The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination
Abstract
1. Introduction
- an increase in the solubility and bioavailability of metal ions in the soil substrate;
- an increase in the potential of plants to accumulate pollutants in terrestrial organs and to develop large biomass;
- the physiological tolerance of plants to stress, caused by high concentrations of pollutants.
2. Problem Formulation
- Testing a new potential chemical inducer of a compound from the class of bisphosphonates—synthetic phosphorus-containing complexones. Organophosphorus compounds, such as hydroxyethylidene diphosphonic acid (HEDP), are capable of forming stable water-soluble complex compounds with many heavy metals in pHs of >9. As an analogy of natural pyrophosphates, HEDP is involved in more than 60 biochemical cellular reactions by regulating ionic calcium and phosphorus exchange. Additionally, organophosphorus is considerably less toxic to living systems and organisms than carboxyl-containing complexes. There is a known study on the use of HEDP for the phytoextraction of Cd, where HEDP has shown greater efficiency [40]. However, there are no studies on the effectiveness of using HEDP for polymetallic contaminants.
- The application of combined treatment with various functional corrections that allows simultaneous stimulation of the absorption of heavy metal ions, photosynthesis, and biomass growth. The complex scheme should be based on a combination of treatments with a chelating agent, an iron complexonate, and hormonal supplements. Taking into account the pronounced manifestation of the antagonism of metal ions during the phytoextraction of multicontaminated soils, the authors of this work put forward the hypothesis that the correction of iron deficiency can have a positive effect on (the overall efficiency of the process) stimulation of