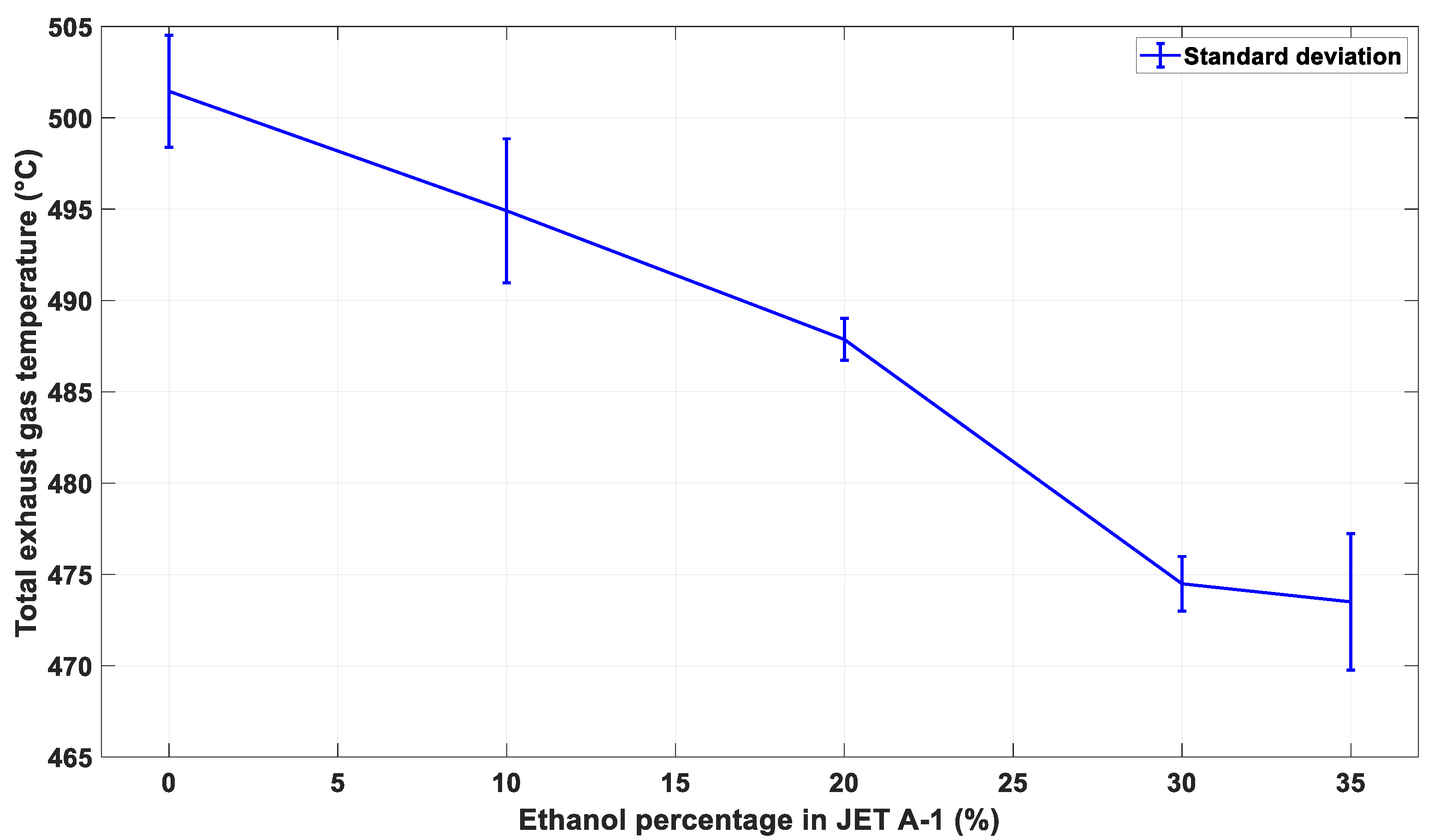
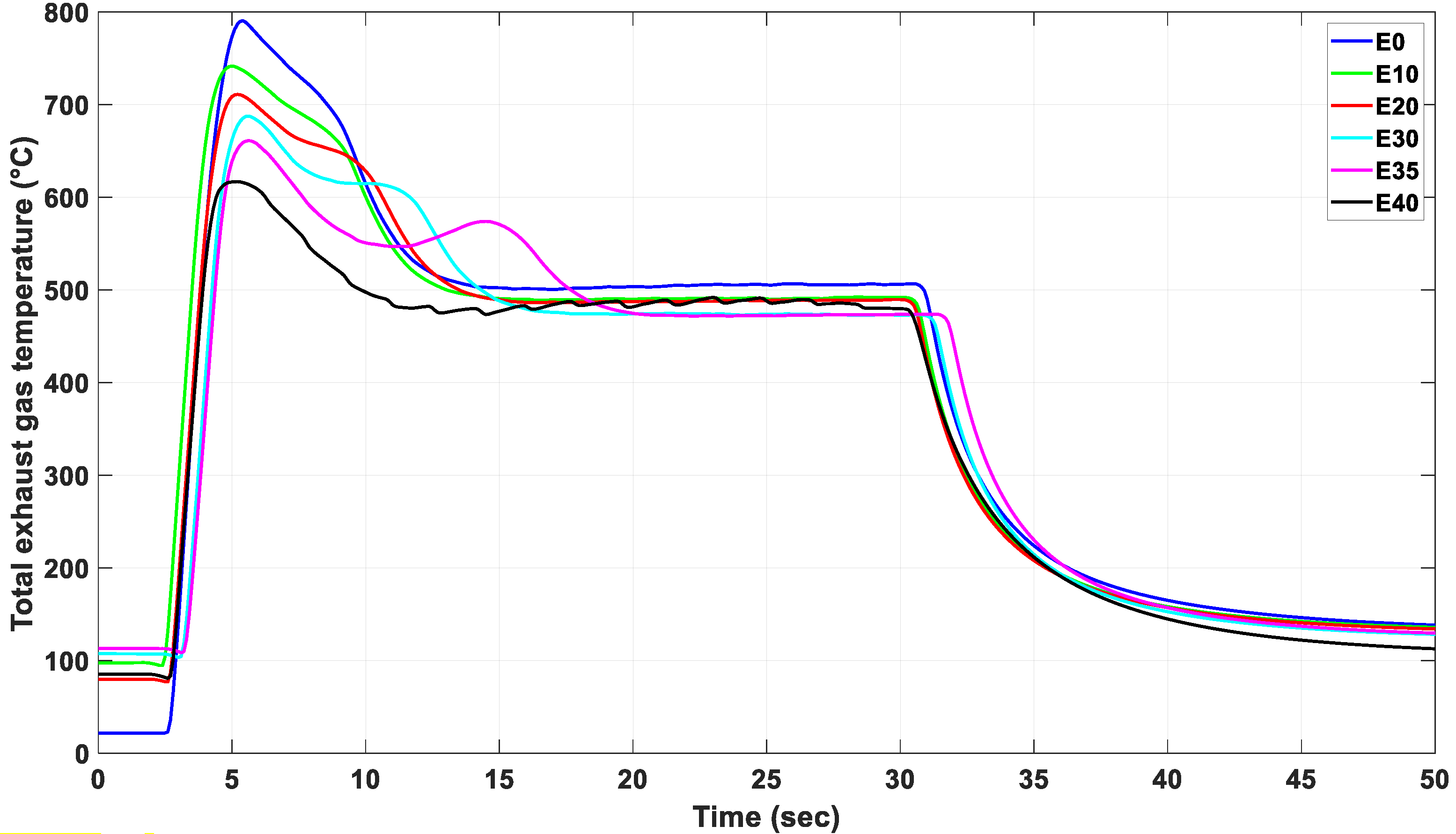
3.3. The Effect of Alternative Fuel on Stable Exhaust Gas Temperature of the Engine
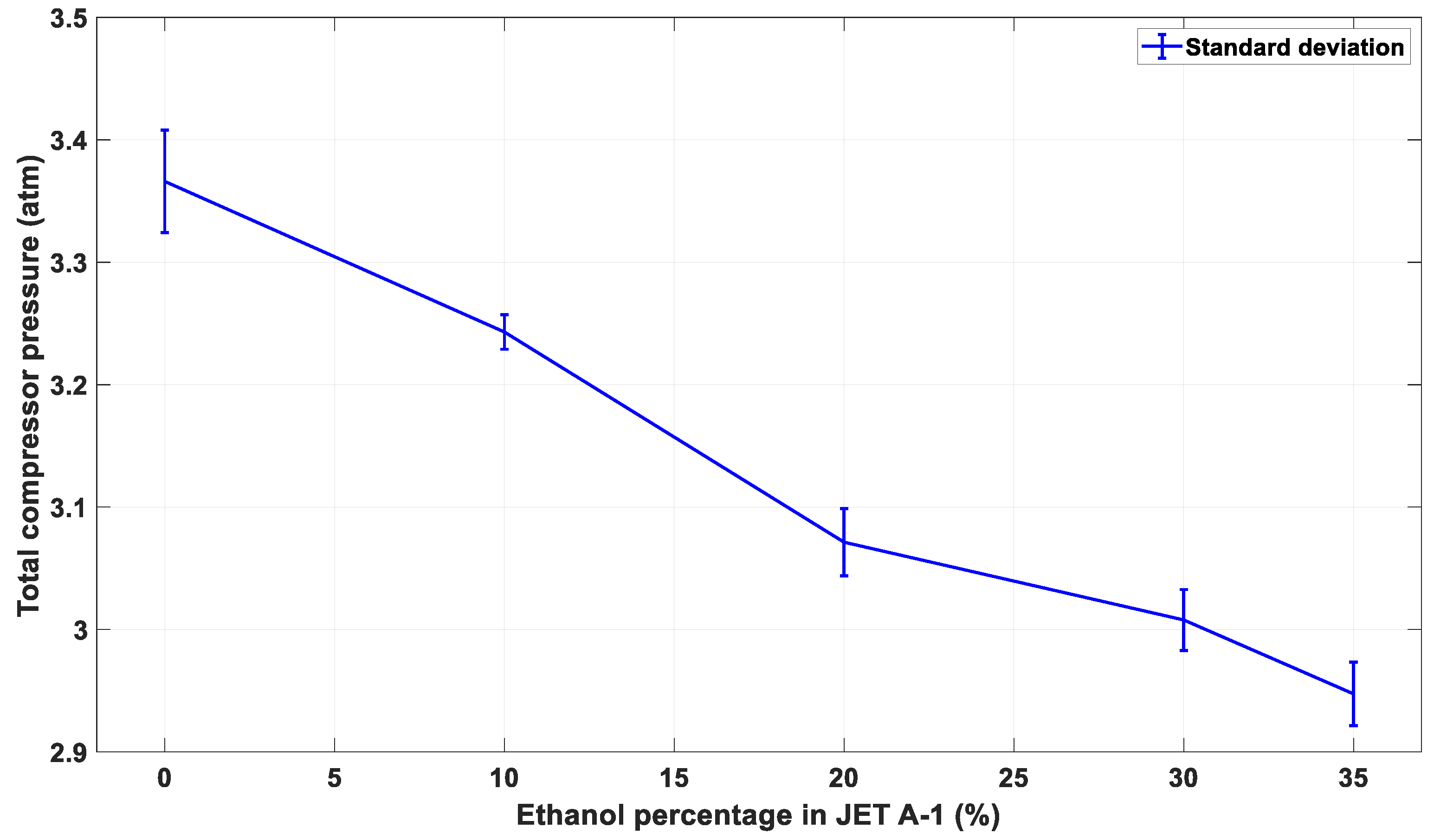
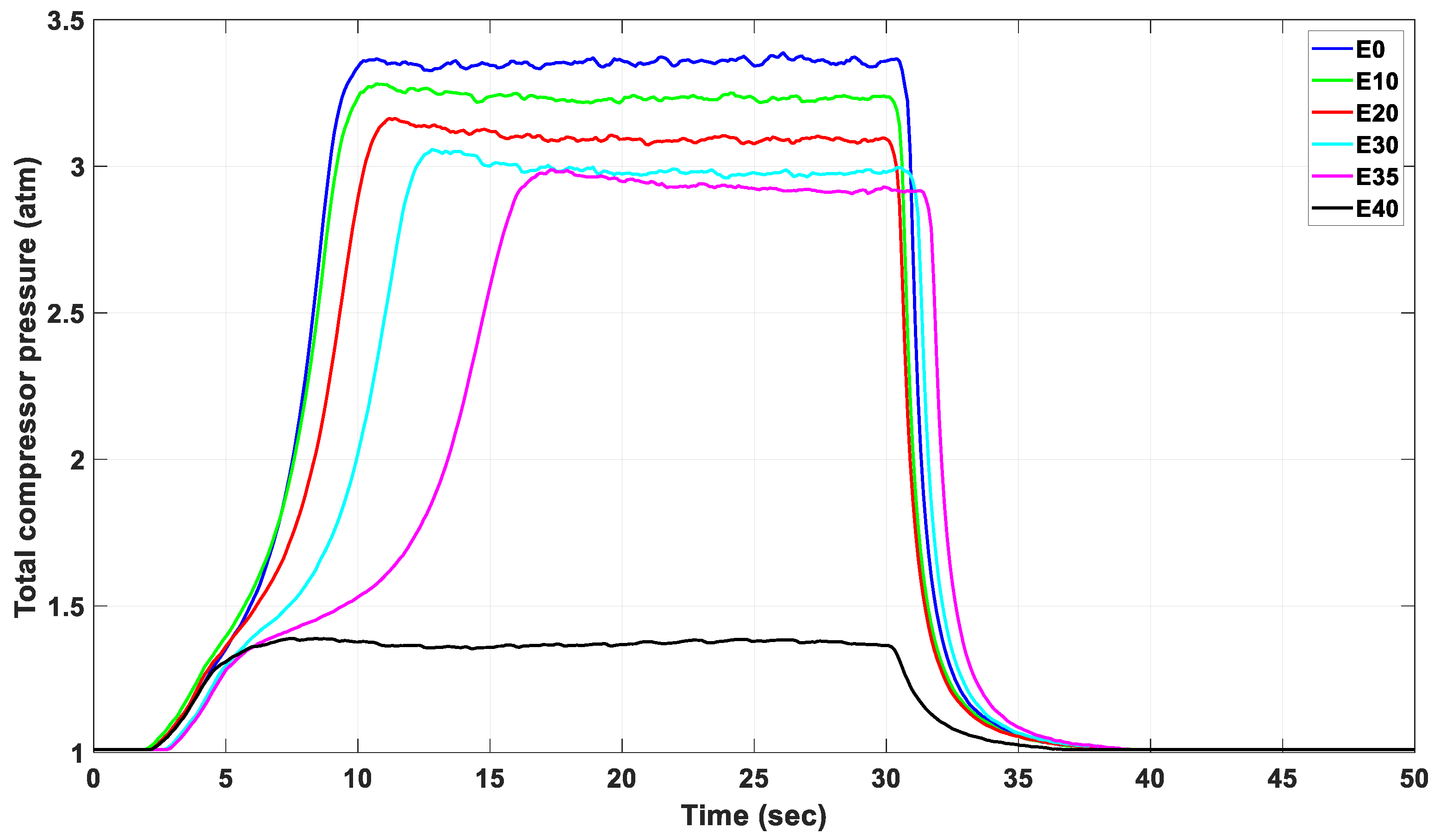
3.4. The Effect of Alternative Fuel on the Stable Compressor Pressure of the Engine
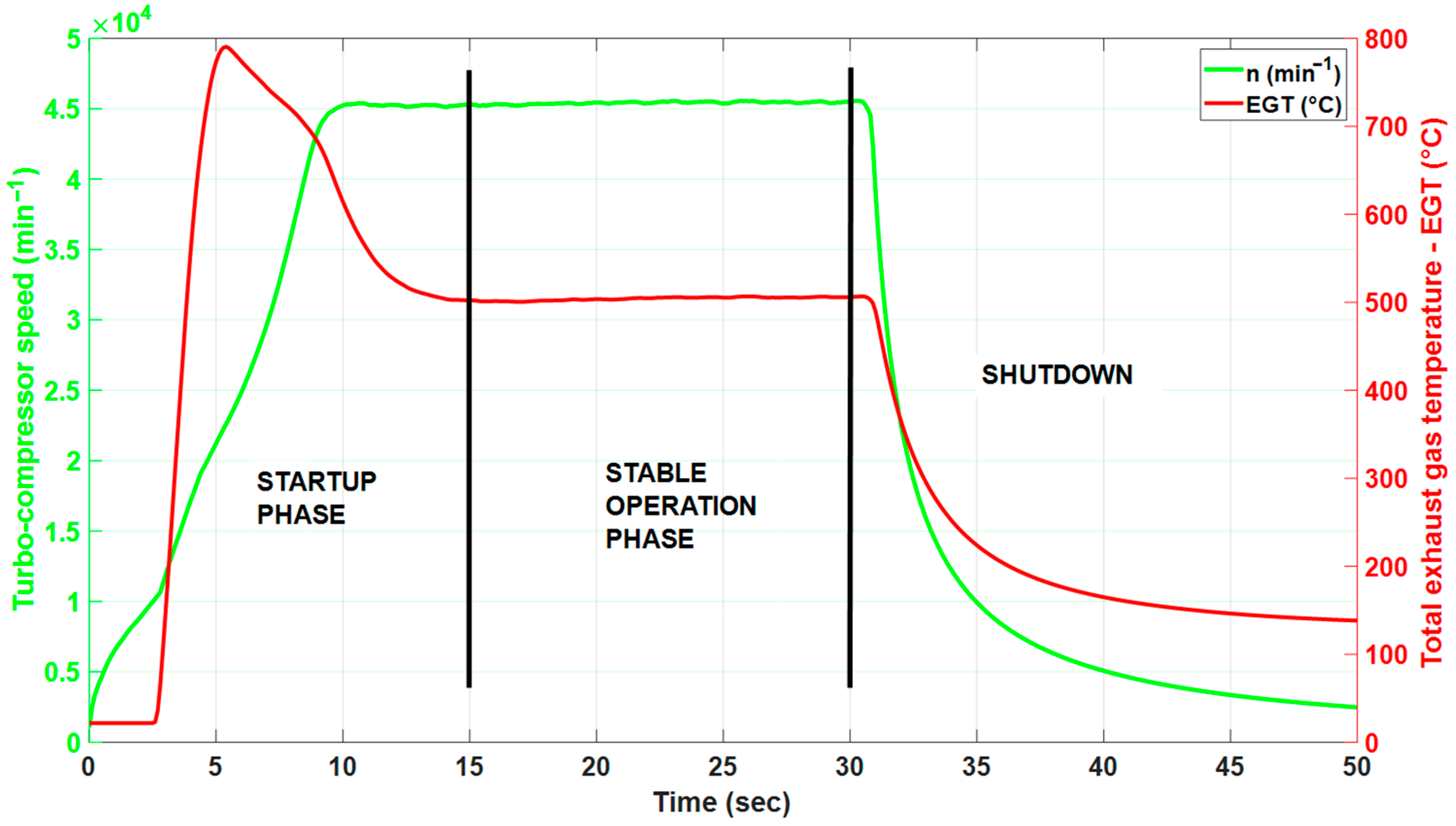