photosynthesis and can lead to an improvement of the general physiological state of the latter. Furthermore, the additional use of PGRs can also reduce the stress caused by high concentrations of pollutants and can compensate for the negative effect of the latter on biomass growth.
3. Materials and Methods
3.1. Materials
3.1.1. Research Objects
3.1.2. Reactive and Preparators
- Universal soil “SELIGER-AGRO EXO” (of the company “Seliger Agro”, Tver, Russia). The main characteristics of the soil are shown in Table 1.
- Nitrogen-phosphorus-potassium fertilizer brand “Antey” “Earth Force” according to RF Specification TU # 2186-002-38522882-2016. Fertilizer contains 21% nitrogen, 11% phosphorus (P2O5), 11% potassium.
- Universal preparation “Zavyaz” (“Orton” LLC, Pushkino, Russia), containing 5% mass sodium salts of (GA).
- Preparation “Kornevin” (“SELHOZEKOSERVICE” LLC, Moscow, Russia), containing IAA (in form 4 (indole-3yl) butyric acid) at a concentration of 5 g/kg.
- Pure Ni(NO3)2 ⋅ 6H2O (nickel nitrate 6-aqueous) for analysis.
- Pure CuSO4 ⋅ 5H2O (copper (II) sulfate 5-water).
- Pure Cd(NO3)2 ⋅ 4H2O (cadmium nitrate 4-aqueous).
- Na (FeEDDHA) in form of 0.1% solution prepared at the Laboratory of Institute for Chemical Reagents and High Purity Chemical Substances of National Research Center “Kurchatov Institute”—IREA.
- Na2EDTA prepared at the Laboratory of Institute for Chemical Reagents and High Purity Chemical Substances of the National Research Center “Kurchatov Institute”—IREA.
- K2HEDP—an aqueous solution with a mass content of the target component of 28.3% was provided by the Laboratory of Institute for Chemical Reagents and High Purity Chemical Substances of National Research Center “Kurchatov Institute”—IREA.
3.2. Methods
- Ni(NO3)2 ⋅ 6H2O—38.2 mg per 1 vegetation pot;
- CuSO4 ⋅ 5H2O—23.18 mg per 1 vegetation pot;
- Cd(NO3)2 ⋅ 4H2O—15.65 mg per 1 vegetation pot.
Method for Determination of Metals in Plants
3.3. Statistical Analysis
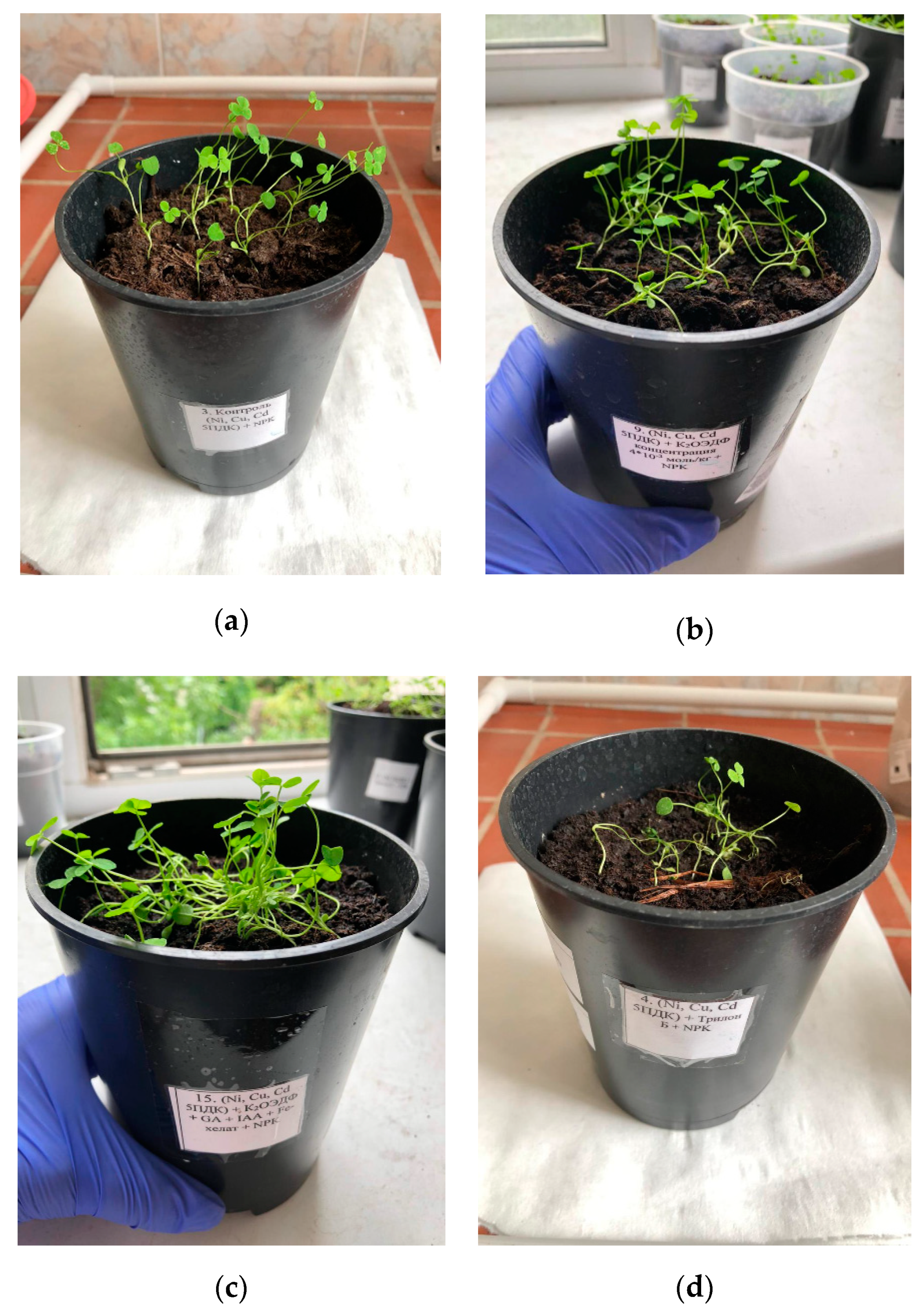
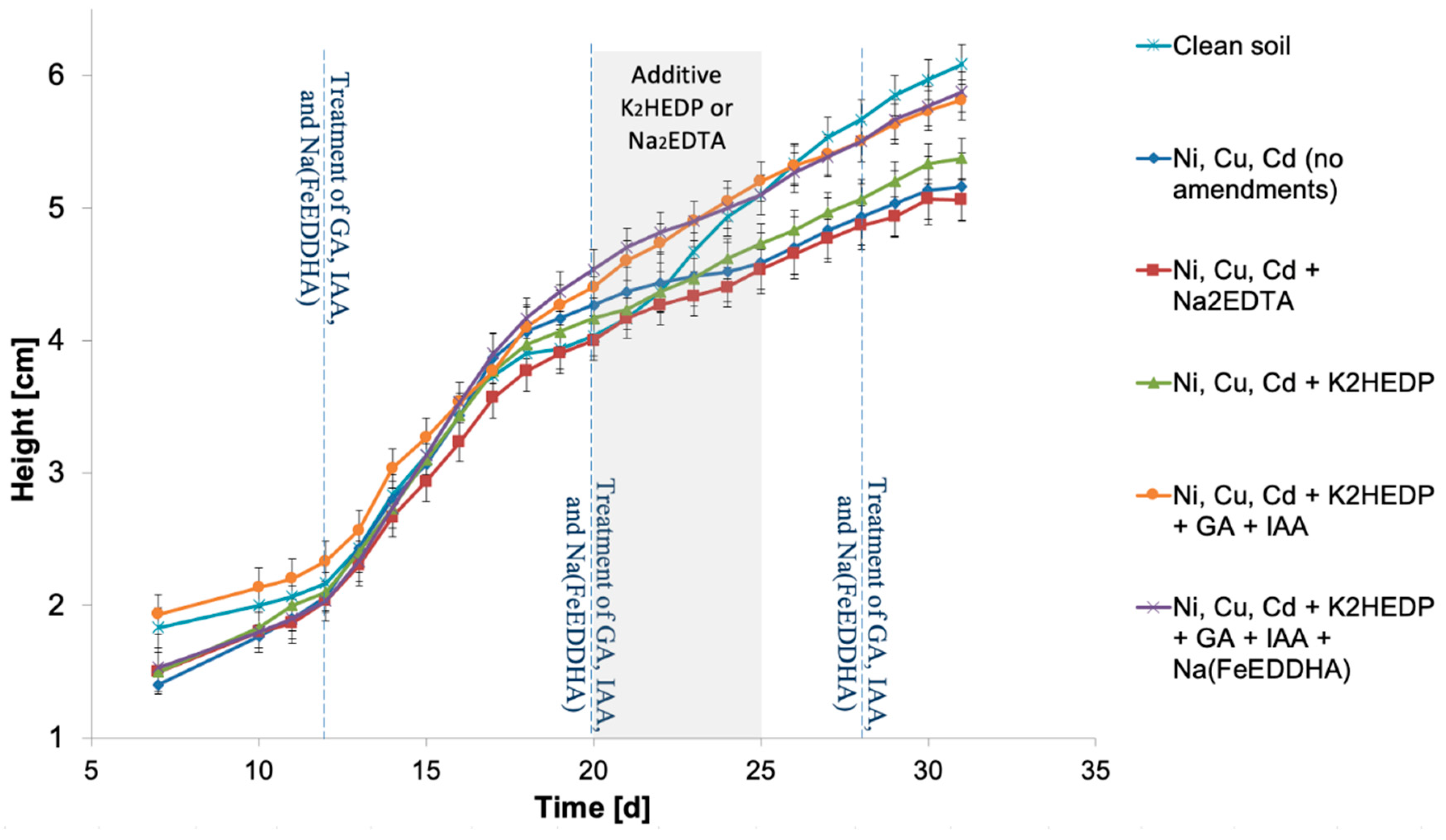
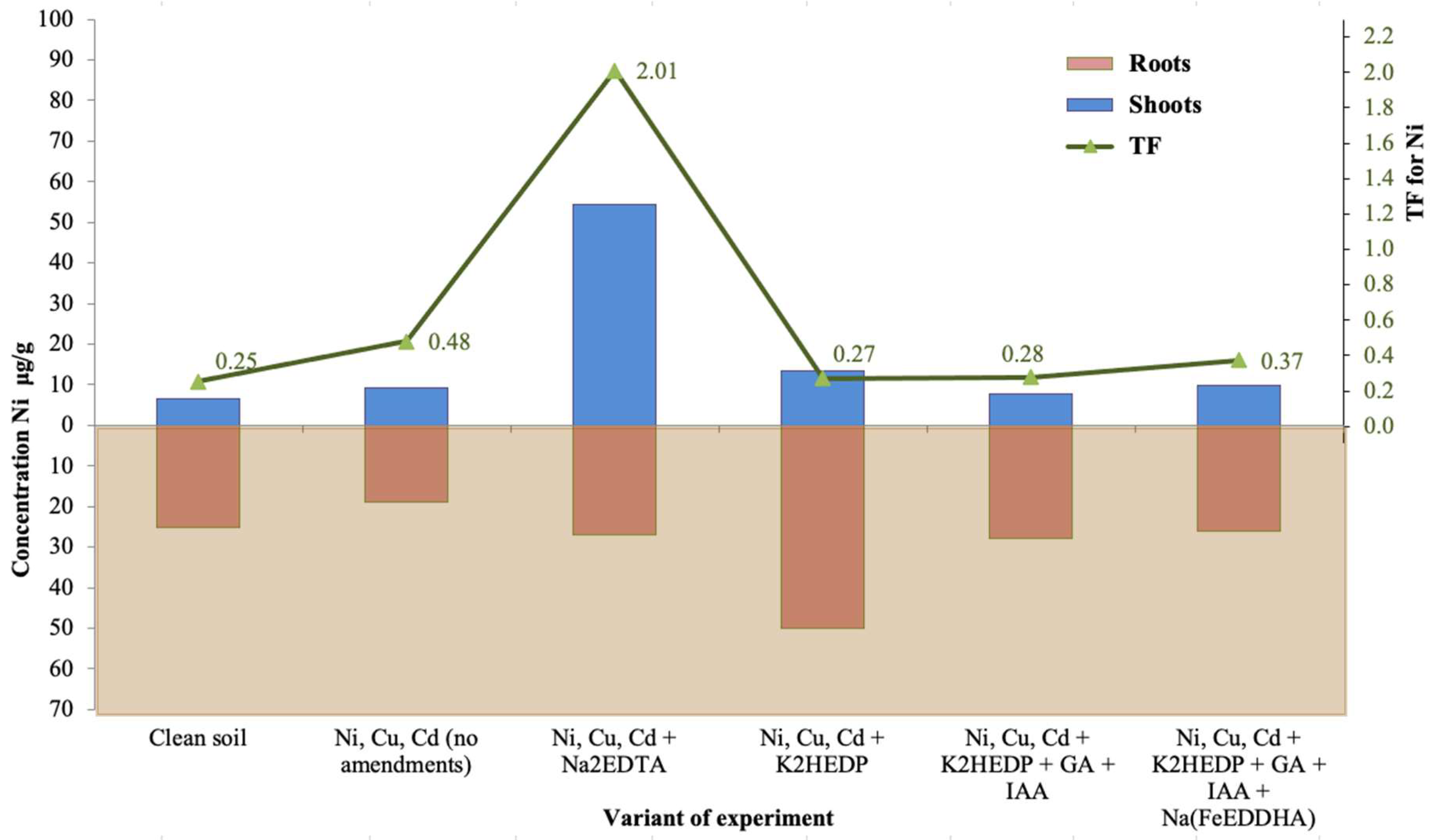
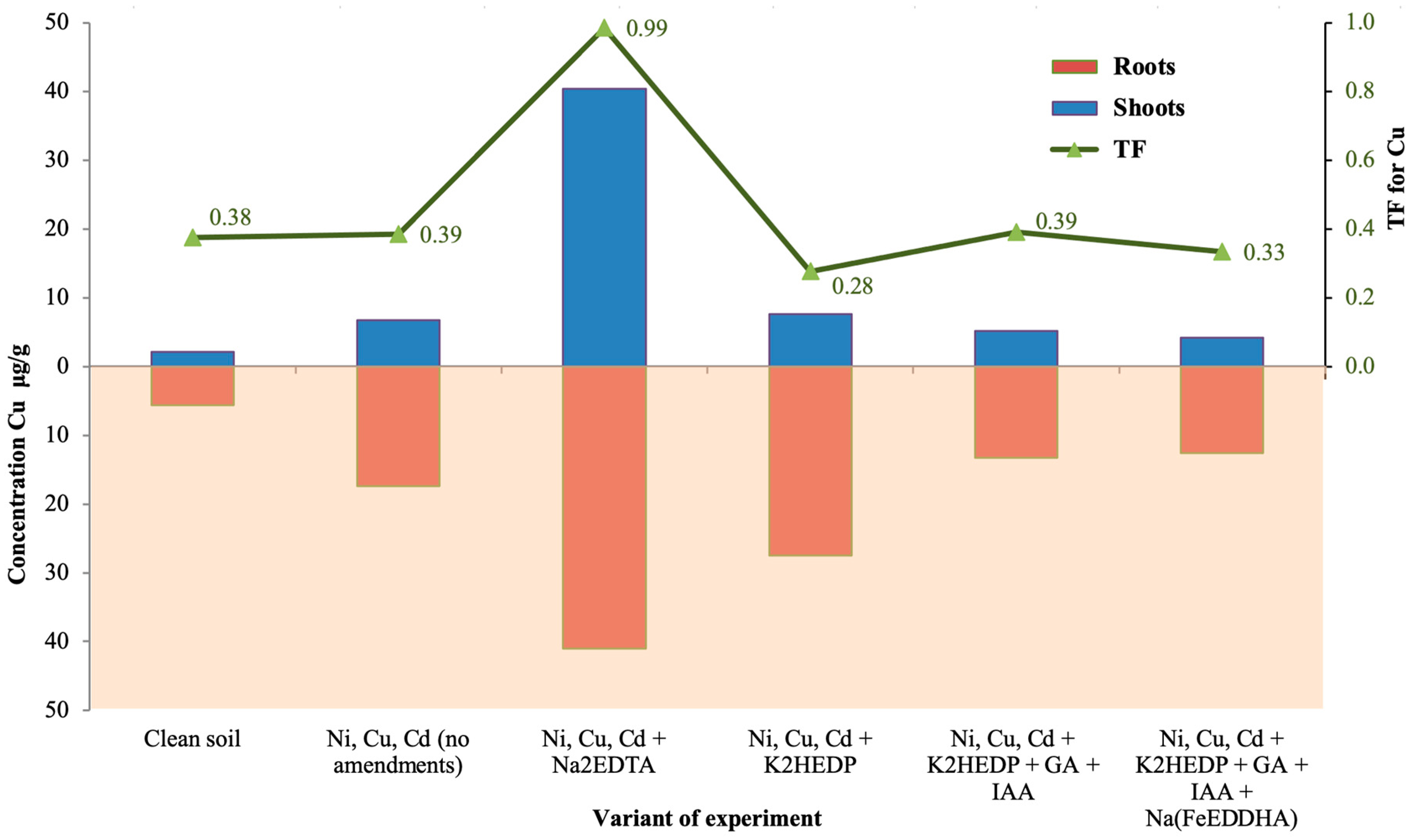
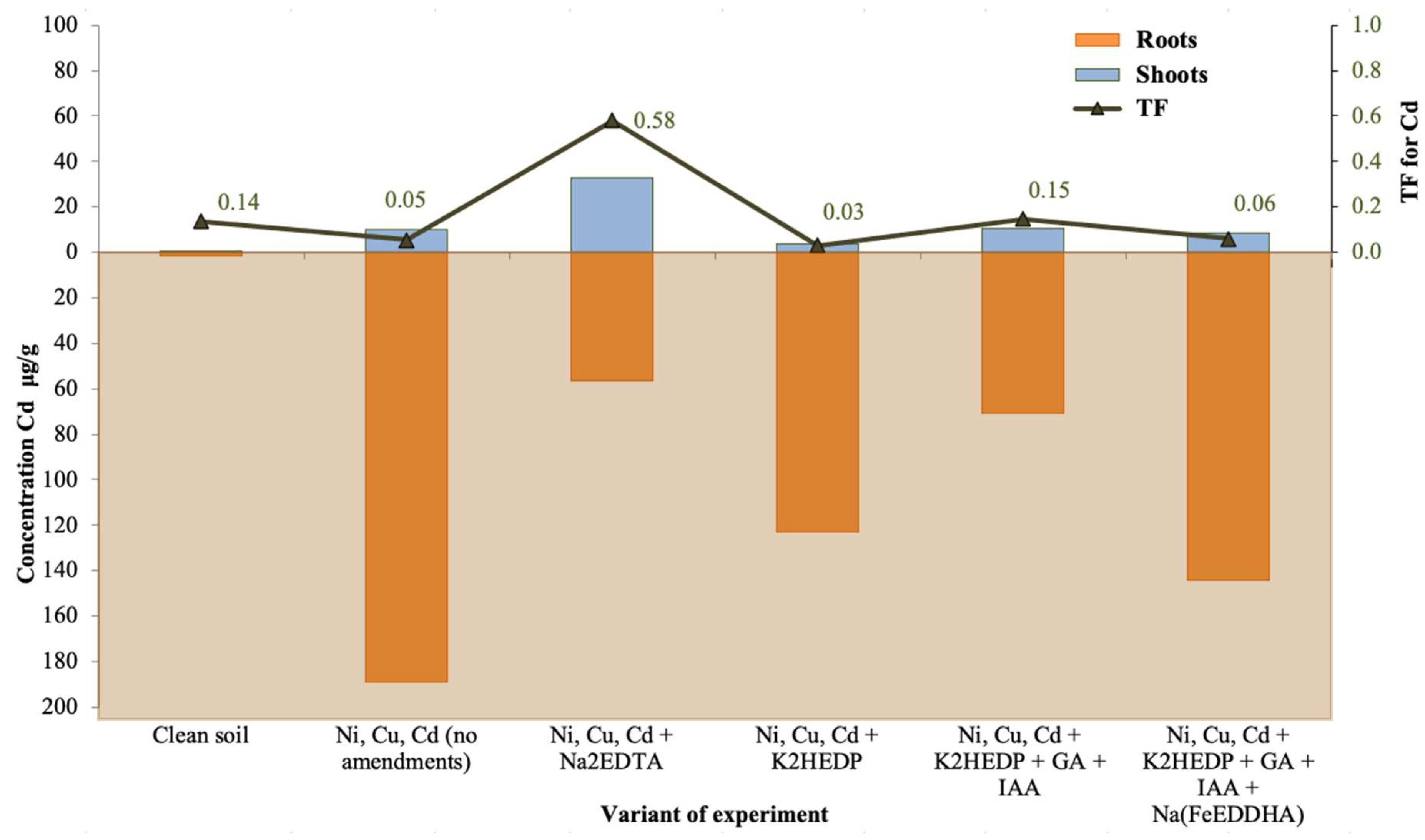
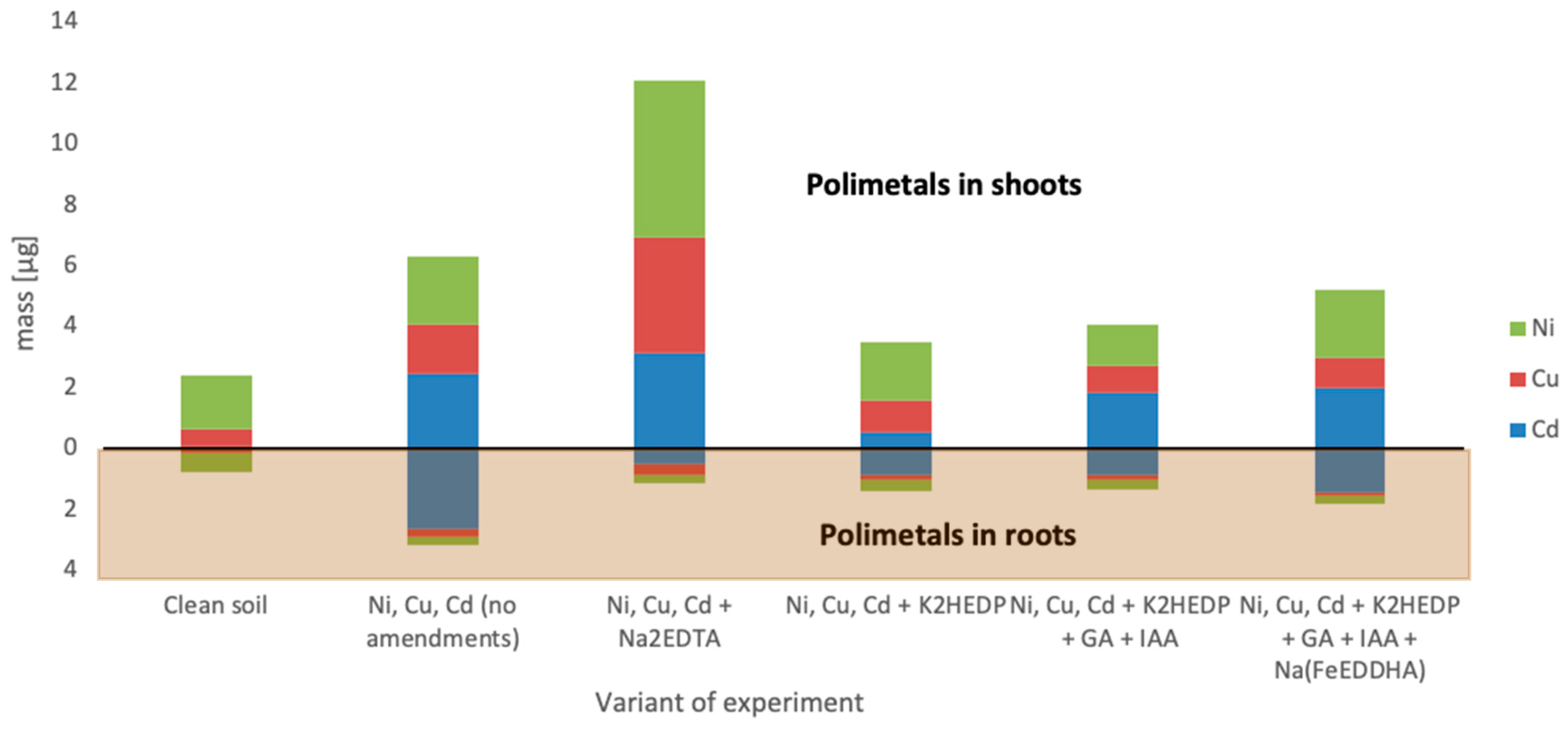
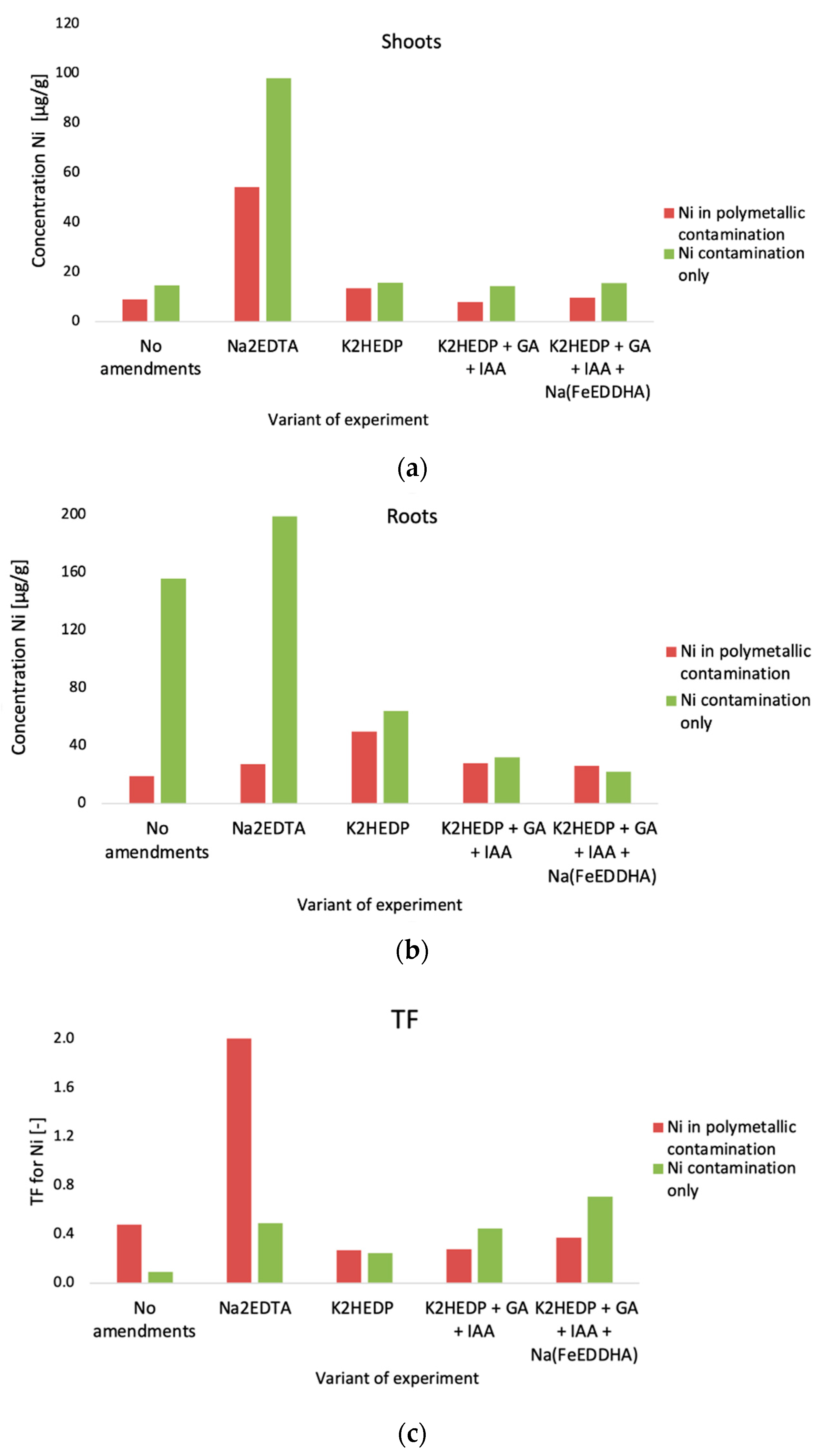
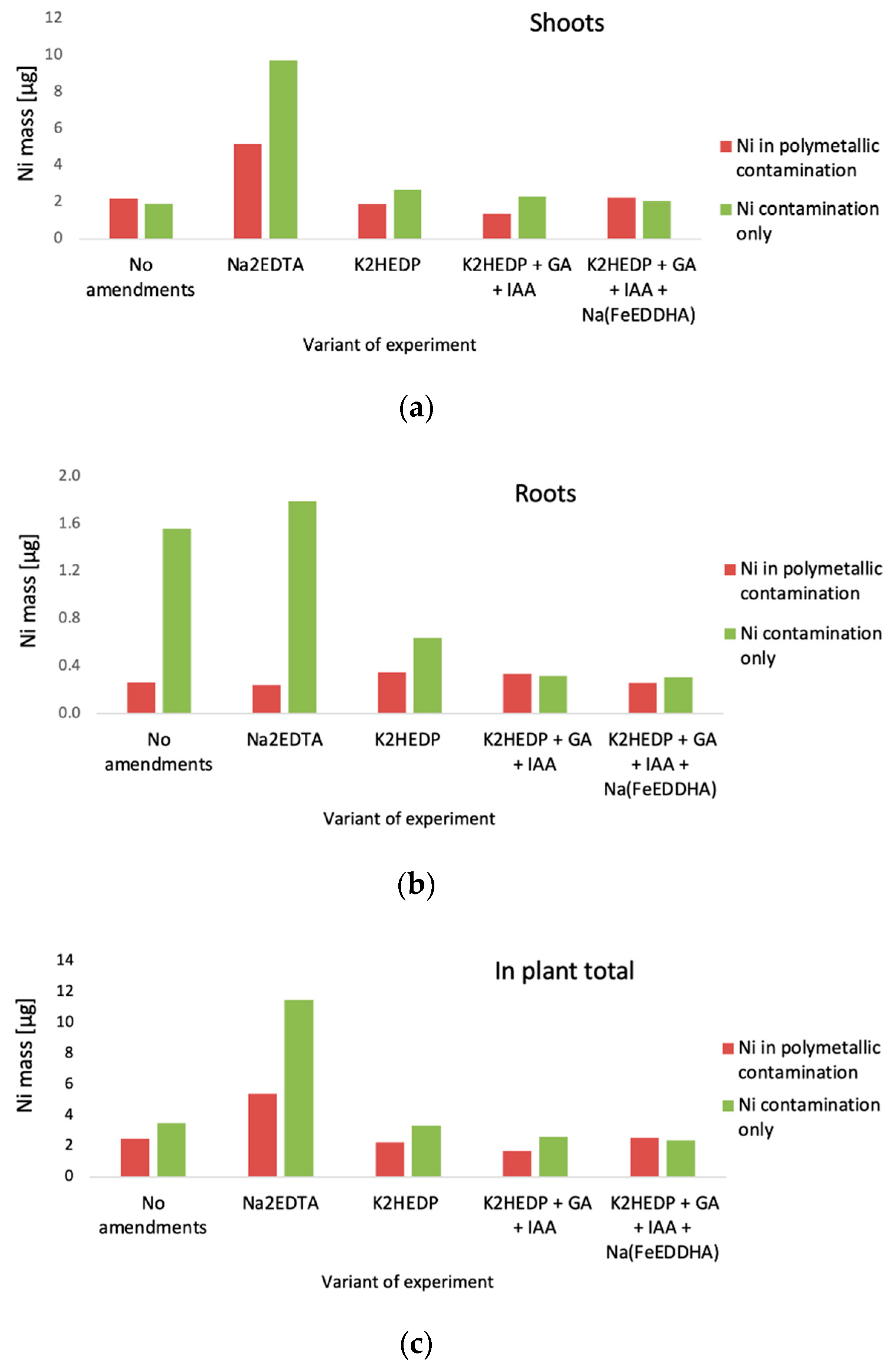
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Makarova, A.; Tarasova, N.; Meshalkin, V.; Kukushkin, I.; Kudryavtseva, E.; Kantyukov, R.; Reshetova, E. Analysis of the management system in the field of environmental protection of Russian chemical companies. Int. J. Qual. Res. 2018, 12, 43–62. [Google Scholar] [CrossRef]
- Nandillon, R.; Lahwegue, O.; Miard, F.; Lebrun, M.; Gaillard, M.; Sabatier, S.; Battaglia-Brunet, F.; Morabito, D.; Bourgerie, S. Potential use of biochar, compost and iron grit associated with Trifolium repens to stabilize Pb and as on a multi-contaminated technosol. Ecotoxicol. Environ. Saf. 2019, 182, 109432. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Hu, Z.; Huang, L.; Tang, S.; Holm, P.E. Influence of alkaline silicon-based amendment and incorporated with biochar on the growth and heavy metal translocation and accumulation of vetiver grass (Vetiveria zizanioides) grown in multi-metal-contaminated soils. J. Soils Sediments 2019, 19, 2277–2289. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Ha, N.T.; Nga, T.T.H.; Minh, N.N.; Anh, B.T.K.; Hang, N.T.A.; Duc, N.A.; Nhuan, M.T.; Kim, K.-W. Uptake of arsenic and heavy metals by native plants growing near Nui Phao multi-metal mine, northern Vietnam. Appl. Geochem. 2019, 108, 104368. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Zhang, Y.; Wang, J.; Ullah, H.; Zhou, M.; Peng, L.; He, A.; Zhang, X.; Yan, X.; et al. Spatial Distributions, Sources, Potential Risks of Multi-Trace Metal/Metalloids in Street Dusts from Barbican Downtown Embracing by Xi’an Ancient City Wall (NW, China). Int. J. Environ. Res. Public Health 2019, 16, 2992. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X.; Chen, C.; Bate, B. Emerging sustainable technologies for remediation of soils and groundwater in a municipal solid waste landfill site—A review. Chemosphere 2019, 227, 681–702. [Google Scholar] [CrossRef]
- Fedotov, P.S. Estimating the bioavailability of trace metals/metalloids and persistent organic substances in terrestrial environments: Challenges and need for multidisciplinary approaches. Pure Appl. Chem. 2014, 86, 1085–1095. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Lemmel, F.; Maunoury-Danger, F.; Fanesi, A.; Leyval, C.; Cébron, A. Soil Properties and Multi-Pollution Affect Taxonomic and Functional Bacterial Diversity in a Range of French Soils Displaying an Anthropisation Gradient. Microb. Ecol. 2019, 77, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release Processes in Soil—The Basis of Phytoremediation Efficiency. In Phytoremediation; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Gemmany, 2018; pp. 91–112. [Google Scholar] [CrossRef]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted phytoremediation of a multi-contaminated soil: Investigation on arsenic and lead combined mobilization and removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef]
- Pandey, J.; Pandey, U. Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environ. Monit. Assess. 2009, 148, 61–74. [Google Scholar] [CrossRef]