3.5. The Effect of Alternative Fuel on the Start-Up of the Engine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| 2-EH | 2-Ethylhexanol |
| AVGAS | Aviation Gas |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| E | Ethanol |
| EGT | Exhaust Gas Temperature |
| FF | Fuel Flow |
| GHG | Greenhouse Gas |
| H2O | Water |
| HC | Hydrocarbon |
| HEFA | Hydro-processed Esters and Fatty Acids |
| HHV | Higher Heating Value |
| JET A | Jet Fuel |
| MPM-20 | Small Turbojet Engine |
| n | Rotational Speed of the Engine |
| N2 | Nitrogen |
| NI | National Instruments |
| NOx | Nitrogen Oxide |
| p2 | Total Compressor Pressure |
| PL | Phase Level |
| PM | Particulate Matter |
| PME | Palm Methyl Ester |
| PR | Phase Range |
| SPK | Synthesized Paraffinic Kerosene |
References
- Greenhouse Gas Emission Statistics-Emission Inventories. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/1180.pdf (accessed on 25 February 2021).
- Blakey, S.; Rye, L.; Wilson, C.W. Aviation gas turbine alternative fuels: A review. Proc. Combust. Inst. 2011, 33, 2863–2885. [Google Scholar] [CrossRef]
- Airbus–Global Market Forecast. Available online: https://www.airbus.com/content/dam/corporate-topics/financial-and-company-information/GMF-2019-Press-Release.pdf (accessed on 15 April 2020).