- Guarino, C.; Sciarrillo, R. The effectiveness and efficiency of phytoremediation of a multicontaminated industrial site: Porto Marghera (Venice Lagoon, Italy). Chemosphere 2017, 183, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Z.; Wang, D.-Q.; Zhang, X.-H. Chelator-induced phytoextraction of zinc and copper by rice seedlings. Ecotoxicology 2014, 23, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Li, F.; Yang, F.; Chen, Y.; Jin, H.; Leng, Y.; Wang, J. Chemical reagent-assisted phytoextraction of heavy metals by Bryophyllum laetivirens from garden soil made of sludge. Chemosphere 2020, 253, 126574. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ji, X.; Luo, X. Phytoremediation of Heavy Metal Pollution: A Bibliometric and Scientometric Analysis from 1989 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 4755. [Google Scholar] [CrossRef]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melián, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury- and chromium-contaminated soil: A review. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. The Influence of Different Growth Stages and Dosage of EDTA on Cd Uptake and Accumulation in Cd-Hyperaccumulator (Solanum Nigrum L.). Bull. Environ. Contam. Toxicol. 2009, 82, 348. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Zhang, X.; Chen, J.; Huo, Z.; Mao, Y. Influence of alkyl polyglucoside, citric acid, and nitrilotriacetic acid on phytoremediation in pyrene-Pb co-contaminated soils. Int. J. Phytoremediation 2018, 20, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Schulin, R.; Nowack, B. Uptake of Metals during Chelant-Assisted Phytoextraction with EDDS Related to the Solubilized Metal Concentration. Environ. Sci. Technol. 2006, 40, 2753–2758. [Google Scholar] [CrossRef]
- Hasegawa, H.; Rahman, I.M.M.; Nakano, M.; Begum, Z.A.; Egawa, Y.; Maki, T.; Furusho, Y.; Mizutani, S. Recovery of toxic metal ions from washing effluent containing excess aminopolycarboxylate chelant in solution. Water Res. 2011, 45, 4844–4854. [Google Scholar] [CrossRef] [PubMed]
- Asemave, K. Greener Chelators for Recovery of Metals and Other Applications. Org. Med. Chem. Int. J. 2018, 6, 555694. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, C.; Guo, Z.; Huang, Z.; Peng, C.; Zeng, P.; Xiao, X.; Xian, Z. Removal of cadmium, lead, and zinc from multi-metal–contaminated soil using chelate-assisted Sedum alfredii Hance. Environ. Sci. Pollut. Res. 2019, 26, 28319–28327. [Google Scholar] [CrossRef]
- Pinto, I.S.S.; Neto, I.F.F.; Soares, H.M.V.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Bano, A.; Fuller, M.P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 2010, 80, 457–462. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Benitez, T.; Gardea-Torresdey, J.L. Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 2005, 61, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Wang, W. Factors affecting metal toxicity to (and accumulation by) aquatic organisms—Overview. Environ. Int. 1987, 13, 437–457. [Google Scholar] [CrossRef]
- Lanaras, T.; Moustakas, M.; Symeonidis, L.; Diamantoglou, S.; Karataglis, S. Plant metal content, growth responses and some photosynthetic measurements on field-cultivated wheat growing on ore bodies enriched in Cu. Physiol. Plant 1993, 88, 307–314. [Google Scholar] [CrossRef]
- Walker, C.D.; Webb, J. Copper in plant: Forms and behavior. In Copper in Soils and Plants; Loneragan, J.F., Robson, A.D., Graham, R.D., Eds.; Academic Press: Sydney, Australia, 1981; pp. 189–212. [Google Scholar]
- Schickler, H.; Caspi, H. Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol. Plant 1999, 105, 39–44. [Google Scholar] [CrossRef]
- Ait Ali, N.; Bernal, M.P.; Ater, M. Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant. Soil 2002, 239, 103–111. [Google Scholar] [CrossRef]
- Liu, J.; Reid, R.J.; Smith, F.A. The mechanism of cobalt toxicity in mung beans. Physiol. Plant 2000, 110, 104–110. [Google Scholar] [CrossRef]