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254. [Google Scholar] [CrossRef]
- Turner, J.; Lewis, A.G.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.; Edwards, P.P. Alcohol fuels for spark-ignition engines: Performance, efficiency and emission effects at mid to high blend rates for binary mixtures and pure components. Automob. Eng. 2018, 232, 36–56. [Google Scholar] [CrossRef]
- Tian, Z.; Zhen, X.; Wang, Y.; Liu, D.; Li, X. Comparative study on combustion and emission characteristics of methanol, ethanol and butanol fuel in TISI engine. Fuel 2020, 259. [Google Scholar] [CrossRef]
- Dandu, M.S.R.; Nanthagopal, K. Tribological aspects of biofuels–A review. Fuel 2019, 258, 116066. [Google Scholar] [CrossRef]
- Turner, J.W.G.; Pearson, R.J.; Holland, B.; Peck, R. Alcohol-based fuels in high performance engines. SAE Trans. 2007, 55–69. [Google Scholar] [CrossRef]
- Goldemberg, J. The ethanol program in Brazil. Environ. Res. Lett. 2006, 1, 014008. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coelho, S.T. History, evolution, and environmental impact of biodiesel in Brazil: A Review. Renew. Sustain. Energy Rev. 2017, 75, 168–179. [Google Scholar] [CrossRef]
- Grahn, M.; Hansson, J. Prospects for domestic biofuels for transport in Sweden 2030 based on current production and future plans. Wiley Interdiscip. Rev. Energy Environ. 2015, 4, 290–306. [Google Scholar] [CrossRef][Green Version]
- De Simio, L.; Gambino, M.; Iannaccone, S. Effect of ethanol content on thermal efficiency of a spark-ignition light-duty engine. ISRN Renew. Energy 2012. [Google Scholar] [CrossRef][Green Version]
- Tibaquirá, J.E.; Huertas, J.I.; Ospina, S.; Quirama, L.F.; Niño, J.E. The effect of using ethanol-gasoline blends on the mechanical, energy and environmental performance of in-use vehicles. Energies 2018, 11, 221. [Google Scholar] [CrossRef]
- Myung, C.-L.; Choi, K.; Cho, J.; Kim, K.; Baek, S.; Lim, Y.; Park, S. Evaluation of regulated, particulate, and BTEX emissions inventories from a gasoline direct injection passenger car with various ethanol blended fuels under urban and rural driving cycles in Korea. Fuel 2020, 262. [Google Scholar] [CrossRef]
- Sandalcı, T.; Karagöz, Y.; Orak, E.; Yüksek, L. An experimental investigation of ethanol-diesel blends on performance and exhaust emissions of diesel engines. Adv. Mech. Eng. 2014, 6. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S.; Laurinaitis, K. Effect of jet A-1/ethanol fuel blend on HCCI combustion and exhaust emissions. J. Energy Eng. 2018, 144. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Lu, Y.; Zhang, C.; Zhang, X.; Zhang, C.; Roskilly, A.P. Experimental study of the gaseous and particulate matter emissions from a gas turbine combustor burning butyl butyrate and ethanol blends. Appl. Energy 2017, 195, 693–701. [Google Scholar] [CrossRef]
- Shauck, M.E.; Tubbs, J.; Zanin, M.G. Certification of a Carburater Aircraft Engine on Ethanol Fuel. Available online: https://afdc.energy.gov/files/pdfs/2896.pdf (accessed on 25 February 2021).
- Iliev, S. A Comparison of Ethanol, Methanol and Butanol Blending with Gasoline and Relationship with Engine Performances and Emissions. Ann. DAAAM Proc. 2018, 29. [Google Scholar] [CrossRef]
- Huynh, T.T.; Le, M.D.; Duong, D.N. Effects of butanol–gasoline blends on SI engine performance, fuel consumption, and emission characteristics at partial engine speeds. Int. J. Energy Environ. Eng. 2019, 10, 483–492. [Google Scholar] [CrossRef]
- Litt, J.S.; Chin, J.C.; Liu, Y. Simulating the Use of Alternative Fuels in a Turbofan Engine; National Aeronautics and Space Administration, Glenn Research Center: Cleveland, OH, USA, 2013. [Google Scholar]
- Gawron, B.; Białecki, T.; Dzięgielewski, W.; Kaźmierczak, U. Performance and emission characteristic of miniature turbojet engine FED Jet A-1/alcohol blend. J. KONES 2016, 23. [Google Scholar] [CrossRef]
- Mendez, C.J.; Parthasarathy, R.N.; Gollahalli, S.R. Performance and emission characteristics of butanol/Jet A blends in a gas turbine engine. Appl. Energy 2014, 118, 135–140. [Google Scholar] [CrossRef]