- Doncheva, S.; Stoyanova, Z.; Georgieva, K.; Nedeva, D.; Dikova, R.; Zehirov, G.; Nikolova, A. Exogenous succinate increases resistance of maize plants to copper stress. J. Plant Nutr. Soil Sci. 2006, 169, 247–254. [Google Scholar] [CrossRef]
- Dan, T.V.; KrishnaRaj, S.; Saxena, P.K. Cadmium and Nickel Uptake and Accumulation in Scented Geranium (Pelargonium sp. ‘Frensham’). Water Air Soil Pollut. 2002, 137, 355–364. [Google Scholar] [CrossRef]
- Toxicity of Cadmium in Plants and Strategies to Reduce its Effects. Case Study: The Tomato. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362019000300010&lng=en&tlng=en (accessed on 29 January 2021).
- Samadder, S.R.; Prabhakar, R.; Khan, D.; Kishan, D.; Chauhan, M.S. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Sci. Total Environ. 2017, 580, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Y.; Hao, S.; Xu, W.; Shen, K.; Long, Z. Leaching Behaviour and Enhanced Phytoextraction of Additives for Cadmium-Contaminated Soil by Pennisetum sp. Bull. Environ. Contam. Toxicol. 2020, 104, 658–667. [Google Scholar] [CrossRef]
- Przydatek, G.; Kanownik, W. Impact of small municipal solid waste landfill on groundwater quality. Environ. Monit. Assess. 2019, 191, 169. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Kudryashova, V.I. Accumulation of Heavy Metals by Wild Plants. Ph.D. Thesis, N. P. Ogarev Mordovia State University, Saransk, Russia, 27 November 2003. [Google Scholar]
- Tribis, L.I. Phytoextraction of Nickel and Copper and Respirometric Indicators of the State of Microbial Communities in Technogenic Soils and Soils Contaminated with Heavy Metals. Ph.D. Thesis, Russian State Agrarian University—Moscow Timiryazev Agricultural Academy, Moscow, Russia, 21 March 2016. [Google Scholar]
- Seliger Agro. Available online: http://www.seliger-agro.ru/exo.html (accessed on 13 January 2021).