- Gawron, B.; Białecki, T. Impact of a Jet A-1/HEFA blend on the performance and emission characteristics of a miniature turbojet engine. Int. J. Environ. Sci. Technol. 2018, 15, 1501–1508. [Google Scholar] [CrossRef]
- Yang, J.; Xin, Z.; Corscadden, K.; Niu, H. An overview on performance characteristics of bio-jet fuels. Fuel 2018, 237, 916–936. [Google Scholar] [CrossRef]
- Vivian, J.; Seeber, A.; Dorrington, G.E. Tests of alternative fuel blends in micro-turbojet engines. In Asia-Pacific International Symposium on Aerospace Technology; Engineers Australia: Cairns, Australia, 2015. [Google Scholar]
- Gawron, B.; Białecki, T.; Król, A. The effect of adding 2-ethylhexanol to jet fuel on the performance and combustion characteristics of a miniature turbojet engine. J. Kones 2018, 25, 101–109. [Google Scholar] [CrossRef]
- Gires, E.; Abu Talib, A.R.; Ahmad, M.T.; Idris, A. Performance test of a small-scale turbojet engine running on a palm oil biodiesel-jet a blend. In Proceedings of the 28th International Congress of the Aeronautical Sciences, Brisbane, Australia, 23–28 September 2012. [Google Scholar] [CrossRef]
- Mendez, C.; Parthasarathy, R.; Gollahalli, S. Performance and emission characteristics of a small-scale gas turbine engine fueled with ethanol/Jet A blends. In Proceedings of the 50th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Nashville, Tennessee, 9–12 January 2012. [Google Scholar] [CrossRef]
- Yakovlieva, A.; Boichenko, S.; Lejda, K. Evaluation of jet engine operation parameters using conventional and alternative jet fuels. Int. J. Sustain. Aviat. 2019, 5, 230–248. [Google Scholar] [CrossRef]
- Főző, L.; Andoga, R.; Madarász, L. Mathematical model of a small Turbojet Engine MPM-20. In Studies in Computational Intelligence vol. 313: International Symposium of Hungarian Researchers on Computational Intelligence and Informatics; Springer: Heidelberg, Germany, 2010; pp. 313–322. ISBN 978-3-642-15220-7-. [Google Scholar] [CrossRef]
- Andoga, R.; Madarász, L.; Fozo, L.; Lazar, T.; Gašpar, V. Innovative approaches in modeling, control and diagnostics of small turbojet engines. Acta Polytech. Hung. 2013, 10, 81–99. [Google Scholar] [CrossRef]
- Minteer, S. (Ed.) Alcoholic Fuels; CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300: Boca Raton, FL, USA, 2006; p. 130. ISBN 0-8493-3944-8. [Google Scholar]
- Basile, A.; Iulianelli, A.; Dalena, F.; Veziroglu, T.N. Ethanol; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128114582. [Google Scholar]
- Ha, D.M.; Park, S.H.; Lee, S. The Measurement of Flash Point of Water-Methanol and Water-Ethanol Systems Using Seta Flash Closed Cup Tester. Fire Sci. Eng. 2015, 29, 39–43. [Google Scholar] [CrossRef][Green Version]
- Torres-Jimenez, E.; Svoljsăak-Jerman, M.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol− biodiesel blends for diesel engines. Energy Fuels 2010, 24. [Google Scholar] [CrossRef]
- Madarász, L.; Andoga, R.; Fozo, L. Intelligent Technologies in Modeling and Control of Turbojet Engines. In New Trends in Technologies: Control, Management, Computational Intelligence and Network Systems; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Standard: Crude Petroleum and Petroleum Products. Determination of Density. Oscillation U-Tube Method. Available online: https://www.mystandards.biz/standard/stneniso-12185-1.9.2001.html (accessed on 15 February 2021).
- Standard: Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. Available online: https://stn-online.sk/eshop/public/standard_detail.aspx?id=95790&p=true (accessed on 15 February 2021).
- Standard: Petroleum Products. Determination of Distillation Characteristics at Atmospheric Pressure. Available online: https://www.mystandards.biz/standard/stneniso-3405-1.8.2011.html (accessed on 15 February 2021).
- Jaffe, R.L.; Taylor, W. The Physics of Energy; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- BS EN ISO 1716:2018: Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). Standard: HongKong, China, 2018.
- Standard Specification for Aviation Turbine Fuels. Available online: https://www.astm.org/Standards/D1655.htm (accessed on 15 February 2021).
- Ertekin, A.; Sridhar, N. Performance of Elastomeric Materials in Gasoline-Ethanol Blends- a Review; National Association of Corrosion Engineers: Houston, TX, USA, 2009. [Google Scholar]
- Boichenko, S.; Lejda, K.; Yakovlieva, A.; Kuszewski, H.; Vovk, O. Experimental study on antiwear properties for blends of jet fuel with bio-components derived from rapeseed oil. Prace Inst. Lotnictwa 2016. [Google Scholar] [CrossRef]





| Systematic Name | Ethanol—Hydroxyethane |
|---|---|
| Rational name | Ethanol (99.9%) |
| CAS Registry No. | 64-17-5 |
| Summary formula | C2H6O |
| Molecular weight | 46.07 g/mol |
| Melting point | −114.4 °C |
| Freezing temperature | −130.5 °C |
| Boiling point | 78.3 °C (1013 hPa) |
| Density (at 20 °C) | 0.789 g/cm3 |
| Flash point | 12.7 °C (<0.1% of water) 13.57 °C (10% of water) 19.33 °C (50% water) |
| Burning temperature | 30 °C |
| Ignition temperature | 366 °C |
| Explosion limits Kinematic viscosity | 3.4–15% of volume 1.5 mm2/s (at 20 °C, 1 atm) |
| Dynamic viscosity | 1.184 mPa·s (at 20 °C, 1 atm) |
| Specific heat Higher heating value | 2 460 J/(kg·K) 29.7 MJ/kg |
| Parameter—Abbreviation | Sensor | Unit | Basic Calibrated Error |
|---|---|---|---|
| Fuel Flow—FF | Badger-meter MN-2 flow meter | (l.min−1) | ±1% |
| Rotational speed of the engine—n | Optical speed meter | (min−1) | ±0.2% |
| Total exhaust gas temperature—EGT | Calibrated K thermocouple | (°C) | ±0.75% |
| Total compressor pressure—p2 | QBE2002-P10 | (atm) | ±0.4% |
| Concentration | Units | E0 | E10 | E20 | E30 | E40 | |
|---|---|---|---|---|---|---|---|
| Test | |||||||
| Density (at 15 °C) | (kg/m3) | 814.1 | 810.6 | 808.2 | 805.8 | 803.6 | |
| Viscosity (at 20 °C) | (mm2/s) | 2.052 | 1.885 | 1.863 | 1.820 | 1.774 | |
| Flashpoint in a closed container | (°C) | 61 | <16 | <16 | <16 | <16 | |
| Water reaction | PR * | Degree | 1 | 1 | 1 | 1 | 1 |
| PL ** | 2 | 3 | 3 | 3 | 3 | ||
| Crystallisation | (°C) | −56.3 | <−105 | <−105 | <−105 | <−105 | |
| Acidity | (mg KOH/100 mL) | 0.17 | 0.15 | 0.14 | 0.12 | 0.08 | |
| Ethanol Percentage in JET A-1 (%) | HHV (MJ/dm3) | HHV (MJ/kg) |
|---|---|---|
| 0 | 37.61 | 46.2 |
| 10 | 36.26 | 44.55 |
| 20 | 34.92 | 42.9 |
| 30 | 33.58 | 41.25 |
| 35 | 32.9 | 40.42 |
| 40 | 32.23 | 39.6 |
| Test Day | Runs | Operational Time (s) | EGT max (°C) | nmax (min−1) | Tested Blend | Note |
|---|---|---|---|---|---|---|
| 1 | 3 | 30 | 812 | 45,500 | E0 | 3 consecutive runs with E0 |
| 1 | 1 | 30 | 690 | 45,000 | E10 | --- |
| 1 | 1 | 10 | 753 | 45,000 | E10 | Run for only 10 s—discarded |
| 1 | 1 | 30 | 735 | 45,000 | E10 | --- |
| Total | 6 | The end of the testing day | ||||
| 2 | 1 | 30 | 731 | 44,700 | E10 | |
| 2 | 1 | 30 | 602 | 21,000 | E10/E20 | Engine achieved only 20,000 (min−1)—discarded |
| 2 | 1 | 30 | 710 | 44,000 | E20 | --- |
| 2 | 1 | 30 | 690 | 44,000 | E20 | --- |
| Total | 10 | The end of the testing day | ||||
| 3 | 1 | 15 | 570 | 19,000 | E20 | Engine achieved only 20,000 (min−1)—discarded |
| 3 | 1 | 30 | 716 | 44,000 | E20 | --- |
| 3 | 1 | 30 | 703 | 45,000 | E0/E20 | Remnants of the fuel blend caused the speed to rise—discarded |
| Total | 13 | The end of the testing day | ||||
| 4 | 1 | 30 | 626 | 43,500 | E30 | Double spike in EGT, difficult startup—discarded |
| 4 | 1 | 30 | 687 | 43,000 | E30 | --- |
| 4 | 1 | 30 | 687 | 42,700 | E30 | --- |
| 4 | 1 | 30 | 687 | 43,000 | E30 | --- |
| 4 | 1 | 50 | 680 | 45,000 | E0/E30 | Remnants of the fuel blend caused the speed to rise from 43k to 45k—discarded |
| Total | 18 | The end of the testing day | ||||
| 5 | 1 | 20 | 610 | 17,000 | E40 | Stabilised only at 17,000 (min−1) |
| 5 | 1 | 20 | 617 | 22,000 | E40 | Stabilised only at 22,000 (min−1) |
| 5 | 1 | 30 | 617 | 22,000 | E40 | Stabilised only at 22,000 (min−1) |
| 5 | 1 | 60 | 680 | 45,200 | E0/E40 | Remnants of the fuel blend caused the speed to rise from 21k to 45k—discarded |
| 5 | 1 | 30 | 674 | 42,500 | E35 | --- |
| 5 | 1 | 30 | 660 | 42,500 | E35 | --- |
| 5 | 1 | 30 | 660 | 42,500 | E35 | --- |
| 5 | 1 | 60 | 655 | 45,200 | E0/E35 | Remnants of the fuel blend caused the speed to rise from 21k to 45k—discarded |