| # | Characteristic Name | Characteristic Value |
|---|---|---|
| 1 | Packaging volume | 60 L |
| 2 | Origin of soil | Natural high-moor peat, neutralized with lime, with the addition of complex mineral fertilizer |
| 3 | The type of plants for which this type of soil is applicable | Ornamental, deciduous, herbaceous |
| 4 | Mass fraction of water | Up to 70% |
| 5 | Acidity (pH KCl) | 5–6 |
| 6 | Nitrogen content (NH4 + NO3) | 100–180 mg/L |
| 7 | Phosphorus content (P2O5) | 135–255 mg/L |
| 8 | Potassium content (K2O) | 115–215 mg/L |
| 9 | Packaging weight | 18 kg |
| Variant of Experiment | Experiment Description | Amendments | Application Time | Application Method |
|---|---|---|---|---|
| Clean soil | The experiment uses only universal soil and fertilizer | No amendments | - | - |
| Ni, Cu, Cd/Ni (no amendments) | The experiment uses only universal soil contaminated with heavy metals and fertilizer | No amendments | - | - |
| Ni, Cu, Cd/Ni + Na2EDTA | Na2EDTA is added to heavy metal contaminated universal soil with fertilizer | Na2EDTA (12.06 g of the reactive was diluted in 600 mL of distilled water) | From 20 to 25 days after planting the seeds | The solution was added in an amount of 20 mL by watering into each vegetation pot |
| Ni, Cu, Cd/Ni + K2HEDP | K2HEDP is added to heavy metal contaminated universal soil with fertilizer | K2HEDP (2 mL of 28.3% solution was diluted in 1 L of distilled water) | From 20 to 25 days after planting the seeds | The solution was added in an amount of 10–20 mL by watering into each vegetation pot |
| Ni, Cu, Cd/Ni + K2HEDP + GA + IAA | K2HEDP is added to heavy metal contaminated universal soil with fertilizer with exogenous treatments with PGRs (GA and IAA) | K2HEDP (2 mL of 28.3% solution was diluted in 1 L of distilled water) | From 20 to 25 days after planting the seeds | The solution was added in an amount of 10–20 mL by watering into each vegetation pot |
| GA (0.2 g of universal preparation “Zavyaz” was diluted in 1 litre of distilled water) | 12, 20, and 28 days after planting the seeds | The solution was sprayed until the ground was moistened | ||
| IAA (0.7 g preparation “Kornevin” was diluted in 1 L of distilled water) | 12, 20, and 28 days after planting the seeds | The solution was added in an amount of 10 mL by watering into each vegetation pot | ||
| Ni, Cu, Cd/Ni + K2HEDP + GA + IAA + Na(FeEDDHA) | K2HEDP is added to heavy metal contaminated universal soil with fertilizer with exogenous treatments with PGRs (GA and IAA) and Na (FeEDDHA) | K2HEDP (2 mL of 28.3% solution was diluted in 1 L of distilled water) | From 20 to 25 days after planting the seeds | The solution was added in an amount of 10–20 mL by watering into each vegetation pot |
| GA (0.2 g of universal preparation “Zavyaz” was diluted in 1 L of distilled water) | 12, 20, and 28 days after planting the seeds | The solution was sprayed until the ground was moistened | ||
| IAA (0.7 g preparation “Kornevin” was diluted in 1 L of distilled water) | 12, 20, and 28 days after planting the seeds | The solution was added in an amount of 10 mL by watering into each vegetation pot | ||
| Na (FeEDDHA) solution of concentration 0.001% | 12, 20 and 28 days after planting the seeds | The solution was sprayed until the ground was moistened |
| Samples | Shoot Turgor | Shoot Growth | Signs of Chlorosis | Sheet Plates |
|---|---|---|---|---|
| Clean soil | High | Natural | No | Green |
| Ni, Cu, Cd (no amendments) | High | Minor oppression | No | Green |
| Ni, Cu, Cd + Na2EDTA | Low | Significant oppression | Yes | Pale green |
| Ni, Cu, Cd + K2HEDP | High | Significant oppression | No | Green |
| Ni, Cu, Cd + K2HEDP + GA + IAA | High | Average oppression | No | Green |
| Ni, Cu, Cd + K2HEDP + GA + IAA + Na(FeEDDHA) | High | Minor oppression | No | Green |
| Variant of Experiment | Shoots | Roots | ||||||
|---|---|---|---|---|---|---|---|---|
| Mass 1 [g] | Concentration 2 [μg/g] | Mass [g] | Concentration [μg/g] | |||||
| Ni | Cu | Cd | Ni | Cu | Cd | |||
| Clean soil | 0.278 | 6.4 | 2.1 | 0.19 | 0.024 | 25.3 | 5.6 | 1.4 |
| Ni, Cu, Cd (no amendments) | 0.24 | 9.1 | 6.7 | 10.1 | 0.014 | 19 | 17.4 | 189 |
| Ni, Cu, Cd + Na2EDTA | 0.10 | 54.3 | 40.4 | 32.9 | 0.009 | 27 | 41 | 56.7 |
| Ni, Cu, Cd + K2HEDP | 0.14 | 13.5 | 7.6 | 3.7 | 0.007 | 49.9 | 27.5 | 123 |
| Ni, Cu, Cd + K2HEDP + GA + IAA | 0.18 | 7.8 | 5.2 | 10.4 | 0.012 | 28 | 13.3 | 70.9 |
| Ni, Cu, Cd + K2HEDP + GA + IAA + Na(FeEDDHA) | 0.23 | 9.7 | 4.2 | 8.5 | 0.010 | 26 | 12.6 | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, A.; Nikulina, E.; Avdeenkova, T.; Pishaeva, K. The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination. Sustainability 2021, 13, 2432. https://doi.org/10.3390/su13052432
Makarova A, Nikulina E, Avdeenkova T, Pishaeva K. The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination. Sustainability. 2021; 13(5):2432. https://doi.org/10.3390/su13052432
Chicago/Turabian StyleMakarova, Anna, Elena Nikulina, Tatiana Avdeenkova, and Ksenia Pishaeva. 2021. "The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination" Sustainability 13, no. 5: 2432. https://doi.org/10.3390/su13052432
APA StyleMakarova, A., Nikulina, E., Avdeenkova, T., & Pishaeva, K. (2021). The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination. Sustainability, 13(5), 2432. https://doi.org/10.3390/su13052432