| Total | 26 | The end of the testing day | ||||
| Indicator | Maximum FF (L/min) | Mean(L/min) | Variance | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Fuel Blend | ||||||
| E0 | 1.166 | 1.130 | 0.0003278 | 0.0181 | 8.5337 × 10−4 | |
| E10 | 1.164 | 1.124 | 0.0002135 | 0.0146 | 6.8883 × 10−4 | |
| E20 | 1.145 | 1.116 | 0.0002354 | 0.0153 | 7.2324 × 10−4 | |
| E30 | 1.136 | 1.117 | 0.0000852 | 0.0092 | 4.3531 × 10−4 | |
| E35 | 1.145 | 1.126 | 0.0000477 | 0.0069 | 3.2588 × 10−4 | |
| E40 | 0.638 | 0.618 | 0.0001162 | 0.0107 | 6.2240 × 10−4 | |
| Indicator | Maximum Speed (min−1) | Mean (min−1) | Variance | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Fuel Blend | ||||||
| E0 | 45556.7 | 45280.9 | 38054.0 | 195.0 | 9.1 | |
| E10 | 44873.2 | 44684.6 | 5963.2 | 77.2 | 3.6 | |
| E20 | 43780.4 | 43514.1 | 22511.4 | 150.0 | 7.0 | |
| E30 | 43299.8 | 42944.4 | 36454.7 | 190.9 | 9.0 | |
| E35 | 43008.0 | 42480.9 | 25084.5 | 158.3 | 7.4 | |
| E40 | 22446.6 | 20251.0 | 3737437.9 | 1933.2 | 111.6 | |
| Indicator | Maximum EGT (°C) | Mean (°C) | Variance | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Fuel Blend | ||||||
| E0 | 506.7 | 501.4 | 9.4 | 3.0 | 0.17 | |
| E10 | 501.5 | 494.9 | 15.5 | 3.9 | 0.22 | |
| E20 | 489.6 | 487.8 | 1.3 | 1.1 | 0.06 | |
| E30 | 477.7 | 474.4 | 2.2 | 1.4 | 0.08 | |
| E35 | 478.8 | 473.4 | 14.0 | 3.7 | 0.21 | |
| E40 | 510.4 | 487.9 | 69.6 | 8.3 | 0.54 | |
| Indicator | Maximum Pressure (atm) | Mean (atm) | Variance | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|---|
| Fuel Blend | ||||||
| E0 | 3.434 | 3.366 | 0.001761 | 0.041 | 0.0019 | |
| E10 | 3.280 | 3.243 | 0.000198 | 0.014 | 0.0006 | |
| E20 | 3.118 | 3.071 | 0.000766 | 0.027 | 0.0013 | |
| E30 | 3.052 | 3.007 | 0.000618 | 0.024 | 0.0011 | |
| E35 | 3.033 | 2.947 | 0.000676 | 0.026 | 0.0012 | |
| E40 | 1.418 | 1.334 | 0.005004 | 0.070 | 0.0040 | |
| Parameter | No. of Measurement | Integral Value (°C.sec) | Peak EGT (°C) | Mean EGT (°C) | Peak Time (sec) | Settling Time (sec) | Overshoot Value (%) | |
|---|---|---|---|---|---|---|---|---|
| Fuel Blend | ||||||||
| E0 | 1. 2. 3. | 7074.2 7455.0 6133.6 | 790.4 813.0 769.8 | 504.2 500.9 498.3 | 2.70 2.90 3 | 10.09 10.26 9.07 | 56.7 62.3 54.4 | |
| Mean | 6887.6 | 791.1 | 501.1 | 2.86 | 9.81 | 57.8 | ||
| E10 | 1. 2. 3. | 7198.5 7529.3 7159.0 | 677.7 741.5 731.5 | 492.6 490.7 499.7 | 2.30 2.70 2.40 | 10.69 10.87 10.06 | 37.5 51.1 46.3 | |
| Mean | 7295.6 | 716.9 | 494.3 | 2.46 | 10.54 | 45.0 | ||
| E20 | 1. 2. 3. | 7662.4 7559.2 8145.7 | 710.9 691.8 718.0 | 487.9 486.0 488.0 | 2.50 2.50 2.80 | 11.16 11.72 11.71 | 45.6 42.3 47.1 | |
| Mean | 7789.1 | 706.9 | 487.3 | 2.6 | 11.53 | 45.0 | ||
| E30 | 1. 2. 3. | 7900.9 8163.8 7964.6 | 688.0 687.6 681.8 | 477.1 473.8 473.7 | 2.50 2.50 2.60 | 11.91 12.67 12.72 | 44.2 45.1 43.9 | |
| Mean | 8009.7 | 685.8 | 474.8 | 2.53 | 12.43 | 44.4 | ||
| E35 | 1. 2. 3. | 7905.6 9503.8 9974.9 | 674.7 660.9 661.2 | 478.3 472.7 469.3 | 2.60 2.40 2.40 | 12.66 15.91 16.53 | 41.0 39.8 40.8 | |
| Mean | 9128.1 | 665.6 | 473.4 | 2.46 | 15.03 | 40.5 | ||
| E40 | 1. 2. 3. | 3753.5 5306.4 4645.2 | 610.6 614.1 616.8 | 496.9 480.3 486.5 | 2.20 2.80 2.50 | 5.79 8.52 7.52 | 22.8 27.8 26.7 | |
| Mean | 4568.3 | 613.8 | 487.9 | 2.5 | 7.28 | 25.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andoga, R.; Főző, L.; Schrötter, M.; Szabo, S. The Use of Ethanol as an Alternative Fuel for Small Turbojet Engines. Sustainability 2021, 13, 2541. https://doi.org/10.3390/su13052541
Andoga R, Főző L, Schrötter M, Szabo S. The Use of Ethanol as an Alternative Fuel for Small Turbojet Engines. Sustainability. 2021; 13(5):2541. https://doi.org/10.3390/su13052541
Chicago/Turabian StyleAndoga, Rudolf, Ladislav Főző, Martin Schrötter, and Stanislav Szabo. 2021. "The Use of Ethanol as an Alternative Fuel for Small Turbojet Engines" Sustainability 13, no. 5: 2541. https://doi.org/10.3390/su13052541
APA StyleAndoga, R., Főző, L., Schrötter, M., & Szabo, S. (2021). The Use of Ethanol as an Alternative Fuel for Small Turbojet Engines. Sustainability, 13(5), 2541. https://doi.org/10.3390/su13052541









